Abstract
The aims of this paper were to compare the predictive validity of three pressure ulcer (PU) risk scales—the Norton scale, the Braden scale, and the Waterlow scale—and to choose the most appropriate calculator for predicting PU risk in surgical wards of India. This is an observational prospective cohort study in a tertiary educational hospital in New Delhi among 100 surgical ward patients from April to July 2011. The main outcomes measured included sensitivity, specificity, positive predictive value (PVP) and negative predictive value (PVN), and the area under the curve of the receiver operating characteristic (ROC) curve of the three PU risk assessment scales. Based on the cutoff points found most appropriate in this study, the sensitivity, specificity, PVP, and PVN were as follows: the Norton scale (cutoff, 16) had the values of 95.6, 93.5, 44.8, and 98.6, respectively; the Braden scale (cutoff, 17) had values of 100, 89.6, 42.5, and 100, respectively; and the Waterlow scale (cutoff, 11) had 91.3, 84.4, 38.8, and 97, respectively. According to the ROC curve, the Norton scale is the most appropriate tool. Factors such as physical condition, activity, mobility, body mass index (BMI), nutrition, friction, and shear are extremely significant in determining risk of PU development (p < 0.0001). The Norton scale is most effective in predicting PU risk in Indian surgical wards. BMI, mobility, activity, nutrition, friction, and shear are the most significant factors in Indian surgical ward settings with necessity for future comparison with established scales.
Keywords: Pressure ulcer, Norton scale, Braden scale, Waterlow scale, Predictor of pressure ulcer
Introduction
The incidence of pressure ulcers (PUs) is much higher (12–66 %) for surgical patients (6–12 %) as compared to long-term care facilities (2.2–23.9 %) or acute care settings (0.4–38 %) [1, 2]. Pressure ulcers have been known to cause serious functional limitations, emotional burden [3], considerable social and mental impacts, and a significant reduction in quality of life. Pressure ulcers cause pain, discomfort, and distress that are not always recognized or adequately treated [4]. They lead to complications such as osteomyelitis and sepsis, with the mortality rate of sepsis approaching 50 % [5–7].
Studies show that treatment of PUs is about 2.5 times costlier than prevention [8]. The cost of treatment for one PU in the acute care settings has been reported to range from US $2,000 to US $30,000 [9], with an average daily inpatient cost to the facility of US $80.42 [10]. It is estimated that around 95 % of PUs may be prevented [11], with the primary method of prevention widely believed to be good nursing care [5, 12].
Therefore, accurate identification of at-risk patients by a reliable and valid PU risk calculator is the first step to PU prevention. Of the 40 PU risk assessment scales (PURASs) available [13–15], the Braden scale [6], the Norton scale [16], and the Waterlow scale [17], having undergone validation in most parts of the world, are the most commonly adopted [18, 19]. In India, no evidence-based PU risk calculator is currently used in the clinical settings. Nurses rely on their clinical judgement to predict the risk of PU development and determine preventive nursing interventions. In this study, approximately 100 participants fulfilling the inclusion criteria were enrolled and assessed for their risk of developing PUs using the Norton, Braden, and Waterlow scales and followed up to check the applicability of these scales in our hospitals and decide on a suitable cutoff for the patient population characteristic of our surgical wards.
Aim
The aims of this study were to compare the predictive validity of three PU risk scales—the Norton scale, the Braden scale, and the Waterlow scale—and to choose the most appropriate calculator for predicting PU risk in surgical wards of India.
Methodology
This prospective observational cohort study was conducted in the general surgical wards of tertiary care hospitals in New Delhi after obtaining clearance from the Institutional Ethics Committee. The pilot study was conducted in February, enrolling ten patients. Based on its success, the rest of the study was carried out during the months from April to July 2011. As the design worked well, no changes were made in the original proposal and the patients of the pilot study were included in the major study.
All patients admitted to surgery units who qualified the age and the hours since admission criteria were approached for enrolment after obtaining informed consent. This was followed by a thorough skin assessment to ensure freedom from any preexisting pressure sores.
Inclusion Criteria
The inclusion criteria are the following: postoperative admission to the surgical ward within the last 24 h; age above 14 years; provision of informed consent; and no preexisting pressure sores at the time of enrolment.
