Abstract
Background:
Acanthamoeba- bacteria interactions enable pathogenic bacteria to tolerate harsh conditions and lead to transmission to the susceptible host. The present study was aimed to address the presence of bacterial endosymbionts of Acanthamoeba isolated from recreational water sources of Tehran, Iran. To the best of our knowledge this is the first study regarding occurrence of bacteria in environmental Acanthamoeba spp. in Iran.
Methods:
A total of 75 samples of recreational water sources were collected. Samples were cultured on non- nutrient agar 1.5% plates. Positive Acanthamoeba spp. were axenically grown. DNA extraction and PCR reaction was performed using JDP1-2 primers. All positive samples of Acanthamoeba were examined for the presence of endosymbionts using staining and molecular methods. The PCR products were then sequenced in order to determine the genotypes of Acanthamoeba and bacteria genera.
Results:
Out of 75 samples, 16 (21.3%) plates were positive for Acanthamoeba according to the morphological criteria. Molecular analysis revealed that Acanthamoeba belonged to T4 and T5 genotypes. Five isolates (35.7%) were positive for bacterial endosymbionts using staining method and PCR test. Sequencing of PCR products confirmed the presence of Pseudomonas aeruginosa and Agrobacterium tumefasiens.
Conclusion:
The presence of Acanthamoeba bearing pathogenic endosymbionts in water sources leads us to public health issues including improved sanitation and decontamination measures in recreational water sources in order to prevent amoebae-related infection. To the best of our knowledge this is the first report regarding the isolation of A. tumefasiens from Acanthamoeba in Iran and worldwide.
Keywords: Acanthamoeba, Endosymbionts, Recreational waters, Pseudomonas aeruginosa, Agrobacterium tumefasiens
Introduction
Free- Living Amoebae (FLA) include potentially pathogenic protozoan parasites such as Acanthamoeba, Balamuthia and Naegleria (1, 2). These amoebae could lead to severe diseases such as painful keratitis, fatal encephalitis and cutaneous ulcers (3). Recently there are several reports regarding other free-living amoebae as potentially pathogenic parasites including Paravahlkampfia francinae and Vahlkampfia spp. (4, 5). These mentioned amoebae could be the cause of primary amoebic meningoencephalitis (PAM) mimicking Naegleria fowleri-related infection and keratitis, respectively (5). It is worthy to mention that there are also report regarding mixed infection of Acanthamoeba and bacteria in keratitis patients (6). These protozoan parasites are distributed in different niches such as lakes, soil, wastewater and clay (1, 2).
It should be mention that beside the direct pathogenic effect of free-living amoebae, they could be a carrier of pathogenic microorganisms such as bacteria and viruses. There are also various opportunistic pathogens such as Non-tuberculous Mycobacteria (NTM), Pseudomonas and Legionella that could exist in the same ecological niches as Acanthamoeba (7–9). Indeed, a wide range of bacteria could resist the intracellular killing of amoebae and they could survive and even exploit Acanthamoeba for their multification such as Pseudomonas putida, Pasteurella pneumotropica, Aeromonas salmonicida, Legionella pneumophila serogroup 1, L. pneumophila serogroup 3, L. pneumophila serogroup 6 (7, 10). Bacteria could remain in amoebae cyst form, this could be a problematic health aspect, since Acanthamoeba cysts are very resistance to harsh environment and could tolerate many adverse conditions such as high osmolarity, various ranges of temperature and pH (7, 11). Thus, uptake of bacteria by amoebae could lead to endosymbiosis relationship. Interestingly, pathogenic bacteria are shown to have the ability to survive in Acanthamoeba cytoplasm despite non-pathogenic bacteria, which they were used only as food sources. It is interesting to note that some endosymbiont environmental Chlamydiae alter the growth speed and/or motility of Acanthamoeba, this could be due to mutual relationship between amoebae and environmental Chlamydiae (12, 13).
