Abstract
Objective
Deep brain stimulation (DBS) is an effective treatment for movement disorders and a promising therapy for treating epilepsy and psychiatric disorders. Despite its clinical success, the efficiency and selectivity of DBS can be improved. Our objective was to design electrode geometries that increased the efficiency and selectivity of DBS.
Approach
We coupled computational models of electrodes in brain tissue with cable models of axons of passage (AOPs), terminating axons (TAs), and local neurons (LNs); we used engineering optimization to design electrodes for stimulating these neural elements; and the model predictions were tested in vivo.
Main results
Compared with the standard electrode used in the Medtronic Model 3387 and 3389 arrays, model-optimized electrodes consumed 45–84 % less power. Similar gains in selectivity were evident with the optimized electrodes: 50 % of parallel AOPs could be activated while reducing activation of perpendicular AOPs from 44–48 % with the standard electrode to 0–14 % with bipolar designs; 50 % of perpendicular AOPs could be activated while reducing activation of parallel AOPs from 53–55 % with the standard electrode to 1–5 % with an array of cathodes; and, 50 % of TAs could be activated while reducing activation of AOPs from 43–100 % with the standard electrode to 2–15 % with a distal anode. In vivo, both the geometry and polarity of the electrode had a profound impact on the efficiency and selectivity of stimulation.
Significance
Model-based design is a powerful tool that can be used to improve the efficiency and selectivity of DBS electrodes.
Keywords: deep brain stimulation, power efficiency, selectivity, electrode designs
1. Introduction
Deep brain stimulation (DBS) is an established therapy for neurological disorders, wherein 100–200 Hz electrical pulses are delivered to specific regions of the brain. DBS is effective in treating movement disorders, including Parkinson’s disease and essential tremor (Limousin et al., 1999, Montgomery, 1999) and shows promise for treating epilepsy and psychiatric disorders (Kuhn et al., 2010, Mayberg et al., 2005). Despite the clinical success of DBS, some aspects of the therapy require further development.
The energy efficiency of stimulation is one aspect of DBS that can be improved. Implantable pulse generators (IPGs) powered by non-rechargeable primary batteries last 3–5 years (Ondo et al., 2007), which is much shorter than the required duration of treatment. Consequently, patients must undergo repeated IPG replacement surgeries, obligating them to incur repeatedly the risks associated with surgery, which include infection (Boviatsis et al., 2010, Bronstein et al., 2011) and misprogramming (Okun et al., 2005). Rechargeable IPGs, which have longer predicted battery lives, are an alternative to primary-cell devices. In some cohorts, a majority of the patients were highly satisfied with the rechargeable stimulators (McAuley et al., 2013, Timmermann et al., 2013, Waln and Jimenez-Shahed, 2014); whereas in other cohorts, some patients felt that the burden of recharging was not worth the extra battery life (Harries et al., 2012, Lam and Rosenow, 2010). Whether a patient prefers a primary-cell or rechargeable IPG, improved efficiency can impact the cost and acceptability of DBS.
There are a number of approaches for improving stimulation efficiency including modifying the stimulation waveform (Foutz and McIntyre, 2010, Sahin and Tie, 2007, Wongsarnpigoon and Grill, 2010) or altering the material properties of the electrode (Cogan, 2008, Merrill et al., 2005). Examples of latter include surface roughening (Norlin et al., 2002) and electrode coatings (Cogan, 2008). Additionally, efficiency may be improved by increasing the area and/or perimeter of the electrode (Golestanirad et al., 2013, Howell and Grill, 2014, Wei and Grill, 2005).
Another aspect of DBS that requires development is stimulation selectivity, or the ability to activate target neural elements over non-target elements, as suboptimal lead placement is a common failure mode of DBS (Ellis et al., 2008, Okun et al., 2008, Okun et al., 2005). Lead deviations of 2–3 mm can preclude some or all potential clinical benefits (Bronstein et al., 2011, Ellis et al., 2008, Okun et al., 2008, Okun et al., 2005), and in some cases, generate side-effects by stimulation of non-target neural elements (Okun et al., 2008). For example, DBS of cerebellar afferents in the ventral intermediate nucleus of thalamus masks tremor-associated burst activity in essential tremor (Birdno et al., 2012). Activation of cerebellar afferents, however, may be limited by activation of non-target projection neurons in the ventral caudal nucleus of thalamus, which can cause uncomfortable paresthesias (Keane et al., 2012). Small lead misplacements can sometimes be overcome by reprogramming (Kuncel and Grill, 2004), but larger misplacements may require an additional surgery to reposition the lead (Ellis et al., 2008).
Improvements in selectivity may be possible by altering the configuration (i.e., the geometry and polarity) of the stimulation electrodes. For example, with a standard clinical lead, such as the Medtronic Model 3387 or Model 3389, selectivity may be improved by altering the geometry of the individual electrodes (Butson and McIntyre, 2006), segmenting the electrodes into many smaller electrodes (Buhlmann et al., 2011, Martens et al., 2011), or by combining the electrodes in multipolar configurations to shape the volume of tissue activated (Keane et al., 2012).
The goal of the current study was to design electrodes that improved the efficiency and selectivity of DBS. We coupled a computational model of a cylindrical electrode array in brain tissue with cable models of neural elements and used engineering optimization to design electrode configurations that increased the efficiency and selectivity of DBS. Subsequently, prototype electrodes were tested in vivo. Electrodes with greater stimulation efficiency will increase the lifetime of primary-cell IPGs and increase the recharge intervals of rechargeable IPGs, and electrodes with greater stimulation selectivity may reduce the sensitivity of clinical outcomes to malpositioning of the electrode, thereby reducing side effects. Preliminary results of this study were presented in a conference paper (Howell and Grill, 2013).
2. Methods
2.1. Computational model of DBS
We constructed a finite element method model in COMSOL Multiphysics v3.4 (COMSOL Inc., Burlington, MA) consisting of a three-contact cylindrical electrode array surrounded by a cubic (60 mm × 60 mm × 60 mm) volume of brain tissue (0.2 S/m (Gabriel et al., 2009)) (figure 1a). A 0.5 mm thick layer of tissue immediately surrounding the electrode represented the glial scar (0.1 S/m (Grill and Mortimer, 1994)), and the electrical conductivities of the encapsulation and brain tissue were homogeneous, isotropic and purely resistive (Bossetti et al., 2008). The origin in the model was located at the geometric center of electrode 2.
Figure 1.
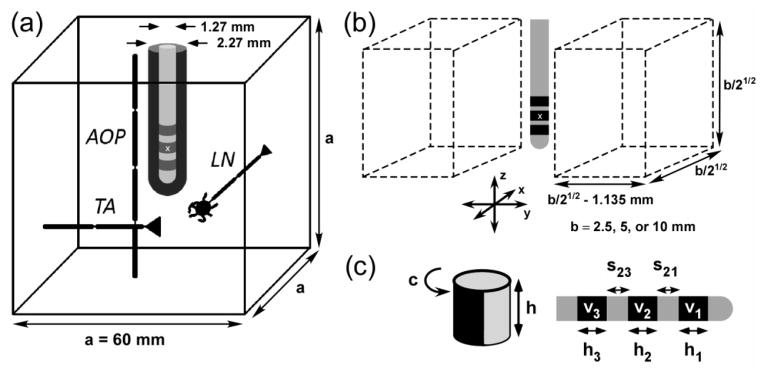
A computational model for design and analysis of deep brain stimulation (DBS) electrodes. (a) A finite element method model of an electrode array surrounded by encapsulation tissue (0.1 S/m) and brain tissue (0.2 S/m) was coupled to cable models of neural elements: AOPs = axons of passage, TAs = terminating axons, and LNs = local neurons. (b) Neural elements were distributed in two rectangular prisms that were displaced on either side of the x-z plane. (c) The three-electrode array (right) was parameterized using eight variables: 3 electrode heights (h1, h2, and h3), 2 interelectrode spacings (s21 and s23), and 3 applied potentials (v1, v2, and v3). In monopolar stimulation (left), electrodes 1 and 3 were modeled as insulating boundaries, and electrode 2 was parameterized by its height, h, and normalized circumference, c. The origin (x) is located at the geometric center of electrode 2.
A normal current density of 0 A/mm2 (i.e., a Neumann boundary condition) was imposed on the electrode shaft to model a perfect insulator. Fixed potentials of X V (i.e., Dirichlet boundary conditions), where − 10 < X < 10, were imposed individually on the three contacts, and 0 V was imposed on the outer tissue boundary. The electric potentials were calculated by solving Laplace’s equation,
| (1) |
where ∇ is the divergence operator, σ is the conductivity and J⃑ is the current density. The injected current was calculated by integrating the current density over the surface of the electrode,
| (2) |
where E⃑ is the electric field.
The NEURON simulation environment (v7.1) (Carnevale and Hines, 1997) was used to implement cable models of neural elements, including myelinated axons of passage (AOPs) (McIntyre et al., 2002), terminating axons (TAs), and local neurons (LNs) (McIntyre et al., 2004). The axons of all neural elements were 2.5 μm in diameter (Assaf et al., 2008, Barazany et al., 2009) and 40 mm in length. AOPs, TAs, and LNs were placed in the tissue according to defined reference points, which were the middle node of Ranvier, the axon terminal, and the center of the soma, respectively. Populations consisted of 100 elements with axons oriented parallel (z direction) and perpendicular (x direction) to the electrode shaft (figure 1b). The two halves of the population were uniformly distributed in two rectangular prisms displaced by ± 1.135 mm on either side of the z = 0 plane (figure 1b). This displacement included the radius of the lead (0.635 mm) plus a 0.5 mm thick region representing the glial scar that forms after chronic implantation (Haberler et al., 2000). The region of glial scar contained no neural elements because chronic inflammation within and around this region can lead to neurodegeneration and/or cell death (McConnell et al., 2009).
Modeling studies (Butson et al., 2007, Keane et al., 2012, Kuncel et al., 2008) predict that neural elements up to 3 mm from the electrode surface are activated at typical DBS parameter settings (e.g., 3 V, 90 μs, and 130–185 Hz) (Kuncel and Grill, 2004). In DBS for the treatment of psychiatric disorders, stimulation amplitudes up to 10 V are required to achieve a therapeutic effect (Holtzheimer and Mayberg, 2011), and at these higher stimulation amplitudes, elements > 3 mm from the electrode surface may be activated (Butson et al., 2007). To encompass this range of distances, the dimensions of the rectangular prisms were chosen so that the furthest neural element in the population had a least one point on its axon or soma that was < 2.5 mm, 5 mm, or 10 mm from the origin.
The source driving polarization of the neural membrane is proportional to the spatial centered second difference of the potentials, δ2Φ (Rattay, 1986), so the potentials were calculated using ~ 1 million 3rd order Lagrangian elements, as 3rd order elements prevented large discontinuities when calculating δ2Φ (Howell et al., 2014). Refinement of the mesh (i.e., splitting elements into smaller elements) from ~320,000 to 1 million cubic elements resulted in changes of < 1 % in the mean absolute relative difference of both the interpolated potentials and corresponding stimulation thresholds of model neurons.
