Abstract
To understand how the presynaptic proteins synapsin and Rab3a may interact in the regulation of the synaptic vesicle cycle and the release process, we derived a double knockout (DKO) mouse lacking both synapsin II and Rab3a. We found that Rab3a deletion rescued epileptic-like seizures typical for synapsin II gene deleted animals (SynII(−)). Furthermore, action potential evoked release was drastically reduced in DKO synapses, although spontaneous release remained normal. At low Ca2+ conditions, quantal content was equally reduced in Rab3a(−) and DKO synapses, but as Ca2+ concentration increased, the increase in quantal content was more prominent in Rab3a(−). Electron microscopy analysis revealed that DKO synapses have a combined phenotype, with docked vesicles being reduced similar to Rab3a(−), and intraterminal vesicles being depleted similar to SynII(−). Consistently, both SynII(−) and DKO terminals had increased synaptic depression and incomplete recovery. Taken together, our results suggest that synapsin II and Rab3a have separate roles in maintaining the total store of synaptic vesicles and cooperate in promoting the latest steps of neuronal secretion.
INTRODUCTION
The synapsin family and Rab3a are presynaptic proteins which reversibly bind to synaptic vesicles. Synapsins, in their dephosphorylated state, are thought to tether vesicles to actin filaments, thus preventing vesicles from becoming releasable, while synapsin phosphorylation is thought to promote availability of vesicles for release (Greengard et al., 1993). In mammals, synapsins are encoded by three genes, synapsin I, II, and III (Greengard et al., 1993; Kao et al., 1998). Synapsin deficiency produces epileptic seizures (Rosahl et al., 1995; Etholm and Heggelund, 2009) and synaptic vesicle depletion (Li et al., 1995; Samigullin et al., 2004; Gitler et al., 2004). Although it is known that different synapsin isoforms have different ATP binding properties (Hosaka and Sudhof, 1998) and represent targets for different protein kinases (Hosaka et al., 1999; Jovanovic et al., 2001; Chi et al., 2001,2003; Cousin et al., 2003; Murthy, 2001 for review), specific roles of different synapsin isoforms in neuronal secretion are not fully understood. The regulation of release time-course by synapsin I (Hilfiker et al., 1998; Coleman and Bykhovskaia, 2009a) suggests its function in the latest stages of exocytosis. In contrast, synapsin II appears to have a specific role in preventing synaptic depression and maintaining the reserve pool of vesicles (Rosahl et al., 1995; Coleman et al., 2008; Gitler et al., 2008).
Rab3a is a small GTP binding protein, which is thought to promote vesicle mobilization and targeting to the active zones. Rab3a associates with vesicles in its GTP bound form (Mizoguchi et al., 1990), but becomes detached upon GTP to GDP hydrolysis during or after exocytosis (Fischer von Mollard et al., 1991). Several studies suggested that Rab3a is a positive regulator of vesicle mobilization and transmitter release (Oberhauser et al., 1992; Geppert et al., 1994; Nonet et al., 1997; Leenders et al., 2001; Coleman et al., 2007; Coleman and Bykhovskaia, 2009b), suggesting a role of Rab3a in targeting vesicles to active zones. Interactions of Rab3a with docking machinery (Wang et al., 1997; Schoch et al., 2002; van Weering et al., 2007; Graham et al., 2008) provide a molecular basis for this Rab3a function. These studies notwithstanding, the role of Rab3a in exocytosis still remains somewhat controversial. Thus, the size of the releasable pool of vesicles at hippocampal synapses was found to be unaffected by Rab3a gene deletion (Geppert et al., 1997), and Rab3a was demonstrated to inhibit exocytosis in neuroendocrine cells (Holz et al., 1994; Johannes et al., 1994; Thiagarajan et al., 2004).
Synapsins and Rab3a were found to interact on synaptic vesicles (Giovedi et al., 2004a), and this interaction affects the function of both proteins (Giovedi et al., 2004b). This finding brings up a question of how synaptic transmission would be affected if both synapsin and Rab3a were deleted? Up to this point, in studying presynaptic mechanisms, mice with single gene deletions or with deleted protein families have been derived. Thus, mutants lacking all three synapsin isoforms (Gitler et al., 2004) and several Rab3 genes (Schluter et al., 2004) have been created and investigated. It is now a timely task to create a mutant that would combine gene deletions of different families, specifically synapsin and Rab3a. That was the goal of the present study, to examine neuronal transmission in synapses lacking both synapsin and Rab3a.
An additional rationale for such a study was our earlier finding that at the mouse diaphragm synapse synapsin and Rab3a have opposite effects on some properties of quantal release and synaptic ultrastructure. Synapsin II, which is a stronger effector of transmitter release at our preparation than synapsin I, was shown to inhibit quantal release at low Ca2+ conditions (Samigullin et al., 2004; Coleman et al., 2008), while Rab3a was shown to promote it (Coleman et al., 2007, 2009b). The above studies also revealed that synapsin II maintains the pool of intraterminal vesicles, while Rab3a maintains the pool of docked vesicles.
Thus, the question arose of how the transmission at the diaphragm synapse would be affected if both synapsin II and Rab3a are deleted. It should be noted that although only the interaction of Rab3a with synapsin I C-terminal domain was demonstrated directly, the interaction of synapsin II with Rab3a has also been detected (Giovedi et al., 2004a). Thus, in the present study we derived double knockout (DKO) animals lacking both synapsin II and Rab3a (Syn II(−)/Rab3a(−) DKO) and investigated exocytosis at the DKO diaphragm synapse.
EXPERIMENTAL PROCEDURES
Animals
Mice homozygous for the Syn2tm1Sud (Syn II(−)) and Rab3atm1Sud (Rab3a(−)) targeted mutation were purchased from Jackson Laboratory (strains B6;129S-Syn2tm1Sud/J and B6;129S-Rab3atm1Sud). The SynII (−)/Rab3a(−) DKO mice were obtained by serial breeding and genotyping. Specifically, the two homozygous strains (Rab3a−/− and Syn II −/−) were crossed to generate double heterozygous (Rab3a+/−;Syn II+/−) animals and then double heterozygous mice were intercrossed to generate the double homozygous strain (Rab3a−/−;Syn II −/−). For genotyping, tail DNA was extracted and analyzed by PCR. Genotyping protocols and primers were used as described in The Jackson Laboratory Genotyping Protocols (Id 457 and 1221). In addition, the genotype of the DKO breeding colony was confirmed using a commercial genotyping service (Transnetyx). The DKO breeders obtained this way were used to start the colony, and the experiments were performed on the first generation of their progeny. The control colony (WT) started from the strain B6;129F2/J (Jackson Laboratory), a genetic match to the KO, was the same as in all of our previous studies (Samigullin et al., 2004; Coleman et al., 2008, Coleman and Bykhovskaia, 2009a,b). All the animals were kept at standard conditions at room temperature and on a 12h dark/light cycle. The husbandry conditions were identical for all the strains under the study. All the experiments were performed on adult animals (3–6 months age) in accordance with the guidelines of the Animal Care and Use Committee of the Lehigh University and the Universidad Central del Caribe and the National Institutes of Health of the US Public Health Service. All the behavioral experiments were performed in the first quarter of the light cycle. For electrophysiology and ultrastructural analysis, mice were anaesthetized with diethyl ether (5–10 ml for 3–5 min) and killed by decapitation.
Electrophysiology
The diaphragms of adult mice were rapidly dissected and the phrenic nerves were isolated. The hemi-diaphragm was pinned to Sylgard and superfused with saline solution (150 mM NaCl, 5 mM KCl, 5 mM MgCl2, 11 mM D-glucose, 5 mM HEPES, pH 7.4) with variable CaCl2 concentration (0.2, 0.5, 1, and 2 mM). The phrenic nerve was stimulated via suction electrode with suprathreshold electric potentials. Excitatory postsynaptic potentials (EPSPs) were recorded focally from visualized endplates (4 μM 4-Di-2-Asp applied for 2 minutes) under ×60 long-distance (2 mm) water immersion objective (Nikon) using manually bent electrodes with 5–10 μm diameter tips. To minimize the variability between endplates, we recorded exclusively from type I terminals identified by their compact round shape, as described in (Coleman et al., 2007, 2008, 2009b). This technique ensured that responses from uniform single nerve endplates were recorded. During the recordings in physiological [Ca2+]o (2 mM), preparations were treated with μ–conotoxin GIIIB (Bachem, 2 μM in the bath for 20 minutes) to prevent muscle twitching. In every experiment spontaneous activity was recorded for at least five minutes.
Quantal analysis
A temporal interval of 50 ms after the nerve action potential was analyzed. Two methods were employed for the evaluation of the quantal content in synaptic responses: direct counts and amplitude analysis. The method of direct counts was used either to detect spontaneous quanta or to detect synchronous quanta at 0.2 and 0.5 mM [Ca2+]o when synaptic transmission was low, and quantal counts were reliable. Quanta were counted as inflections or peaks of EPSP traces using in-house software (Bykhovskaia 2008). At high-output synapses or at physiological Ca2+ concentration, quantal content was calculated as the ratio between EPSP amplitude and quantal amplitude. Quantal amplitude was calculated as the average amplitude of spontaneous events.
