Abstract
Background:
Free-living amoebae belonging to the genus Acanthamoeba have an environmental distribution. Amoebic keratitis due to these protozoan parasites continue to rise in Iran and worldwide. In Iran, there are various researches regarding both morphological and molecular identification of Acanthamoeba spp. in environmental and clinical samples. However, there is no thorough review about Acanthamoeba genotypes and their distribution in environmental sources such as water, dust and biofilm in Iran. Besides, according to increasing cases of Amoebic keratitis in the region awareness regarding the pathogenic potential of these sight-threatening amoebae is of utmost importance.
Methods:
We conducted a thorough review based on the database sources such as MEDLINE, PubMed and Google scholar. No restrictions were placed on study date, study design or language of publication. We searched all valuable and relevant information considering the occurrence of the Acanthamoeba in both environmental and clinical samples.
Results:
According to our thorough review Acanthamoeba belonging to T4 genotype is the most prevalent type strain in environmental and clinical samples in several regions in Iran and worldwide, however, there are reports regarding Acanthamoeba belonging to other genotypes such as T2, T3, T5, T6 and T11 and the mentioned point could leads us to more researches with the goal of presenting the real genotype dominance of Acanthamoeba and related disease in the country.
Conclusion:
Overall, the present review will focus on present status of genotypes of Acanthamoeba in Iran during recent years.
Keywords: Acanthamoeba spp., Genotypes, Acanthamoeba keratitis, Encephalitis, Iran
Introduction
Free-living amoebae include families with potential pathogenic ability. Vahlkampfiids, Acanthamoebidae and Techamoebidae are among the important free-living amoebae (FLA) and they could lead to severe disease including Amoebic Keratitis (AK), granulomatose encephalitis and cutaneous ulcers (1–3). Free-living amoebae belonging to Acanthamoeba genus are distributed in many environmental sources such as soil, clay, dust, fresh waters, mineral springs, sea, water-air interface, spas, Jacuzzis and hot springs (4, 5). However, despite the widespread distribution of Acanthamoeba in the environmental sources the incidence of disease related to these genera is not high. Thus, it is reasonable to predict that not all people are susceptible to such infections (6, 7). Indeed, the high-risk people can be divided in two categories: contact lens wearers and immunosuppressed patients including HIV positive patients, graft patients, patients undergoing corticosteroid and chemotherapy, pregnant women, diabetes, cirrhosis and lupus patients. Corneal trauma even in microscopic form can lead to Acanthamoeba keratitis (8, 9). This is due to increased mannose levels in healing epithelium of cornea. Indeed the mannose level of cornea in healing epithelium is twofold more than normal cornea and thus the capacity of Acanthamoeba binding is much higher in this situation (10–12).
Morphological criteria classified Acanthamoeba at the genus level. Acanthamoeba trophozoites are flat in shape with large karyo-some and multiple food vacuoles. The resistant cysts are double walled with star shape endocyst and round ectocyst. However, various shape of endocyst may be seen in culture including triangular, square, and round forms. To date, Acanthamoeba has been divided into 18 different genotypes (T1–T18) being most of them the cause of human infections such as severe keratitis (12, 13). The genetic typing is based on 18S rRNA gene and the sequencing of Diagnostic fragment 3 (DF3) of 18S rRNA gene (14). Previously only T4, T3 and T2 were introduced as a causal agent of Acanthamoeba related infections; however, later it was shown that many genotypes could lead to Acanthamoeba keratitis (AK) including T11, T13, T15, etc. Unfortunately, most infections because of Acanthamoeba show poor prognosis and the treatment using topical propamidin isethionate (0.1%) and neosporin is still challenging (1, 2, 13). Diagnosis of AK is mainly based on cultivation of corneal scrapes, contact lenses on non-nutrient agar along with heat killed Eschershia coli, and molecular based analysis. Confocal microscopy could be non-invasive diagnosis approaches, which could be helpful for AK diagnosis and monitoring, however by using this microscopy-based test, it is not possible to differentiate between various free-living amoebae and therefore culture of corneal specimen could be the gold standard (15).
Overall, the present research aimed to review the occurrence of Acanthamoeba spp. In addition, their genotypes based on 18S rRNA gene in environmental samples and clinical sources such as corneal scrape contact lenses and their paraphernalia. In addition, the present review highlights the increasing trend of amoebic keratitis in the country, which needs improved education regarding this opportunistic free-living protozoan.