Exclusion Criteria
The exclusion criteria are the following: postoperative admission to the surgical ward more than 24 h before enrolment; preexisting pressure sores at the time of enrolment; active skin disease that would interfere with PU assessment; hospital stay of <72 h; and physical constraints to skin assessment.
There were 240 admissions to the general surgical ward during the period of the study. Of these 100 patients, (which include the patients of the pilot study), fulfilling all the inclusion criteria were ultimately enrolled and assessed for the study.
The enrolled patients were assessed for their risk of development of pressure sores. The scoring was done by three independent assessors from the research team instead of the nurse involved in direct care of the patient to avoid certain sources of bias and error [20]. The assessors were trained in the scoring of their respective scales to ensure a high rate of accuracy. They used physical appraisal and chart review to determine the appropriate rating. The assessment was done within 24 h postoperatively for patients who underwent surgery. Patients who were on conservative treatments were also assessed within 24 h of admission to the ward.
Scoring by different scales was carried out independently and at separate times, and the assessors were blinded to each other’s scores to prevent any bias. The Norton Plus scale uses five criteria to score patients: physical condition, mental condition, activity, mobility, and incontinence. It makes deductions in the Norton score for associated conditions such as diabetes, hypertension, fever, low hematocrit, low hemoglobin and albumin, and changes in mental status and concurrent use of five or more medications. A Norton Plus rating below 10 implies high risk, 11–15 implies moderate risk, and above 15 implies low risk of developing PUs.
The Braden scale is a summated rating scale made up of six subscales: activity, mobility, sensory perception, nutrition, moisture, and friction and shear. These measure functional capabilities of the patients that contribute to either higher intensity and duration of pressure or lower tissue tolerance for pressure. It is scored from 1 to 4 (1 for low level of functioning and 4 for the highest level or no impairment). The total scores range from 6 to 23. Patients with a total score of 15 or 16 are considered to be at mild risk, 13 or 14 equals moderate risk, and 12 or less equals high risk of developing PUs.
The Waterlow scale evaluates patients on age, sex, body build, appetite, continence of urine and feces, mobility, skin appearance in risk areas, and special risks (disorders associated with tissue malnutrition, neurological deficits, medication, recent surgery, or trauma). Scores are totalled to produce a summary score from 3 (best prognosis) to 45 (worst prognosis). Scores of 10+ denote risk of developing a PU, 15+ high risk, and 20+ very high risk.
After the initial scoring, the patients were inspected for the development of any PUs daily in the morning between 8 a.m. and 11 a.m. The occurrence of any PU was staged according to the National Pressure Ulcer Advisory Panel Staging System, and the characteristics of the ulcer were noted in terms of number and site, along with the date of development in the record sheet. Stage 1 ulcers were classified as present only if they were seen on two consecutive days to differentiate them from transient reactive hyperaemia. They were further observed for progression to higher stages by continuing skin assessments, along with other patients who had not yet developed any ulcers until discharge or transfer (or death in the case of one patient). The occurrence of ulcers was reported to the head nurse for provision of suitable measures.
Raw data were compiled in Microsoft excel sheets. Statistical tools available in Microsoft excel were used to analyze the data and calculate the sensitivity, specificity, positive predictive value (PVP), and negative predictive value (PVN). These parameters were then used for evaluating the predictive validity of each assessment scale. Cohen’s kappa was calculated to assess inter-scale agreement.
Results
The sensitivity, specificity, PVP, and PVN of all the scales along with the generally accepted values of the cutoff scores in the literature are different from what we observed in our study (Table 1).
Table 1.