The interpolated potentials were used in NEURON to stimulate the neural elements. To isolate the effect of electrode geometry during model optimization, neural elements were stimulated with a 100 μs monophasic rectangular pulse. However, biphasic stimulation was used in our experiments, and for that reason, the performance of all optimal designs was quantified post hoc during stimulation with a short 100 μs cathodic phase followed by a long 900 μs anodic phase. Since the conductivities were linear, the potentials at a specific stimulation voltage were calculated by multiplying the 1 V solutions by a scalar, and the threshold stimulation voltage to activate each model axon was calculated using a bisection algorithm (relative error < 1 %).
2.2. Design of electrodes with increased stimulation efficiency and selectivity
The computational model of DBS was used with engineering optimization to design electrode geometries that could efficiently activate AOPs, TAs, and LNs; selectively activate parallel AOPs over perpendicular AOPs; or selectively activate parallel TAs over AOPs. The three-electrode array was parameterized using eight parameters (figure 1c): three electrode heights (h1, h2, and h3 ≥ 0.1 mm), two inter-electrode spacings (s21 and s23 ≥ 0.1 mm), and three applied potentials (v1, v2, and v3). In preliminary work (Howell and Grill, 2013), we allowed the radius of the electrode to vary between 0.1 and 1 mm. The optimal radius for efficient activation of AOPs was > 0.635 mm. However, a larger diameter electrode, although potentially more efficient, would damage more tissue upon implantation, so r was held constant at 0.635 mm. If the optimal solution identified by the genetic algorithm (GA, see below) resembled a single contact (i.e., s21 and s23 were equal to 0.1 mm), then contacts 1 and 3 were treated as insulation, contact 2 was parameterized by its height (h) and normalized circumference (c), and a post hoc brute force search was conducted on the reduced parameter space: 1 mm ≤ h ≤ 15 mm in increments of 0.5 mm and 0.1 ≤ c ≤ 1 in increments of 0.1.
For each electrode configuration (i.e., geometry and polarity), we calculated the electric potentials, stimulation thresholds for activation of each neural element, and subsequently, the cost functions quantifying efficiency or selectivity. The cost function for stimulation efficiency was the average power required to activate 50 % of the population of neural elements (P50):
| (3) |
where V(t) is the applied voltage and PW is the duration of the rectangular pulse. The efficiency of the optimal electrode design was quantified by calculating a relative power usage (RPU), which is 100 times the ratio of P50 with the optimal design to P50 with the standard electrode (height = 1.5 mm and radius = 0.635 mm) that is used in the Medtronic Model 3387, the Medtronic Model 3389, and the Boston Scientific Vercise arrays. Selectivity was quantified by constructing a curve, p(x), of the proportion of a population of neural elements activated versus selected proportions of a population of other neural elements and minimizing or maximizing the (normalized) area under the curve:
| (4) |
We used a genetic algorithm (GA) to search the parameter space and optimize the cost function (MATLAB v2008b, Global Optimization Toolbox). The initial geometry was selected randomly within the bounds of the parameter space. Each generation consisted of 20 solutions: the 2 fittest solutions and 18 progeny, which were generated by mating (i.e., randomly swapping parameters between) nine random pairs of parent vectors. Mating was conducted by creating a random binary vector (of 0s and 1s) for each vector pair and exchanging the elements of the parent vectors where the elements of the corresponding binary vectors were equal to 1. Mutations were introduced by adding random numbers to each element of the progeny vector. The random numbers for each element were generated from a Gaussian distribution with a mean of zero and a standard deviation equal to the difference in the parameter between the first two generations. The standard deviation of the Gaussian distribution for each parameter decreased linearly so that it reached zero at the maximum number of generations. The GA terminated when the average change in the cost function was < 0.1 % over 100 iterations, or after 150 iterations.
Optimizations were repeated ten times on three independent populations of 100 neural elements to ensure that the results were not the result of a specific population, and a post hoc brute force search was conducted on a subset of the parameter space surrounding the best GA solution to determine whether a better solution existed.
The sensitivity of the efficient electrode designs to changes in model parameters was assessed by quantifying how RPU changed when the 900 μs charge-balancing phase was removed from the stimulus pulse (i.e., monophasic stimulation), when the encapsulation tissue was replaced with brain tissue, when the diameter of target axons was increased from 2.5 μm to 15 μm in increments of 2.5 μm, and when the load of the electrode tissue interface (ETI) impedance was included. For the ETI analysis, the double-layer capacitance (Cdl) and Faradaic resistance (Rf) were set to the minimum or maximum values measured in vivo (see Sections 2.3 and 3.4.1). The sensitivity of the selective electrode designs to changes in model parameters was assessed when the 900 μs charge-balancing phase was removed from the stimulus pulse (i.e., monophasic stimulation) and when the axons were rotated about the y axis in the xz plane. The angle of rotation for each fiber was randomly sampled from a normal distribution with mean = 0 ° and standard deviation = 7.5 °.
Simulations were also conducted to determine whether the electrodes of the 3387, the 3389, and the Vercise arrays could be configured to approximate the potentials of the novel electrode configurations that resulted from the optimization. The 3387 and 3389 have four electrodes, 1.5 mm in height and 0.635 mm in radius, with (edge-to-edge) inter-electrode spacings of 1.5 mm and 0.5 mm, respectively. The Vercise array has eight electrodes with the same height, radius, and inter-electrode spacing of the 3389 array. If the standard clinical array did not achieve an RPU or AUC that was within 5 % of the corresponding optimal configuration, further simulations were conducted to assess whether an array with more electrodes and a smaller inter-electrode spacing could achieve RPU and AUC to within 5 % of the optimal configurations.
2.3. Measurements of stimulation efficiency and selectivity in vivo
Two sets of prototype electrodes were fabricated to test the model-based designs in cats (figure 2). Prototypes were fabricated by sputter depositing a chrome adhesion layer followed by a gold metal layer onto a planar polyimide substrate, and planar substrates were wrapped around and bound to a rigid steel shaft to form cylindrical electrode arrays. The set of electrode designs for measuring efficiency in vivo consisted of a long cylinder (LC) with a height of 6 mm, a long hemi-cylinder (LHC) electrode with a height of 6 mm, and the standard electrode with a height of 1.5 mm. The LC, LHC, and standard electrode all had radii of 0.635 mm. Selectivity was measured in vivo using a five-electrode array that could be configured to approximate the potentials produced by the optimal electrode configurations. Each electrode had a height of 1 mm and a radius of 0.635 mm, and the inter-electrode spacing was 0.2 mm. Since the size of the neural tissue targeted in the cat was small compared to that of humans, we focused on the optimal configurations for stimulating neural elements ≤ 2.5 mm from the origin.
Figure 2.
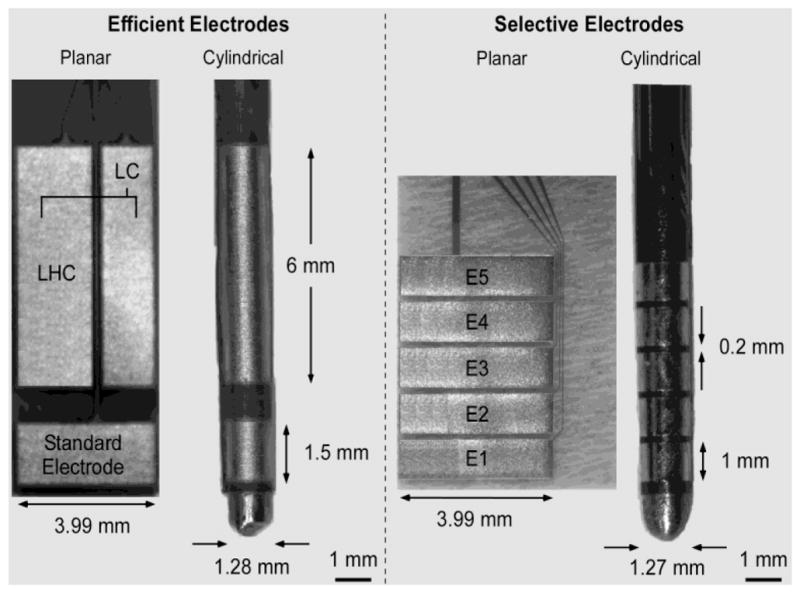
Prototype electrodes for experimental measurement of stimulation efficiency and selectivity. Photomicrographs of two sets of planar and cylindrical electrode designs for measuring the efficiency (left) and selectivity (right) of electrical stimulation in vivo. LHC = long hemicylinder, LC = long cylinder, and E = electrode. The standard electrode has the geometry of the contacts used in the Medtronic Model 3387 and 3389 arrays, and the Boston Scientific Vercise system array.
Acute in vivo experiments were conducted in anesthetized cats using a protocol approved by the Institutional Animal Care and Use Committee at Duke University. Anesthesia was induced with ketamine HCL (Ketaset 35 mg/kg IM, supplemented as required at 15 mg/kg during surgical preparation) and maintained with alpha chloralose (65 mg/kg IV, supplemented at 15 mg/kg). In one set of experiments, the planar electrode designs were oriented parallel or perpendicular to the cat sciatic nerve (figure 3a), and an isolated stimulator was used to deliver a 0.5 Hz train of voltage-regulated, asymmetric pulses with a short 100 μs cathodic phase followed by a long 900 μs anodic phase. Performance was assessed using a repeated measures design: in each animal, we tested all the efficient or selective planar electrode designs, and we repeated the experiments for measuring efficiency and selectivity in a total of seven and five cats, respectively.
Figure 3.
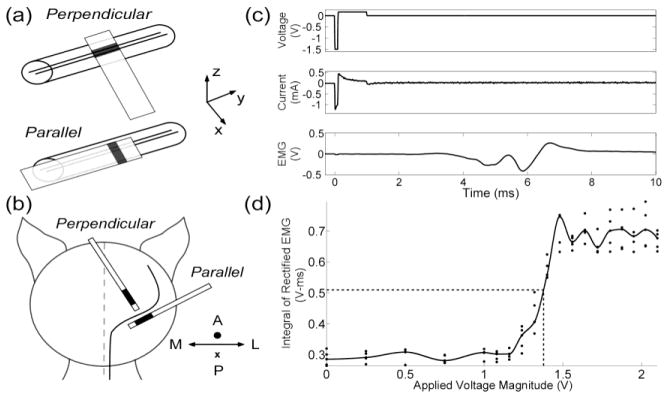
Measuring the efficiency and selectivity of prototype electrodes in vivo. (a) Planar electrode designs (see figure 2) were oriented parallel or perpendicular to the sciatic nerve. (b) Cylindrical electrode designs were implanted parallel or perpendicular to corticospinal axons in the posterior limb of the internal capsule. A = anterior, P = posterior, M = medial, and L = lateral. (c) The applied voltage, applied current, and evoked electromyographic responses (EMGs) were recorded during stimulation. (d) The EMG was rectified and integrated, and a fitted spline was used to calculate the voltage (and average power) required to evoke a half maximal response.