Methods
We conducted a systematic review based on the database sources such as MEDLINE, PubMed, Scopus and Google scholar. No restrictions were placed on study date, study design or language of publication. We searched all valuable and relevant information considering the occurrence of the Acanthamoeba in both environmental and clinical samples. We referred to the information databases of Medline, PubMed, Scopus and Google scholar and the used keywords were the combinations of Acanthamoeba and Amoebic Keratitis, and words associated with environmental sources such as; Acanthamoeba and soil, Acanthamoeba and water, Acanthamoeba and dust sources, Acanthamoeba plus treatment. We also referred to some information in books associated with issue of Acanthamoeba and health hazard Abstracts and full articles that were written in English and relevant to the topic were enrolled in this study and critically studied in detail. We excluded studies and reports with minimal importance on the topics.
Acanthamoeba distribution in the environmental sources in Iran
There are various researches, which has conducted in Iran as following. The first research regarding environmental Acanthamoeba distribution was conducted by Rezaeian et al. These investigators showed the presence of Acanthamoeba and Naegleria in water samples of Kazeroon city using morphological key (15). However, at that time, there was no report regarding various genotypes of Acanthamoeba genus and these amoebas classified using morphological based criteria. Later, another morphological-based research was done by Rezaeian et al. and the results revealed that 46.25% of environmental samples contained Acanthamoeba spp. Interestingly, all of the soil samples (5 out of 5) were positive in the culture. In addition, out of 61 dust samples, 28 of them contained Acanthamoeba (16). Consequently, Niyyati et al. performed a sequencing based test for the mentioned environmental sources and the genotypes were belonged to T2, T6, T4 and T11 (17). The predominant type was assigned to T4 strain. It is important to mention that all of isolated genotypes have been reported as a cause of AK.
Nazar et al. revealed that Acanthamoeba present in pond water sources of all districts of Tehran, Iran. Genotyping of strains showed that they were belonged to T4 and T5 genotype, both genotypes has been identified as corneal pathogens mainly in soft contact lens wearers (18). In another research, Niyyati et al. showed the presence of potentially pathogenic Acanthamoeba spp. in recreational river waters of Tehran, Iran. All of the examined waters were associated with human activity. Briefly, 55 water samples from 10 major rivers were analyzed for FLA and identified by morphological-based criteria, PCR amplification and sequencing analysis. The percent of positive FLA isolates was 27.3%. Acanthamoeba, assigned to the T4 and T15 genotype were then identified using sequencing of DF3 region (19). Other researchers showed the presence of thermotolerant Acanthamoeba in hot spring of northwester Iran (20–22). Interestingly, thermo-tolerance Acanthamoeba introduce as amoebae with pathogenic potential.
In this regard, Mirjalili et al. conducted a survey regarding the pathogenic potential of Acanthamoeba T4 type using osmo-tolerance and thermo-tolerance assay. These researches revealed that not all of T4 types are pathogens (23). Further studies are needed for pathogenic assay regarding various genotypes. Acanthamoeba spp. isolated from various environmental sources is shown in Fig. 1, 2.
Fig. 1:
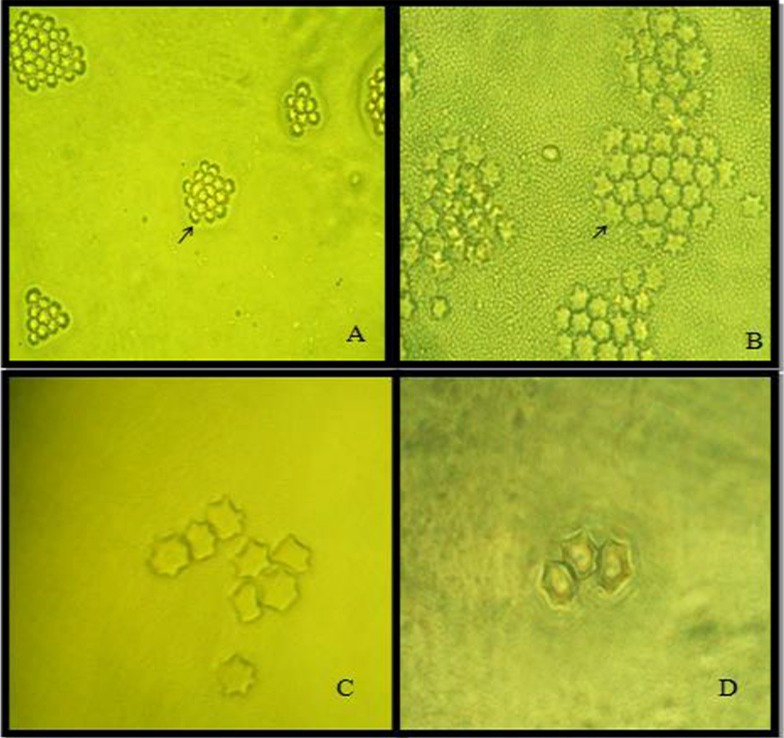