Electron Microscopy
Preparations were fixed for electron microscopy as described in (Samigullin et al., 2004; Coleman et al., 2007, 2008). Samples were cut from the area surrounding the phrenic nerve. After overnight incubation in physiological saline, samples were post-fixed in 1% osmium tetroxide, dehydrated through a graded series of ethanol and acetone, and embedded in Embed 812 epoxy resin (Electron Microscopy Sciences). Thin sections (75–100 nm) were collected on Formvar/carbon coated copper slot grids, and contrasted with lead citrate. Samples were examined on a Joel 2000 FX transmission electron microscope at 150 kV. Micrographs were analyzed using Scion Image software (NIH) and Photoshop 10.0. To calculate vesicle density, we measured the area of the terminal excluding mitochondria. Terminals were divided into three layers: docked (0–50nm from the presynaptic membrane), membrane (50–150nm from the presynaptic membrane), and intraterminal (>150nm).
For consistency, we analyzed only the boutons which were unambiguously identified as belonging to type I endplates, according to their compact shape, irregularly positioned clefts, non-proximity to actin-myosin fibers, and a lack of dense filamentous matrix (Padykula and Gauthier, 1970; Samigullin et al., 2004; Coleman et al., 2008). To enable fast location of nerve endplates at the diaphragm muscle, prior to the fixation we have visualized the endplates as described above and labeled the region containing several type I endplates by three cactus needles that surrounded our region of interest. These needles were easily located in the embedded sample.
Behavioral analysis
Seizure activity was induced in mature adult mice (aged 5 months or older) by a mechanical stimulation (lifting by the tail) and monitored visually. Four trials have been performed, and the percent of animals demonstrating seizures was determined for each strain in each trial. Motor activity was tested using a TruScan system (Coulbourn Instruments) that recorded movements of the mice employing infrared sensors. Mice were placed into the center of the scanning chamber and allowed to move freely for 10 minutes, while the moving and resting time was recorded in 30 s intervals. Non-invasive vital parameters measurements were performed at the UCC Behavioral Testing Facility using the MouseOx™ system (STARR Life Science). We have measured: 1) real time cardiac pulse rate (aka heart rate, beats per minute); 2) breath rate per minute; 3) breath distention that provides a non-invasive indication of the effort a subject requires to generate each breath; and 4) real time percent oxygen saturation of hemoglobin in arterial blood. The recordings were made after the parameter stabilization was achieved, which normally took 10–15 minutes.
Statistical analysis
Statistical significances were determined by one-way ANOVA, independent two-sided t-test, or Kolmogorov-Smirnov test.
RESULTS
The Syn II(−)/Rab3a(−) DKO mice proved to be viable and fertile. However, the proportion of DKO homozygotes in the progeny from the cross of double heterozygotes was approximately twice lower than would be expected from Mendelian frequencies, and the progeny death in the DKO colony was observed frequently, indicating a reduced viability. The DKO animals had normal breath rate (154±30 per minute versus 162±21 in WT) and arterial oxygen saturation (89.9±1.3% versus 91.1±1.9% in WT) but their breath distention was significantly increased (45.1±13.0 μm versus 20.2±3.6 μm in WT), indicating a possible abnormality in the diaphragm function; in addition, the heart rate of DKO animals was significantly increased (717±33 per minute in DKO versus 637±25 in WT).
We performed behavioral, physiological and ultrastructural assays of the SynII(−)/Rab3a(−) DKO in comparison with Syn II(−) and Rab3a(−), with the goal to discriminate between the following possibilities. First, Rab3a and synapsin II could control some characteristics of synaptic transmission via a physical interaction within the same pathway. In this case, we would expect that the loss of either protein would cause the same effect, while the loss of the second protein would cause no additional effect. In other words, we would expect that the phenotypes of Syn II(−), Rab3a(−), and SynII(−)/Rab3a(−) DKO would be similar. Second, Rab3a and synapsin II could control different characteristics of synaptic transmission acting independently of each other via different pathways. In this case, we would expect that some characteristics of synaptic transmission or ultrastructure would depend on one protein but not the other. Third, some characteristics of synaptic transmission could be controlled by synapsin II and Rab3a via their independent actions, in which case the effect of these two proteins would be additive. Finally, the functional interaction between these proteins could be more complex, with one protein strengthening or weakening the functioning of the other protein. Thus, behavioral, electrophysiological, and ultrastructural characteristics of Rab3a(−), Syn II (−), and Syn II(−)/Rab3a(−) DKO were examined and compared to test these possibilities (Table 1).
Table 1.
Effects of single and double deletions on different characteristics of synaptic transmission, ultrastructure, and behavior.
| Characteristic | Rab3a (−) | SynII (−) | DKO |
|---|---|---|---|
| 1. Controlled by one protein independently of the other: the same characteristic appears in a single KO and in the DKO | |||
| Docked vesicles | Decreased | Unchanged | Decreased |
| Intraterminal vesicles | Unchanged | Decreased | Decreased |
| Depression | Unchanged | Increased | Increased |
| Recovery from depression | Unchanged | Incomplete | Incomplete |
| 2. Opposing effects of Syn II and Rab3a compensate for each other in DKO | |||
| Spontaneous synaptic activity | Decreased | Increased | Unchanged |
| 3. Rab3a loss precludes the effect of Syn II loss | |||
| Quantal content | Decreased | Increased | Strongly decreased |
| Quantal content at low Ca2+ | Strongly decreased | Strongly increased | Strongly decreased |
| Seizure activity | Unchanged | Increased | Slightly increased |
| 4. Syn II loss precludes the effect of Rab3a loss | |||
| Locomotor activity | Decreased | Increased | Increased |
Synapsin II and Rab3a have independent roles in maintaining the pool of synaptic vesicles
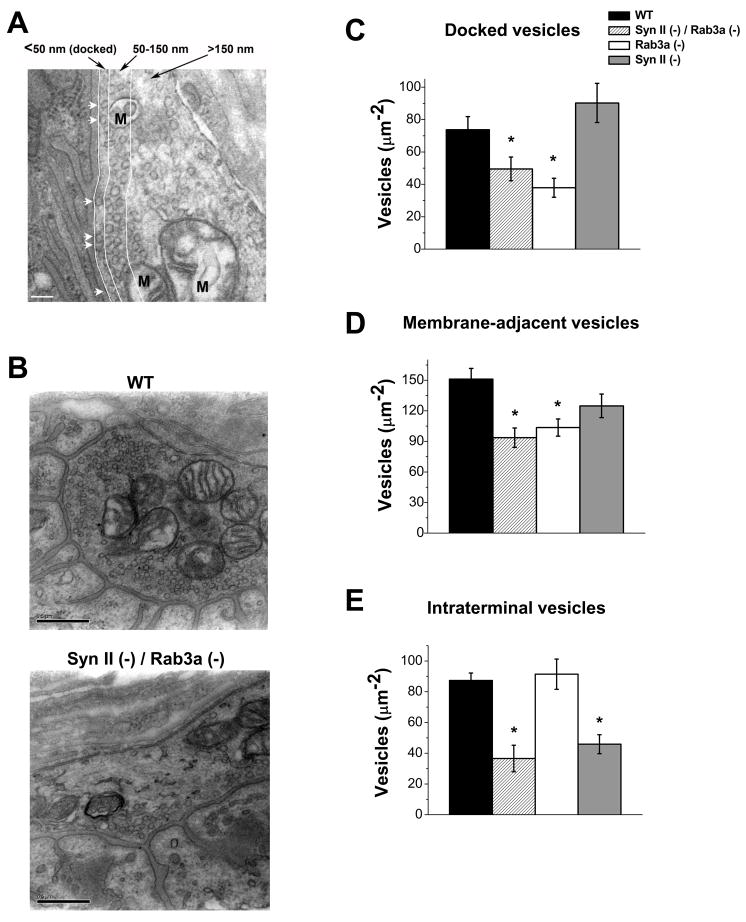
First, we investigated the distribution of vesicles in DKO synaptic terminals, including docked and intraterminal vesicles, as described in (Coleman et al., 2007). Synaptic boutons were subdivided into three layers: docked, membrane (within 150 nm from the plasma membrane), and intraterminal (Fig. 1A; Coleman et al., 2007);. We found that DKO boutons are substantially depleted of vesicles (Fig. 1, B–E), and this includes all three layers. We have already shown (Coleman et al., 2007, 2008) that Syn II deletion produces a selective depletion of intraterminal vesicles (Fig. 1, C–E, gray bars), while Rab3a deletion produces a selective depletion of docked vesicles (Fig. 1, C–E, white bars). Thus, the DKO boutons demonstrate a combined phenotype, with docked vesicles being depleted similar to Rab3a(−) and intraterminal vesicles being depleted similar to Syn II(−). Thus, the roles of Syn II and Rab3a in maintaining the total pool of synaptic vesicles appear to be complementary, with Rab3a maintaining the pool of docked vesicles, and Syn II maintaining the pool of intraterminal vesicles. We conclude that the roles of these two proteins in maintaining vesicle pools are independent (Table 1).
Fig. 1. Synapsin II and Rab3a have separate roles in maintaining the pool of synaptic vesicles within the boutons.
A. An electron micrograph showing three pools of vesicles (separated by white lines): those docked to the membrane (white arrows), those positioned at a distance of 50–150 nm from the presynaptic membrane, and those positioned at a distance greater than 150 nm. Mitochondria (marked “M”) were excluded from the analysis. Scale bar is 100nm.
B. Micrographs showing WT and Syn II (−) / Rab3a (−) DKO boutons. Vesicles are abundant in the WT terminal, while in the Syn II (−) / Rab3a (−) DKO terminal vesicles are noticeably depleted. Scale bars are 500nm.
