Abstract
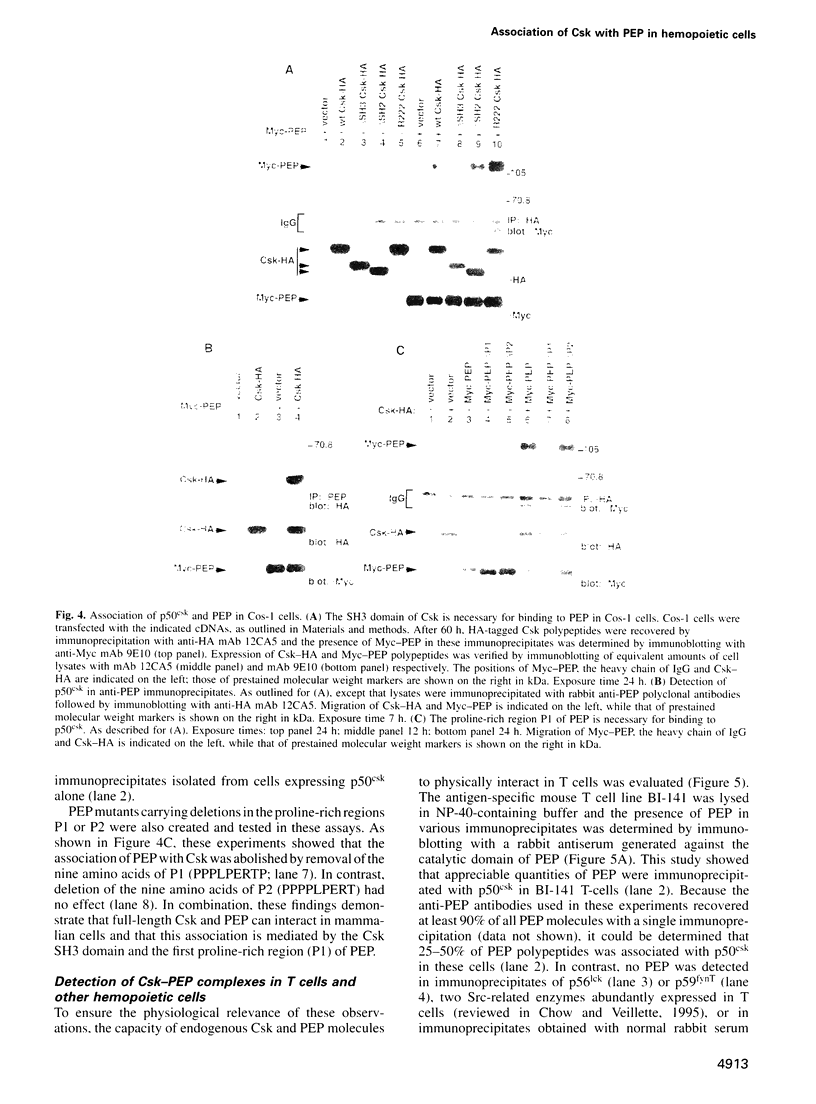
p50csk is a tyrosine protein kinase (TPK) that represses the activity of Src family TPKs. We previously showed that Csk is a potent negative regulator of antigen receptor signaling in T lymphocytes and that its Src homology (SH) 3 and SH2 domains are required to inhibit these signals. To test the idea that the Csk SH3 and SH2 domains mediate interactions with other cellular proteins, we attempted to identify Csk-associated polypeptides using the yeast two-hybrid system. The results of our experiments demonstrated that Csk physically associates with PEP, a protein tyrosine phosphatase (PTP) expressed in hemopoietic cells. Further analyses revealed that this interaction was mediated by the Csk SH3 domain and by a proline-rich region (PPPLPERTP) in the non-catalytic C-terminal portion of PEP. The association between Csk and PEP was documented in transiently transfected Cos-1 cells and in a variety of cells of hemopoietic lineages, including T cells. Additional analyses demonstrated that the association between Csk and PEP is highly specific. Together, these data indicated that PEP may be an effector and/or a regulator of p50csk in T cells and other hemopoietic cells. Moreover, they allowed the identification of PEP as the first known ligand for the Csk SH3 domain.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergman M., Joukov V., Virtanen I., Alitalo K. Overexpressed Csk tyrosine kinase is localized in focal adhesions, causes reorganization of alpha v beta 5 integrin, and interferes with HeLa cell spreading. Mol Cell Biol. 1995 Feb;15(2):711–722. doi: 10.1128/mcb.15.2.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman M., Mustelin T., Oetken C., Partanen J., Flint N. A., Amrein K. E., Autero M., Burn P., Alitalo K. The human p50csk tyrosine kinase phosphorylates p56lck at Tyr-505 and down regulates its catalytic activity. EMBO J. 1992 Aug;11(8):2919–2924. doi: 10.1002/j.1460-2075.1992.tb05361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowtell D. D., Langdon W. Y. The protein product of the c-cbl oncogene rapidly complexes with the EGF receptor and is tyrosine phosphorylated following EGF stimulation. Oncogene. 1995 Oct 19;11(8):1561–1567. [PubMed] [Google Scholar]
- Charest A., Wagner J., Shen S. H., Tremblay M. L. Murine protein tyrosine phosphatase-PEST, a stable cytosolic protein tyrosine phosphatase. Biochem J. 1995 Jun 1;308(Pt 2):425–432. doi: 10.1042/bj3080425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow L. M., Davidson D., Fournel M., Gosselin P., Lemieux S., Lyu M. S., Kozak C. A., Matis L. A., Veillette A. Two distinct protein isoforms are encoded by ntk, a csk-related tyrosine protein kinase gene. Oncogene. 1994 Dec;9(12):3437–3448. [PubMed] [Google Scholar]