Previous studies in Iran were mainly focused on isolation of Acanthamoeba spp. from water sources, however there were no previous researches regarding survey of Acanthamoeba in chlorinated water an also Amoebae-endosymbionts in this region and thus the main aim of the present research was to address the occurrence of bacterial endosymbionts of Acanthamoeba isolated from man-made recreational water sources using staining and molecular based methods.
Material and Methods
Study area and sample collection
A total of 75 samples of recreational water sources including 40 samples of ponds and 35 samples from indoor swimming pools were collected from Tehran, Iran. All samples were transferred to Dep. Parasitology and mycology, School of medicine, Shahid Beheshti University of Medical Sciences within few days.
Sample processing (filtration, cultivation and cloning)
Four hundred milliliters of water sample filtered using nitrate membranes. Each sample was cultured into non nutrient agar plates. All plates were then sealed and incubated at room temperature for up to 1 month. Positive plated were then submitted to cloning according to our previous study (14). Briefly, a single cyst was transferred to a fresh medium and adopted to axenic situation within weeks. This allows no contaminants in the medium. Cleaned plates were then examined for intracellular bacteria using inverted microscopy and gram staining as following.
Microscopic bacterial - endosymbionts detection
Cloned plates washed with sterile normal saline and slides were then prepared from Acanthamoeba-positive plates. Gram staining was applied according to previous researches (15).
DNA extraction, PCR amplification and nucleotide sequencing of Acanthamoeba spp.
DNA extraction was performed using modified phenol-chloroform method according to our previous study (14). For genotyping of Acanthamoeba spp. PCR analysis was done using genus-specific primer pairs called JDP1-2 primers. These primers could detect a diagnostic fragment of all 18 genotypes of Acanthamoeba spp. (16). The PCR reaction was prepared in 30 μl Ampliqone (Taq DNA Polymerase Master Mix Red, Denmark). Briefly, 25 μl of master mix with 10 ng DNA templates and 20 pmol primers were mixed to achieve a volume of 30 μl. PCR product were then submitted to sequencing.
Molecular identification of bacterial-endosymbionts
All Acanthamoeba-positive samples were submitted to PCR using bacteria-universal primers. These primes could amplify a fragment of 16S rRNA gene of various bacteria including Pseudomonas, Agrobacterioum and several other genera. The nucleotide sequences of the primers were as following: Forward: 5-TCG ACA ACA GAG TTT GAT CCT GGC TCA G -3 and Reverse: 5-ATC CAA GCT TAA GGA GGT GAT CCA GCC-3. These primers correspond to 1100 bp DNA sequence of 16S rRNA gene of several bacteria (17).
The PCR amplification was carried out in a total volume of 30 μl. Thermal profile involved 40 s at 94°C, 90 s at 59.6°C and 120 s at 72°C for 35 cycles.
Sequences analysis for Acanthamoeba and bacterial endosymbionts
Sequences derived from the amoebae and their endosymbionts were tested against all available nucleotide sequences in the GenBank database. The DNA sequences have been deposited in the Genetic sequence database at the National Center for Biotechnical Information (NCBI) using the Sequin program (version 10.3). (GenBank ID for Acanthamoeba: KJ504214-KJ504227 and GenBank ID for bactertia: KJ563278- KJ563281). Multialign were performed for Acanthamoeba genotypes.
Results
Out of 75 samples, 16 (21.3%) plates were positive for Acanthamoeba according to the morphological criteria. Acanthamoeba trophozoites were flat in shape and cysts were double walled with star shape endocysts. Ten samples (25%) of pond and 4 samples (11.4%) of swimming pool waters were found to be positive for Acanthamoeba genus in non-nutrient agar medium.