C. Rab3a (−) boutons have a significantly reduced density of docked vesicles, while Syn II (−) boutons have no significant change in docking compared to WT. Syn II (−) / Rab3a (−) DKO boutons have a significant reduction in the density of docked vesicles, similar to the Rab3a (−).
D. Rab3a (−) boutons have a significantly reduced density of vesicles in the vicinity of the presynaptic membrane, while Syn II (−) boutons have no significant change compared to WT. Syn II (−) / Rab3a (−) DKO boutons have a significant reduction in the density of vesicles in the vicinity of the presynaptic membrane, similar to the Rab3a (−).
E. Rab3a (−) boutons have a normal density of intraterminal vesicles, while Syn II (−) boutons have a significant depletion of intraterminal vesicles compared to WT. Syn II (−) / Rab3a (−) DKO boutons also have a significant reduction in the density of intraterminal vesicles, similar to the Syn II (−). Data collected from 10 WT, 10 Rab3a (−), 10 Syn II (−), and 9 Syn II (−) / Rab3a (−) DKO terminals. * p ≤ 0.05.
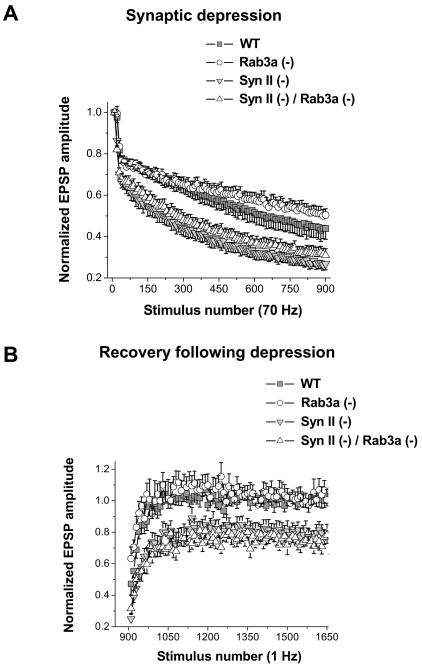
Intraterminal vesicle depletion may be associated with increased synaptic depression and delayed recovery following the depression. Thus, we investigated depression and recovery in all the four lines. In these experiments, 900 stimuli delivered at 70 Hz frequency were followed by 750 stimuli delivered at 1 Hz frequency. EPSPs recorded at the high stimulation frequency were used to monitor the depression (Fig. 2A), while EPSPs recorded during a subsequent low-frequency stimulation were used to monitor the kinetics of recovery (Fig. 2B). Consistent with the depleted intraterminal vesicles, both Syn II(−) and DKO synapses demonstrated increased synaptic depression (Fig. 2A) and incomplete recovery after depression (Fig. 2B), neither of which was observed in Rab3a(−). These results confirm that the kinetics of both depression and recovery depend largely on intraterminal vesicles, as it is similarly affected in Syn II(−) and DKO synapses and unaffected in Rab3a(−) synapses (Table 1).
Fig. 2. Syn II(−) and Syn II (−) / Rab3a (−) DKO endplates have increased synaptic depression and incomplete recovery.
A. Normalized EPSPs during a 70 Hz train of stimuli. Rab3a (−) preparations demonstrate slightly decreased synaptic depression, while both Syn II (−) and Syn II (−) / Rab3a (−) DKO preparations demonstrate increased synaptic depression compared to WT. Each data point represents the average of 10 responses.
B. Recovery rate. EPSPs were recorded every second immediately following the high-frequency stimulation. WT and Rab3a (−) preparations demonstrate complete recovery following depression, while both Syn II (−) and Syn II (−) / Rab3a (−) DKO have impaired recovery. Each data point represents a single response. Data collected from 11 WT, 12 Rab3a (−), 13 Syn II (−), and 12 Syn II (−) / Rab3a (−) DKO endplates.
Evoked synaptic transmission is significantly reduced in Syn II(−)/Rab3a(−) DKO
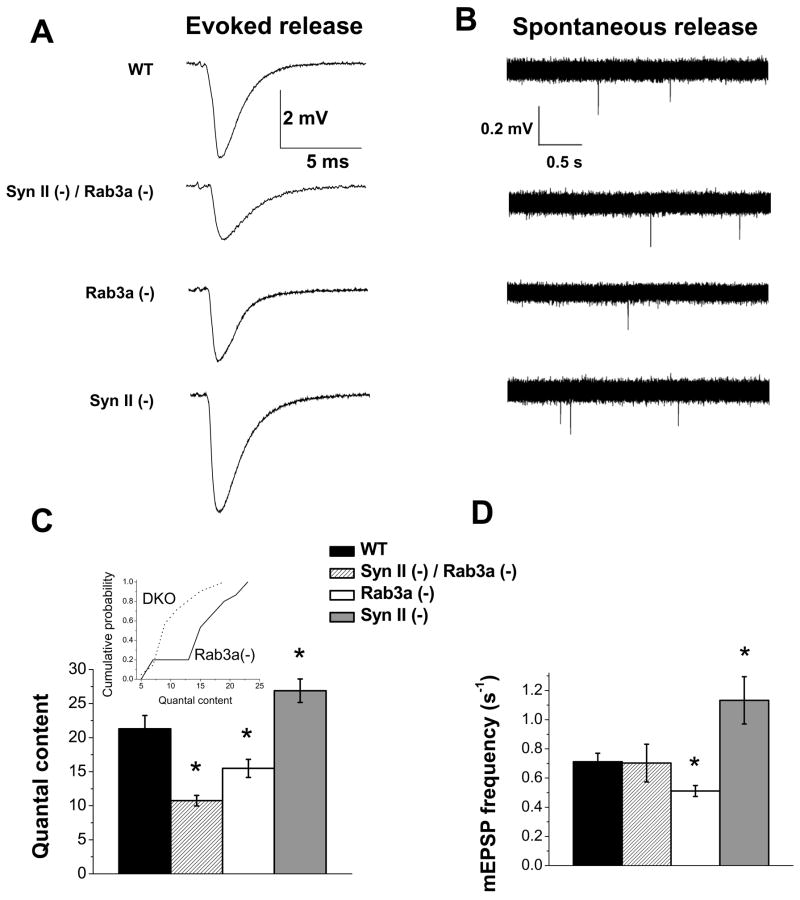
We next investigated how action potential evoked and spontaneous release are affected in DKO synapses. We found that evoked synaptic transmission is dramatically reduced (by 50%) in DKO synapses (Fig. 3A, C, striped bar). A single Rab3a deletion produced a more subtle inhibition of release (Fig. 3C, white bar; Coleman et al., 2007). In contrast, synapsin II deletion produced a subtle but significant increase in release (Fig. 3C, gray bar). Thus, single gene deletions of Rab3a or synapsin II have opposite effects on release, with synapsin II being a release inhibitor and Rab3a being a release promoter, and, strikingly, a double deletion produces a release inhibition which significantly exceeds the effect of each single deletion. To ensure that this effect is highly significant, we performed additional statistical tests to compare the Rab3a(−) and DKO strains (p<0.001 according to Kolmogorov-Smirnov test, Fig. 3C inset; p<0.005 according to independent t-test; one-way ANOVA was employed for the initial comparison of the four strains).
Fig. 3. Synapsin II and Rab3a have opposite effects on transmitter release, and the effect of the double deletion on evoked release is beyond the effect of single deletions.
A–B. Representative traces of excitatory postsynaptic potentials (EPSPs, A) and miniature excitatory postsynaptic potentials (mEPSPs, B) from WT, Syn II (−) / Rab3a (−) DKO, Rab3a (−), and Syn II (−) preparations.
C. Rab3a(−) endplates have significantly reduced quantal content, while Syn II (−) endplates have significantly increased quantal content. Syn II (−)/Rab3a (−) DKO endplates have impaired quantal content, and this effect is stronger than the effect of a single Rab3a deletion. The inset shows cumulative quantal content plots for Rab3a(−) and SynII(−)/Rab3a(−) DKO strains used in the Kolmogorov-Smirnov test. Data collected from 16m WT, 15 Rab3a, 23 Syn II (−), and 20 Syn II (−) / Rab3a (−) DKO endplates. Preparations were stimulated at 0.2Hz frequency and at least 100 stimuli were delivered. Recordings were taken at physiological (2mM) extracellular Ca2+. * p ≤ 0.05.
D. Rab3a (−) endplates have significantly reduced spontaneous release, while Syn II (−) have significantly increased spontaneous release. Syn II (−) / Rab3a (−) DKO endplates have spontaneous release similar to WT. Data collected from 55 WT, 90 Rab3a (−), 23 Syn II (−), and 20 Syn II (−) / Rab3a (−) DKO endplates. * p ≤ 0.05.
Interestingly, a very different effect of the double deletion on spontaneous neurosecretion was observed (Fig. 3B, D). Although the effect of single Rab3a or synapsin II deletions on spontaneous release did not differ from the effect of these deletions on evoked release (Fig. 3D, white and gray bars, respectively), spontaneous activity of the DKO endplates was similar to that of WT (Fig. 3D, striped and black bars, respectively). Thus, the activities of Rab3a and synapsin II in the regulation of spontaneous release are opposing and cancel each other when combined. This pattern clearly contrasts the observed functional interaction of these proteins in regulating evoked basal release (Fig. 3C).