Acanthamoeba spp. cysts isolated from water, soil and dust (star shape cysts are shown in clump) (Magnification: A: x10; B, C, D:x40) (Figures are from Pathogenic free living amoebae in human book, Rezaeian M and Niyyati M, 2009)
Fig. 2:
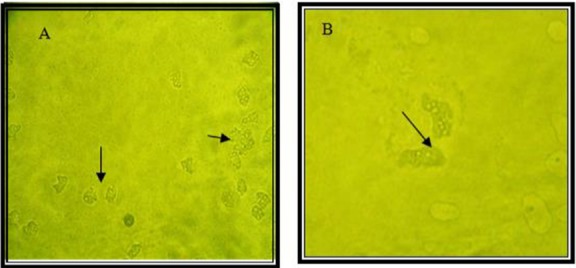
Acanthamoeba spp. trophozoites isolated from soil and dust (flat shape trophozoites are shown) (Magnification: A: x10, B: x40)
Hospital dust and biofilm contamination to Acanthamoeba
Rezaeian et al. was the first to report the presence of Acanthamoeba in hospital dusts in Tehran, Iran. Interestingly, Acanthamoeba T4 type was isolated from ophthalmology wards in Tehran, Iran. This finding could be a health hazard for high-risk people including contact lens wearers and patients with eye surgery (16). The occurrence of FLA in immunodeficiency wards of hospitals in Tehran, Iran were also investigated by Lasjerdi et al. (20). Briefly, 70 dust and biofilm samples from immunodeficiency wards of university hospitals were collected and tested for the presence of FLA using culturing and molecular approaches. Out of 70 samples, 37 (52.9%) showed positive culture. The most prevalent isolate were belonged to Acanthamoeba T4 genotype. Presence of the T4 genotype on medical instruments, including an oxygen mask in an isolation room of an immunodeficiency ward, should be of concern for health authorities. Another genotype was belonged to T5 corresponding to A. lenticulata (24).
Overall, these results reflect a clear need for improved disinfection, especially where high-risk people, such as those who are immune-suppressed or undergo eye surgery such as LASIC surgeries, are served.
Acanthamoeba-related disease in Iran
The only reported disease related to Acanthamoeba in Iran is AK. Encephalitis due to Acanthamoeba has not been reported yet, mainly due to lack of knowledge regarding Acanthamoeba as an agent of central nervous system infections. So far, there are 150 cases of AK in Iran, although it seems that these numbers is lower than the true cases. The first cases of AK in Iran were reported in a soft contact lens wearer (15). In 2007, a ten year survey regarding AK was reported by Rezaeian et al. whom showed among 142 patients, 49 (34.5%) present with AK. The most common age was between 15–25 yr (75.5%). Interestingly, 44 patients (89.79%) were contact lens wearers for cosmetic purposes or visual corrections. Among them 41 patients (93.18%) wore soft contact lenses and three patients were hard contact lens wearers (25). Among 50 keratitis patient, 13 were positive for Acanthamoeba (26). Three species including A. griffin, A. palesinensis and A. castellanii were identified in their samples. Another study revealed Acanthamoeba as a causal agent in 15 (30%) of 50 keratitis samples. Among these clinical isolates, 13 (86.7%) belonged to female patients and 2 (13.3%) were male. All positive specimens belonged to soft contact lens wearers and only one belonged to a patient with a history of hard contact lens usage. Regarding genotype identification, 13 (86.7%) of these isolates belonged to T4 genotype. However, it is important to mention there was a mixed genotype belonging to Acanthamoebae T4 and T11 genotypes in one patient. Other genotypes identified in the clinical specimens were T11 (13.3%) and T3 (6.7%) (17). Another survey of the 90 asymptomatic contact lens wearers, 9 (10%) were positive for FLA outgrowth. Morphological analysis revealed that 3 isolates were belonged to Hartmannella genus according to small round cysts and 6 isolates were belonged to Acanthamoeba genus based on the star shape of endocysts. Sequencing revealed that Acanthamoeba belonged to T4, T3 and T5 genotype (27). Acanthamoeba isolated from contact lenses or corneal scarping is presented in Fig. 3–4.