- Chow L. M., Fournel M., Davidson D., Veillette A. Negative regulation of T-cell receptor signalling by tyrosine protein kinase p50csk. Nature. 1993 Sep 9;365(6442):156–160. doi: 10.1038/365156a0. [DOI] [PubMed] [Google Scholar]
- Chow L. M., Jarvis C., Hu Q., Nye S. H., Gervais F. G., Veillette A., Matis L. A. Ntk: a Csk-related protein-tyrosine kinase expressed in brain and T lymphocytes. Proc Natl Acad Sci U S A. 1994 May 24;91(11):4975–4979. doi: 10.1073/pnas.91.11.4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow L. M., Veillette A. The Src and Csk families of tyrosine protein kinases in hemopoietic cells. Semin Immunol. 1995 Aug;7(4):207–226. doi: 10.1006/smim.1995.0026. [DOI] [PubMed] [Google Scholar]
- Cloutier J. F., Chow L. M., Veillette A. Requirement of the SH3 and SH2 domains for the inhibitory function of tyrosine protein kinase p50csk in T lymphocytes. Mol Cell Biol. 1995 Nov;15(11):5937–5944. doi: 10.1128/mcb.15.11.5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. B., Ren R., Baltimore D. Modular binding domains in signal transduction proteins. Cell. 1995 Jan 27;80(2):237–248. doi: 10.1016/0092-8674(95)90406-9. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Howell B. The when and how of Src regulation. Cell. 1993 Jun 18;73(6):1051–1054. doi: 10.1016/0092-8674(93)90634-3. [DOI] [PubMed] [Google Scholar]
- Davidson D., Fournel M., Veillette A. Oncogenic activation of p59fyn tyrosine protein kinase by mutation of its carboxyl-terminal site of tyrosine phosphorylation, tyrosine 528. J Biol Chem. 1994 Apr 8;269(14):10956–10963. [PubMed] [Google Scholar]
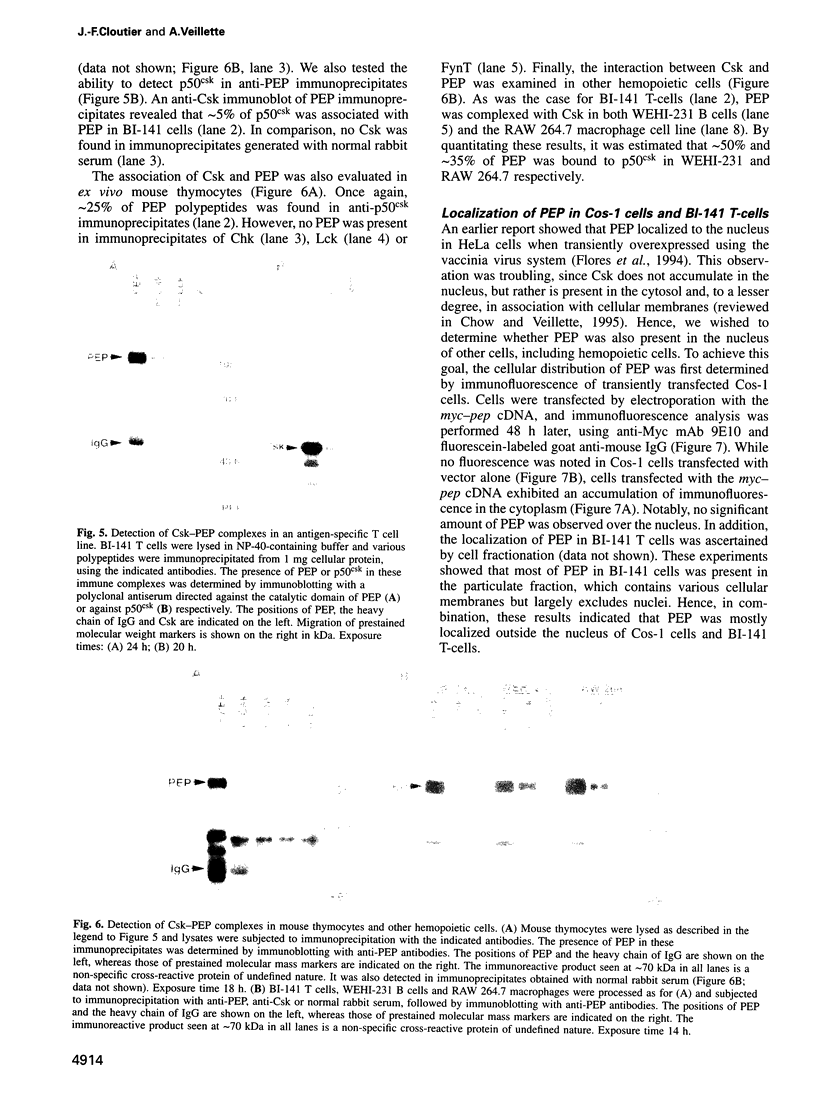
- Evan G. I., Lewis G. K., Ramsay G., Bishop J. M. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985 Dec;5(12):3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S., Kasahara C., Rickles R. J., Schreiber S. L. Specific interactions outside the proline-rich core of two classes of Src homology 3 ligands. Proc Natl Acad Sci U S A. 1995 Dec 19;92(26):12408–12415. doi: 10.1073/pnas.92.26.12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields S., Song O. A novel genetic system to detect protein-protein interactions. Nature. 1989 Jul 20;340(6230):245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- Flores E., Roy G., Patel D., Shaw A., Thomas M. L. Nuclear localization of the PEP protein tyrosine phosphatase. Mol Cell Biol. 1994 Jul;14(7):4938–4946. doi: 10.1128/mcb.14.7.4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gout I., Dhand R., Hiles I. D., Fry M. J., Panayotou G., Das P., Truong O., Totty N. F., Hsuan J., Booker G. W. The GTPase dynamin binds to and is activated by a subset of SH3 domains. Cell. 1993 Oct 8;75(1):25–36. [PubMed] [Google Scholar]