Parameters of all scales at generally acceptable cutoff scores
| Norton | Norton+ | Braden | Waterlow | |
|---|---|---|---|---|
| Cutoff | 15 | 10 | 16 | 10 |
| Sensitivity | 82.61 | 52.17 | 86.96 | 95.65 |
| Specificity | 98.70 | 100 | 93.511 | 74.02 |
| PVP | 48.72 | 50 | 44.44 | 34.38 |
| PVN | 95 | 87.5 | 96 | 98.28 |
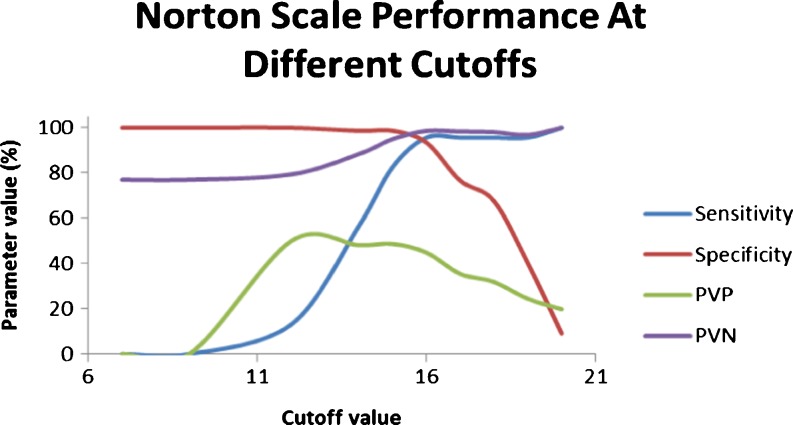
Figure 1 (Table 2) shows the performance of the Norton scale at different cutoff scores. The cutoff score of 16 seems to be the most appropriate for our settings as it gives high sensitivity (95.6) with reasonably high specificity (93.5), PVP (44.8), and PVN (98.6). For the Norton Plus scale, a cutoff score of 13 enhances the sensitivity (95.6) of the scale, but reduces the specificity (85.7) and has a PVP of 40 and a PVN of 98.5.
Fig. 1.
Norton scale performance at different cutoff scores
Table 2.
Norton scale
| Cutoff | Sensitivity | Specificity | PVP | PVN |
|---|---|---|---|---|
| 20.00 | 100.00 | 9.09 | 19.83 | 100.00 |
| 19.00 | 95.65 | 40.26 | 24.44 | 96.88 |
| 18.00 | 95.65 | 67.53 | 31.88 | 98.11 |
| 17.00 | 95.65 | 76.62 | 35.48 | 98.33 |
| 16.00 | 95.65 | 93.51 | 44.90 | 98.63 |
| 15.00 | 82.61 | 98.70 | 48.72 | 95.00 |
| 14.00 | 56.52 | 98.70 | 48.15 | 88.37 |
| 12.00 | 13.04 | 100.00 | 50.00 | 79.38 |
| 9.00 | 0.00 | 100.00 | NA | 77.00 |
| 7.00 | 0.00 | 100.00 | NA | 77.00 |
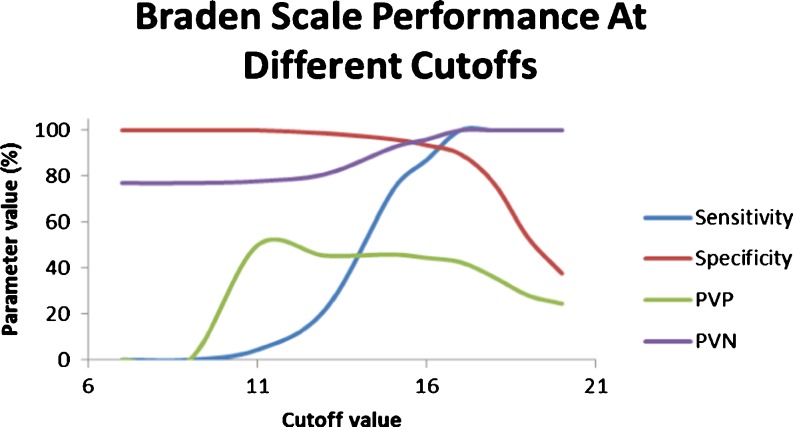
Figure 2 (Table 3) shows the performance of the Braden scale at different cutoff scores, with a cutoff score of 17 providing very high sensitivity (100) and reasonable specificity (89.6), PVP (42.5), and PVN (100).
Fig. 2.
Braden scale performance at different cutoff scores
Table 3.