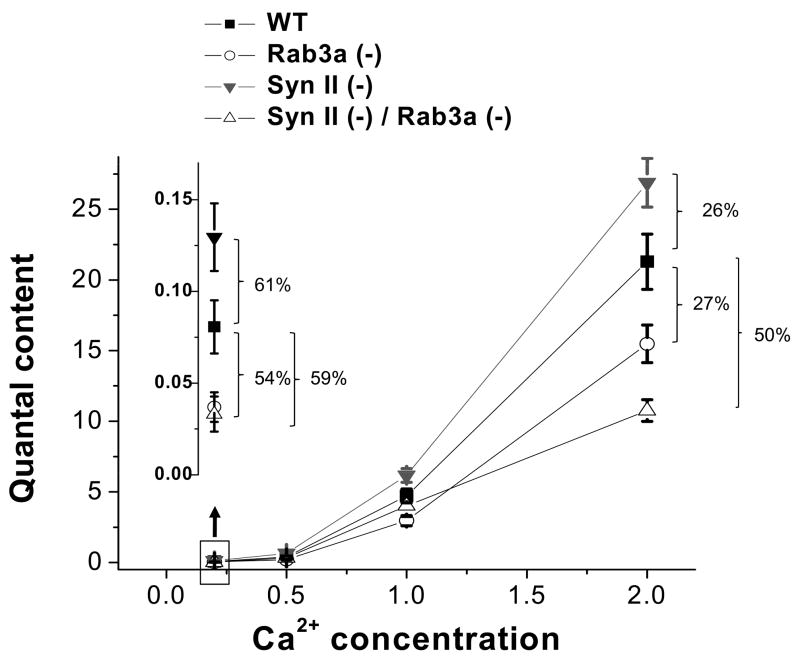
To further explore the functional interaction of synapsin II and Rab3a in regulating evoked neurosecretion, we monitored action potential evoked release at different concentrations of extracellular Ca2+. As we reported earlier (Coleman et al., 2007, 2008., 2009b), at low extracellular Ca2+ (0.2 mM), both Rab3a and synapsin II single gene deletions have a very prominent effect on quantal content (Fig. 4, inlay). As Ca2+ concentration increases, the effects of single gene deletions become less prominent (Fig. 4). This is not the case for the SynII(−)/Rab3a(−) DKO phenotype. The increase in Ca2+ does not mitigate the effect of the double deletion on quantal release (Fig. 4), and the release in DKO at normal Ca2+ conditions remains strongly inhibited.
Fig. 4. An increase in Ca2+ concentration mitigates the effect of single gene deletion but not the effect of the double gene deletions.
Quantal content of WT, Rab3a (−), Syn II (−), and Syn II (−) / Rab3a (−) DKO preparations at different extracellular Ca2+ concentrations. Inlay shows quantal content at the lowest Ca2+ level tested, 0.2mM (boxed area).
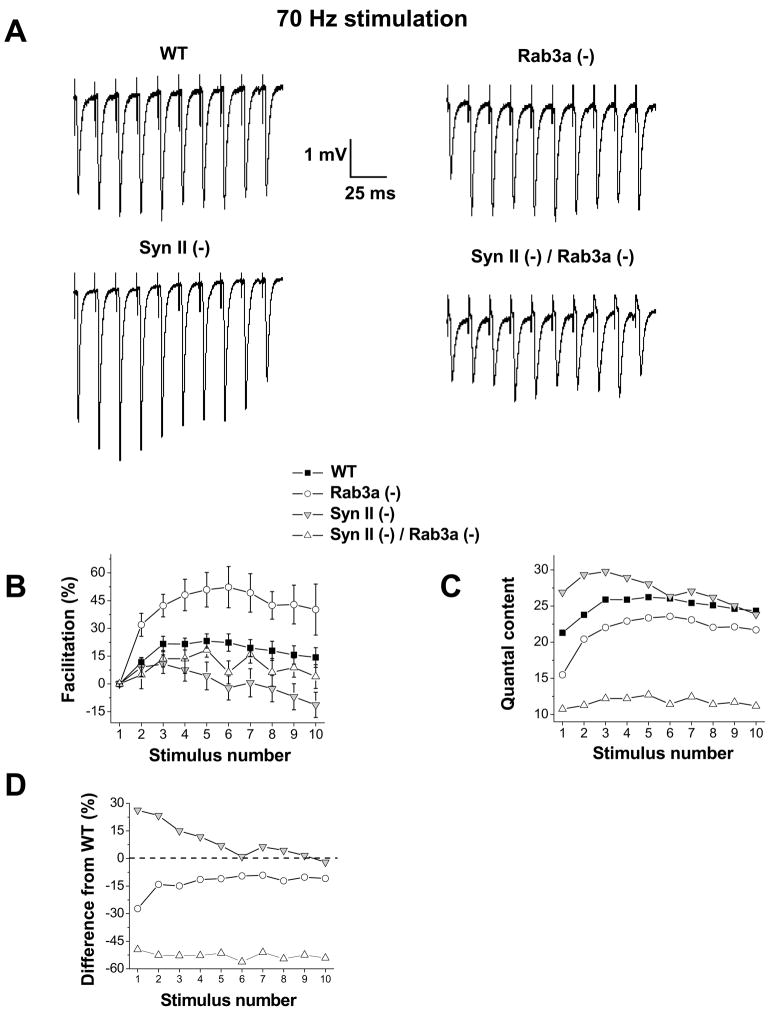
A somewhat similar effect was observed when the nerve was stimulated repetitively (at 70 Hz; Fig. 5) at normal Ca2+ conditions (2 mM). Rab3a(−) synapses, which have a slightly reduced basal release, demonstrated a stronger synaptic enhancement than WT, and thus the maximal release observed in Rab3a(−) around 5–7th stimulus approached that observed in WT (Fig. 5 B,C; Coleman et al., 2009b). Syn II (−) synapses, which have increased basal release, demonstrated a weaker enhancement than WT, and, correspondingly, the maximal release observed in Syn II(−) around 5–7th stimulus was similar to that observed in WT (Fig. 5B,C). This trend was not observed in Syn II(−)/Rab3a(−) DKO synapses. Even though the basal release in these synapses was substantially reduced compared to WT, facilitation in DKO was not increased, and it was even slightly decreased, although this effect was not significant (Fig. 5B). Thus, as a synapse was stimulated repetitively, the effects of single Rab3a or synapsin II deletions were mitigated, and this did not occur at Syn II(−)/Rab3a(−) DKO synapses (Fig. 5C,D). We suggest that Ca2+ accumulation resulting from repetitive stimulation, mitigates the effect of single synapsin II or Rab3a deletions, but it is unable to mitigate the effect of the double deletion.
Fig. 5. High-frequency stimulation mitigates the effect of single gene deletions but not the effect of the double gene deletion.
A. EPSP traces during a 70 Hz train of stimuli in WT, Rab3a (−), Syn II (−), and Syn II (−) / Rab3a (−) DKO preparations. Facilitation typically occurs within the initial 5 stimuli prior to the onset of synaptic depression.
B. Synaptic facilitation during repetitive stimulation. Rab3a (−) preparations demonstrate an increased facilitation, while Syn II (−) preparations tend to have a deceased facilitation compared to WT.
C. Rab3a (−) preparations have reduced initial quantal content. However, during repetitive stimulation, the release levels in Rab3a(−) tend to increase and approach that of WT. Syn II (−) preparations have increased initial quantal content, while during repetitive stimulation Syn II (−) release levels tend to decrease and become similar to WT levels. Syn II (−) / Rab3a (−) DKO preparations have strongly reduced quantal content compared to WT, and repetitive stimulation does not mitigate this release impairment.
D. Percent difference between the knockout preparations and WT under repetitive stimulation conditions. Data collected from 11 WT, 12 Rab3a (−), 13 Syn II (−), and 12 Syn II (−) / Rab3a (−) DKO endplates.
Rab3a deletion rescues epileptic seizures, which are typical for Syn II (−)
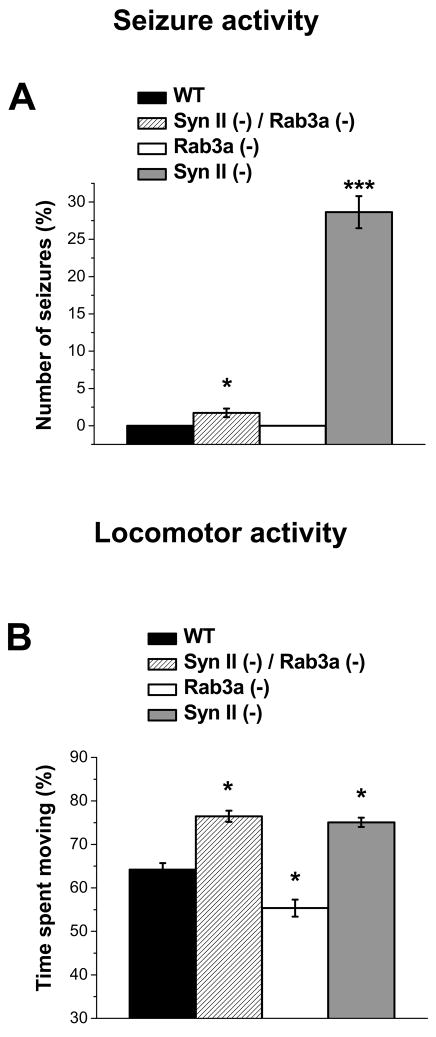
Strikingly, we found that DKO animals had only occasional seizure activity, while in Syn II (−) animals the seizure activity was very prominent (Fig 6). Specifically, 25–30% of Syn II (−) mice showed strong full body seizures, similar to those described in (Etholm and Heggelund, 2009), immediately after being provoked (Fig. 6A, gray bar). Such seizures were never observed in either WT or Rab3a(−) animals (Fig. 6A, black and white bars, respectively). The Syn II(−)/Rab3a(−) DKO animals did show some seizure activity, but it was observed infrequently (2–3%). Thus, Rab3a gene deletion rescued epileptic seizures, which are prominent in Syn II (−) animals, and which are, in general, typical for synapsin gene deleted animals (Rosahl et al., 1995; Etholm and Heggelund, 2009).
