Abstract
Background:
Surgery is the preferred treatment for hydatid cyst (cystic echinococcosis, CE). At present, various scolicidal agents have been used for inactivation of protoscoleces during surgery, but they are associated with adverse side effects. The aim of the present study was to evaluate the scolicidal effects of amphotricin B, Silver nano particles, Foeniculum vulgare Mill, essential oil and hypertonic saline against protoscoleces of hydatid cyst on an in vitro model.
Methods:
Protoscoleces were aseptically aspirated from the naturally infected livers of sheep and goats. Various concentrations of AmB (2.5–20 mg/ml), Ag-NPs (0.5–4 mg/ml), F. vulgare essential oil (0.125–1 mg/ml) and hypertonic saline (10–20%) were used for 5–60 min. Eosin exclusion test was used to determine the viability of protoscoleces.
Results:
Maximum protoscolicidal effect of AmB and Ag-NPs was found at concentrations of 20 and 4 mg/mL, resulting in only 82.3% and 71.6% of the protoscoleces after 60 min of incubation, respectively. In contrast, F. vulgare essential oil at concentration of 1 mg/ml and hypertonic saline 20% killed 100% protoscoleces after 5 and 10 min of exposure, respectively.
Conclusion:
The results indicated weak scolicidal activity of AmB and Ag-NPs; whereas F. vulgare essential oil had potent scolicidal activity against protoscoleces of hydatid cyst that revealed the potential of F. vulgare as a natural source for the production of new scolicidal agent for use in hydatid cyst surgery. However, further studies will be needed to confirm these results by checking the essential oil and its active component in the in vivo model.
Keywords: Fungizone, Ag-Nps, Fennel, Hypertonic saline, Cystic echinococcosis
Introduction
Cystic echinococcosis (Hydatid cyst, CE) is a parasitic infection caused by the larval stage of dog tapeworm Echinococcus granulosus. It has been identified as a major public health and economic problem in developing countries including Iran (1). The most common form of treatment is open surgical removal of the cysts combined with chemotherapy using albendazole and/or mebendazole before and after surgery (2). However, if there are cysts in multiple organs or tissues, or the cysts are in risky locations, surgery becomes impractical. For inoperable cases such as these, chemotherapy and/or PAIR (puncture-aspiration-injection-reaspiration) are recommended as alternative treatments (3, 4). Nevertheless, it has been proven that, albendazole and mebendazole used in treatment of hydatid cysts exhibited various adverse effects such as hepatotoxicity, severe leucopenia, thrombocytopenia and alopecia (4).
At present, to reduce the risk of intraoperative spillage of the cyst contents (protoscoleces) and subsequently recurrence of CE and secondary infection, which is observed in nearly 10% of the postoperative cases, the use of effective scolicidal agents are necessary (5, 6). So far, different scolicidal agents such as hypertonic saline, silver-nitrate, cetrimide, and ethanol have been used for inactivation of protoscoleces during surgery, but most of them are associated with adverse side effects such as hypernatremia, sclerosane colangititis, metabolic acidosis, liver necrosis and methaemoglobinaemia (7, 8). Thus, the development of new scolicidal agents with high efficacy in a shorter time of exposure, scolicidal ability inside a cyst, lower toxicity, higher availability and ability to be prepared rapidly is an urgent need for surgeons (9).
Amphotericin B (Fungizone, AmB) is known to be a polyene antifungal agent that binds to sterols (cholesterol and ergosterol) in the fungal cell wall, forming transmembrane channels resulting in osmotic lysis and death of the organism (10). AmB has also been used as a drug of last resort in otherwise-untreatable parasitic protozoan infections such as visceral leishmaniasis and primary amoebic meningoencephalitis (11, 12). Up to now, in several investigations, activity of AmB against larval stage Echinococcus multilocularis and Schistosoma mansoni have been reported (13, 14). However, there is no study on scolicidal effets of AmB on protoscoleces of E. granulosus.
Silver nanoparticles (Ag-NPs or nanosilver) are one of the most widely used nanoparticles, most notably serving as an antimicrobial agent for medical applications. The toxicity of nanosilver may be explained by the interaction of nanoparticles with microbes involving silver ion release and particle cellular internalization (15, 16). Foeniculum vulgare Mill. (Apiaceae) commonly known as Fennel, is a popular medicinal plant with various pharmacological activities mentioned in traditional Iranian medicine and modern phytotherapy such as antioxidant, anti-inflammatory, antimutagenic, antimicrobial, gastroprotective, hepatoprotective and memory enhancing (17).