Fig 3:
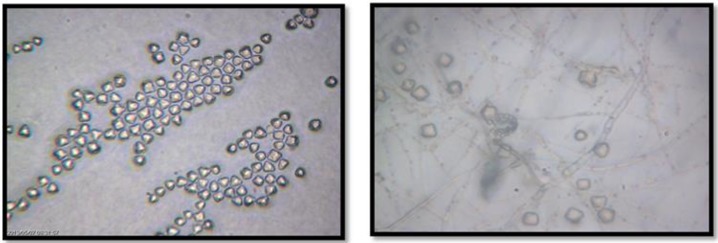
Acanthamoeba spp. cysts isolated from Acanthamoeba keratitis (triangular shape cysts are shown) (Magnification: x10)
Fig 4:
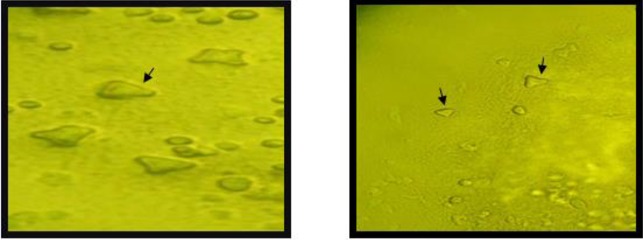
Acanthamoeba cysts isolated from Acanthamoeba keratitis (triangular shape cysts are shown) (Magnification: right: x10, left: x40)
So far, other infections due to Acanthamoeba spp. have not been reported in Iran. This is due to lack of knowledge regarding these opportunistic protozoan parasites in this region. It should be noted that Memari et al. reported that Acanthamoeba belonging to potentially pathogenic T3, T4 and T5 genotypes could be colonize in nasal mucosa of cancer patients which this could be a serious health hazard for developing GAE (28).
Regarding treatment approaches Khojaste et al. conducted a study targeting the gene profile of Acanthamoeba T4 type in trophozoites and cysts. The result revealed that three genes including heat shock protein 70 (hsp70), actin-I and elongation factor-1alpha (EF-1α) were differentially expressed during Acanthamoeba differentiation and they could be the novel target for treatment (29).
A recent research by Niyyati et al. also reported that tap and filtrated waters could be the sources of pathogenic free-living amoebae and this could render contact lens wearers for developing AK (30).
Conclusion
The ubiquity of Acanthamoeba spp. in environmental sources such as fresh waters, hot springs and tap waters and recreational soil sources and also the occurrence of the amoebae in the dust and biofilm samples of hospitals and clinical settings in Iran and around the world could be a health concern specially for those who are within high risk groups such as contact lens wearers and immunosuppressed patients. On the other hand, AK continues to rise in Iran mainly in female soft contact lens wearers with a history of poor maintenance of their lenses. There are no reports of Acanthamoeba encephalitis in the region yet and thus this point reflects the need for more researches in suspected encephalitis patients.
Here, according to our thorough review Acanthamoeba belonging to T4 genotype is the most prevalent type strain in environmental and clinical samples in several regions in Iran and worldwide, however, there are reports regarding Acanthamoeba belonging to other genotypes such as T2 and the mentioned point could leads us to more researches with the goal of presenting the real genotype dominance of Acanthamoeba and related disease in this country. Additionally researches regarding new treatment strategies for Acanthamoeba-related infections are an utmost priority topic as such infections are manifest with poor prognosis.
Acknowledgments
Thanks are due to Tehran University of Medical Sciences and Shahid Beheshti University of Medical Sciences for supporting the grants for Acanthamoeba research during recent years.
The authors highly appreciate the kind comments and helps of Dr. Mehdi Mohebali and Mr. Alireza Latifi. The authors declare that there is no conflict of interests.