Fig. 6. The effect of synapsin II and Rab3a on seizure and locomotor activity, with the epileptic seizures typical for Syn II(−) animals being partially rescued by the Rab3a gene deletion.
A. WT and Rab3a (−) mice have no seizure activity, while Syn II (−) mice have prominent seizures. Syn II (−)/Rab3a (−) DKO animals have a very low frequency of seizures, demonstrating a partial rescue of the Syn II (−) phenotype by the Rab3a deletion. Each bar is the average of 4 separate trials. Data collected from 54 WT, 27 Rab3a (−), 18 Syn II (−), and 44 Syn II (−) / Rab3a (−) DKO mice. * p ≤ 0.05, *** p ≤ 0.0005
B. Rab3a (−) mice spend significantly more time resting than WT mice, while both the Syn II (−) and the Syn II (−)/Rab3a (−) DKO mice spend significantly more time moving than WT mice. Data was collected for 10 minutes in 10 s bins from 18 WT, 16 Rab3a (−), 16 Syn II (−), and 18 Syn II (−) / Rab3a (−) DKO mice. * p ≤ 0.05.
To explore further the effect of the double deletion at the behavioral level, we tested the locomotor activity. We found that the locomotor activity in SynII(−)/Rab3a(−) DKO (Fig. 1B, striped bars) and in Syn II(−) animals (Fig. 1B, gray bars) were similarly increased compared to WT animals (the results for males and females were pooled together since we did not detect any significant difference between the sexes). The observed increase in the locomotor activity in Syn II(−) animals agrees with the results reported by Dyck et al., (2009). The increase in locomotor activity in DKO and Syn II(−) animals was opposing to the effect of Rab3a gene deletion (Fig. 1B, white bar). The decrease in motor activity of freely moving Rab3a(−) animals agrees with the trend observed by D’Adamo et al. (2004), although in the latter study this effect was not found to be statistically significant and was not consistent with motor activity tested using the running wheel.
Thus, we found that Syn II and Rab3a functional interactions may manifest themselves at the behavioral level, with the deletion of one protein preventing the effect of the deletion of the other protein. The significance of this finding is illustrated by the ability of the Rab3a deletion to mitigate the effect of synapsin II deletion on seizure susceptibility.
DISCUSSION
Aiming to understand the functional interaction between two proteins that govern the preparation of synaptic vesicles for the release process, synapsin and Rab3a, we derived a double knockout mouse lacking both synapsin II and Rab3a, and compared its synaptic ultrastructure and function with that of single knockouts. At the behavioral level, we discovered that simultaneous Rab3a deletion rescued epileptic seizures which are typical for synapsin I and synapsin II knockout animals. This finding warrants a systematic study of the effects of synapsin/Rab3a functional interaction at different types of synapses. Here, we have initiated this study employing the mouse diaphragm synapse, an excellent model system for rigorously monitoring subtle changes in the release process. We found that basal synaptic transmission is noticeably reduced in DKO synapses, and this finding cannot be explained by either independent or additive effects of the two individual deletions. In contrast, the effect of the two deletions on synaptic vesicle distribution was combined, with synapsin II maintaining intraterminal vesicles and Rab3a maintaining docked vesicles, and consequentially, DKO synapses being severely and uniformly depleted. Thus, a complex functional interaction of synapsin II and Rab3a in regulating transmitter release cannot be explained by the regulation of vesicle docking. In contrast, it is plausible that these proteins may functionally interact at the latest stages of Ca2+ -dependent vesicle priming and fusion.
Synapsin-dependent epileptic seizures
We have demonstrated that epileptic seizures typical for synapsin I and II knockouts (Rosahl et al., 1993, 1995; Li et al., 1995; Etholm and Heggelund, 2009) can be rescued by Rab3a gene deletion. Since synapsin levels are modified in animal models of epilepsy (Morimoto et al., 1998; Suemaru et al., 2000; Sato and Abe, 2001, Liu et al., 2008) and since synapsin mutation was identified in a human family with epilepsy (Garcia et al. 2004), it is likely that some forms of epilepsy may result from impairments in presynaptic vesicle cycling, possibly in inhibitory GABAergic nerve terminals (Terada et al., 1999; Hirsch et al., 1999; Gitler et al., 2004) This suggestion is further strengthened by an observation that mice lacking a vesicle-associated protein SV2 demonstrated severe epileptic seizures (Crowder et al., 1999). Our finding that this type of epileptic seizure is rescued by Rab3a gene deletion warrants a systematic investigation of the effect of Rab3a regulation on synapsin deficient terminals in both glutamatergic and GABAergic synapses of the central nervous system. The Syn II(−)/Rab3a(−) DKO animals generated here represent an advantageous model system for such a study.
Such an investigation was, however, out of the scope of the present study. Here, we present the first report of synaptic transmission at the Syn II(−)/Rab3a(−) DKO synapses at both the functional and ultrastructural levels employing the diaphragm synapse. This synapse represents the most advantageous model system for the initial study of synaptic transmission in Syn II(−)/Rab3a(−) DKO, since at this synapse the actions of synapsin II and Rab3a have already been documented (Samigullin et al., 2004; Hirsh et al., 2002; Sons and Plomp, 2006; Coleman et al., 2007, 2008, 2009b), and since it enables assessing the magnitude of quantal release with a remarkable degree of rigor by providing an opportunity to record synaptic responses from individual singly innervated endplates.
The nature of synapsin II/Rab3a functional interaction
Since molecular interaction of synapsins and Rab3a on synaptic vesicles has been demonstrated (Giovedi et al., 2004a,b), we questioned whether this interaction reveals itself in our experiments. A molecular interaction of these two proteins in a single signaling pathway would be expected to produce their cooperative effect, where the loss of each protein affects a synaptic/behavioral characteristic in the same direction. Furthermore, in this case the effect of a double deletion would be either similar or stronger than the effects of single deletions. It is important to note that in our experiments we did not observe a single characteristic at either the synaptic or behavioral level corresponding to this pattern.
The comparison of the patterns of functional interactions between synapsin II and Rab3a in regulating different synaptic or behavioral characteristics falls into several categories (Table 1). First, each of these proteins regulates synaptic vesicle distribution, and their actions in this function appear to be independent. Second, both proteins regulate the rate of transmitter release in an opposing and Ca2+-dependent manner, and their actions in regulating spontaneous release are additive, suggesting possible “push-pull” dynamics. The most intriguing finding is that the double gene deletion produces a very strong inhibitory effect on the action potential evoked release and at physiological conditions this effect surpasses the effect of single Rab3a deletion. It is important to note that in regulating quantal content the Rab3a deletion appears to prevent the effect of the synapsin II deletion, and this is also the case for the regulation of epileptic seizures.
Synapsin II and Rab3a have independent roles in maintaining the pool of synaptic vesicles
Our data show no evidence of synapsin II/Rab3a interaction in regulating vesicle clustering. We found that synapsin II selectively maintains the pool of intraterminal synaptic vesicles (the reserve pool), and thus synapsin II deficiency is manifested in depleted intraterminal vesicles, increased synaptic depression, and incomplete recovery following depression. Rab3a deficiency has no effect on either of these characteristics. However, Rab3a deletion affects the pool of docked (releasable) vesicles.
The Syn II(−)/Rab3a(−) DKO appears to have a combined phenotype in maintaining these vesicle pools. Indeed, intraterminal layers were equally depleted in Syn II (−) and Syn II(−)/Rab3a(−) DKO terminals, and, consistently, these two lines demonstrated an equal enhancement in synaptic depression and an equal impairment in the recovery from depression. Furthermore, membrane-adjacent vesicles and docked vesicles were depleted to an equal degree in Rab3a(−) and SynII(−)/Rab3a(−) DKO terminals, showing no evidence that synapsin II/Rab3a interaction had any effect on vesicle docking.
In spite of that, basal evoked release at Syn II(−)/Rab3a(−) DKO synapses was found to be significantly lower than at Rab3a(−) terminals, and this finding is the most intriguing.
Synapsin II/Rab3a functional interaction may regulate final Ca2+-dependent stages of exocytosis
It is of interest that synapsin II and Rab3a have opposing effects on synaptic transmission, and these effects are the most prominent at low Ca2+ conditions (Coleman et al., 2007,2008,2009b). We must note that the increased basal release in Syn II(−) at normal Ca2+ was not detected in our earlier studies, where it was reported that basal release in Syn II(−) at physiological Ca2+ was normal (Samigullin et al.,2004; Coleman et al., 2008). The reason for this discrepancy is that at the studies cited above we performed the recordings at normal Ca2+ conditions without discriminating between type I and type II terminals. This increased variability between individual experiments, and thus a subtle effect of synapsin II gene deletion on basal quantal release was overlooked.