- Gross J. A., Appleby M. W., Chien S., Nada S., Bartelmez S. H., Okada M., Aizawa S., Perlmutter R. M. Control of lymphopoiesis by p50csk, a regulatory protein tyrosine kinase. J Exp Med. 1995 Feb 1;181(2):463–473. doi: 10.1084/jem.181.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell B. W., Cooper J. A. Csk suppression of Src involves movement of Csk to sites of Src activity. Mol Cell Biol. 1994 Aug;14(8):5402–5411. doi: 10.1128/mcb.14.8.5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamoto A., Soriano P. Disruption of the csk gene, encoding a negative regulator of Src family tyrosine kinases, leads to neural tube defects and embryonic lethality in mice. Cell. 1993 Jun 18;73(6):1117–1124. doi: 10.1016/0092-8674(93)90641-3. [DOI] [PubMed] [Google Scholar]
- Klages S., Adam D., Class K., Fargnoli J., Bolen J. B., Penhallow R. C. Ctk: a protein-tyrosine kinase related to Csk that defines an enzyme family. Proc Natl Acad Sci U S A. 1994 Mar 29;91(7):2597–2601. doi: 10.1073/pnas.91.7.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren H. S., Handwerger B. S., Wunderlich J. R. Identification of macrophage-like characteristics in a cultured murine tumor line. J Immunol. 1975 Feb;114(2 Pt 2):894–897. [PubMed] [Google Scholar]
- Matthews R. J., Bowne D. B., Flores E., Thomas M. L. Characterization of hematopoietic intracellular protein tyrosine phosphatases: description of a phosphatase containing an SH2 domain and another enriched in proline-, glutamic acid-, serine-, and threonine-rich sequences. Mol Cell Biol. 1992 May;12(5):2396–2405. doi: 10.1128/mcb.12.5.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nada S., Okada M., MacAuley A., Cooper J. A., Nakagawa H. Cloning of a complementary DNA for a protein-tyrosine kinase that specifically phosphorylates a negative regulatory site of p60c-src. Nature. 1991 May 2;351(6321):69–72. doi: 10.1038/351069a0. [DOI] [PubMed] [Google Scholar]
- Neet K., Hunter T. The nonreceptor protein-tyrosine kinase CSK complexes directly with the GTPase-activating protein-associated p62 protein in cells expressing v-Src or activated c-Src. Mol Cell Biol. 1995 Sep;15(9):4908–4920. doi: 10.1128/mcb.15.9.4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oetken C., Couture C., Bergman M., Bonnefoy-Bérard N., Williams S., Alitalo K., Burn P., Mustelin T. TCR/CD3-triggering causes increased activity of the p50csk tyrosine kinase and engagement of its SH2 domain. Oncogene. 1994 Jun;9(6):1625–1631. [PubMed] [Google Scholar]