Braden scale
| Cutoff | Sensitivity | Specificity | PVP | PVN |
|---|---|---|---|---|
| 20.00 | 100.00 | 37.66 | 24.47 | 100.00 |
| 19.00 | 100.00 | 53.25 | 28.05 | 100.00 |
| 18.00 | 100.00 | 76.62 | 35.94 | 100.00 |
| 17.00 | 100.00 | 89.61 | 42.59 | 100.00 |
| 16.00 | 86.96 | 93.51 | 44.44 | 96.00 |
| 15.00 | 73.91 | 96.10 | 45.95 | 92.50 |
| 13.00 | 21.74 | 98.70 | 45.45 | 80.85 |
| 11.00 | 4.35 | 100.00 | 50.00 | 77.78 |
| 9.00 | 0.00 | 100.00 | NA | 77.00 |
| 7.00 | 0.00 | 100.00 | NA | 77.00 |
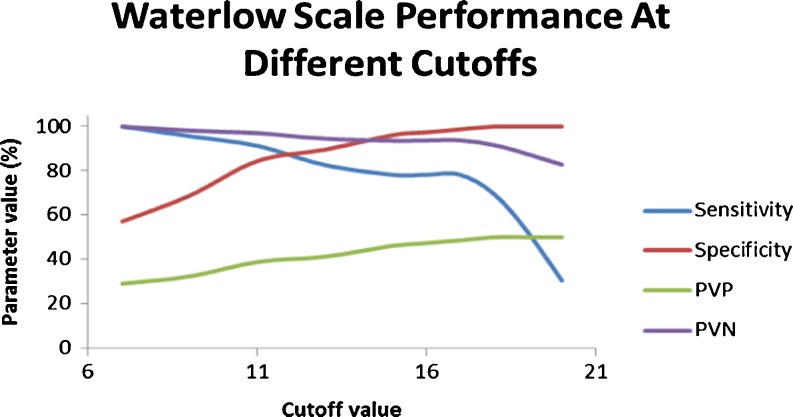
Figure 3 (Table 4) shows the performance of the Waterlow scale at different cutoff scores, depicting a cutoff score of 11 to provide adequate sensitivity (91.3) as well as specificity (84.4), PVP (38.8), and PVN (97).
Fig. 3.
Waterlow scale performance at different cutoff scores
Table 4.
Waterlow scale
| Cutoff | Sensitivity | Specificity | PVP | PVN |
|---|---|---|---|---|
| 20.00 | 30.43 | 100.00 | 50.00 | 82.80 |
| 19.00 | 52.17 | 100.00 | 50.00 | 87.50 |
| 18.00 | 69.57 | 100.00 | 50.00 | 91.67 |
| 17.00 | 78.26 | 98.70 | 48.65 | 93.83 |
| 16.00 | 78.26 | 97.40 | 47.37 | 93.75 |
| 15.00 | 78.26 | 96.10 | 46.15 | 93.67 |
| 13.00 | 82.61 | 89.61 | 41.30 | 94.52 |
| 11.00 | 91.30 | 84.42 | 38.89 | 97.01 |
| 9.00 | 95.65 | 68.83 | 32.35 | 98.15 |
| 7.00 | 100.00 | 57.14 | 29.11 | 100.00 |
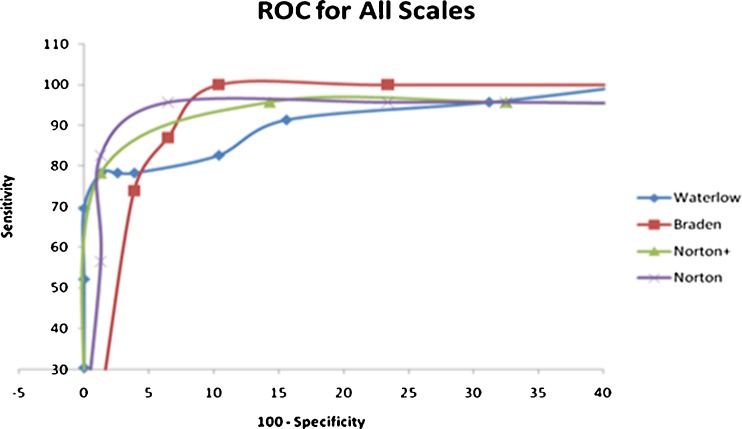
The receiver operating characteristic (ROC) curve plots the sensitivity against the specificity and is a widely used measure for the predictive utility of a scale. The ROC for all the scales based on our data is plotted in Fig. 4. It shows that the Norton scale can provide the highest sensitivity without compromising the specificity. The Norton scale is hence the most appropriate tool to be used for risk assessment in our settings. On calculation of the Cohen’s kappa values, the Norton and Braden scales have a higher agreement among each other than with the Waterlow scale (0.80 vs. 0.46 and 0.47).