In a second set of experiments, brain surgery was performed under 1 % isoflurane anesthesia, and the electrode was implanted parallel or perpendicular to axons in the posterior limb of the internal capsule (Duerden et al., 2011) (figure 3b) – located 7 mm anterior, 10.5 mm lateral, and 10 mm above the interaural line (Nicholson, 1965, Snider and Niemer, 1961). Stimulus trains consisted of 30 voltage-regulated rectangular pulses with a short 300 μs cathodic phase followed by a long 700 μs anodic phase delivered at 350 Hz. Voltage amplitudes were varied between 0 V and 5 V, focusing the majority of values between the stimulation threshold for evoking an electromyographic response (EMG) and the amplitude at which the EMG was maximal. Voltage amplitudes were repeated five times. Cdl, Rf, and the access resistance (Ra) of each electrode were determined by using nonlinear regression to fit a three-element Randles equivalent circuit to the average current transient (Equation 5) recorded at each voltage amplitude (figure 3c).
| (5) |
| (6) |
Vo is the amplitude of the applied voltage. The efficiency and selectivity of the cylindrical electrode designs were measured in ten and five cats, respectively.
Measurements were taken using an isolated, low noise amplifier (SR560, Stanford Research, Sunnyvale, CA): the applied voltage was measured from the output of the isolated stimulator, and the current was measured from the voltage drop across a 25 Ω resistor in series with the implanted electrode and tissue load. The measured current waveforms were averaged across five repetitions, and the product of the voltage and current waveforms was used to calculate the average electrical power transferred over time (figure 3c). EMGs were measured differentially between two 29-gauge multi-stranded stainless steel wires (Cooner Wire, Chatsworth, CA). In the sciatic nerve experiments, the EMG electrodes were implanted in the ipsilateral peroneus muscles, and in the brain experiments, the EMG electrodes were implanted in the contralateral extensor digitorum muscles. EMGs were AC coupled, amplified by a gain of between 100 and 500, and bandpass filtered with lower and upper cutoff frequencies of 3 Hz and 3 kHz, respectively (SR560). The EMG was rectified and integrated to quantify the average magnitude of the signal over time. Data were sampled and stored using the MATLAB Data Acquisition toolbox (Mathworks, Natick, MA).
Stimulation efficiency was quantified by calculating the average power required to evoke a half maximal EMG (P50) and comparing it to P50 of the standard clinical electrode used in the 3387, 3389, and Vercise arrays (figure 2a). Stimulation selectivity was measured by calculating the ratio of the voltage required to evoke a half maximal EMG (V50) when oriented parallel to the target fibers to V50 when oriented perpendicular to the target fibers.
The location of implanted electrodes was determined from post mortem histology. Animals were perfused with 10 % formalin (~ 4 % formaldehyde). The brain was removed and immersed in a 10 % formalin (~ 4 % formaldehyde) solution for two days, immersed in a 30 % sucrose solution for one week, and frozen in optimal cutting temperature (OCT) medium at −80 °C. 30 μm thick brain slices were stained using 0.1 % cresyl violet, and electrode placement was determined by overlaying images of the stained tissue with a brain slice of the target region from a cat brain atlas (www.brainmuseum.org).
2.4. Statistical analysis
We fitted a spline to the EMG data to calculate V50 or P50 (figure 3d). The dependent variable in our statistical analysis was the natural logarithm of the ratio of V50 or P50 of one electrode in one orientation to the V50 or P50 of a second (same) electrode in the same (second) orientation. Three statistical tests were conducted. First, for a given orientation and electrode design, a Lilliefors test was conducted on the distributions of ln(V50) or ln(P50) across experiments to test the null hypothesis that ln(V50) or ln(P50) was normally distributed. Second, if there was not enough evidence to reject the null hypothesis of the Lilliefors test across all designs and orientations, a repeated measures analysis of variance (ANOVA) was conducted to test the hypothesis that ln(V50) or ln(P50) across all electrodes in both orientations came from the same normal distribution. Finally, if the null hypothesis of the ANOVA was rejected, post hoc paired comparisons were made using Fisher’s least significant difference test. Data are presented as the mean ± standard error, and all statistical tests were conducted at a 95 % confidence level (α < 0.05).
3. Results
We used a computational model of DBS and engineering optimization to design electrodes intended to increase the efficiency and selectivity of DBS. The efficiency and selectivity of the prototype electrodes were subsequently measured in vivo. Biphasic stimulation was used in the experiments; therefore, unless stated otherwise, the performance of the optimal designs reflects stimulation with a short 100 μs cathodic phase followed by a long 900 μs anodic phase.
3.1. Computational analysis of efficient electrode designs
Optimizations of the eight design parameters were conducted to determine what three-electrode configurations (figure 1) minimized the power required to stimulate parallel or perpendicular neural elements (AOPs, TAs, and LNs). Optimal solutions yielded parameters sets where s21 and s23 were much smaller than h1, h2, and h3; and s21 and s23 were > h1 and h3, and v1 and v3 were close to 0 V. Since these solutions resembled monopolar stimulation with a single electrode, electrodes 1 and 3 were replaced with insulation, and electrode 2 was optimized with parameters c and h.
Electrodes with relatively large (height to diameter) aspect ratios were more efficient at stimulating neural elements oriented perpendicular to the electrode shaft (figure 4a). For example, long electrodes with h between 3.5 and 10 mm and c between 0.5 and 0.8 consumed 45–84 % less power than the standard clinical electrode when activating populations of perpendicular AOPs, TAs, and LNs. In contrast, electrodes with relatively small aspect ratios were more efficient for stimulating parallel neural elements. The geometry of the standard electrode was close to optimal for stimulating parallel AOPs and TAs, and altering h and c decreased power consumption by only 14 % when stimulating parallel LNs (figure 4a).
Figure 4.
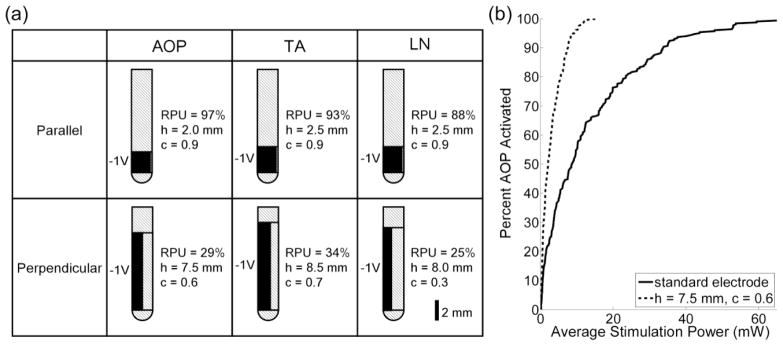
Optimal monopolar electrode geometries for efficiently activating neural elements ≤ 5 mm from the origin. (a) Optimal geometries and corresponding relative power usage (RPU) when stimulating a population of AOPs (column 1), TAs (column 2), and LNs (column 3) oriented either parallel (row 1) or perpendicular (row 2) to the electrode shaft. AOP = axon of passage, TA = terminating axon, LN = local neuron, h = height, c = normalized circumference, and RPU is 100 times the ratio of the average stimulation power required to activate 50 % of the neural elements (P50) with the optimal design to P50 with the standard clinical electrode (see section 2.2) in a cathodic configuration. (b) Input-output curves of average stimulation power versus percentage of perpendicular AOPs activated for electrodes: the electrode in row 2, column 1 of part a and the standard electrode in a cathodic configuration.
Four additional analyses were conducted to assess how sensitive the efficiency of the optimal electrode geometries were to changes in the model parameters. Since the solutions were qualitatively similar across the different neural elements, analyses were conducted only for stimulation of AOPs. The efficiency of the optimal designs was sensitive to an increase in fiber diameter above 5 μm and the presence of the ETI, but insensitive to the presence of encapsulation tissue and changing the waveform from biphasic to monophasic (Table 1).
Table 1.
Sensitivity of stimulation efficiency to changes in model parametersa
| Case | Baselineb (RPU) | Monophasic Stimulation | RPU | ETIc | No ET | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fiber diameter (μm) | |||||||||||
|
| |||||||||||
| Distance (mm) | Orientation | 5 | 7.5 | 10 | 12.5 | 15 | Min. | Max. | |||
| 2.5 | parallel | 96 | 94 | 98 | 106 | 111 | 106 | 108 | 101 | 107 | 90 |
| 5 | parallel | 97 | 96 | 94 | 93 | 93 | 94 | 92 | 97 | 93 | 98 |
| 10 | parallel | 74 | 72 | 70 | 68 | 66 | 65 | 64 | 78 | 67 | 80 |
| 2.5 | perpendicular | 55 | 56 | 69 | 75 | 77 | 74 | 73 | 70 | 70 | 61 |
| 5 | perpendicular | 29 | 29 | 36 | 39 | 42 | 41 | 43 | 46 | 37 | 35 |
| 10 | perpendicular | 20 | 20 | 23 | 25 | 26 | 27 | 27 | 39 | 28 | 23 |
Stimulation of axons of passage. See Section 2.2 for a summary of the sensitivity analyses.
Biphasic stimulation of axons with diameters of 2.5 μm and ET present. RPU = relative power usage (see Section 2.2).
The effect of the ETI was assessed at the minimum Cdl and Rf, or maximum Cdl and Rf values.
Abbreviations: ETI = electrode-tissue interface, ET = encapsulation tissue.
3.2. Computational analysis of selective electrode designs
3.2.1. Selectivity based on orientation
Optimizations of the eight design parameters were conducted to determine electrode configurations that maximized selectivity of parallel AOPs over perpendicular AOPs and vice versa. An asymmetric bipolar configuration was the optimal solution for selectively activating parallel AOPs over perpendicular AOPs (figure 5a). When neural elements were placed up to 2.5 mm, 5 mm, and 10 mm from the origin, asymmetric bipolar configurations activated 50 % of target parallel AOPs while limiting activation of non-target perpendicular AOPs to 0 %, 14 %, and 8 %, respectively, whereas the standard electrode activated almost 50 % of the non-target elements (figure 5).
Figure 5.
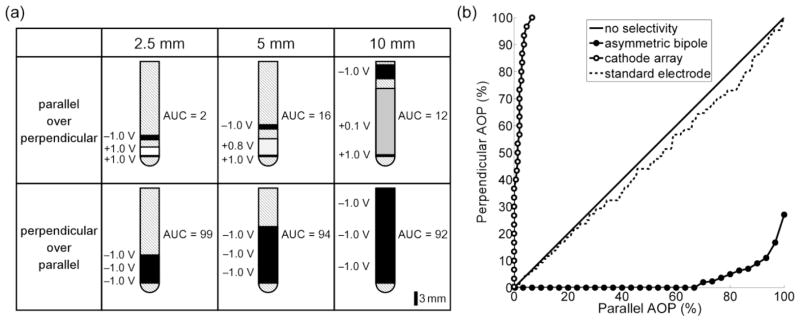
Optimal three-electrode configurations for selectively activating axons of passage (AOPs) based on their orientation. (a) Optimal electrode configurations and corresponding normalized area under curve (AUC, see Equation 4) when stimulating parallel AOPs over perpendicular AOPs (row 1) and perpendicular AOPs over parallel AOPs (row 2). Elements were placed ≤ 2.5 mm (column 1), 5 mm (column 2), and 10 mm (column 3) from the origin. The configurations shown in rows 1 and 2 are referred to as asymmetric bipoles and cathodes arrays, respectively. Note, in row 2, the edge-to-edge spacings between electrodes are all 0.1 mm, which could not be resolved in the image. (b) Curves of the percent of perpendicular AOPs activated versus select percentages of parallel AOPs when elements were placed ≤ 2.5 mm from the origin. For comparison, the standard clinical electrode (see section 2.2) was used to stimulate AOPs in a cathodic configuration.