The aim of the present study was to evaluate the scolicidal effects of AmB, Ag-NPs, F. vulgare essential oil and hypertonic saline against protoscoleces of hydatid cyst on in vitro model.
Materials and Methods
Chemicals
Colloidal Ag-NPs was obtained from Behban Shimi Pharmacy, Tehran, Iran, and were stored at room temperature (25°C) until testing. Amphotericin B, Eosin stain and Tween 20 were prepared from Sigma-Aldrich, (St. Louis, MO, USA). All the other chemicals and solvents used were of the highest purity commercially available.
Plant materials
F. vulgare seeds were collected from Baft District in June 2013, Kerman Province, Iran. The identities were confirmed by a botanist at the Botany Department of Shahid Bahonar University, Kerman, Iran. A voucher specimen of the plant materials was deposited at the Herbarium of Department of Pharmacognosy of School of Pharmacy, Kerman University of Medical Science, Kerman, Iran.
Isolation of the essential oil
Crushed seeds of F. vulgare (100 g) were subjected to hydro-distillation for 3 h using an all-glass Clevenger-type apparatus. The Essential oil obtained was dried over anhydrous sodium sulfate and stored in darkness at 4°C in airtight glass vials closed under nitrogen gas until testing.
Drug dilutions
For the preparation of dilutions of F. vulgare essential oil, 0.1 ml of the essential oil was dissolved in 9.7 ml of normal saline. To enhance the dispersal of the essential oil in normal saline, 0.2 ml of Tween 20 was added to the test tube. The resulting solution was mixed adequately by a magnetic stirrer. Serial dilution was then made to obtain the essential oil at 0.125, 0.25, 0.5 and 1 mg/ml. Twenty milligrams of AmB dissolved in 1 ml of normal saline and serial dilution was subsequently made to obtain AmB at 1.25, 2.5, 5, 10 and 20 mg/ml. To prepare dilutions of the Ag-NPs, 4 mg of colloidal Ag-NPs was in 1mL of normal saline and serial dilution was made to get Ag-NPs at concentrations of 1.25, 2.5, 5, 10 and 20 mg/mL. For the preparation of dilutions of hypertonic saline, 20g NaCl was dissolved in 100 ml of normal saline. The selection of dilutions of the AmB, Ag-NPs and F. vulgare essential oil was based on initial tests, which also exhibited that Tween 20 plus normal saline had no effect on the viability of protoscoleces.
Collection of protoscoleces
The protoscoleces of E. granulosus were collected from the livers of naturally infected sheep and goats slaughtered at Kerman abattoir, southeastern Iran and carried to the Parasitology Laboratory at the Department of Parasitology and Mycology, Kerman University of Medical Sciences (Kerman, Iran). The hydatid fluid aspirated by a 50 ml syringe and aseptically transferred into a flask was left to set for 30 min for protoscoleces to settle down. The supernatant was discarded and the protoscoleces were washed two times with PBS (pH 7.2) solution. The number of protoscoleces per milliliter was adjusted as 2× 103 protoscoleces in 0.9% NaCl solution with at least 90% viability rate.
Scolicidal effects against protoscoleces
In the present study, various concentrations of AmB, Ag-NPs, F. vulgare essential oil and hypertonic saline were used for 5, 10, 20, 30 and 60 min. At first, 0.5 ml of the protoscoleces (2× 103/ml) solution was placed in test tubes. Then 0.5 ml of various concentrations of tested drugs was added to each test tube. Tubes were gently mixed and then incubated at 37 °C for 5, 10, 20, 30 and 60 min. At the end of each incubation time the upper phase was carefully removed so as not to interrupt the protoscoleces. Fifty μl of 0.1% eosin stain was then added to the remaining settled protoscoleces and mixed gently. The upper portion of the solution was discarded and remaining pellet of protoscoleces was then smeared on a glass slide, covered with a cover glass and examined under a light microscope (18). The percentages of dead protoscoleces were determined by counting 100 protoscoleces. In addition, normal saline containing Tween 20 and 10, 15 and 20% hypertonic saline were used as control groups.