Fig. 4.
Receiver operating characteristic (ROC) curve
The physical conditions of patients graded as per the Norton scale, where 1 = bad, 2 = poor, 3 = fair, and 4 = good, showed that patients who developed PUs had an average score of 2.13 (SD = 0.55) as compared to an average score of 2.97 (SD = 0.51) in those without ulcers, making it an extremely significant factor for the development of PUs (p < 0.0001; Table 5)
Table 5.
Significance of various parameters in determining sore formation
| Parameter | Parameter values for patients | p value | |||
|---|---|---|---|---|---|
| With sores | Without sores | ||||
| Average | SD | Average | SD | ||
| Physical condition | 2.13 | 0.55 | 2.97 | 0.51 | <0.0001 |
| Activity | 1.30 | 0.76 | 3.48 | 0.82 | <0.0001 |
| Mobility | 2.39 | 0.66 | 3.47 | 0.58 | <0.0001 |
| Build | 2.52 | 0.90 | 0.78 | 1.17 | <0.0001 |
| Nutrition | 1.17 | 0.49 | 2.04 | 0.77 | <0.0001 |
| Friction and shear | 1.39 | 0.58 | 2.57 | 0.51 | <0.0001 |
The build of patients as coded by the Waterlow scale, where 0 = average BMI, 1 = above average BMI, 2 = obesity, and 3 = below-average BMI, showed that patients with PUs had an average score of 2.52 (SD= 0.90) as compared to an average score of 0.78 (SD = 1.17) of those without ulcers, making obesity and below average BMI extremely significant (p < 0.0001)
Mobility coded as in the Braden scale, where 1 = completely immobile, 2 = very limited, 3 = slightly limited, and 4 = no limitation, showed that patients with PUs had an average score of 2.39 (SD = 0.66) as compared to an average score of 3.47 (SD= 0.58) in those without ulcers, making it an extremely significant factor (p < 0.0001).
The activity of patients as per the Braden scale, where 1 = bedfast, 2 = chair fast, 3 = walks occasionally, and 4 = walks frequently, showed that patients with PU development had an average score of 1.30 (SD= 0.76) as compared to an average score of 3.48 (SD= 0.82), making it an extremely significant factor (p < 0.0001)
The nutritional status of patients with and without PUs as per the Braden scale, where 1 = very poor, 2 = probably inadequate, 3 = adequate, and 4 = excellent, showed that patients with PU development had an average score of 1.17 (SD= 0.49) as compared to an average score of 2.04 (SD= 0.77) in those without ulcers, making it extremely significant (p < 0.0001).
Friction and shear as per the Braden scale, where 1 = problem, 2 = potential problem, and 3 = no apparent problem, showed that patients with PU development had an average score of 1.39 (SD= 0.58) as compared to an average score of 2.57 (SD= 0.51) in those without ulcers, making it extremely significant (p < 0.0001).
Table 6 shows the incidence of PUs in patients with specific conditions such as low albumin (41 %), more than five medications (46 %), diabetes (50 %) as per the Norton scale, and smoking (34 %) and more than 2 h on the OR table (82 %) as per the Waterlow scale. The incidence is much higher as compared to the general incidence of 23 %, proving them to be significant in PU development. Mental state, incontinence, and hematocrit turned out to be insignificant.
Table 6.
Incidence of pressure ulcers in specific conditions
| Category | Incidence | n |
|---|---|---|
| General | 23 | 100 |
| Low albumin | 41.30435 | 46 |
| >5 medications | 46.875 | 32 |
| Smokers | 34.28571 | 35 |
| Diabetics | 50 | 4 |
| Major surgery | 82.35294 | 17 |
Discussion
The status of risk factors is likely to change over time, but repeat assessments of risk factors were not considered necessary because of the anticipated improvement in patients post-surgery, although they might have been helpful in defining these changes and the frequency with which risk assessments should be performed. However, because most PUs occur within the first few weeks in a health care facility [20], identification of risk factors present on admission is critical for targeting appropriate preventive interventions [21].