The optimal configuration for selectively activating perpendicular AOPs over parallel AOPs was an array of cathodes. When neural elements were placed up to 2.5 mm, 5 mm, and 10 mm from the origin, an array of cathodes activated 50 % of target perpendicular AOPs while limiting activation of non-target parallel AOPs to 1 %, 3 %, and 5 %, respectively, whereas cathodic monopolar stimulation with the standard electrode activated more than 50 % of the non-target elements (figure 5).
Additional analyses were conducted to assess how sensitive the performance of the optimal tripolar electrode geometries was to changes in the waveform shape and axon orientation. Aside from selective stimulation of parallel AOP over perpendicular AOP that were placed ≤ 10 mm from the origin, the selectivity of the optimal electrode designs was insensitive to shifts in the axon orientation and changing the waveform from biphasic to monophasic (Table 2).
Table 2.
Sensitivity of stimulation selectivity to changes in model parametersa
| Distance (mm) | Orientations | Baselinea (AUC) | AUC | |
|---|---|---|---|---|
| Monophasic Stimulation | Angular Shiftsb | |||
| 2.5 | parallel over perpendicular | 2 | 0 | 6 |
| 5 | parallel over perpendicular | 16 | 17 | 19 |
| 10 | parallel over perpendicular | 12 | 22 | 23 |
| 2.5 | perpendicular over parallel | 99 | 99 | 100 |
| 5 | perpendicular over parallel | 94 | 93 | 93 |
| 10 | perpendicular over parallel | 92 | 91 | 89 |
Biphasic stimulation of axons with diameters of 2.5 μm. AUC = area under curve (see Section 2.2)
Shifts in axon orientations sampled from a normal distribution (mean = 0°, standard deviation = 7.5°).
3.2.2. Selectivity based on type of neural element
Optimization of the eight design parameters was conducted to identify electrode configurations that maximized selectivity of parallel TAs over parallel and perpendicular AOPs. An anode distal to the target population was the optimal solution for selectively activating TAs over AOPs. When neural elements were placed up to 2.5 mm, 5 mm, and 10 mm from the origin, distal anodes activated 50 % of target parallel TAs while limiting activation of non-target parallel AOPs to 2 %, 15 %, and 2 %, respectively; whereas monopolar anodic stimulation with the standard electrode activated 64 %, 43 %, and 100 % of non-target elements, respectively (figure 6). Selectivity was greatest when distal anodes were used to activate parallel TAs over perpendicular AOPs (figure 6a).
Figure 6.
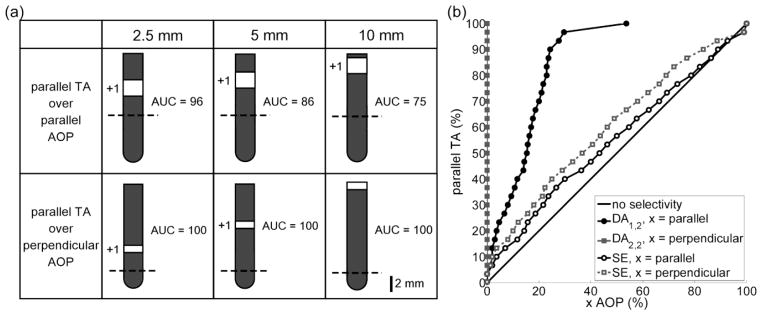
Optimal electrode configurations for selectively activating terminating axons (TAs) over axons of passage (AOPs). (a) Optimal electrode configurations and corresponding normalized area under the curve (AUC, see Equation 4) when stimulating parallel TAs over parallel AOPs (row 1) and parallel TAs over perpendicular AOPs (row 2) when neural elements were placed ≤ 2.5 mm (column 1), 5 mm (column 2), and 10 mm (column 3) from the origin. The dashed line denotes z = 0. (b) The percentage of parallel TAs versus selected percentages of parallel or perpendicular AOPs for the distal anodes in row 1, column 2 (DA1,2) and row 2, column 2 (DA2,2) in part a. For comparison, the standard electrode (SE) in an anodic configuration was used to stimulate parallel TAs over AOPs.
3.3. Efficiency and selectivity of clinical arrays
We conducted additional simulations to determine whether the 3387, 3389, and Vercise arrays could be configured to approximate the performance of the optimal electrode configurations (figures 4a, 5a, and 6a). The clinical arrays performed on par with the asymmetric bipole configurations and distal anode configurations but worse than the efficient monopolar configurations and selective cathode array configurations (Table 3).
Table 3.
Using electrode arrays to approximate the optimal model electrode configurationsa
| Description | Performance (RPU or AUC)b of Arrays | ||||||
|---|---|---|---|---|---|---|---|
| Case | Orientation | Element | Optimalc | 3387 | 3389 | Vercise | 12-electrode |
| Efficiency | parallel | AOP | 97 | 100 | 100 | 100 | 95 |
| Efficiency | parallel | TA | 93 | 100 | 100 | 100 | 87 |
| Efficiency | parallel | LN | 88 | 100 | 100 | 100 | 77 |
| Efficiency | perp. | AOP | 29 | 161 | 284 | 283 | 32 |
| Efficiency | perp. | TA | 34 | 161 | 282 | 282 | 39 |
| Efficiency | perp. | LN | 25 | 161 | 284 | 283 | 36 |
| Selectivity | parallel over perp. | AOP over AOP | 16 | 28 | 11 | 11 | 13 |
| Selectivity | perp. over parallel | AOP over AOP | 94 | 84 | 84 | 86 | 96 |
| Selectivity | parallel over parallel | TA over AOP | 86 | 86 | 86 | 86 | 86 |
| Selectivity | parallel over perp. | TA over AOP | 100 | 99 | 99 | 99 | 100 |
Biphasic stimulation of neural elements ≤ 5 mm from origin.
Relative power usage (RPU) for efficiency and area under curve (AUC) for selectivity (see Section 2.2).
See figures 4a, 5a, and 6a for optimal 3-electrode configurations.
Abbreviations: AOP = axon of passage, TA = terminating axon, LN = local neuron, perp. = perpendicular.
We also tested a 12-electrode array whose contacts were approximately 0.9 mm in height and spaced by 0.1 mm. The 12-electrode array was able to achieve a performance that was relatively close to the performance of all ten optimal electrode configurations (Table 3). It should be noted that in a few cases the clinical and 12-electrode arrays performed marginally better than the optimal designs. Using more than three contacts increased the total perimeter of the configurations, which decreased the electrode impedance (Howell and Grill, 2014, Wei and Grill, 2005), leading to slight improvements in performance.
3.4. Measuring stimulation efficiency and selectivity in vivo
3.4.1. Efficient prototype electrodes
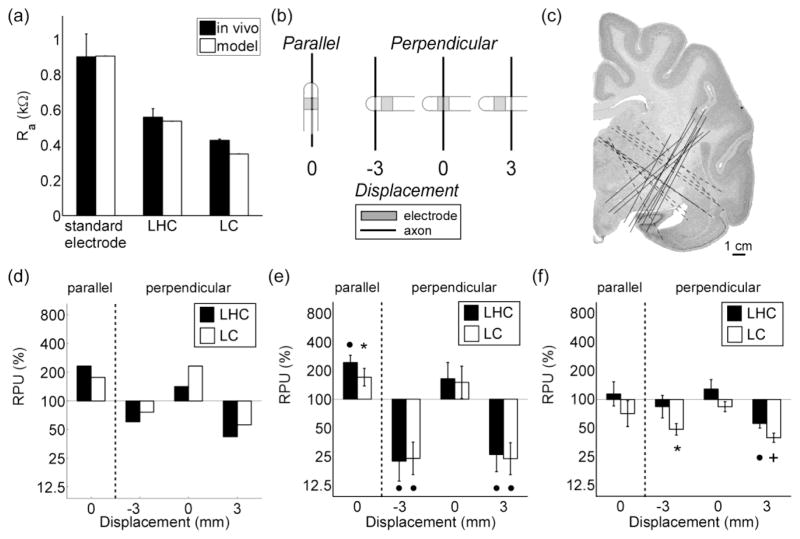
The electrical properties (i.e., Ra, Rf, and Cdl) of LHC, LC, and the standard electrode were determined by fitting Equation 5 to the current transient recorded during voltage-regulated stimulation (figure 3c). The relationship between Ra and the electrode geometry observed in vivo was predicted by the computational model (figure 7a). Rf values were between 21 kΩ and 3.9 × 103 kΩ, corresponding to a range of specific Faradaic resistances (i.e., rf) between 1.3 kΩ-cm2 and 490 kΩ-cm2; and the range of Cdl values was between 0.64 and 6.9 μF, corresponding to specific double-layer capacitances (i.e., cdl) between 6.8 and 41 μF/cm2.
Figure 7.
Measuring the stimulation efficiency of optimized electrode designs in vivo. (a) The access resistance (Ra) of the prototype electrodes measured in vivo compared to Ra of the electrodes calculated from the computational model. LC = long cylinder and LHC = long hemicylinder. (b) Electrodes were oriented either parallel or perpendicular to target axons. In the perpendicular orientation, electrodes were displaced ± 3 mm from the long axis of the axon bundle. (c) Locations of electrode tracks in the brain stimulation experiments. (d) Computational results. Relative power usage (RPU) is 100 times the ratio of the average power required to activate 50 % of the AOPs (P50) with the optimal design to P50 with the standard electrode in a cathodic configuration. RPU in the (e) sciatic nerve (n = 7) and (f) brain (n = 10) stimulation experiments is the same as part c, except P50 is the average power required to evoke a half maximal EMG. A repeated-measures ANOVA revealed a significant effect of electrode geometry on the relative P50 that depended on the orientation and distance of the electrode in parts d (p = 0.0105) and e (p = 0.0242), and post hoc comparisons were made using a Fisher’s least significant difference (FLSD) test. An asterisk, bullet, and plus sign denote a p value of < 0.05, < 0.01, and < 0.001, respectively, in the FLSD test.
We quantified the stimulation efficiency of each prototype electrode with respect to the standard electrode by calculating its RPU. Parallel to the sciatic nerve (n = 7), LHC and LC were less efficient than the standard electrode, as their RPU was significantly greater than 100 %, which agreed with the results from the computational model (figure 7c). However, when oriented perpendicular to the sciatic nerve, LHC and LC performed on par with the standard electrode (figure 7d). This unexpected result was attributed to differences in the distributions of neural elements between the model and cat sciatic nerve. Perpendicular axons in the model were located near the electrode, along the shaft, and below the tip of the electrode (Figure 1b), whereas target fascicles in the experiments only lay adjacent to the electrode (Figure 7b). To emulate better the distributions of perpendicular axons in the computational model, we displaced the stimulating electrode ± 3 mm mediolaterally from the long axis of the sciatic nerve (figure 7b). When oriented perpendicular to the nerve and displaced by ± 3 mm, the RPU of LHC and LC were significantly < 100 %, indicating that they were more efficient than the standard electrode (figure 7d). This dependence of stimulation efficiency on displacement was also observed in the model (figure 7c).