Viability test
In this investigation, eosin exclusion test was used to determine the viability of protoscoleces (19). After exposure to the eosin 0.1% (1 g of eosin powder in 1000 ml distilled water), alive protoscoleces remained colorless and showed characteristic muscular movements and flame cell activity ( Fig. 1 ), while dead protoscoleces absorbed eosin and colored red ( Fig. 2 ).
Fig. 1:
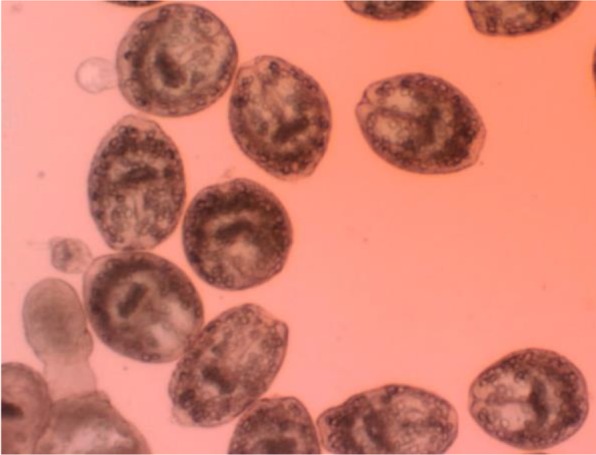
Live protoscoleces of Echinococcus granulosus after exposure with 0.1% eosin
Fig. 2:
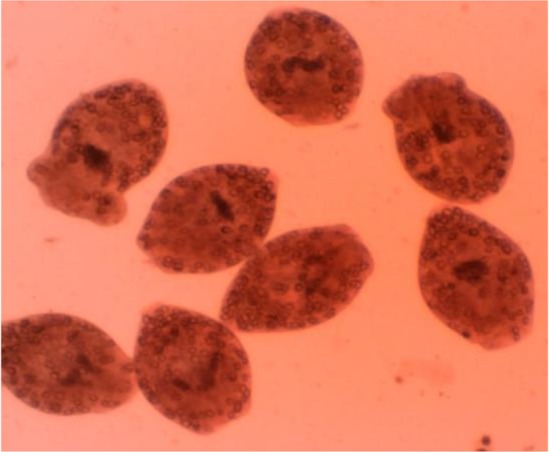
Dead protoscoleces of Echinococcus granulosus after exposure to tested drugs and with 0.1% eosin
Statistical analysis
In the present study, all the experiments were performed in triplicate. Moreover, data analysis was done using SPSS statistical package (version 17.0) (SPSS Inc., Chicago, IL, USA). Differences between test and control groups were analyzed by t-test. In addition, P<0.05 was considered statistically significant.
Results
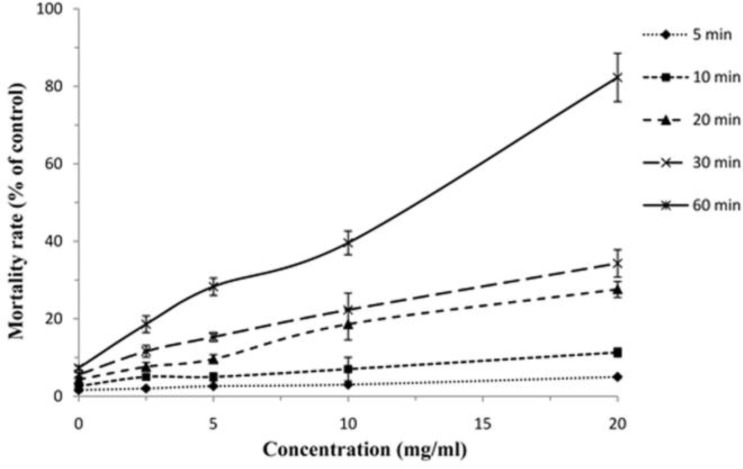
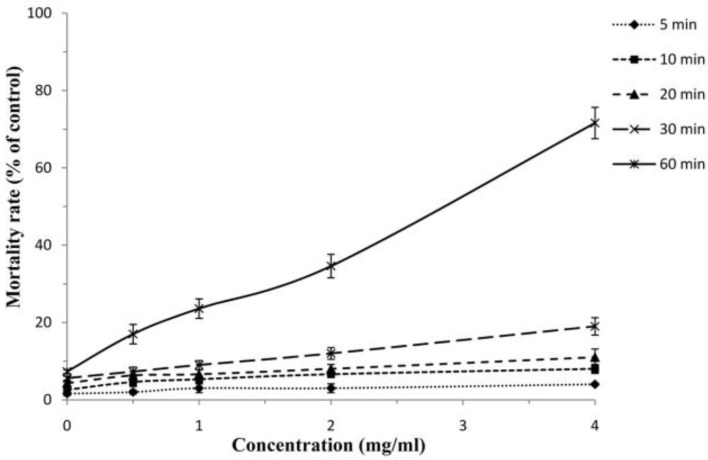
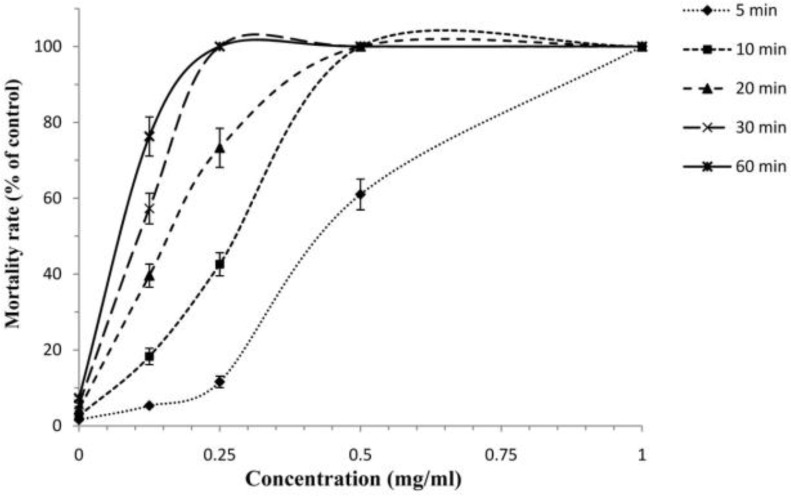
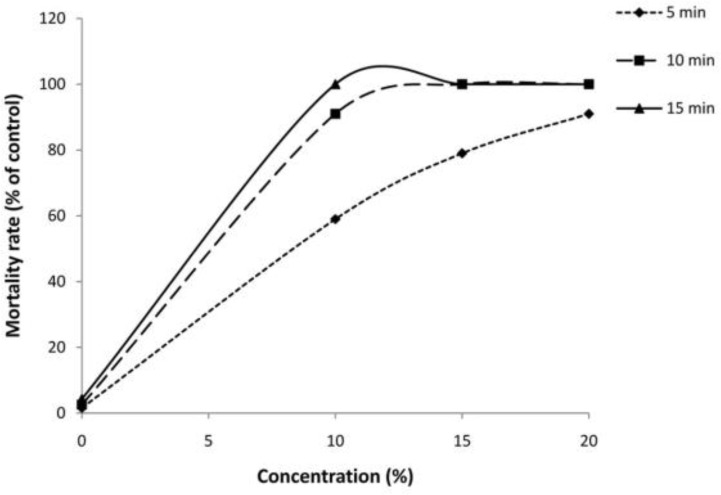