At low Ca2+ conditions, basal evoked release in Rab3a(−) and Syn II(−)/Rab3a(−) DKO synapses was found to be similar. This is not surprising given that these two lines have similar density of docked vesicles. However, an increase in extracellular Ca2+ concentration partially rescued the effect of Rab3a deletion in single Rab3a(−) animals. Similarly, we found that Rab3a deficiency can be partially mitigated by repetitive stimulation at a high frequency, presumably due to Ca2+ accumulation at the active zones. This was not the case for the Syn II(−)/Rab3a(−) DKO synapses: neither repetitive stimulation nor an increase in Ca2+ concentration was able to mitigate the effect of the double gene deletion on quantal content.
It is of interest that at both Rab3a(−) and Syn II(−) synapses, deviations in the short-term synaptic enhancement can be explained by a modified initial amount of release, as in general it is accepted that a decreased release would produce an increase in facilitation, and vice versa. However, this pattern does not apply to DKO synapses: although an initial amount of release in this synapse is decreased by approximately twice, facilitation is not increased. Whereas in both Syn II(−) and Rab3a(−) synapses the deviations in the amount of release are mitigated by repetitive stimulation and become negligible after 5–6 stimuli, the DKO synapses retain a significantly decreased release even when being stimulated repetitively.
These results indicate a rather complex functional interaction of synapsin II and Rab3a in regulating the action potential evoked release, including its Ca2+ dependence and short-term plasticity. Both proteins affect the release, their effects are opposing and can be mitigated by increased Ca2+ levels. The effect of the double deletion is inhibitory, similar to the effect of Rab3a deletion, and the effect of the double deletion cannot be mitigated by the increased Ca2+ levels. This pattern (Table 1) contrasts to the action of these two proteins in the regulation of the rate of spontaneous release, where opposing effects of Rab3a and synapsin II cancel out in Syn II(−)/Rab3a(−) DKO synapses. Furthermore, this pattern also contrasts to the action of these two proteins in the regulation of vesicle docking, where synapsin II has no effect at the physiological Ca2+ concentration, and the effect of the double deletion coincides with the effect of the Rab3a deletion. Thus, our results indicate a complex functional interaction of synapsin II and Rab3a at the late Ca2+-dependent stages of exocytosis.
Importantly, this complex functional interaction between synapsin II and Rab3a is specific to action potential evoked release, and does not manifest itself in the regulation of spontaneous release. Although synapsin II and Rab3a deletions have opposing effects on the rate of spontaneous synaptic activity, these effects are additive producing a normal rate of spontaneous release in the Syn II(−)/Rab3a(−) DKO. This differential interaction of Rab3a and synapsin II in regulating evoked and spontaneous release may be explained by the studies reporting that spontaneous neurosecretory quanta are released from an isolated pool of vesicles (Sara et al., 2005; Fredj and Burrone, 2009), which implies different successions of preparatory release steps for spontaneous and evoked neurosecretion.
Notably, the pattern of the regulation of release by synapsin II and Rab3a is different from the pattern of the regulation of vesicle docking by these proteins, except for the single Rab3a deletion (Table 1). Thus, synapsin II deletion did not affect docking but increased both evoked and spontaneous release. Furthermore, the double gene deletion reduced vesicle docking similar to the Rab3a deletion, however it reduced the evoked release to a larger extent and did not affect the spontaneous release. These results point out to a likely role of synapsin II and its functional interaction with Rab3a in the regulation of the latest stages of Ca2+-dependent exocytosis that are downstream from vesicle docking.
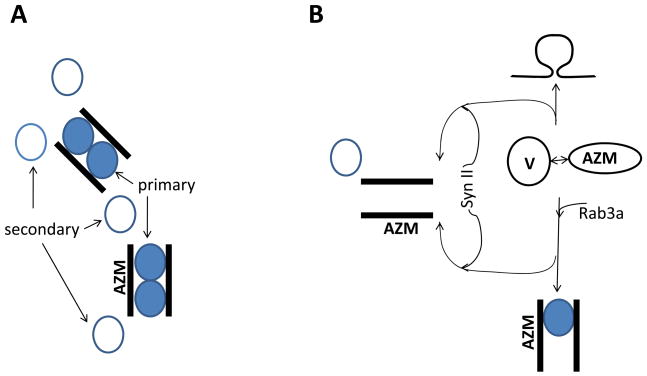
The observed effects of synapsin II and Rab3a on release may be explained parsimoniously by a model that involves a pool of vesicles in the immediate proximity to Ca2+ channels, as was proposed in several experimental and modeling studies (Shahrezaei and Delaney, 2004; Wadel et al., 2007; Pan and Zucker, 2009) and observed recently by electron tomography (Nagwaney et al., 2009). As was demonstrated by the latter study, the arrangement of docked vesicles at the diaphragm synapse involves “primary” vesicles enclosed by the bands of active zone material and “secondary” vesicles at the periphery of active zones (Fig. 7A). The “primary vesicles” are situated in the immediate proximity to the clusters of Ca2+ channels, and this structural heterogeneity creates a basis for the functional heterogeneity between docked vesicles, which has been previously proposed in a number of studies (Neher 1998 for review). Furthermore, it was proposed that vesicle “superpriming” or targeting to the clusters of Ca2+ channels is mediated by Rab3a (Schluter et al., 2006, Gracheva et al., 2008; Coleman and Bykhovskaia, 2009b). In view of our results, it is an attractive hypothesis that synapsin II targets vesicles to the “secondary” pool. In other words, we propose that upon an interaction with the active zone material, a vesicle may either fuse with the plasma membrane producing a spontaneous neurosecretory quantum, or become temporarily locked in a docked “primary” or “secondary” state in the anticipation of an action potential (Fig. 7B), and Rab3a and synapsin II would mediate the choice between these options. It is also likely that a small proportion of “primary” and “secondary” vesicles can be released in a spontaneous or asynchronous mode that is uncoupled from the stimulus. This model predicts that in the absence of Rab3a the pool of “primary” vesicles will be reduced, and that will be manifested by the reduction in the number of docked vesicles, as well as a decrease in the rate of evoked and spontaneous release. Furthermore, this effect will be the most pronounced at low Ca2+ levels, since Ca2+ elevation may induce release of some of “secondary” vesicles and thus partially mitigate Rab3a deficiency. These are the results that have been observed in our studies (Coleman et al., 2009b, and the present study). The synapsin II deficiency would reduce the number of vesicles that are diverted from the “primary” to the “secondary” pool, and that would increase the rate of evoked and spontaneous release. Again, this effect would be the most pronounced at low Ca2+ levels, since Ca2+ elevation would enhance release probabilities among secondary vesicles and thus partially mitigate vesicle heterogeneity. Finally, in Rab3a(−)/Syn II(−) DKO synapses, vesicles of both “primary” and “secondary” pools will be reduced. Instead of becoming docked, a part of the vesicle pool will be diverted to the asynchronous release mode. That would produce a significant reduction in the evoked but not in the spontaneous release, just as it was observed experimentally. Although this model is at present highly speculative, it is based on experimental evidence, and it provides a parsimonious explanation for the presented data.
Fig. 7. Hypothetical vesicle cycle diagram showing possible roles of the proteins synapsin II and Rab3a in the preparatory steps for the release process.
A. Cartoon showing the organization of docked vesicles around bands of active zone material (AZM) observed by electron tomography (Nagwaney et al., 2009). The diagram shows “primary” vesicles (gray) enclosed between the bands of AZM and “secondary” vesicles observed at the periphery of AZM. Most likely, primary vesicles would have higher release probabilities being in the immediate vicinity of Ca2+ channel clusters.
B. A hypothetical model proposing that upon interacting with AZM, a vesicle can be either docked as “primary”, or as “secondary”, or fused with the plasma membrane releasing neurotransmitter. Rab3a promotes vesicle targeting to AZM and docking. Synapsin II positively regulates the pathway that diverts the vesicles from other pathways to become docked as “secondary”.
Our results suggest that synapsin II is likely to regulate the latest stages of exocytosis. Such a role has been already proposed for synapsin I, as synapsin I was demonstrated to affect directly the time-course of release (Hilfiker et al., 1998). However, synapsin II unlike synapin I, does not affect the release time-course (Coleman and Bykhovskaia, 2009a). Thus, the present study represents the first evidence of a synapsin II action in the latest stages of exocytosis. This synapsin II-dependent mechanism interacts functionally with Rab3a-dependent vesicle targeting.
It is notable that Rab3a precludes the excitation-enhancing effect of synapsin II loss at the level of seizure activity as well as at the level of the transmitter release (Table 1). Both evoked and spontaneous release components are increased in Syn II (−) terminals, decreased in Rab3a(−) terminals, and either decreased (evoked) or unchanged (spontaneous) in Rab3a(−)/Syn II(−) DKO terminals. A thorough examination of both evoked and spontaneous activity in central glutamatergic and GABAergic synapses coupled with systematic study of seizure activity in the transgenic lines under the study is required to address the question of whether effects on spontaneous or action potential evoked release components underlie the observed functional interaction of synapsin II and Rab3a in the regulation of seizure activity.