In brain stimulation experiments (n = 10), LHC performed on par with the standard electrode when oriented both parallel and perpendicular to axons in the posterior IC, whereas LC was more efficient than the standard electrode when oriented perpendicular to the target axons and displaced ± 3 mm from their long axis (figure 7e). Post-mortem histology confirmed that the electrode tracts passed through the posterior IC in all experiments (figure 7f).
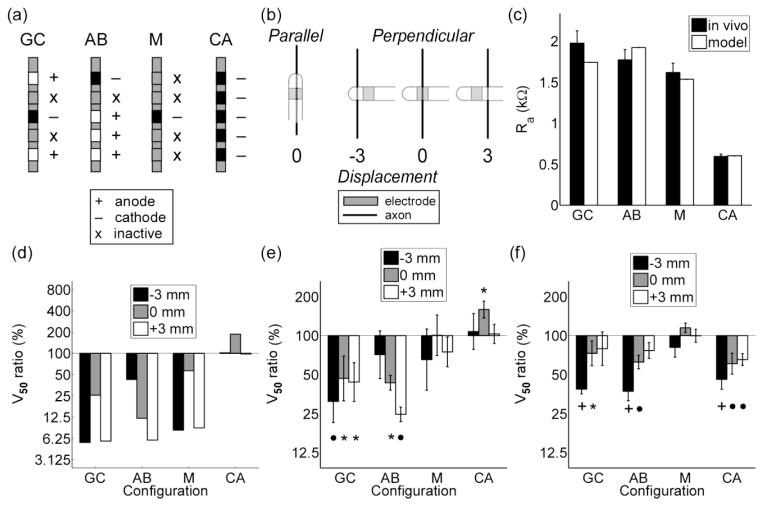
3.4.2. Selective prototype electrodes
We used a five-electrode array (figure 2) to measure the selectivity of an asymmetric bipole and cathode array, which were the two optimal electrode configurations for activating AOPs based on their orientation (figure 5a). We also used the five-electrode array to measure the selectivity of a guarded cathode, which is a cathode surrounded by two anodes, and a single electrode or monopole (figure 8a). The guarded cathode was tested because its selectivity was similar to that of the asymmetric bipole when neural elements were placed ≤ 2.5 mm from the origin (results not shown), and the monopolar configuration was tested because it was expected to have no orientation-dependent selectivity (figure 5b). We confirmed that the relationship between Ra and the electrode configuration predicted by the computational model was observed in vivo (figure 8c).
Figure 8.
Measuring the stimulation selectivity of optimal electrode designs in vivo. (a) Four electrode configurations were tested: GC = guarded cathode, AB = asymmetric bipole, M = monopole, and CA = an array of cathodes. (b) Electrode configurations were oriented parallel or perpendicular to target axons. Electrodes in the perpendicular orientation were displaced ± 3 mm from the long axis of the axons. (c) The access resistance (Ra) of the electrode configurations measured in vivo compared to Ra calculated from the model. (d) The voltage required to activate 50 % of modeled AOPs (V50) in the parallel orientation to V50 in the perpendicular orientation. V50 is the same in (e) the sciatic nerve (n = 5) and (f) brain (n = 5) stimulation experiments, except V50 denotes the voltage required to evoke a half maximal EMG. A repeated-measures ANOVA revealed a significant effect of electrode configuration on the V50 ratio that depended on the orientation and distance of the electrode in parts e (p = 0.0280) and f (p = 0.0288). Post hoc comparisons were made using a Fisher’s least significant difference (FLSD) test. An asterisk, bullet, and plus sign denote a p value of < 0.05, < 0.01, and < 0.001, respectively, in the FLSD test.
The selectivity of the four configurations was quantified using the ratio of V50 in the parallel orientation to V50 in the perpendicular orientation. In the sciatic nerve experiments (n = 5), the V50 ratio of the guarded cathode and the asymmetric bipole were significantly less than 100 when oriented parallel to the axon, the V50 ratio of the monopolar configuration did not differ significantly between the two orientations, and the V50 ratio of an array of cathodes was greater than 100 when oriented parallel to the axons (figure 8e). The selectivity of all four configurations depended on their displacement from the long axis of the nerve. Similar results were observed in brain experiments (n = 5), with the exception of the array of cathodes, which had significantly smaller V50 ratio when oriented parallel to the axon (figure 8f). The selectivity for each configuration measured in vivo compared well with the predictions from the model-based analysis (figure 8d).
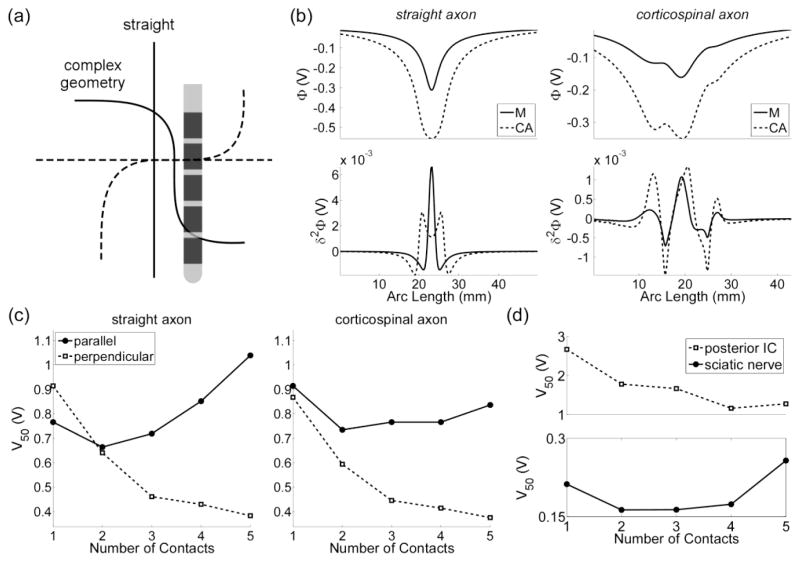
In the brain experiments, the array of cathodes activated parallel AOPs selectively over perpendicular AOPs (figure 8f). We predicted that this unexpected result might be explained by the geometry of the targeted corticospinal axons, which are not straight, like the axons in the sciatic nerve. We used anatomical images from a cat atlas (Snider and Niemer, 1961) and spline fits to approximate the trajectory of CS axons, and additional simulation and experiments were conducted to test our prediction (figure 9). With straight axons, cathode array configurations consisting of 4 or 5 electrodes had larger stimulation thresholds than a monopolar configuration when oriented parallel to the modeled AOPs, whereas with curved CS axons, all thresholds with cathode arrays were less than the monopolar threshold (figure 9c). Similar results were observed in two additional experiments (figure 9d).
Figure 9.
The effect of axon geometry on stimulation thresholds. (a) A straight axon and corticospinal axon with bends oriented parallel (solid line) and perpendicular (dashed line) to a five-electrode array. (b) The potentials (top) and the second difference of the potentials (δ2Φ, bottom) when either one (M) or five contacts (CA) in a cathodic configuration was used to stimulate the target axons. (c) The voltage required to activate 50 % of a population of modeled axons (V50) when the array was oriented either parallel or perpendicular to the axons. (d) The voltage required to evoke a half maximal EMG (V50) when the array was oriented parallel to the posterior internal capsule (IC, top) and sciatic nerve (bottom).
4. Discussion
We combined computational models of electric potentials and neurons and used engineering optimization to design novel electrode configurations for efficient and selective DBS. In vivo measurements of EMGs evoked by either peripheral nerve or brain stimulation were used to quantify the performance of prototype electrodes. Electrode geometry and polarity had profound effects on the efficiency and selectivity of stimulation, which depended on the orientation and type of the neural elements, and the optimal electrode designs exhibited significant improvements in efficiency and selectivity compared to the standard clinical electrode. Further, the results suggest that there is no universal electrode geometry or configuration that is optimal for all DBS targets, but demonstrate the potential performance improvements that can accrue from target-specific electrode designs.
4.1. Principles for electrode design
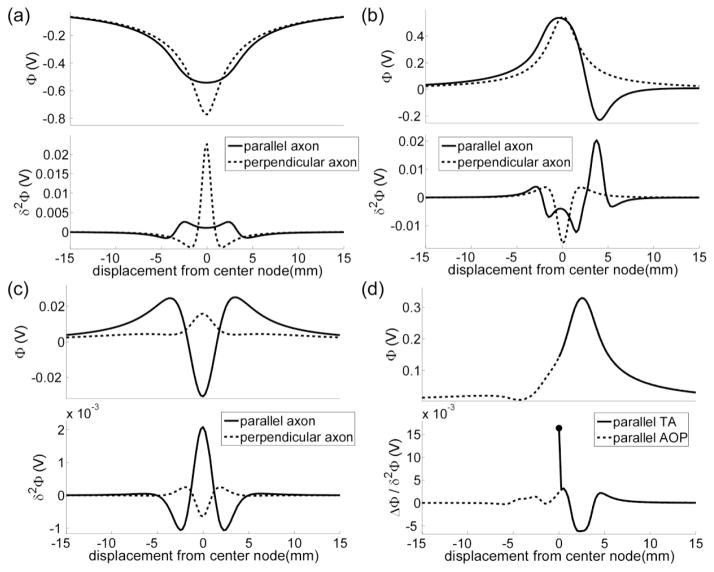
The increases in efficiency of some, but not all, of the efficient designs could be explained by decreases in the electrode impedance, and the increases in efficiency and selectivity were better explained by examining the second difference of the potentials (δ2Φ). Therefore, we analyzed δ2Φ of the optimal electrode designs to understand the origin of the results and how they might translate into principles for electrode design.
The results reveal that efficiency can be improved by using long cylindrical electrodes with relatively large (height to diameter) aspect ratios to stimulate perpendicular neural elements (figure 4a). Increasing h increases electrode area, which reduces Ra, reduces Rf, and increases Cdl. Therefore, the efficiency of longer electrodes could be explained, in part, by a decrease in the electrode impedance. The other factor that explained the efficiency of the long cylindrical electrodes was their proximity to the neural elements. Increasing h brought the electrode surface closer to excitable regions (e.g., nodes of Ranvier or the axon hillock) of perpendicular neural elements distributed along the insulated region of the shaft. Since the magnitude of the potentials is greatest near the electrode surface, the stimulation thresholds of these distal elements were decreased.