The findings revealed that among tested drugs, F. vulgare essential oil and hypertonic saline had potent scolicidal effects against protoscoleces of hydatid cysts in a dose-dependent and also time-dependent manner, while AmB and Ag-NPs demonstrated weak scolocidal activities on protoscoleces of hydatid cysts (Fig. 3 and 4). F. vulgare essential oil at the concentration of 1 mg/ml after 5 min of exposure killed 100% protoscoleces. Similarly, the mean of mortality rate of protoscoleces after 10 min of exposure to concentration of 0.5 mg/ml was 100%. The scolicidal effect of F. vulgare essential oil at the concentration of 0.25 mg/ml was 11.6, 42.6, 73.3, 100 and 100% after 5, 10, 20, 30 and 60 min of incubation, respectively. These values for the concentration of 0.125 mg/ml were 5, 18.3, 39.6, 57.3 and 73.6% respectively (Fig. 5). In contrast, the maximum protoscolicidal effect of AmB and Ag-NPs was found at the concentrations of 20 and 4 mg/ml, resulting in only 82.3% and 71.6% of the protoscoleces after 60 min of incubation, respectively. The mortality rate of protoscoleces in the negative control group was 7.1% after 60 min of exposure, while the scolicidal power of 10, 15 and 20% hypertonic saline as the positive controls was 100% after 15, 10 and 10 min of application (Fig. 6). Results also revealed that among tested drugs, the scolicidal effect of various concentrations of F. vulgare essential oil was extremely significant (P<0.05) compared to the control group at all exposure times.