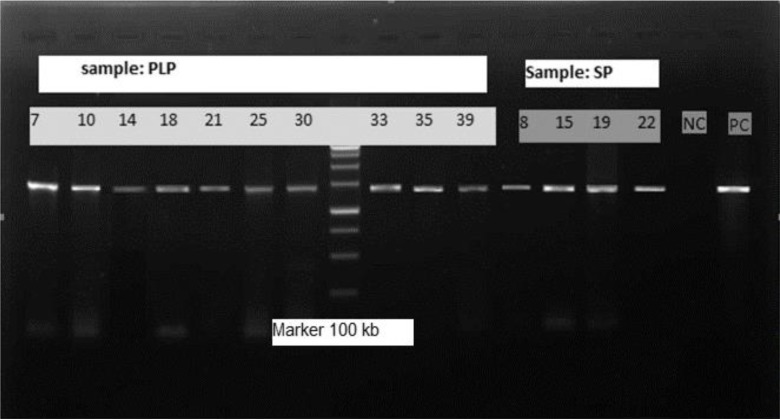
Of 16 Acanthamoeba isolate, 14 were cloned successfully. Sequences analysis revealed that Acanthamoeba belonged to T4 and T5 genotypes (Fig. 1) (Table 1). T5 genotype has been isolated from swimming pools. Five isolates (35.7%) of Acanthamoeba were positive for bacterial endosymbionts in their cytoplasm using microscopic observation and gram staining method. Gram staining revealed the presence of bacteria readily in the host amoebae cytoplasm (Fig. 2). Many rod shaped bacteria were localized in the cytoplasm. It should be mention that 4 isolated bacteria showed an approximately 1100 bp band (Fig. 3). However, one strain were failed in sequencing even with repeated PCR reaction. Sequencing of PCR products verified the presence of three Pseudomonas aeruginosa and one Agrobacterium tumefasiens in Acanthamoeba T4 genotypes (Table 1). The result of multialign is shown in Fig. 4 which it shows >5% dissimilarity in 18S rRNA gene. Isolation of Agrobacterium tumefasiens from Acanthamoeba is for the first time worldwide.
Fig. 1:
500 bp PCR product electrophoresis of positive Acanthamoeba
(Marker: 100 bp, NC: Negative control, PC: Positive control, SP: Swimming pool, PLP: pond water)
Table 1:
Acanthamoeba genotypes isolated from recreational waters and their related endosymbionts
| Isolates code | Genotypes | Accession Number | Endosymbiont code | Isolated bacteria | Accession Number |
|---|---|---|---|---|---|