The specificity, sensitivity, PVP, and PVN were used as indicators of the accuracy and utility of the different risk assessment scales for comparison. At the values generally accepted in the literature, all scales show relatively high PVN and specificity, but low PVP and a slightly low sensitivity. Because all these parameters depend very strongly on the cutoff values used for predicting at-risk patients, the performance of all the scales at different cutoffs was analyzed and compared.
The performance of Norton Plus has been shown separately for the purpose of comparison with older studies as it has been a recent modification in the Norton scale. At lower cutoffs, specificity is high, but sensitivity is low. Because the Norton Plus scale is used only for assessing high-risk cases, high sensitivity is more important than specificity and, hence, a higher cutoff score is recommended.
The reason for the lower sensitivity and specificity of the scales in the study could be due to the assessment done by the research team who have very limited contact with the patients rather than the nurses involved in direct care of the patient who may be able to rate the patients more accurately. Determination of more accurate values for sensitivity and specificity would require withholding preventive intervention and allowing PUs to develop in vulnerable patients. The cutoff scores that were optimal for this study turned out to be different from the generally accepted values in the literature; this may be due to inherent differences in our study population and the populations researched during in the development of these tools. The primary difference in the populations is likely to be in terms of gradually improving functional status in the surgical ward after operation or suitable conservative interventions as opposed to gradually deteriorating functional status in the typical long-term care facility. Whereas most of our subjects were short-stay patients with high functional status and low risk, others were high-risk patients with high level of dependence who stayed for longer periods; some even returned to the hospital or died.
In our study, the Norton and Braden scales appear to have a greater agreement with each other as compared with the Waterlow scale. Braden was also found to show good inter-observer reliability in the Netherlands in the study by Bours et al. [22]. The Waterlow scale showed poor inter-observer reliability according to the study by Kelly [23]. It is possible that the Waterlow score is inherently less reliable than Norton or Braden, but other factors such as training on the use of the tool will affect the reliability.
Reduced mobility and activity turned out to be significant risk factors; this supports the study by Lindgren et al. [24] which showed immobility to be a major risk factor in hospitalized patients. In another such study, a significantly higher risk was found for patients with reduced mobility in the immediate postoperative period [25].
The role of nutrition has been controversial in previous studies. Although this study supports poor nutrition as a risk factor, there may be confusion over how to assess nutrition. Being underweight and obese, as expected [26], turned out to be significant predictors of PU development. Friction and shear were very significant, supporting the conceptual scheme of Kring [6] and Defloor [26]. Low albumin (<3.3 g/dL) was a significant risk factor for the development of PUs; it supports the studies by Papantonio et al. [27], Lewicki et al. [25], and Feuchtinger et al. [28]. Although the use of multiple drugs (more than five in our study) has been found to be significant in the development of PU by various studies, further research on the type of medications is needed. The role of smoking in the development of PUs has been controversial. Whereas one study [28] found smoking as a significant risk factor, another refuted it [29]. However, our study found smoking to be a significant risk factor, but further research on specifics such as duration, frequency, and amount of smoking needs to be conducted. Diabetics, as expected [28], turned out to be at higher risk of ulcer development in our study, although the total number of diabetic patients was small, affecting the reliability of the statistics.
A comparison of the Norton, Braden, and Waterlow scales has determined their applicability, which can be precisely used for the prevention of PUs and may lead to a significant reduction in the incidence and prevalence of PUs in Indian health care settings, hence saving costs of treatment. Because none of the scales are 100 % sensitive and specific, they should be adopted along with clinical judgement to predict PU development and in individualizing a turning, repositioning schedule and using pressure relieving aids for effective prevention. The sample size for this study was small; similar studies should be done with larger and randomized sampling to obtain more accurate, generalizable results.