Fig. 3:
Scolicidal effects of AmB against protoscoleces of Echinococcus granulosus at various concentrations following different exposure times
Fig. 4:
Scolicidal effects of Ag-Nps against protoscoleces of Echinococcus granulosus at various concentrations following different exposure times
Fig. 5:
Scolicidal effects of F. vulgare essential oil against protoscoleces of Echinococcus granulosus at various concentrations following different exposure times
Fig. 6:
Scolicidal effects of hypertonic saline against protoscoleces of Echinococcus granulosus at various concentrations following different exposure times
Discussion
The aim of the present study was to evaluate the scolicidal effects of amphotricin B, Silver nano particles, Foeniculum vulgare Mill. essential oil and hypertonic saline against protoscoleces of hydatid cyst on an in vitro model. Findings demonstrated that maximum protoscolicidal effect of AmB and Ag-NPs was found at concentrations of 20 and 4 mg/mL, resulting in only 82.3% and 71.6% of the protoscoleces after 60 min of incubation, respectively. In contrast, F. vulgare essential oil at concentration of 1 mg/ml and hypertonic saline 20% killed 100% protoscoleces after 5 and 10 min of exposure, respectively.
Although, in several investigations, scolicidal activity of hypertonic saline (20), silver nitrate and mannitol, cetrimide, ethyl alcohol (95%), H2O2 and 10% providone iodine, albendazole, chlorhexidine gluconate, Selenium NPs and some plant extracts (20, 21) have been proven. However, they are associated with adverse effects and their efficacy is controversial (7, 8).
Our findings demonstrated that AmB had no significant scolicidal activity even at high concentrations and long times on protoscoleces of hydateid cysts; whereas, Reuter et al. (13) reported that AmB effectively inhibits the growth of E. multilocularis metacestodes in a tissue culture system. This difference between previous study and present investigation could be related to type of studied species and also method of work.
Although the Ag-nitrate is one of the most effective scolicidal agents; but in the present study Ag-NPs were found to be less effective against protoscoleces of hydatid cysts. Similar to our findings Choi et al. (22) exhibited that Ag-NPs are not as effective as Ag ions in killing Escherichia coli bacteria. Since ancient times, plant-derived compounds and plants extracts have been used as a valuable natural resource of traditional remedy (23).
Essential oils contain a large number of components, and it is likely that their mode of action involves several targets in the cell of microorganisms (24). In this investigation, we reported that F. vulgare essential oil had remarkable scolicidal effects in compared with positive control; so that, at concentrations of 1 and 0.5 mg/ml killed 100% protoscoleces after 5 and 10 min, respectively. These findings are comparable with scolicidal activity of 20% hypertonic saline (15 min), 20% silver nitrate (20 min), 0.5–1% cetrimide (10 min), H2O2 3% (15 min) and 95% ethyl alcohol (15 min).
So far in the various study, antibacterial, antiviral, antifungal and antiparasitic effects of F. vulgare have been demonstrated (25, 26). Moreover, qualitative phytochemical analysis of F. vulgare has previously been carried out by Kaur and Arora that showed the presence of alkaloids, flavonoids, tannins, saponins and cardiac glycosides in this plant. Purified alkaloids as well as their synthetic derivatives are used as medicinal agents for their various biological effects such as analgesic, antispasmodic and bactericidal (27). Flavonoids have also been reported to possess antibacterial activity, which could be attributed to their ability to form complex with extracellular, soluble proteins and bacterial cell walls (28). Therefore, phytoconstituents in the plant materials could be responsible for their antimicrobial activity though their exact mode of action is poorly understood. In the case of cytotoxicity effects of F. vulgare essential oil, Erdogan Orhan et al. (25) demonstrated that essential oil of F. vulgare had no significant cytotoxicity against vero cells on an in vitro model. In addition, estragole, contained in the essential oil of F. vulgare was less cytotoxic against hepG2 human hepatoma cell line (29). Thus, F. vulgare and its constituents appear to have a low level of toxicity and could be considered safe in low concentrations.