| MN(AC)7-PW-IR | T4 | KJ504214 | MN-Endo5 | P. aeroginosa | KJ563278 |
| MN(AC)10-PW-IR | T4 | KJ504215 | -------- | -------- | |
| MN(AC)14-PW-IR | T4 | KJ504216 | -------- | -------- | |
| MN(AC)18-PW-IR | T4 | KJ504217 | MN-Endo32 | P. aeroginosa | KJ563279 |
| MN(AC)21-PW-IR | T4 | KJ504218 | -------- | -------- | |
| MN(AC)25-PW-IR | T4 | KJ504219 | -------- | -------- | |
| MN(AC)30-PW-IR | T4 | KJ504220 | -------- | -------- | |
| MN(AC)33-PW-IR | T4 | KJ504221 | MN-Endo1 | P. aeroginosa | KJ563280 |
| MN(AC)35-PW-IR | T4 | KJ504222 | -------- | -------- | |
| MN(AC)39-PW-IR | T4 | KJ504223 | -------- | -------- | |
| MN(AC)8-SPW-IR | T5 | KJ504224 | MN-Endo11 | A.tumefacience | KJ563281 |
| MN(AC)15-SPW-IR | T4 | KJ504225 | - | No sequencing | -------- |
| MN(AC)22-SPW-IR | T5 | KJ504226 | -------- | -------- | |
| MN(AC)19-SPW-IR | T4 | KJ504227 | -------- | -------- |
Fig. 2:
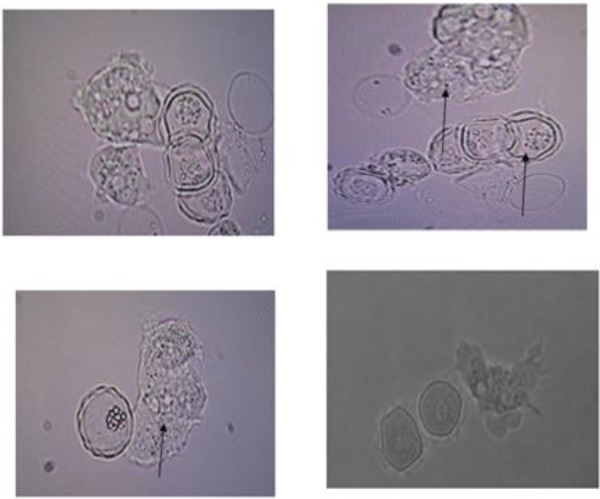
Cloned Acanthamoeba spp. cysts and trophozoites containing rod shaped-bacteria (magnification x400)
Fig. 3:
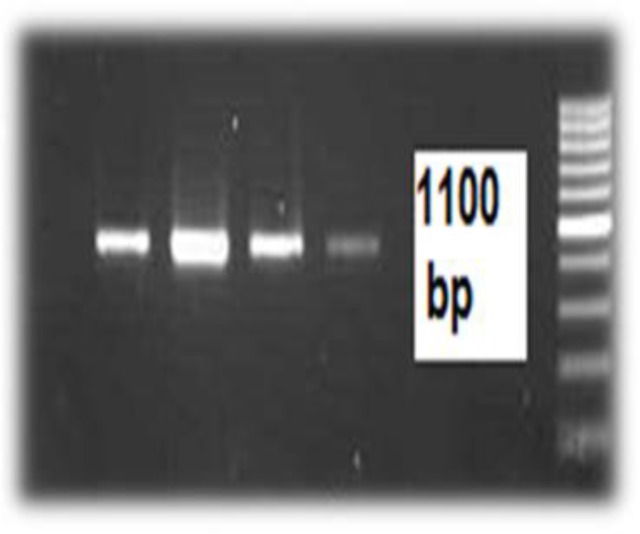
1100 bp PCR product electrophoresis of positive endosymbionts (four isolates) (Marker: 1kb)
Fig. 4:
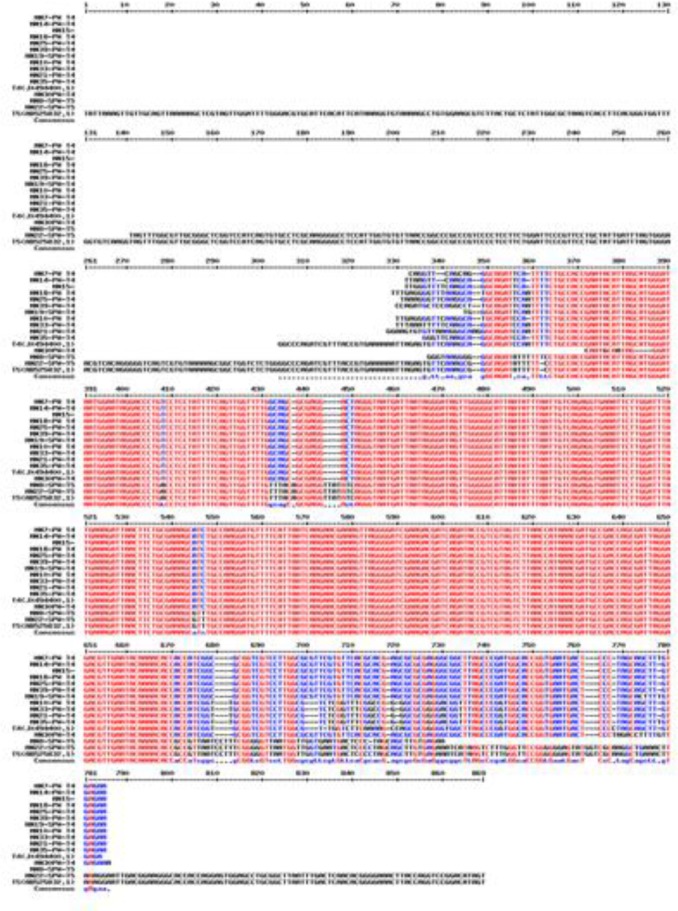
Multialign of isolated Acanthamoeba belonginig to T4 and T5 genotypes (multalign software, France)
Discussion
The present study revealed that Acanthamoeba could harbor potentially pathogenic bacteria in recreational water sources such as pond and chlorinated water including swimming pools.