Increasing h also decreased the gradient of the potentials along the electrode shaft, which translated to a reduction in δ2Φ (figure 10a). This explains why LC and LHC, despite having a smaller Ra, were less efficient than the standard electrode in activating parallel AOPs, and it also explains why longer electrodes, or, similarly, an array of closely spaced cathodes, were selective in activating perpendicular AOPs over parallel AOPs (figure 5a). The selectivity of long cylindrical electrodes has been studied in a computational model of electrical stimulation of the retina (Rattay and Resatz, 2004), and a longer electrode, the Model 3391, is currently used to stimulate the anterior internal capsule in DBS for the treatment of obsessive compulsive disorder.
Figure 10.
The effect of electrode geometry and polarity on the force driving polarization of the neural membrane. Shown are the potentials and second difference of the potentials (δ2Φ) generated by (a) a long cylindrical electrode (figure 4a), (b) an asymmetric bipole (figure 5a), (c) a guarded cathode (figure 8a), and (d) a distal anode (figure 6a) across the nodes of Ranvier of an axon of passage (AOP). The filled circle in d denotes the first difference of the potentials (ΔΦ) at the terminal of a terminating axon (TA).
The results also revealed that multipolar configurations, such as an asymmetric bipole and a guarded cathode, can improve selectivity when activating neural elements based on their orientation. This can be explained by considering how δ2Φ = (Φn+1−2Φn + Φn−1) depends on orientation, where n denotes the nth node of Ranvier. Parallel to the electrode shaft, the potentials change sign (figures 10b and 10c), and δ2Φ can be as large as |Φn+1+2Φn+Φn−1|, where | | denotes the absolute value. However, perpendicular to the electrode shaft, the potentials are either positive, negative, or zero. Since the potentials perpendicular to the electrode do not change sign (figures 10b and 10c), δ2Φ is only as large as 2|Φn|. Bipolar and tripolar electrode configurations are currently used in spinal cord stimulation for the treatment of chronic pain (Holsheimer et al., 1998, Holsheimer and Wesselink, 1997, North et al., 2002, Sankarasubramanian et al., 2011) and may hold promise for increasing spatial targeting of the therapeutic elements in DBS for essential tremor (Keane et al., 2012).
A single electrode in an anode configuration distal to the target population can improve selective activation of TAs over AOPs (figure 6a). The selectivity of the distal anode can be explained in two parts. First, the excitability of a TA is different from an AOP (Rubinstein, 1993) because the force driving polarization at the axon terminal is proportional to the first difference of the potentials (ΔΦ) (Rattay, 1999). For example, when an anode is centered at the origin, ΔΦ in the z direction is positive when z > 0, zero when z = 0, and negative when z < 0. Therefore, half the terminals of a population of parallel TAs (in the z direction) are depolarized, while the other half are hyperpolarized. As the anode is displaced progressively in the positive z direction, terminals that were previously hyperpolarized are now depolarized. Because |ΔΦ|, in general, is greater than |δ2Φ|, TAs are activated at lower stimulation thresholds.
ΔΦ is symmetric, and displacing a cathode in the negative z direction can also reduce the stimulation thresholds of TAs. Therefore, the second reason the distal anode configuration is selective for activating TAs over AOPs is that an anode depolarizes AOPs less than a cathode, as the maximum δ2Φ of the former is markedly less than that of the latter. In other words, a distal anode strongly depolarizes the terminals of TAs, reducing their stimulation thresholds, while also minimizing depolarization of AOPs (figure 10d). Although the performance of the distal anode for selective stimulation of TAs over AOPs has not been measured in vivo, anodes, in general, have been studied for selective microstimulation of LNs over AOPs (Lu et al., 2008, McIntyre and Grill, 2000, Wang et al., 2012) and selective stimulation of local retinal ganglion cells over passing axons of distal retinal ganglion cells (Schiefer and Grill, 2006).
4.2. Differences between computational and in vivo results
The goal of this study was to design electrodes that improved the efficiency and selectivity of DBS, which employs chronically implanted electrodes. However, we tested the predictions of our model in acute experiments, where the glial scar had not yet formed. Removing the model glial scar did not have a marked impact on the model predictions (Table 1); therefore, the presence or absence of the glial scar did not explain differences between the predicted and observed results.
The majority of alpha motor axons in cat peripheral nerves have diameters < 10 μm (Fabricius et al., 1994). Although our model predicted that the RPU of longer electrodes increased with increasing fiber diameter (Table 1), LC and LHC were still more efficient for stimulation of perpendicular neural elements. In fact, the measured RPUs were markedly smaller than what was predicted for stimulation of perpendicular axons < 2.5 mm from the electrode (Table 1), and we believe this underestimation of efficiency may be due to differences in the electrical properties between nerve and brain.
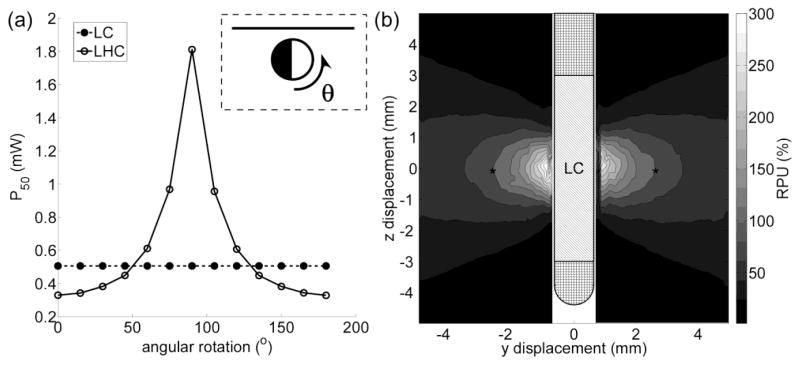
In the brain experiments, the LHC, rather than being more efficient, was less efficient than the LC (figure 7f). Since most axons in the brain have diameters < 4 μm (Assaf et al., 2008, Barazany et al., 2009, Yagishita et al., 1994), this discrepancy could not be explained by changes in fiber diameter. We predicted that LHC was less efficient than LC because of its axial asymmetry; therefore, additional simulations were conducted to assess how the performance of LHC varied with axial rotations (figure 11a). Rotating LHC about its axis markedly reduced its efficiency; thereby the difference between model and experiment could be explained by suboptimal alignment of LHC with the target axons.
Figure 11.
Evaluating the stimulation efficiency of the long cylinder (LC) and long hemicylinder (LHC) in cathodic configurations. (a) P50 of LC and LHC at different rotations about their axes. P50 is the average power required to activate 50 % of axons of passage (AOPs) oriented perpendicular to the electrode shaft. Inset: cross-section of LHC at an angular rotation (θ) of 0° with respect to a target AOP (solid line) and the direction of rotation. (b) The efficiency of LC in activating individual perpendicular AOPs. The relative power usage (RPU) is 100 times the ratio of the average power required to activate an individual AOP with the LC to the average power required to activate an individual AOP with the standard electrode (see section 2.2) in a cathodic configuration. The asterisks denote the equi-value contour of RPU = 100.
Another difference between the predicted and observed results was that the efficiency of the LC and the standard electrode were not significantly different when oriented parallel to the target brain axons (figure 7e). Recall, long electrodes, despite having a smaller impedance than the standard electrode, were expected to be less efficient because increasing h decreased δ2Φ parallel to the electrode axis (figure 10a), resulting in increased stimulation thresholds. Indeed, the computational model was able to reproduce the relationship between Ra and electrode geometry (figure 7a), as well as the impact of the ETI impedance on the predicted efficiency. However, the model did not account for the heterogeneous and anisotropic electrical properties of the tissue, which can alter the shape of the potential distribution and resulting excitation thresholds (Chaturvedi et al., 2010).
The results of the experiments to measure selectivity were consistent with many of the model predictions (figures 5 and 8e). In the sciatic nerve experiments, the asymmetric bipolar and guarded cathode configurations were selective in activating parallel AOPs over perpendicular AOPs. Moreover, the monopolar configuration showed no selectivity based on orientation, and the array of cathodes was selective in activating perpendicular AOPs over parallel AOPs. Similar results were observed in brain experiments, except for the array of cathodes, which was selective in activating parallel AOPs over perpendicular AOPs (figure 8f). We predicted that this unexpected result might be explained by the geometry of the targeted corticospinal axons.
Many corticospinal (CS) axons in the IC originate from the motor cortex (Lemon, 2008). In cats, CS axons travel from rostral to caudal, and as they pass through the IC, they course medially to form the cerebral peduncle (Snider and Niemer, 1961). Our electrode designs were implanted in the posterior IC, caudal to the optic chiasm and rostral to the cerebral peduncle, so we predicted that there would be bends in CS axons rostral and caudal to the target region.
The bends in the CS axons had an impact on the model predictions. When axons were straight and oriented parallel to an array of cathodes, decreases in electrode impedance that resulted from increasing the number of stimulation electrodes did not progressively decrease V50 (figure 9c) because decreases in δ2Φ parallel to the cathode array (figure 10a) were eventually large enough to counteract the effect of decreasing impedance. This effect was observed when stimulating the sciatic nerve, where axons are straight, but not when stimulating axons with bends in the posterior IC (figure 9d), which the model predicted (figures 9c). Therefore, the geometry of the axon is a crucial parameter to consider in future studies.
4.3. Considerations for a versatile electrode array
No single configuration of electrodes was both optimally efficient and selective at stimulating neural elements across the cases tested, suggesting there is no universal electrode geometry or configuration that is optimal for all DBS targets. Fabrication of a multitude of different electrode designs for different DBS applications is not practical. Instead, an array with many electrodes (Keane et al., 2012) can be used to approximate closely the potential distributions of optimal configurations.
A 12-electrode array could be configured so that it, in most cases, performed on par with the optimal configurations (Table 3). Indeed, by increasing the number of contacts, the versatility of an array in approximating many optimal configurations can be improved, but most clinical arrays are limited to < 10 electrodes due to physical limitations (e.g., the number of wires that can fit inside the shaft). As new technologies emerge, it will be possible to construct arrays with tens to hundreds of electrodes on a flexible substrate (Viventi et al., 2011), where thin film metal traces, rather than wires, are used to connect the electrodes to the stimulator.
4.4. Limitations
One limitation of this study was the choice of animal model for testing the electrode designs. Targets in DBS for movement disorders, including the subthalamic nucleus and the ventral intermediate nucleus of thalamus, span a length scale of 1–10 mm in humans (Keane et al., 2012, Slavin et al., 2006), and the neural volumes activated at therapeutic stimulation levels are predicted to span similar length scales (Butson et al., 2007). In contrast, the cat sciatic nerve (Grill and Mortimer, 1996) and the posterior IC (Nicholson, 1965) span a length scale of 1–3 mm, and in these tissues, EMG activity in a single muscle group can be evoked by activating a small population of axons that occupy a length scale of < 1 mm.
The smaller volumes of tissue in the cat were a challenge for assessing the efficiency of electrodes that were intended for use in humans. For example, compared to the standard electrode, LC was more efficient at activating > 90 % of perpendicular AOPs but less efficient at activating a small subset of the AOPs located near the center of the electrode (figure 11b). To emulate the presence of perpendicular axons distal to the electrode (i.e., along the shaft insulation and below the shaft tip), we displaced the electrodes ± 3 mm when oriented perpendicular to the target axons. While many of the qualitative trends observed experimentally could be reproduced with the model (figures 7d and 8d), experiments in larger animals may provide more accurate evaluation of improved electrode designs.