Various departments should conduct their own studies to determine the optimal cutoff scores for their PURAS based on their patient population and care settings as the recommended cutoff scores vary based on different patient groups and different care settings [6]. The age structure in our study was comparatively younger as compared to most of other studies done in developed countries on older populations; further research on the risk factors affecting this age structure should be done. The relationship of the amount of time spent on the OR table, the use of preventive overlays, the degree and kind of manipulations, and the relationship of primary diagnosis with PU development should be explored to identify the exact risk factors applicable to Indian health care settings.
To conclude, the ROC for all the scales based on our data shows that the Norton scale can provide the highest sensitivity without compromising the specificity. The Norton scale is, hence, the most appropriate tool to be used for risk assessment in our settings. Physical condition, BMI, mobility, activity, nutrition, friction, and shear turned out to be very significant in the development of PUs in the Indian scenario. Low albumin, more than five medications, smoking, and diabetes had increased association with ulcer development in Indian surgical wards. These factors can be used to devise a specific PURAS for use in Indian surgical wards and can be further validated in future studies.
Although we tried for a sample representative of all types of patients admitted to general surgical wards, because of the informed consent of patients and organizational constraints, a selection bias might have occurred.
References
- 1.Bolton L. Which pressure ulcer risk assessment scales are valid for use in the clinical setting? J Wound Ostomy Continence Nurs. 2007;34(4):368–381. doi: 10.1097/01.WON.0000281653.32955.9b. [DOI] [PubMed] [Google Scholar]
- 2.Cuddigan J, Ayello EA, Sussman C. Pressure ulcers in America: prevalence, incidence, and implications for the future. Reston, VA: National Pressure Ulcer Advisory Panel; 2001. [DOI] [PubMed] [Google Scholar]
- 3.Thein HH, Gomes T, Krahn MD, Wodchis WP. Health status utilities and the impact of pressure ulcers in long-term care residents in Ontario. Qual Life Res. 2010;19(1):81–89. doi: 10.1007/s11136-009-9563-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spilsbury K, Nelson A, Cullum N, Iglesias C, Nixon J, Mason S. Pressure ulcers and their treatment and effects on quality of life: hospital inpatient perspectives. J Adv Nurs. 2007;57(5):494–504. doi: 10.1111/j.1365-2648.2006.04140.x. [DOI] [PubMed] [Google Scholar]
- 5.Davis K. Pressure sores: aetiology, risk factors and assessment scales. Br J Nurs. 1994;3:258–261. doi: 10.12968/bjon.1994.3.6.256. [DOI] [PubMed] [Google Scholar]
- 6.Kring DL. Reliability and validity of the Braden Scale for predicting pressure ulcer risk. J Wound Ostomy Continence Nurs. 2007;34(4):399–406. doi: 10.1097/01.WON.0000281656.86320.74. [DOI] [PubMed] [Google Scholar]
- 7.Bergstrom N, Braden B, Laguzza A, Holman V. The Braden scale for predicting pressure sore risk. Nurs Res. 1987;36:205–210. doi: 10.1097/00006199-198707000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Gelfand JM, et al. et al. In: Fitzpatrick’s dermatology in general medicine. Freedberg IM, et al.et al., editors. New York: McGraw-Hill Professional; 2003. p. 1256. [Google Scholar]
- 9.The National Pressure Ulcer Advisory Panel (1989) Pressure ulcers prevalence, cost and risk assessment: consensus development conference statement. Decubitus 2(2):24–28 [PubMed]
- 10.Alterescu V. The financial costs of inpatient pressure ulcers to an acute care facility. Decubitus. 1989;2(3):14–23. [PubMed] [Google Scholar]