References
- 1. Cabral FM, Cabral G. Acanthamoeba spp. as Agents of Disease in Humans. Clin Microbiol Rev. 2003; 16 ( 2): 273– 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schuster FL, Visvesvara GS. Free-living amoebae as opportunistic and non-opportunistic pathogens of humans and animals. Int J Parasitol. 2004; 34 ( 9): 1001– 27. [DOI] [PubMed] [Google Scholar]
- 3. Visvesvara GS, Moura H, Schuster FL. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol Med Microbiol. 2007; 50 ( 1): 1– 26. [DOI] [PubMed] [Google Scholar]
- 4. Lorenzo-Morales J, Miranda CA, Jimenez C, Tejedor ML, Valladares B, Ortega-Rivas A. Evaluation of Acanthamoeba isolates from environmental sources in Tenerife, Canary Islands, Spain. Ann Agric Environ Med. 2005; 12 ( 2): 233– 6. [PubMed] [Google Scholar]
- 5. Rahdar M, Niyyati M, Salehi M, Feghhi M, Makvandi M, Pourmehdi M, Farnia S. Isolation and Genotyping of Acanthamoeba Strains from Environmental Sources in Ahvaz City, Khuzestan Province, Southern Iran. Iran J Parasitol. 2012; 7 ( 4): 22– 6. [PMC free article] [PubMed] [Google Scholar]
- 6. Cursons R T, Brown T J, Keys E A, Moriarty K M, Till D. Immunity to pathogenic free-living amoebae: role of cell-mediated immunity. Infect Immun. 1980; 29 ( 2): 408– 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kiderlen AF, Laube U, Radam E, Tata PS. Oral infection of immunocompetent and immunodeficient mice with Balamuthia mandrillaris amebae. Parasitol Res. 2007; 100 ( 4): 775– 82. [DOI] [PubMed] [Google Scholar]
- 8. Visvesvara GS, Moura Hercules, Schuster FL. (2007) Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri and Sappinia diploidea. Immunol Med Microbiol. 2007; 50 ( 1): 1– 26. [DOI] [PubMed] [Google Scholar]
- 9. Kamel AG, Faridah H, Yusof S, Norazah A, Nakisah MA. A case of trauma related Acanthamoeba keratitis. Trop Biomed. 2004; 21 ( 2): 135– 8. [PubMed] [Google Scholar]
- 10. Niyyati M, Rezaie S, Rahimi F, Mohebali M, Maghsood AH, Farnia SH, Rezaeian M. Molecular Characterization and Sequencing of a Gene Encoding Mannose Binding Protein in an Iranian isolate of Acanthamoeba castellanii as a Major Agent of Acanthamoeba keratitis. Iran J Public Health. 2008; 37 ( 2): 9– 14. [Google Scholar]
- 11. Niyyati M, Rezaei S, Babaie Z, Rezaeian M. Molecular identification and sequencing of Mannose Binding Protein gene of an Iranian isolate of Acanthamoeba palestinensis. Iran J Parasitol. 2010; 5 ( 1): 1– 5. [PMC free article] [PubMed] [Google Scholar]
- 12. Panjawani N, Marchant J, Cubillose I, Garate M. Biochemical characterization and functional studies of Acanthamoeba mannose-binding protein. Infect Immunol. 2005; 73 ( 9): 5775– 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Magnet A, Henriques-Gil N, Galván-Diaz AL, Izquiedo F, Fenoy S, del Aguila C. Novel Acanthamoeba18S rRNA gene sequence type from an environmental isolate. Parasitol Res. 2014; 113 ( 8): 2845– 50. [DOI] [PubMed] [Google Scholar]
- 14. Schroeder JM, Booton GC, Hay J, Niszl IA, Seal DV, Markus MB, Fuerst PA, Byers Tj. Use of subgenic 18S ribosomal DNA PCR and sequencing for genus and genotype identification of Acanthamoebae from humans with keratitis and from sewage sludge. J Clin Microbiol. 2001; 39 ( 5): 1903– 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rezaeian M, Niyyati M. Pathogenic free-living amoeba in humans. Tehran University of medical Sciences Publication, Tehran, Iran: 2009. [Google Scholar]
- 16. Rezaeian M, Niyyati M, Farnai Sh, Rahimi F, Motevalli haghi A. Isolation of Acanthamoeba Spp. from Different Environmental Sources. Iran J of Parasitol. 2008; 3: 44– 47. [Google Scholar]