This is the first study regarding detection of Acanthamoeba in swimming pools in Iran and the result showed that contamination of pools are less than ponds. The present study showed that 35.7% of amoebae contain intracellular bacteria. Other researches showed that 24% of environmental amoebae could harbor bacteria (18). Additionally, out 24% of environmental Acanthamoeba and 26% of clinical Acanthamoeba contain intracellular bacteria (19). In accordance, several bacteria have been reported that could live in the free-living amoebae as endosymbionts including Acanthamoeba and Naegleria (20–22), however this is the first report of Agrobacterium tumefasience in Acanthamoeba spp. worldwide. Agrobacterium is a potentially pathogenic Gram-negative bacterium responsible for systematic human infection especially in imunocompromised individuals (23, 24). Agrobacterium spp. are ubiquitous in natural and man-made water sources and basically they are harmful for plants (24).
The present study confirmed that P. aeroginosa presents in cytoplasm of the host amoebae. This is important since both of organisms are corneal pathogen and they may increase the chance of AK in appropriate situation (25). To this end there are several reports regarding severe keratitis due to mixed Acanthamoeba and Pseudomonas infection in cosmetic contact lens wearers with poor prognosis (26, 27). Complicated cases of AK also may be due to coexistence of corneal bacterial pathogens such as Pseudomonas with Acanthamoeba and lack of response to proper treatment may reflect the presence of amoebae-endosymbionts. In addition, according to previous studies this interaction may lead to increased virulence of bacteria and also may affect the pathogenicity of amoebae (25, 26, 28). This issue needs to clarify by more researches. To this end, the focus of recent researches has shifted from direct pathogenic effects of Acanthamoeba toward their role as carriers of pathogenic bacteria. Overall, amoebae could be an ideal replicative niche for bacterial communications and could act as reservoir. The long interaction between amoebae and bacteria could lead to adaptation behavior towards an intracellular lifestyle (29). A previous research also revealed that although addition of disinfectants may influence amoebal density, but it seems that FLA can re-colonize in treated waters within a short period of time (30).
Conclusion
The present study reports for the first time the occurrence of novel endosymbiotic bacteria (such as A. tumefasciences) in environmental Acanthamoeba strains. Further researches regarding the relationship between bacteria and their host amoeba and their effect on pathogenicity of either amoebae or bacteria is of utmost importance.
Acknowledgment
Dr. Maryam Niyyati was supported by a Research Grant from the National Elites Foundation for distinguished Young associated Professors. The present research was funded by the project (no: 1391-1-125-1092) from the Cellular and Molecular Biology Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran. The authors declare that they have no conflicts of interest.