Another limitation was the choice of straight axon trajectories in the model analysis. Although the trajectory of an axon is never perfectly straight, it was a reasonable approximation. In DBS of the internal globus pallidus, activation of AOPs that course approximately perpendicular to the electrode array has been observed at therapeutic stimulation settings (Johnson and McIntyre, 2008). In subthalamic nucleus DBS, activation of AOPs in the anterior internal capsule, which course approximately parallel to the electrode array in humans (Miocinovic et al., 2006), improved PD symptoms in rats (Gradinaru et al., 2009). And, evidence from non-human primates suggest that axons from the lenticular fasciculus, which course approximately perpendicular to the electrode array, may also be therapeutic targets (Miocinovic et al., 2006). As a further example, in DBS of the ventral intermediate nucleus of the thalamus, TAs from the cerebellothalamic tract that course approximately parallel to the implanted array are predicted to be the therapeutic targets (Birdno et al., 2012). Therefore, while the results do not reveal an optimal configuration for any particular region, they do identify what types of configurations may improve efficiency and/or selectivity for different possible orientations of neural elements that might be encountered across anatomical targets.
Although the simplified FEM model did not account for heterogeneity or anisotropy of brain tissues, it reproduced the dependence of Ra on the electrode configuration (figures 7a and 8c) and had two advantages: model solution times were less than thirty minutes, allowing us to study a very large parameter space of possible electrode designs; and the effects of electrode geometry and polarity on the potentials could be studied in isolation from other variables, such as heterogeneities in the conducting volume, that may also affect the potential distribution (Astrom et al., 2006). The simplified model, however, did not reproduce all of the experimental findings. It is possible that discrepancies between the model and experiments may be due to anisotropy and heterogeneity in the conductivity of brain tissue and/or the geometry of the target neural elements (figure 9), and future computational models should account for these features when designing electrodes for specific DBS applications (Butson et al., 2007, Chaturvedi et al., 2010, Frankemolle et al., 2010).
5. Conclusion
Model-based engineering optimization was used to design electrodes that improved the efficiency and selectivity of DBS. Electrodes with heights greater than the standard clinical electrode contact were the most efficient for stimulating neural elements oriented perpendicular to the long axis of the electrode, and electrodes with geometries similar to the standard clinical electrode were the most efficient for stimulating parallel neural elements. Bipolar and tripolar electrode configurations were the most selective for activating parallel axons over perpendicular axons. An array of cathodes with short interelectrode spacing, or equivalently, an elongated cylindrical electrode, was the most selective for activating perpendicular axons over parallel axons; and an anode displaced from the center of the target region was the most selective for selectively activating terminating axons over passing axons. The performance of these designs could not be explained solely by differences in their electrical properties, suggesting that field-shaping effects from changing electrode geometry and polarity can be on par with or greater than the effects of decreasing electrode impedance.
Acknowledgments
This work was supported by a Ruth L. Kirschstein F31 NS079105 Individual Predoctoral Fellowship and by Grant R01 NS040894 from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health.
References
- Assaf Y, Blumenfeld-Katzir T, Yovel Y, Basser PJ. Axcaliber: A method for measuring axon diameter distribution from diffusion MRI. Magnetic Resonance in Medicine. 2008;59:1347–1354. doi: 10.1002/mrm.21577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrom M, Johansson JD, Hariz MI, Eriksson O, Wardell K. The effect of cystic cavities on deep brain stimulation in the basal ganglia: a simulation-based study. J Neural Eng. 2006;3:132–8. doi: 10.1088/1741-2560/3/2/007. [DOI] [PubMed] [Google Scholar]
- Barazany D, Basser PJ, Assaf Y. In vivo measurement of axon diameter distribution in the corpus callosum of rat brain. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdno MJ, Kuncel AM, Dorval AD, Turner DA, Gross RE, Grill WM. Stimulus features underlying reduced tremor suppression with temporally patterned deep brain stimulation. Journal of Neurophysiology. 2012;107:364–383. doi: 10.1152/jn.00906.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossetti CA, Birdno MJ, Grill WM. Analysis of the quasi-static approximation for calculating potentials generated by neural stimulation. J Neural Eng. 2008;5:44–53. doi: 10.1088/1741-2560/5/1/005. [DOI] [PubMed] [Google Scholar]
- Boviatsis EJ, Stavrinou LC, Themistocleous M, Kouyialis AT, Sakas DE. Surgical and hardware complications of deep brain stimulation. A seven-year experience and review of the literature. Acta Neurochir (Wien) 2010;152:2053–62. doi: 10.1007/s00701-010-0749-8. [DOI] [PubMed] [Google Scholar]
- Bronstein JM, Tagliati M, Alterman RL, Lozano AM, Volkmann J, Stefani A, Horak FB, Okun MS, Foote KD, Krack P, Pahwa R, Henderson JM, Hariz MI, Bakay RA, Rezai A, Marks WJ, Jr, Moro E, Vitek JL, Weaver FM, Gross RE, DeLong MR. Deep Brain Stimulation for Parkinson Disease: An Expert Consensus and Review of Key Issues. Arch Neurol. 2011;68:165. doi: 10.1001/archneurol.2010.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhlmann J, Hofmann L, Tass PA, Hauptmann C. Modeling of a segmented electrode for desynchronizing deep brain stimulation. Front Neuroeng. 2011;4:15. doi: 10.3389/fneng.2011.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butson CR, Cooper SE, Henderson JM, McIntyre CC. Patient-specific analysis of the volume of tissue activated during deep brain stimulation. Neuroimage. 2007;34:661–70. doi: 10.1016/j.neuroimage.2006.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butson CR, McIntyre CC. Role of electrode design on the volume of tissue activated during deep brain stimulation. J Neural Eng. 2006;3:1–8. doi: 10.1088/1741-2560/3/1/001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnevale NT, Hines ML. The NEURON Book. Cambridge: Cambridge University Press; 1997. [Google Scholar]
- Chaturvedi A, Butson CR, Lempka SF, Cooper SE, McIntyre CC. Patient-specific models of deep brain stimulation: influence of field model complexity on neural activation predictions. Brain Stimul. 2010;3:65–7. doi: 10.1016/j.brs.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogan SF. Neural stimulation and recording electrodes. Annu Rev Biomed Eng. 2008;10:275–309. doi: 10.1146/annurev.bioeng.10.061807.160518. [DOI] [PubMed] [Google Scholar]
- Duerden EG, Finnis KW, Peters TM, Sadikot AF. Three-dimensional somatotopic organization and probabilistic mapping of motor responses from the human internal capsule. J Neurosurg. 2011;114:1706–14. doi: 10.3171/2011.1.JNS10136. [DOI] [PubMed] [Google Scholar]
- Ellis TM, Foote KD, Fernandez HH, Sudhyadhom A, Rodriguez RL, Zeilman P, Jacobson CEt, Okun MS. Reoperation for suboptimal outcomes after deep brain stimulation surgery. Neurosurgery. 2008;63:754–60. doi: 10.1227/01.NEU.0000325492.58799.35. discussion 760–1. [DOI] [PubMed] [Google Scholar]
- Fabricius C, Berthold C, Rydmark M. Dimensions of individual alpha and gamma motor fibres in the ventral funiculus of the cat spinal cord. Journal of anatomy. 1994;184:319. [PMC free article] [PubMed] [Google Scholar]
- Foutz TJ, McIntyre CC. Evaluation of novel stimulus waveforms for deep brain stimulation. Journal of Neural Engineering. 2010;7:066008. doi: 10.1088/1741-2560/7/6/066008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankemolle AMM, Wu J, Noecker AM, Voelcker-Rehage C, Ho JC, Vitek JL, McIntyre CC, Alberts JL. Reversing cognitive–motor impairments in Parkinson’s disease patients using a computational modelling approach to deep brain stimulation programming. Brain. 2010;133:746–761. doi: 10.1093/brain/awp315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel C, Peyman A, Grant EH. Electrical conductivity of tissue at frequencies below 1 MHz. Phys Med Biol. 2009;54:4863–78. doi: 10.1088/0031-9155/54/16/002. [DOI] [PubMed] [Google Scholar]
- Golestanirad L, Pollo C, Graham SJ. Analysis of fractal electrodes for efficient neural stimulation. Engineering in Medicine and Biology Society (EMBC), 2013 35th Annual International Conference of the IEEE; 3–7 July 2013; 2013. pp. 791–794. [DOI] [PubMed] [Google Scholar]
- Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical Deconstruction of Parkinsonian Neural Circuitry. Science. 2009;324:354–359. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill WM, Jr, Mortimer JT. Quantification of recruitment properties of multiple contact cuff electrodes. Rehabilitation Engineering, IEEE Transactions on. 1996;4:49–62. doi: 10.1109/86.506402. [DOI] [PubMed] [Google Scholar]
- Grill WM, Mortimer JT. Electrical properties of implant encapsulation tissue. Ann Biomed Eng. 1994;22:23–33. doi: 10.1007/BF02368219. [DOI] [PubMed] [Google Scholar]
- Haberler C, Alesch F, Mazal PR, Pilz P, Jellinger K, Pinter MM, Hainfellner JA, Budka H. No tissue damage by chronic deep brain stimulation in Parkinson’s disease. Ann Neurol. 2000;48:372–6. [PubMed] [Google Scholar]
- Harries AM, Major S, Sandhu M, Honey CR. Rechargeable Internal Neural Stimulators—Is There a Problem with Efficacy? Neuromodulation: Technology at the Neural Interface. 2012;15:214–218. doi: 10.1111/j.1525-1403.2011.00406.x. [DOI] [PubMed] [Google Scholar]
- Holsheimer J, Struijk JJ, Wesselink WA. Analysis of spinal cord stimulation and design of epidural electrodes by computer modeling. Neuromodulation: Technology at the Neural Interface. 1998;1:14–18. doi: 10.1111/j.1525-1403.1998.tb00026.x. [DOI] [PubMed] [Google Scholar]
- Holsheimer J, Wesselink WA. Optimum electrode geometry for spinal cord stimulation: The narrow bipole and tripole. Medical and Biological Engineering and Computing. 1997;35:493–497. doi: 10.1007/BF02525529. [DOI] [PubMed] [Google Scholar]