Acknowledgments
We thank Cynthia A. Bill and Lidia Zueva for their technical assistance in performing the electron microscopy analysis, John Nyby and Seetha Chandrasekhara for assistance in collecting behavioral data, and the UCC Behavioral Testing Facility (Lehier Rojas, Lydia Miranda, and Jenise Segarra) for animal vital parameters measurements. This study was supported by the grants R01 MH061059 and U54 NS039408 (MB), and G12 RR-03035-24 (UCC Behavioral Testing Facility) from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- D’Adamo P, Wolfer DP, Kopp C, Tobler I, Toniolo D, Lipp HP. Mice deficient for the synaptic vesicle protein Rab3a show impaired spatial reversal learning and increased explorative activity but none of the behavioral changes shown by mice deficient for the Rab3a regulator Gdi1. Eur J Neurosci. 2004;19:1895–1905. doi: 10.1111/j.1460-9568.2004.03270.x. [DOI] [PubMed] [Google Scholar]
- Alvarez YD, Ibanez LI, Uchitel OD, Marengo FD. P/Q Ca2+ channels are functionally coupled to exocytosis of the immediately releasable pool in mouse chromaffin cells. Cell Calcium. 2008;43:155–164. doi: 10.1016/j.ceca.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Bykhovskaia M. Making quantal analysis more convenient, fast, and accurate: user-friendly software QUANTAN. J Neurosci Methods. 2008;168:500–513. doi: 10.1016/j.jneumeth.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi P, Greengard P, Ryan TA. Synapsin dispersion and reclustering during synaptic activity. Nat Neurosci. 2001;4:1187–1193. doi: 10.1038/nn756. [DOI] [PubMed] [Google Scholar]
- Chi P, Greengard P, Ryan TA. Synaptic vesicle mobilization is regulated by distinct synapsin I phosphorylation pathways at different frequencies. Neuron. 2003;38:69–78. doi: 10.1016/s0896-6273(03)00151-x. [DOI] [PubMed] [Google Scholar]
- Coleman WL, Bill CA, Bykhovskaia M. Rab3a deletion reduces vesicle docking and transmitter release at the mouse diaphragm synapse. Neuroscience. 2007;148:1–6. doi: 10.1016/j.neuroscience.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Coleman WL, Bill CA, Simsek-Duran F, Lonart G, Samigullin D, Bykhovskaia M. Synapsin II and calcium regulate vesicle docking and the cross-talk between vesicle pools at the mouse motor terminals. J Physiol. 2008;586:4649–4673. doi: 10.1113/jphysiol.2008.154666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman WL, Bykhovskaia M. Synapsin I accelerates the kinetics of neurotransmitter release in mouse motor terminals. Synapse. 2009;63:531–533. doi: 10.1002/syn.20635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman WL, Bykhovskaia M. Rab3a-mediated vesicle recruitment regulates short-term plasticity at the mouse diaphragm synapse. Mol Cell Neurosci. 2009;41:286–296. doi: 10.1016/j.mcn.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Cousin MA, Malladi CS, Tan TC, Raymond CR, Smillie KJ, Robinson PJ. Synapsin I-associated phosphatidylinositol 3-kinase mediates synaptic vesicle delivery to the readily releasable pool. J Biol Chem. 2003;278:29065–29071. doi: 10.1074/jbc.M302386200. [DOI] [PubMed] [Google Scholar]
- Crowder KM, Gunther JM, Jones TA, Hale BD, Zhang HZ, Peterson MR, Scheller RH, Chavkin C, Bajjalieh SM. Abnormal neurotransmission in mice lacking synaptic vesicle protein 2A (SV2A) Proc Natl Acad Sci USA. 1999;96:15268–15273. doi: 10.1073/pnas.96.26.15268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyck BA, Skoblenick KJ, Castellano JM, Ki K, Thomas N, Mishra RK. Behavioral abnormalities in synapsin II knockout mice implicate a causal factor in schizophrenia. Synapse. 2009;63:662–672. doi: 10.1002/syn.20643. [DOI] [PubMed] [Google Scholar]
- Etholm L, Heggelund P. Seizure elements and seizure element transitions during tonic-clonic seizure activity in the synapsin I/II double knockout mouse: a neuroethological description. Epilepsy Behav. 2009;14:582–590. doi: 10.1016/j.yebeh.2009.02.021. [DOI] [PubMed] [Google Scholar]
- Fischer VM, Sudhof TC, Jahn R. A small GTP-binding protein dissociates from synaptic vesicles during exocytosis. Nature. 1991;349:79–81. doi: 10.1038/349079a0. [DOI] [PubMed] [Google Scholar]
- Fredj NB, Burrone J. A resting pool of vesicles is responsible for spontaneous vesicle fusion at the synapse. Nat Neurosci. 2009 doi: 10.1038/nn.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia CC, Blair HJ, Seager M, Coulthard A, Tennant S, Buddles M, Curtis A, Goodship JA. Identification of a mutation in synapsin I, a synaptic vesicle protein, in a family with epilepsy. J Med Genet. 2004;41:183–186. doi: 10.1136/jmg.2003.013680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geppert M, Bolshakov VY, Siegelbaum SA, Takei K, De Camilli P, Hammer RE, Sudhof TC. The role of Rab3A in neurotransmitter release. Nature. 1994;369:493–497. doi: 10.1038/369493a0. [DOI] [PubMed] [Google Scholar]
- Geppert M, Goda Y, Stevens CF, Sudhof TC. The small GTP-binding protein Rab3A regulates a late step in synaptic vesicle fusion. Nature. 1997;387:810–814. doi: 10.1038/42954. [DOI] [PubMed] [Google Scholar]
- Giovedi S, Vaccaro P, Valtorta F, Darchen F, Greengard P, Cesareni G, Benfenati F. Synapsin is a novel Rab3 effector protein on small synaptic vesicles. I. Identification and characterization of the synapsin I-Rab3 interactions in vitro and in intact nerve terminals. J Biol Chem. 2004;279:43760–43768. doi: 10.1074/jbc.M403293200. [DOI] [PubMed] [Google Scholar]
- Giovedi S, Darchen F, Valtorta F, Greengard P, Benfenati F. Synapsin is a novel Rab3 effector protein on small synaptic vesicles. II. Functional effects of the Rab3A-synapsin I interaction. J Biol Chem. 2004;279:43769–43779. doi: 10.1074/jbc.M404168200. [DOI] [PubMed] [Google Scholar]
- Gitler D, Takagishi Y, Feng J, Ren Y, Rodriguiz RM, Wetsel WC, Greengard P, Augustine GJ. Different presynaptic roles of synapsins at excitatory and inhibitory synapses. J Neurosci. 2004;24:11368–11380. doi: 10.1523/JNEUROSCI.3795-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitler D, Cheng Q, Greengard P, Augustine GJ. Synapsin IIa controls the reserve pool of glutamatergic synaptic vesicles. J Neurosci. 2008;28:10835–10843. doi: 10.1523/JNEUROSCI.0924-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracheva EO, Hadwiger G, Nonet ML, Richmond JE. Direct interactions between C. elegans RAB-3 and Rim provide a mechanism to target vesicles to the presynaptic density. Neurosci Lett. 2008;444:137–142. doi: 10.1016/j.neulet.2008.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham ME, Handley MT, Barclay JW, Ciufo LF, Barrow SL, Morgan A, Burgoyne RD. A gain-of-function mutant of Munc18–1 stimulates secretory granule recruitment and exocytosis and reveals a direct interaction of Munc18–1 with Rab3. Biochem J. 2008;409:407–416. doi: 10.1042/BJ20071094. [DOI] [PubMed] [Google Scholar]
- Greengard P, Valtorta F, Czernik AJ, Benfenati F. Synaptic vesicle phosphoproteins and regulation of synaptic function. Science. 1993;259:780–785. doi: 10.1126/science.8430330. [DOI] [PubMed] [Google Scholar]
- Hilfiker S, Schweizer FE, Kao HT, Czernik AJ, Greengard P, Augustine GJ. Two sites of action for synapsin domain E in regulating neurotransmitter release. Nat Neurosci. 1998;1:29–35. doi: 10.1038/229. [DOI] [PubMed] [Google Scholar]
- Hirsch JC, Agassandian C, Merchan-Perez A, Ben-Ari Y, DeFelipe J, Esclapez M, Bernard C. Deficit of quantal release of GABA in experimental models of temporal lobe epilepsy. Nat Neurosci. 1999;2:499–500. doi: 10.1038/9142. [DOI] [PubMed] [Google Scholar]
- Hirsh JK, Searl TJ, Silinsky EM. Regulation by Rab3A of an endogenous modulator of neurotransmitter release at mouse motor nerve endings. J Physiol. 2002;545:337–343. doi: 10.1113/jphysiol.2002.032516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz RW, Brondyk WH, Senter RA, Kuizon L, Macara IG. Evidence for the involvement of Rab3A in Ca(2+)-dependent exocytosis from adrenal chromaffin cells. J Biol Chem. 1994;269:10229–10234. [PubMed] [Google Scholar]
- Hosaka M, Sudhof TC. Synapsins I and II are ATP-binding proteins with differential Ca2+ regulation. J Biol Chem. 1998;273:1425–1429. doi: 10.1074/jbc.273.3.1425. [DOI] [PubMed] [Google Scholar]
- Hosaka M, Hammer RE, Sudhof TC. A phospho-switch controls the dynamic association of synapsins with synaptic vesicles. Neuron. 1999;24:377–387. doi: 10.1016/s0896-6273(00)80851-x. [DOI] [PubMed] [Google Scholar]