Conclusion
The findings demonstrated weak scolicidal activity of AmB and Ag-NPs; whereas F. vulgare essential oil had potent scolicidal activity against protoscoleces of hydatid cyst that revealed the potential of F. vulgare as a natural source for the production of new scolicidal agent for use in hydatid cyst surgery. However, further studies will be needed to confirm these results by checking the essential oil and its active component in the in vivo model.
Acknowledgments
This study was supported by the Research Center for Tropical and Infectious diseases and Vice Chancellor for Research, Kerman University of Medical Sciences (project no.92/176) Kerman, Iran. The authors declare that there is no conflict of interest in this study.
References
- 1. Fasihi Harandi M, Budke CM, Rostami S. The Monetary Burden of Cystic Echinococcosis in Iran. PLOS Negl Trop Dis. 2012; 6 ( 11): e1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Eckert J, Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev. 2004; 17: 107– 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brunetti E, Kern P, Vuitton DA. Writing Panel for the WHO-IWGE Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2010; 114 ( 1): 1– 16. [DOI] [PubMed] [Google Scholar]
- 4. Junghanss T, da Silva AM, Horton J, Chiodini PL, Brunetti E. Clinical management of cystic echinococcosis: state of the art, problems, and perspectives. Am J Trop Med Hyg. 2008; 79 ( 3): 301– 11. [PubMed] [Google Scholar]
- 5. McManus DP, Zhang W, Li J. Echinococcosis. Lancet. 2003; 362: 1295– 304. [DOI] [PubMed] [Google Scholar]
- 6. Besim H, Karayalcin K, Hamamci O. Scolicidal agents in hydatid cyst surgery. HPB Surg. 1998; 10: 347– 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hosseini SV, Ghanbarzadeh K, Barzin Z, Sadjjadi SM, Tanideh N, Mehrabani D. In vitro protoscolicidal effects of hypertonic glucose on protoscolices of hydatid cyst. Korean J Parasitol. 2006; 44 ( 3): 239– 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rajabi M.A. Fatal reactions and methaemoglobinaemia after silver nitrate irrigation of hydatid cyst. Surg Pract. 2009: 13: 2–7. [Google Scholar]
- 9. Adas G, Arikan S, Kemik O, Oner A, Sahip N, Karatepe O. Use of albendazole sulfoxide, and combined solutions as scolicidal agents on hydatid cysts (in vitro study). World J Gastroenterol. 2009; 15: 112– 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. HsuChen CC and Feingold DS. Polyene antibiotic action on lecithin liposomes: effect of cholesterol and fatty acyl chains. Biochem Biophysic Res Comm. 1973; 51: 972– 8. [DOI] [PubMed] [Google Scholar]
- 11. Kafetzis DA, Velissariou IM, Stabouli S, Mavrikou M, Delis D, Liapi G. Treatment of paediatric visceral leishmaniasis: amphotericin B or pentavalent antimony compounds?. Int J Antimicrob Agents. 2005; 25 ( 1): 26– 30. [DOI] [PubMed] [Google Scholar]
- 12. Veerareddy PR, Vobalaboina V, Ali N. Antileishmanial activity, pharmacokinetics and tissue distribution studies of mannose-grafted amphotericin B lipid nanospheres. J Drug Target. 2008; 17 ( 2): 140– 7. [DOI] [PubMed] [Google Scholar]
- 13. Reuter S, Merkle M, Brehm K, Kern P, Manfras B. Effect of amphotericin B on larval growth of Echinococcus multilocularis. Antimicrob Agents Chemother. 2003; 47: 620– 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moné Y, Mitta G, Duval D, Gourbal BE. Effect of amphotericin B on the infection success of Schistosoma mansoni in Biomphalaria glabrata. Exp Parasitol. 2010; 125 ( 2): 70– 75. [DOI] [PubMed] [Google Scholar]