References
- 1. Khan NA. Acanthamoeba: biology and increasing importance in human health. FEMS Microbiol Rev. 2006; 30: 560– 65. [DOI] [PubMed] [Google Scholar]
- 2. Lorenzo-Morales J, Marciano-Cabral F, Lindo JF, Visvesvara GS, Maciver SK. Pathogenicity of amoebae. Exp Parasitol. 2010; 126 ( 1): 2– 3. [DOI] [PubMed] [Google Scholar]
- 3. Khan NA. Pathogenesis of Acanthamoeba infections. Microb Pathog. 2003; 34 ( 6): 277– 85. [DOI] [PubMed] [Google Scholar]
- 4. Visvesvara GS, Sriram R, Qvarnstrom Y, Bandyopadhyay K, Da Silva AJ, Pieniazek NJ, Cabral GA. Paravahlkampfia francinae n. sp. masquerading as an agent of primary amoebic meningoencephalitis. J Eu Microbiol. 2009; 56(4): 357–66. [DOI] [PubMed] [Google Scholar]
- 5. Niyyati M, Lorenzo-Morales J, Rezaie S, Rahimi F, Martín-Navarro CM, Mohebali M, Maghsood AH, Farnia S, Valladares B, Rezaeian M. First report of a mixed infection due to Acanthamoeba genotype T3 and Vahlkampfia in a cosmetic soft contact lens wearer in Iran. Exp Parasitol. 2010; 126 ( 1): 89– 90. [DOI] [PubMed] [Google Scholar]
- 6. Mascarenhas J, Lalitha P, Prajna NV, Srinivasan M, Das M, D'Silva SS, Oldenburg CE, Borkar DS, Esterberg EJ, Lietman TM, Keenan JD. Acanthamoeba, fungal, and bacterial keratitis: a comparison of risk factors and clinical features. Am J Ophthalmol. 2014; 157 ( 1): 56– 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Trabelsi H, Dendana F, Sellami A, Sellami H, Cheikhrouhou F, Neji S, Makni F, Ayadi A. Pathogenic free-living amoebae: epidemiology and clinical review. Pathol Biol (Paris). 2012; 60 ( 6): 399– 405. [DOI] [PubMed] [Google Scholar]
- 8. Delafont V, Mougari F, Cambau E, Joyeux M, Bouchon D, Héchard Y, Moulin L. First evidence of amoebae–mycobacteria association in drinking water network. Environ Sci Technol. 2014; 48 ( 20): 11872– 82. [DOI] [PubMed] [Google Scholar]
- 9. Zeybek Z, Binay AR. Growth ability of Gram negative bacteria in free-living amoebae. Exp Parasitol. 2014; 145 Suppl: S121–6. [DOI] [PubMed] [Google Scholar]
- 10. Medina G, Flores-Martin S, Fonseca B, Otth C, Fernandez Mechanisms associated with phagocytosis of Arcobacter butzleri by Acanthamoeba castellanii. Parasitol Res. 2014; 113 ( 5): 1933– 42. [DOI] [PubMed] [Google Scholar]
- 11. Marciano-Cabral F, Cabral G; Acanthamoeba spp. as agents of disease inhumans. Clin Microbiol Rev. 2003; 16 ( 2): 273– 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Da Rocha-Azevedo B, Tanowitz HB, Marciano-Cabral F. Diagnosis of infections caused by pathogenic free-living amoebae. Interdiscip Perspect Infect Dis. 2009 . ; 251406 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Okude M1, Matsuo J, Nakamura S, Kawaguchi K, Hayashi Y, Sakai H, Yoshida M, Takahashi K, Yamaguchi H. Environmental chlamydiae alter the growth speed and motility of host acanthamoebae. Microbes Environ. 2012; 27 ( 4): 423– 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Niyyati M, Rahimi F, Lasjerdi Z, Rezaeian M. Potentially Pathogenic Free-Living Amoebae in contact lenses of the asymptomatic contact lens wearers. Iran J Parasitol. 2014; 9(1): 14–9. [PMC free article] [PubMed] [Google Scholar]