- 17. Niyyati M, Jacob Lorenzo-Morales B, et al. Genotyping of Acanthamoeba isolates from clinical and environmental specimens in Iran. Exp Parasitol. 2009: 121; 242–245. [DOI] [PubMed] [Google Scholar]
- 18. Nazar M, Haghighi A, Niyyati M, Eftekhar M, Tahvildar-Biderouni F, Taghipour N, Abadi A, Nazemalhosseini Mojarad E, Athari A. Genotyping of Acanthamoeba isolated from water in recreational areas of Tehran, Iran. J Water Health. 2011; 9 ( 3): 603– 8. [DOI] [PubMed] [Google Scholar]
- 19. Niyyati M, Lasjerdi Z, Nazar M, Haghighi A, Nazemalhosseini Mojarad E. Screening of-recreational areas of rivers for potentially pathogenic free-living amoebae in the suburbs of Tehran, Iran. J Water Health. 2012; 10 ( 1): 140– 6. [DOI] [PubMed] [Google Scholar]
- 20. Solgi R, Niyyati M, Haghighi A, Taghipour N, Tabaei SJ, Eftekhar M, Nazemalhosseini Mojarad E. Thermotolerant Acanthamoeba spp. isolated from therapeutic hot springs in Northwestern Iran. J Water Health. 2012; 10 ( 4): 650– 6. [DOI] [PubMed] [Google Scholar]
- 21. Solgi R, Niyyati M, Haghighi A, Nazemalhosseini Mojarad E. Occurrence of thermotolerant Hartmannella vermiformis and Naegleria spp. in hot springs of Ardebil province, Iran. Iran J Parasitol. 2012; 6 ( 2): 47– 52. [PMC free article] [PubMed] [Google Scholar]
- 22. Badirzadeh A, Niyyati M, Babaei Z, Amini H, Badirzadeh H, Rezaeian M. Isolation of Free-Living Amoebae from Sarein Hot Springs in Ardebil Province, Iran. Iran J Parasitol. 2011; 6 ( 2): 1– 8. [PMC free article] [PubMed] [Google Scholar]
- 23. Mirjalali H, Niyyati M, Abed Khojasteh H, Babaei Z, Sharifdini H, Rezaeian M. Acanthamoeba T4 genotype: in vivo and in vitro pathogenicity survey. Iran J Parasitol. 2013: 8 (4): 530–5. [PMC free article] [PubMed] [Google Scholar]
- 24. Lasjerdi Z, Niyyati M, Haghighi A, Shahabi S, Biderouni FT, Taghipour N, Eftekhar M, Nazemalhosseini Mojarad E. Potentially pathogenic free-living amoebae isolated from hospital wards with immunodeficient patients in Tehran, Iran. Parasitol Res. 2011; 109 ( 3): 575– 80. [DOI] [PubMed] [Google Scholar]
- 25. Rezaeian M, Farnia Sh, Niyyati M, Rahimi F. (2007) Amoebic keratitis in Iran (1997–2007). Iran J Parasitol. 2007; 3 ( 2): 1– 6. [Google Scholar]
- 26. Dudley R, Matin A, Alsam S, Sissons J, Maghsood AH, Khan NA. (2005) Acanthamoeba isolates belonging to T1, T2, T3, T4 but not T7 encyst in response to increased osmolarity and cysts do not bind to human corneal epithelial cells. Acta Trop. 2005; 95 ( 2): 100– 8. [DOI] [PubMed] [Google Scholar]
- 27. Niyyati M, Rahmani F, Lasjerdi Z, Rezaeian M. Potentially Pathogenic Free-Living Amoebae in Contact Lenses of the Asymptomatic Contact Lens Wearers. Iran J Parasitol. 2014; 14–19. [PMC free article] [PubMed] [Google Scholar]
- 28. Memari F, Niyyati M, Haghighi A, Seyyed Tabaei SJ, Lasjerdi Z. Occurrence of pathogenic Acanthamoeba genotypes in nasal swabs of cancer patients in Iran. Parasitol Res. 2015; 114 ( 5): 1907– 12 [DOI] [PubMed] [Google Scholar]
- 29. Abedkhojasteh H, Niyyati M, Rezaei S, Mohe-bali M, Farnia S, Kazemi-Rad E, Roozafzoon R, Sianati H, Rezaeian M, Heidari M. Identifying differentially expressed genes in trophozoites and cysts of Acanthamoeba T4 genotype: Implications for developing new treatments for Acanthamoeba keratitis. Eur J Protist. 2015. 10.1016/j.ejop.2014.10.001 [DOI] [PubMed] [Google Scholar]
- 30. Niyyati M, Lasgerdi Z, Lorenzo-Morales J. Detection and Molecular Characterization of Potentially Pathogenic Free-living Amoebae from Water Sources in Kish Island, Southern Iran. Microbiol Insights. 2015; 9:8(Suppl 1): 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]