- 15. Kelly L, Coronado E, Zhao LL, Schatz GC. The Optical properties of metal nanoparticles: The Influence of size, shape and dielectric environment. J Phys Chem B. 2003; 107: 668– 77. [Google Scholar]
- 16. Sondi I, Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci. 2004; 275 ( 1): 177– 82. [DOI] [PubMed] [Google Scholar]
- 17. Rahimi R, Shams Ardekani MR. Medicinal Properties of Foeniculum Vulgare Mill. in Traditional Iranian Medicine and Modern Phytotherapy Chin J Integr Med. 2013; 19 ( 1): 73– 9. [DOI] [PubMed] [Google Scholar]
- 18. Moazeni M, Saharkhiz MJ, Hosseini AA. In vitro lethal effect of ajowan (Trachyspermum ammi L.) essential oil on hydatid cyst protoscoleces. Vet Parasitol. 2012; 187: 203– 8. [DOI] [PubMed] [Google Scholar]
- 19. Smyth JD, Barrett NJ: Procedures for testing the viability of human hydatid cysts following surgical removal, especially after chemotherapy. Trans R Soc Trop Med Hyg. 1980; 74: 649– 52. [DOI] [PubMed] [Google Scholar]
- 20. Mahmoudvand H, Fasihi Harandi M, Shakibaie M, Aflatoonian MR, Makki MS, Jahanbakhsh S. Scolicidal effects of biogenic selenium nanoparticles against protoscolices of hydatid cysts. Int J Surg. 2014. March 28 10.1016/j.ijsu.2014.03.017. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 21. Mahmoudvand H, Sharififar F, Saedi Dezaki E, Ezatpour B, Jahanbakhsh S, Fasihi Harandi M. Protoscolecidal effect of Berberis vulgaris root extract and its main compound, berberine in cystic echinococcosis. Iran J Parasitol. 2014; 9 ( 4): 26– 34. [PMC free article] [PubMed] [Google Scholar]
- 22. Choi O, Deng KK, Kim NJ, Ross L, Surampalli RY, Hu Z. The inhibitory effects of silver nanoparticles, silver ions, and silver chloride colloids on microbial growth. Water Res. 2008; 42 ( 12): 3066– 74. [DOI] [PubMed] [Google Scholar]
- 23. Mahmoudvand H, Sharififar F, Sharifi I, Ezatpour B, Fasihi Harandi M, Makki MS, et al. In vitro inhibitory effect of Berberis vulgaris (Berberidaceae) and its main component, Berberine against different Leishmania species. Iran J Parasitol. 2014; 9 ( 1): 28– 36. [PMC free article] [PubMed] [Google Scholar]
- 24. Kalemba D, Kunicka A. Antibacterial and anti-fungal properties of essential oils. Curr Med Chem. 2003; 10: 813– 29 [DOI] [PubMed] [Google Scholar]
- 25. Erdogan Orhan I, Berrin Ozcelik B, Kartal M, KAN Y. Antimicrobial and antiviral effects of essential oils from selected Umbelliferae and Labiatae plants and individual essential oil components. Turk J Biol. 2012; 36: 239– 46. [Google Scholar]
- 26. Liviu Drăgan L, Györke A, Ferreira J, Pop L, Dunca L, Drăgan M, et al. Effects of Artemisia annua and Foeniculum vulgare on chickens highly infected with Eimeria tenella (phylum Apicomplexa). Acta Vet Scand. 2014; 15 ( 56): 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kaur GJ, Arora DS. Antibacterial and phytochemical screening of Anethum graveolens, Foeniculum vulgare and Trachyspermum ammi. BMC Complement Alternat Med. 2009; 9 ( 30): 1– 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tsuchiya H, Sato M, Miyazaki T, Fuziwara S, Tanigaki S, Ohyama M, et al. Comparative study on the antibacterial activity of phytochemical flavonones against methicillinresistan Staphylococcus aureus. J Ethnopharmacol. 1996; 50: 27– 34. [DOI] [PubMed] [Google Scholar]
- 29. Villarini M, Pagiotti R, Dominici L, Fatigoni C, Vannini S, Levorato S, Moretti M. Investigation of the cytotoxic, genotoxic, and apoptosis-inducing effects of estragole isolated from Fennel (Foeniculum vulgare). J Nat Prod. 2014; 77: 773– 8. [DOI] [PubMed] [Google Scholar]