- Johannes L, Lledo PM, Roa M, Vincent JD, Henry JP, Darchen F. The GTPase Rab3a negatively controls calcium-dependent exocytosis in neuroendocrine cells. EMBO J. 1994;13:2029–2037. doi: 10.1002/j.1460-2075.1994.tb06476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic JN, Sihra TS, Nairn AC, Hemmings HC, Jr, Greengard P, Czernik AJ. Opposing changes in phosphorylation of specific sites in synapsin I during Ca2+-dependent glutamate release in isolated nerve terminals. J Neurosci. 2001;21:7944–7953. doi: 10.1523/JNEUROSCI.21-20-07944.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao HT, Porton B, Czernik AJ, Feng J, Yiu G, Haring M, Benfenati F, Greengard P. A third member of the synapsin gene family. Proc Natl Acad Sci USA. 1998;95:4667–4672. doi: 10.1073/pnas.95.8.4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leenders AG, Lopes da Silva FH, Ghijsen WE, Verhage M. Rab3a is involved in transport of synaptic vesicles to the active zone in mouse brain nerve terminals. Mol Biol Cell. 2001;12:3095–3102. doi: 10.1091/mbc.12.10.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Chin LS, Shupliakov O, Brodin L, Sihra TS, Hvalby O, Jensen V, Zheng D, McNamara JO, Greengard P. Impairment of synaptic vesicle clustering and of synaptic transmission, and increased seizure propensity, in synapsin I-deficient mice. Proc Natl Acad Sci USA. 1995;92:9235–9239. doi: 10.1073/pnas.92.20.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XY, Yang JL, Chen LJ, Zhang Y, Yang ML, Wu YY, Li FQ, Tang MH, Liang SF, Wei YQ. Comparative proteomics and correlated signaling network of rat hippocampus in the pilocarpine model of temporal lobe epilepsy. Proteomics. 2008;8:582–603. doi: 10.1002/pmic.200700514. [DOI] [PubMed] [Google Scholar]
- Mantilla CB, Sieck GC. Invited review: Mechanisms underlying motor unit plasticity in the respiratory system. J Appl Physiol. 2003;94:1230–1241. doi: 10.1152/japplphysiol.01120.2002. [DOI] [PubMed] [Google Scholar]
- Mizoguchi A, Kim S, Ueda T, Kikuchi A, Yorifuji H, Hirokawa N, Takai Y. Localization and subcellular distribution of smg p25A, a ras p21-like GTP-binding protein, in rat brain. J Biol Chem. 1990;265:11872–11879. [PubMed] [Google Scholar]
- Morimoto K, Sato K, Sato S, Suemaru S, Sato T, Yamada N, Hayabara T. Increases in mRNA levels for synapsin I but not synapsin II in the hippocampus of the rat kindling model of epilepsy. Seizure. 1998;7:229–235. doi: 10.1016/s1059-1311(98)80041-1. [DOI] [PubMed] [Google Scholar]
- Murthy VN. Spreading synapsins. Nat Neurosci. 2001;4:1155–1157. doi: 10.1038/nn1201-1155. [DOI] [PubMed] [Google Scholar]
- Nagwaney S, Harlow ML, Jung JH, Szule JA, Ress D, Xu J, Marshall RM, McMahan UJ. Macromolecular connections of active zone material to docked synaptic vesicles and presynaptic membrane at neuromuscular junctions of mouse. J Comp Neurol. 2009;513:457–468. doi: 10.1002/cne.21975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. Vesicle pools and Ca2+ microdomains: new tools for understanding their roles in neurotransmitter release. Neuron. 1998;20:389–399. doi: 10.1016/s0896-6273(00)80983-6. [DOI] [PubMed] [Google Scholar]
- Nonet ML, Staunton JE, Kilgard MP, Fergestad T, Hartwieg E, Horvitz HR, Jorgensen EM, Meyer BJ. Caenorhabditis elegans rab-3 mutant synapses exhibit impaired function and are partially depleted of vesicles. J Neurosci. 1997;17:8061–8073. doi: 10.1523/JNEUROSCI.17-21-08061.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhauser AF, Monck JR, Balch WE, Fernandez JM. Exocytotic fusion is activated by Rab3a peptides. Nature. 1992;360:270–273. doi: 10.1038/360270a0. [DOI] [PubMed] [Google Scholar]
- Padykula HA, Gauthier GF. The ultrastructure of the neuromuscular junctions of mammalian red, white, and intermediate skeletal muscle fibers. J Cell Biol. 1970;46:27–41. doi: 10.1083/jcb.46.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B, Zucker RS. A general model of synaptic transmission and short-term plasticity. Neuron. 2009;62:539–554. doi: 10.1016/j.neuron.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash YS, Miller SM, Huang M, Sieck GC. Morphology of diaphragm neuromuscular junctions on different fibre types. J Neurocytol. 1996;25:88–100. doi: 10.1007/BF02284788. [DOI] [PubMed] [Google Scholar]
- Rosahl TW, Geppert M, Spillane D, Herz J, Hammer RE, Malenka RC, Sudhof TC. Short-term synaptic plasticity is altered in mice lacking synapsin I. Cell. 1993;75:661–670. doi: 10.1016/0092-8674(93)90487-b. [DOI] [PubMed] [Google Scholar]
- Rosahl TW, Spillane D, Missler M, Herz J, Selig DK, Wolff JR, Hammer RE, Malenka RC, Sudhof TC. Essential functions of synapsins I and II in synaptic vesicle regulation. Nature. 1995;375:488–493. doi: 10.1038/375488a0. [DOI] [PubMed] [Google Scholar]
- Sakaba T. Roles of the fast-releasing and the slowly releasing vesicles in synaptic transmission at the calyx of held. J Neurosci. 2006;26:5863–5871. doi: 10.1523/JNEUROSCI.0182-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samigullin D, Bill CA, Coleman WL, Bykhovskaia M. Regulation of transmitter release by synapsin II in mouse motor terminals. J Physiol. 2004;561:149–158. doi: 10.1113/jphysiol.2004.073494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara Y, Virmani T, Deak F, Liu X, Kavalali ET. An isolated pool of vesicles recycles at rest and drives spontaneous neurotransmission. Neuron. 2005;45:563–573. doi: 10.1016/j.neuron.2004.12.056. [DOI] [PubMed] [Google Scholar]
- Sato K, Abe K. An experimental study on the course of trans-synaptic propagation of neural activity and plasticity in the hippocampus in kainate-induced epilepsy. Brain Res Bull. 2001;55:393–400. doi: 10.1016/s0361-9230(01)00519-6. [DOI] [PubMed] [Google Scholar]
- Schluter OM, Schmitz F, Jahn R, Rosenmund C, Sudhof TC. A complete genetic analysis of neuronal Rab3 function. J Neurosci. 2004;24:6629–6637. doi: 10.1523/JNEUROSCI.1610-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluter OM, Basu J, Sudhof TC, Rosenmund C. Rab3 superprimes synaptic vesicles for release: implications for short-term synaptic plasticity. J Neurosci. 2006;26:1239–1246. doi: 10.1523/JNEUROSCI.3553-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch S, Castillo PE, Jo T, Mukherjee K, Geppert M, Wang Y, Schmitz F, Malenka RC, Sudhof TC. RIM1alpha forms a protein scaffold for regulating neurotransmitter release at the active zone. Nature. 2002;415:321–326. doi: 10.1038/415321a. [DOI] [PubMed] [Google Scholar]
- Shahrezaei V, Delaney KR. Consequences of molecular-level Ca2+ channel and synaptic vesicle colocalization for the Ca2+ microdomain and neurotransmitter exocytosis: a monte carlo study. Biophys J. 2004;87:2352–2364. doi: 10.1529/biophysj.104.043380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sons MS, Plomp JJ. Rab3A deletion selectively reduces spontaneous neurotransmitter release at the mouse neuromuscular synapse. Brain Res. 2006;1089:126–134. doi: 10.1016/j.brainres.2006.03.055. [DOI] [PubMed] [Google Scholar]
- Suemaru S, Sato K, Morimoto K, Yamada N, Sato T, Kuroda S. Increment of synapsin I immunoreactivity in the hippocampus of the rat kindling model of epilepsy. Neuroreport. 2000;11:1319–1322. doi: 10.1097/00001756-200004270-00034. [DOI] [PubMed] [Google Scholar]
- Terada S, Tsujimoto T, Takei Y, Takahashi T, Hirokawa N. Impairment of inhibitory synaptic transmission in mice lacking synapsin I. J Cell Biol. 1999;145:1039–1048. doi: 10.1083/jcb.145.5.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiagarajan R, Tewolde T, Li Y, Becker PL, Rich MM, Engisch KL. Rab3A negatively regulates activity-dependent modulation of exocytosis in bovine adrenal chromaffin cells. J Physiol. 2004;555:439–457. doi: 10.1113/jphysiol.2003.056333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Weering JR, Toonen RF, Verhage M. The role of Rab3a in secretory vesicle docking requires association/dissociation of guanidine phosphates and Munc18-1. PLoS ONE. 2007;2:e616. doi: 10.1371/journal.pone.0000616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadel K, Neher E, Sakaba T. The Coupling between Synaptic Vesicles and Ca(2+) Channels Determines Fast Neurotransmitter Release. Neuron. 2007;53:563–575. doi: 10.1016/j.neuron.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Wang Y, Okamoto M, Schmitz F, Hofmann K, Sudhof TC. Rim is a putative Rab3 effector in regulating synaptic-vesicle fusion. Nature. 1997;388:593–598. doi: 10.1038/41580. [DOI] [PubMed] [Google Scholar]