- Pawson T., Gish G. D. SH2 and SH3 domains: from structure to function. Cell. 1992 Oct 30;71(3):359–362. doi: 10.1016/0092-8674(92)90504-6. [DOI] [PubMed] [Google Scholar]
- Peri K. G., Gervais F. G., Weil R., Davidson D., Gish G. D., Veillette A. Interactions of the SH2 domain of lymphocyte-specific tyrosine protein kinase p56lck with phosphotyrosine-containing proteins. Oncogene. 1993 Oct;8(10):2765–2772. [PubMed] [Google Scholar]
- Reske-Kunz A. B., Rüde E. Insulin-specific T cell hybridomas derived from (H-2b x H-2k)F1 mice preferably employ F1-unique restriction elements for antigen recognition. Eur J Immunol. 1985 Oct;15(10):1048–1054. doi: 10.1002/eji.1830151017. [DOI] [PubMed] [Google Scholar]
- Rodrigues G. A., Park M. Dimerization mediated through a leucine zipper activates the oncogenic potential of the met receptor tyrosine kinase. Mol Cell Biol. 1993 Nov;13(11):6711–6722. doi: 10.1128/mcb.13.11.6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabe H., Hata A., Okada M., Nakagawa H., Hanafusa H. Analysis of the binding of the Src homology 2 domain of Csk to tyrosine-phosphorylated proteins in the suppression and mitotic activation of c-Src. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3984–3988. doi: 10.1073/pnas.91.9.3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabe H., Knudsen B., Okada M., Nada S., Nakagawa H., Hanafusa H. Molecular cloning and expression of chicken C-terminal Src kinase: lack of stable association with c-Src protein. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2190–2194. doi: 10.1073/pnas.89.6.2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonson M. S., Wang Y., Herman W. H. Nuclear signaling by endothelin-1 requires Src protein-tyrosine kinases. J Biol Chem. 1996 Jan 5;271(1):77–82. doi: 10.1074/jbc.271.1.77. [DOI] [PubMed] [Google Scholar]
- Veillette A., Bookman M. A., Horak E. M., Bolen J. B. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell. 1988 Oct 21;55(2):301–308. doi: 10.1016/0092-8674(88)90053-0. [DOI] [PubMed] [Google Scholar]
- Veillette A., Caron L., Fournel M., Pawson T. Regulation of the enzymatic function of the lymphocyte-specific tyrosine protein kinase p56lck by the non-catalytic SH2 and SH3 domains. Oncogene. 1992 May;7(5):971–980. [PubMed] [Google Scholar]
- Yamaji Y., Amemiya M., Cano A., Preisig P. A., Miller R. T., Moe O. W., Alpern R. J. Overexpression of csk inhibits acid-induced activation of NHE-3. Proc Natl Acad Sci U S A. 1995 Jul 3;92(14):6274–6278. doi: 10.1073/pnas.92.14.6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q., Co D., Sommercorn J., Tonks N. K. Cloning and expression of PTP-PEST. A novel, human, nontransmembrane protein tyrosine phosphatase. J Biol Chem. 1993 Mar 25;268(9):6622–6628. [PubMed] [Google Scholar]