- Holtzheimer PE, Mayberg HS. Deep Brain Stimulation for Psychiatric Disorders. Annual Review of Neuroscience. 2011;34:289–307. doi: 10.1146/annurev-neuro-061010-113638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell B, Grill WM. Model-based optimization of electrode designs for deep brain stimulation. Neural Engineering (NER), 2013 6th International IEEE/EMBS Conference on; 6–8 Nov. 2013; 2013. pp. 154–157. [Google Scholar]
- Howell B, Grill WM. Evaluation of high-perimeter electrode designs for deep brain stimulation. Journal of Neural Engineering. 2014;11:046026. doi: 10.1088/1741-2560/11/4/046026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell B, Naik S, Grill WM. Influences of interpolation error, electrode geometry, and the electrode-tissue interface on models of electric fields produced by deep brain stimulation. IEEE Transactions on Biomedical Engineering. 2014;61:297–307. doi: 10.1109/TBME.2013.2292025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MD, McIntyre CC. Quantifying the Neural Elements Activated and Inhibited by Globus Pallidus Deep Brain Stimulation. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane M, Deyo S, Abosch A, Bajwa JA, Johnson MD. Improved spatial targeting with directionally segmented deep brain stimulation leads for treating essential tremor. J Neural Eng. 2012;9:046005. doi: 10.1088/1741-2560/9/4/046005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn J, Grundler TO, Lenartz D, Sturm V, Klosterkotter J, Huff W. Deep brain stimulation for psychiatric disorders. Dtsch Arztebl Int. 2010;107:105–13. doi: 10.3238/arztebl.2010.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuncel AM, Cooper SE, Grill WM. A method to estimate the spatial extent of activation in thalamic deep brain stimulation. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2008;119:2148–2158. doi: 10.1016/j.clinph.2008.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuncel AM, Grill WM. Selection of stimulus parameters for deep brain stimulation. Clinical Neurophysiology. 2004;115:2431–2441. doi: 10.1016/j.clinph.2004.05.031. [DOI] [PubMed] [Google Scholar]
- Lam CK, Rosenow JM. Patient Perspectives on the Efficacy and Ergonomics of Rechargeable Spinal Cord Stimulators. Neuromodulation: Technology at the Neural Interface. 2010;13:218–223. doi: 10.1111/j.1525-1403.2009.00269.x. [DOI] [PubMed] [Google Scholar]
- Lemon RN. Descending Pathways in Motor Control. Annual Review of Neuroscience. 2008;31:195–218. doi: 10.1146/annurev.neuro.31.060407.125547. [DOI] [PubMed] [Google Scholar]
- Limousin P, Speelman JD, Gielen F, Janssens M. Multicentre European study of thalamic stimulation in parkinsonian and essential tremor. J Neurol Neurosurg Psychiatry. 1999;66:289–96. doi: 10.1136/jnnp.66.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Chestek CA, Shaw KM, Chiel HJ. Selective extracellular stimulation of individual neurons in ganglia. Journal of Neural Engineering. 2008;5:287. doi: 10.1088/1741-2560/5/3/003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens HCF, Toader E, Decré MMJ, Anderson DJ, Vetter R, Kipke DR, Baker KB, Johnson MD, Vitek JL. Spatial steering of deep brain stimulation volumes using a novel lead design. Clinical Neurophysiology. 2011;122:558–566. doi: 10.1016/j.clinph.2010.07.026. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep Brain Stimulation for Treatment-Resistant Depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- McAuley J, van Gröningen R, Green C. Spinal Cord Stimulation for Intractable Pain Following Limb Amputation. Neuromodulation: Technology at the Neural Interface. 2013;16:530–536. doi: 10.1111/j.1525-1403.2012.00513.x. [DOI] [PubMed] [Google Scholar]
- McConnell GC, Rees HD, Levey AI, Gutekunst CA, Gross RE, Bellamkonda RV. Implanted neural electrodes cause chronic, local inflammation that is correlated with local neurodegeneration. J Neural Eng. 2009;6:056003. doi: 10.1088/1741-2560/6/5/056003. [DOI] [PubMed] [Google Scholar]
- McIntyre C, Grill W. Selective Microstimulation of Central Nervous System Neurons. Annals of Biomedical Engineering. 2000;28:219–233. doi: 10.1114/1.262. [DOI] [PubMed] [Google Scholar]
- McIntyre CC, Grill WM, Sherman DL, Thakor NV. Cellular effects of deep brain stimulation: model-based analysis of activation and inhibition. J Neurophysiol. 2004;91:1457–69. doi: 10.1152/jn.00989.2003. [DOI] [PubMed] [Google Scholar]
- McIntyre CC, Richardson AG, Grill WM. Modeling the excitability of mammalian nerve fibers: influence of afterpotentials on the recovery cycle. Journal of Neurophysiology. 2002;87:995–1006. doi: 10.1152/jn.00353.2001. [DOI] [PubMed] [Google Scholar]
- Merrill DR, Bikson M, Jefferys JG. Electrical stimulation of excitable tissue: design of efficacious and safe protocols. J Neurosci Methods. 2005;141:171–98. doi: 10.1016/j.jneumeth.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Miocinovic S, Parent M, Butson CR, Hahn PJ, Russo GS, Vitek JL, McIntyre CC. Computational Analysis of Subthalamic Nucleus and Lenticular Fasciculus Activation During Therapeutic Deep Brain Stimulation. 2006. [DOI] [PubMed] [Google Scholar]
- Montgomery EB., Jr Deep brain stimulation reduces symptoms of Parkinson disease. Cleve Clin J Med. 1999;66:9–11. doi: 10.3949/ccjm.66.1.9. [DOI] [PubMed] [Google Scholar]
- Nicholson PW. Specific impedance of cerebral white matter. Exp Neurol. 1965;13:386–401. doi: 10.1016/0014-4886(65)90126-3. [DOI] [PubMed] [Google Scholar]
- Norlin A, Pan J, Leygraf C. Investigation of interfacial capacitance of Pt, Ti and TiN coated electrodes by electrochemical impedance spectroscopy. Biomolecular Engineering. 2002;19:67–71. doi: 10.1016/s1389-0344(02)00013-8. [DOI] [PubMed] [Google Scholar]
- North RB, Kidd DH, Olin JC, Sieracki JM. Spinal cord stimulation electrode design: Prospective, randomized, controlled trial comparing percutaneous and laminectomy electrodes—part I: technical outcomes. Neurosurgery. 2002;51:381–390. [PubMed] [Google Scholar]
- Okun MS, Rodriguez RL, Foote KD, Sudhyadhom A, Bova F, Jacobson C, Bello B, Zeilman P, Fernandez HH. A case-based review of troubleshooting deep brain stimulator issues in movement and neuropsychiatric disorders. Parkinsonism Relat Disord. 2008;14:532–8. doi: 10.1016/j.parkreldis.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Okun MS, Tagliati M, Pourfar M, Fernandez HH, Rodriguez RL, Alterman RL, Foote KD. Management of referred deep brain stimulation failures: a retrospective analysis from 2 movement disorders centers. Arch Neurol. 2005;62:1250–5. doi: 10.1001/archneur.62.8.noc40425. [DOI] [PubMed] [Google Scholar]
- Ondo WG, Meilak C, Vuong KD. Predictors of battery life for the Activa Soletra 7426 Neurostimulator. Parkinsonism Relat Disord. 2007;13:240–2. doi: 10.1016/j.parkreldis.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Rattay F. Analysis of models for external stimulation of axons. IEEE Transactions on Biomedical Engineering. 1986;33:974–977. doi: 10.1109/TBME.1986.325670. [DOI] [PubMed] [Google Scholar]
- Rattay F. The basic mechanism for the electrical stimulation of the nervous system. Neuroscience. 1999;89:335–46. doi: 10.1016/s0306-4522(98)00330-3. [DOI] [PubMed] [Google Scholar]
- Rattay F, Resatz S. Effective electrode configuration for selective stimulation with inner eye prostheses. Biomedical Engineering, IEEE Transactions on. 2004;51:1659–1664. doi: 10.1109/TBME.2004.828044. [DOI] [PubMed] [Google Scholar]
- Rubinstein JT. Axon termination conditions for electrical stimulation. IEEE Trans Biomed Eng. 1993;40:654–63. doi: 10.1109/10.237695. [DOI] [PubMed] [Google Scholar]
- Sahin M, Tie Y. Non-rectangular waveforms for neural stimulation with practical electrodes. Journal of Neural Engineering. 2007;4:227. doi: 10.1088/1741-2560/4/3/008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankarasubramanian V, Buitenweg JR, Holsheimer J, Veltink P. Electrode alignment of transverse tripoles using a percutaneous triple-lead approach in spinal cord stimulation. Journal of Neural Engineering. 2011;8:1741–2560. doi: 10.1088/1741-2560/8/1/016010. [DOI] [PubMed] [Google Scholar]
- Schiefer MA, Grill WM. Sites of neuronal excitation by epiretinal electrical stimulation. Neural Systems and Rehabilitation Engineering, IEEE Transactions on. 2006;14:5–13. doi: 10.1109/TNSRE.2006.870488. [DOI] [PubMed] [Google Scholar]
- Slavin KV, Thulborn KR, Wess C, Nersesyan H. Direct Visualization of the Human Subthalamic Nucleus with 3T MR Imaging. AJNR Am J Neuroradiol. 2006;27:80–84. [PMC free article] [PubMed] [Google Scholar]
- Snider RS, Niemer WT. A stereotaxic Atlas of the Cat Brain. University of Chicago Press; 1961. [Google Scholar]
- Timmermann L, Schüpbach M, Hertel F, Wolf E, Eleopra R, Franzini A, Servello D, Skogseid IM, Rumia J, Aliaga AS, Barbe MT, Pauls KAM, Lin JP, Moro E, Lloyd A, Maarouf M. A New Rechargeable Device for Deep Brain Stimulation: A Prospective Patient Satisfaction Survey. European Neurology. 2013;69:193–199. doi: 10.1159/000342236. [DOI] [PubMed] [Google Scholar]
- Viventi J, Kim DH, Vigeland L, Frechette ES, Blanco JA, Kim YS, Avrin AE, Tiruvadi VR, Hwang SW, Vanleer AC, Wulsin DF, Davis K, Gelber CE, Palmer L, Van der Spiegel J, Wu J, Xiao J, Huang Y, Contreras D, Rogers JA, Litt B. Flexible, foldable, actively multiplexed, high-density electrode array for mapping brain activity in vivo. Nat Neurosci. 2011;14:1599–1605. doi: 10.1038/nn.2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waln O, Jimenez-Shahed J. Rechargeable Deep Brain Stimulation Implantable Pulse Generators in Movement Disorders: Patient Satisfaction and Conversion Parameters. Neuromodulation: Technology at the Neural Interface. 2014;17:425–430. doi: 10.1111/ner.12115. [DOI] [PubMed] [Google Scholar]
- Wang Q, Millard DC, Zheng HJ, Stanley GB. Voltage-sensitive dye imaging reveals improved topographic activation of cortex in response to manipulation of thalamic microstimulation parameters. J Neural Eng. 2012;9:026008. doi: 10.1088/1741-2560/9/2/026008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei XF, Grill WM. Current density distributions, field distributions and impedance analysis of segmented deep brain stimulation electrodes. J Neural Eng. 2005;2:139–47. doi: 10.1088/1741-2560/2/4/010. [DOI] [PubMed] [Google Scholar]
- Wongsarnpigoon A, Grill WM. Energy-efficient waveform shapes for neural stimulation revealed with a genetic algorithm. Journal of Neural Engineering. 2010;7:046009. doi: 10.1088/1741-2560/7/4/046009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagishita A, Nakano I, Oda M, Hirano A. Location of the corticospinal tract in the internal capsule at MR imaging. Radiology. 1994;191:455–460. doi: 10.1148/radiology.191.2.8153321. [DOI] [PubMed] [Google Scholar]