- 11.Gunningberg L, Lindholm C, Carlsson M, Sjoden P. Implementation of risk assessment and classification of pressure ulcers as quality indicators for patients with hip fractures. J Clin Nurs. 1999;8(4):396–406. doi: 10.1046/j.1365-2702.1999.00287.x. [DOI] [PubMed] [Google Scholar]
- 12.Pang SM, Wong TK. Predicting pressure sore risk with the Norton, Braden and Waterloo scales in a Hong Kong rehabilitation hospital. Nurs Res. 1998;47(3):147–153. doi: 10.1097/00006199-199805000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Anthony D, Parboteeah S, Saleh M, Papanikolaou P. Norton, Waterlow and Braden scores: a review of the literature and a comparison between the scores and clinical judgement. J Clin Nurs. 2008;17(5):646–653. doi: 10.1111/j.1365-2702.2007.02029.x. [DOI] [PubMed] [Google Scholar]
- 14.Papanikolaou P, Lyne P, Anthony D. Risk assessment scales for pressure ulcers: a methodological review. Int J Nurs Stud. 2007;44(2):285–296. doi: 10.1016/j.ijnurstu.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 15.TorraiBou JE, Garca’-Ferna’ndez FP, Pancorbo-Hidalgo PL, Furtado K. Risk assessment scales for predicting the risk of developing pressure ulcers. In: Romanelli M, editor. Science and practice of pressure ulcer management. London: Springer; 2008. pp. 43–57. [Google Scholar]
- 16.Norton D, McLaren R, Exton-Smith AN (1975) An investigation of geriatric nursing problems in hospitals. London 7 National Corporation for the Care of Old people
- 17.Waterloo J. Pressure sores: a risk assessment card. Nurs Times. 1985;81(48):49–55. [PubMed] [Google Scholar]
- 18.Bridel J. Assessing the risk of pressure sores. Nurs Stand. 1993;7(25):32–35. doi: 10.7748/ns.7.25.32.s47. [DOI] [PubMed] [Google Scholar]
- 19.MacDonald K. The reliability of pressure sore risk assessment tools. Prof Nurse. 1995;11(3):169–172. [PubMed] [Google Scholar]
- 20.Wasson JH, Sox HC, Neff RK, Goldman L. Clinical prediction rules. Applications and methodological standards. N Engl J Med. 1985;13(13):793–799. doi: 10.1056/NEJM198509263131306. [DOI] [PubMed] [Google Scholar]
- 21.Smith DM, Winsemius DK, Besdine RW. Pressure sores in the elderly: can this outcome be improved? J Gen Intern Med. 1991;6(1):81–93. doi: 10.1007/BF02599399. [DOI] [PubMed] [Google Scholar]
- 22.Bours GJ, Halfens RJ, Lubbers M, Haalboom JR. The development of a national registration form to measure the prevalence of pressure ulcers in the Netherlands. Ostomy Wound Manage. 1999;45(28–33):36–38. [PubMed] [Google Scholar]
- 23.Kelly J. Inter-rater reliability and Waterlow’s pressure ulcer risk assessment tool. Nurs Stand. 2005;19(32):86–87. doi: 10.7748/ns2005.04.19.32.86.c3851. [DOI] [PubMed] [Google Scholar]
- 24.Lindgren M, Unosson M, Fredrikson M, Ek AC. Immobility—a major risk factor for development of pressure ulcers among adult hospitalized patients: a prospective study. Scand J Caring Sci. 2004;18(1):57–64. doi: 10.1046/j.0283-9318.2003.00250.x. [DOI] [PubMed] [Google Scholar]
- 25.Lewicki LJ, Mion L, Splane KG, Samstag D, Secic M. Patient risk factors for pressure ulcer during cardiac surgery. AORN J. 1997;65(5):933–942. doi: 10.1016/S0001-2092(06)62976-1. [DOI] [PubMed] [Google Scholar]
- 26.Defloor T. The risk of pressure sores: a conceptual scheme. J ClinNurs. 1999;8:206–216. doi: 10.1046/j.1365-2702.1999.00254.x. [DOI] [PubMed] [Google Scholar]
- 27.Papantonio CT, Wallop JM, Kolodner KB. Sacral ulcers following cardiac surgery: incidence and risks. Adv Wound Care. 1994;7(2):24–36. [PubMed] [Google Scholar]
- 28.Feuchtinger J, Halfens RJ, Dassen T. Pressure ulcer risk factors in cardiac surgery: a review of the research literature. Heart Lung. 2005;34(6):375–385. doi: 10.1016/j.hrtlng.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Olson B, Langemo D, Burd C, Hanson D, Hunter S, Cathcart-Silberberg T. Pressure ulcer incidence in an acute care setting. J Wound Ostomy Continence Nurs. 1996;23(1):15–22. doi: 10.1016/s1071-5754(96)90111-4. [DOI] [PubMed] [Google Scholar]