- 15. Iovieno A, Ledee DR, Miller D, Alfonso EC. Detection of Bacterial Endosymbionts in clinical Acanthamoeba isoletes. Opthalmology. 2010; 117 ( 3): 445– 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schroeder JM, Booton GC, Hay J, Niszl IA, Seal DV, Markus MB, Fuerst PA, Byers TJ. Use of subgenic 18s ribosomal DNA PCR and sequencing for genus and genotype identification of Acanthamoeba from humans with keratitis and from sewage sludge. J Clin Microbiol. 2001; 39 ( 5): 1903– 1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Michel R, Hauroder D. Isolation of a Acanthamoeba strain with Intracellular Burkholderia pickettii infection. Zen Bakteriol 1997, 285: 541–557. [DOI] [PubMed] [Google Scholar]
- 18. Visvesvara GS, Moura H, Schuster FL; Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol Med Microbiol. 2007; 50 ( 1): 1– 26. [DOI] [PubMed] [Google Scholar]
- 19. Fritsche TR, Gautom RK, Seyedirashti S, Bergeron DL, Lindquist TD. Occurrence of bacterial endosymbiont in Acanthamoeba. Clin Microbiol. 1993, 31(5): 1122–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fritsche TR, Horn M, Seyedirashti S, Gautom RK, Schleifer KH, Wagner M. In situ detection of novel bacterial endosymbionts of Acanthamoeba spp. phylogenetically related to members of the order Rickettsiales. Appl Env Microbiol. 1999; 206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lagkouvardos I, Shen J, Horn M. Improved axenization method reveals complexity of symbiotic associations between bacteria and acanthamoebae. Environ Microbiol Rep. 2014; 6 ( 4): 383– 8. [DOI] [PubMed] [Google Scholar]
- 22. Tosetti N, Croxatto A, Greub G. Amoebae as a tool to isolate new bacterial species, to discover new virulence factors and to study the host-pathogen interactions. Microb Pathog. 2014; 77: 125– 30. [DOI] [PubMed] [Google Scholar]
- 23. Xin-an PU, Goodman RN. Induction of necrogenesis by Agrobacterium tumefaciens on grape explants. Physiol Molecul Plant Pathol. 1992; 41 ( 4): 241– 254. [Google Scholar]
- 24. McCullen CA, Binns AN. Agrobacterium tumefaciens and plant cell interactions and activities required for interkingdom macromolecular transfer. Annu Rev Cell Dev Biol. 2006; 22: 101– 27. [DOI] [PubMed] [Google Scholar]
- 25. Miller MJ, Wilson LA, Ahearn DG. Adherence of Pseudomonas aeruginosa to rigid gas-permeable contact lenses. Arch Ophthalmol. 1991; 109 ( 10): 1447– 1448. [DOI] [PubMed] [Google Scholar]
- 26. Dini LA, Cockinos C, Frean JA, Niszl IA, Markus MB. Unusual Case of Acanthamoeba polyphaga and Pseudomonas aeruginosa Keratitis in a Contact Lens Wearer from Gauteng, South Africa. J Clin Microbiol. 2000; 38 ( 2): 826– 829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hong J, Ji J, Xu J, Cao W, Liu Z, Sun X. An unusual case of Acanthamoeba Polyphaga and Pseudomonas aeruginosa keratitis. Diagn Pathol. 2014; 3: 9: 105 10.1186/1746-1596 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lorenzo-Morales J, Martín-Navarro CM, López-Arencibia A, Arnalich-Montiel F, Piñero JE, Valladares B. Acanthamoeba keratitis: an emerging disease gathering importance worldwide? Trends Parasitol. 2013; 29( 4: 181– 7. [DOI] [PubMed] [Google Scholar]
- 29. Tosetti N, Croxatto A, Greub G. Amoebae as a tool to isolate new bacterial species, to discover new virulence factors and to study the host-pathogen interactions. Microb Pathog. 2014; 77: 125– 30. [DOI] [PubMed] [Google Scholar]
- 30. Scheikl U, Sommer R, Kirschner A, Rameder A, Schrammel B, Zweimüller I, Wesner W, Hinker M, Walochnik J. Free-living amoebae (FLA) co-occurring with legionellae in industrial waters. Eur J Protistol. 2014; 50 ( 4): 422– 9. [DOI] [PMC free article] [PubMed] [Google Scholar]