Abstract
Objectives
Patients with solid tumors are at greatest risk for dying from their cancers in the five years following diagnosis. For most malignancies, deaths from other chronic diseases begin to exceed those from cancer at some point. As little is known about the causes of death among long-term survivors of ovarian cancer, we examined causes of death by years from diagnosis.
Methods
The Surveillance, Epidemiology, and End Results (SEER) database was used to identify women diagnosed with ovarian cancer between 1988 and 2012. We compared causes of death by stage, age, and interval time after diagnosis.
Results
A total of 67,385 women were identified. For stage I neoplasms, 13.6% (CI, 13.0-14.2%) died from ovarian cancer, 4.2% (CI, 3.8-4.5%) from cardiovascular disease, 3.6% (CI, 3.3-3.9%) from other causes and 2.6% (CI, 2.4-2.9%) from other tumors; ovarian cancer was the leading cause of death until 7 years after diagnosis after which time deaths are more frequently due to other causes. For those with stage III-IV tumors, 67.8% (CI, 67.3-68.2%) died from ovarian cancer, 2.8% (CI, 2.6-2.9%) from other causes, 2.3% (CI, 2.2-2.4%) from cardiovascular disease and 1.9% (CI, 1.7-2.0%) from other cancers; ovarian cancer was the most frequent cause of death in years 1-15 after which time deaths were more commonly due to other causes.
Conclusions
The probability of dying from ovarian cancer decreases with time. Ovarian cancer remains the most common cause of death for 15 years after diagnosis in women with stage III-IV tumors.
Introduction
There will be an estimated 21,290 cases of ovarian cancer diagnosed in the United States in 2015, which will result in approximately 14,180 deaths. Ovarian cancer has the lowest five year survival rate among gynecologic cancers at 46%.1 Ovarian cancer typically remains asymptomatic in its early stages, and over 70% of patients present with advanced stage disease.2-5 Women with early stage tumors typically respond well to treatment and some sub-groups have greater than 90% five-year survival rates. In contrast, five-year survival is less than 50% for patients with advanced stage disease.6
For most solid tumors, the most common cause of death in the first five years after a diagnosis of cancer is due to cancer itself. As the duration from diagnosis increases, other causes of death become more common7-9. These findings have been reported for cancers of the prostate 10,11, kidney 7, central nervous system 7,12-15, lung 11,16, head and neck 8, and colon.11,17 In a study 217,573 patients with breast, colorectal, lung, and prostate cancer, the probability of long-term survival increased significantly after patients survived a critical time period following diagnosis. The median conditional survival, or likelihood of survival after a specified time from initial diagnosis, increased from initial diagnosis to five years after diagnosis from 18.5 to 42.5 months for breast, 7.5 to 71.5 months for colorectal, 4.5 to 52.5 months for lung, and 24.5 to 34.5 months for prostate cancer.11
Mortality estimates and the specific causes of death are of clinical importance to patients and physicians and can be used for counseling and prognostication as well as to help design more tailored surveillance and survivorship strategies.7,12,13,15,18,19. In contrast to other solid tumors, little is known about the causes of death among long-term survivors of ovarian cancer. We therefore examined survival and cause of death among ovarian cancer patients and estimated the risk of death from various causes based on the duration since diagnosis.
Materials and Methods
Data Source
The National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database was utilized for this analysis.20,21 SEER is a population-based cancer registry that includes data on approximately 28% of the United States population and captures patient demographics, cancer site and stage, and survival. Exemption from the Columbia University Institutional Review Board was obtained.
Patient Characteristics
Women diagnosed with epithelial ovarian cancer between January 1988 and December 2012 were analyzed. The cohort was limited to women with epithelial ovarian tumors based on the World Health Organization (WHO) criteria including serous, mucinous, endometrioid, clear cell, transitional cell carcinomas, and epithelial tumors not otherwise specified.22 Age at diagnosis was categorized into 10-year intervals, race recorded as white, black, and other, and diagnosis stratified by year. The SEER marital status variable was recorded as married, single, and unknown. The SEER registries were categorized as Eastern (Connecticut, New Jersey, Atlanta, rural Georgia, greater Georgia), Central (Detroit, Iowa, Kentucky, Louisiana, Utah), and Western (Alaska, California, Hawaii, Los Angeles, New Mexico, San Francisco, San Jose, Seattle). Tumor grade was grouped as well, moderately, or poorly differentiated, or unknown. Stage was determined based on the American Joint Cancer Committee and FIGO staging criteria as classified by SEER.
Outcomes
The primary outcome of the analysis was death. SEER records cause of death based on death certificate data. Patients were considered to have died from ovarian cancer if the cause of death was reported as ovarian cancer or cancer-related death likely due to ovarian cancer (miscellaneous malignant cancer, female genital tract cancer, cancer of the peritoneum, mesentery or omentum). Additional categories for cause of death included cardiovascular disease (disease of heart, hypertension without heart disease, cerebrovascular diseases, atherosclerosis, aortic aneurysm and dissection), other chronic disease (diabetes mellitus, alzheimers dieseaes, chronic obstructive pulmonary disease and allied conditions, chronic liver disease and cirrhosis, nephritis, nephrotic syndrome and nephrosis), and other cancer (“all malignant cancers” with the exception of ovarian cancer). A category of other was included for patients with codes that did not meet criteria for the above categories.
Statistical Analysis
Data for the cohort is reported descriptively. Causes of death were calculated and reported as rates with 95% confidence intervals (95% CI). Cause of death was further stratified by stage groupings (stage I, stage II, stage III/IV). A sensitivity analysis was performed in which the cohort was stratified by both stage and age (<60 or >60 years of age). Kaplan-Meier curves were developed to examine stage-specific cumulative mortality rates. All statistical tests were two-sided. All analyses were performed with SAS version 9.4 (SAS Institute Inc, Cary, North Carolina).
Results
We identified 67,385 women with epithelial ovarian cancer diagnosed between 1988 and 2012. The cohort included 14,190 stage I, 5302 stage II, 25,194 stage III, and 19,465 patients with stage IV tumors. The clinical and demographic characteristics of the epithelial ovarian cancer patients are displayed (Table 1). Overall, 85.2% of the women were white and serous histology was most common accounting for 50.2% of the tumors. Median follow-up was 6.5 years (IQR, 2.7-11.7 years) for women with stage I neoplasms, 4.3 years (IQR, 1.5-8.8 years) for those with stage II tumors and 1.8 years (IQR, 0.6-3.9) for patients with stage III-IV neoplasms.
Table 1.
Clinical and demographic characteristics of the cohort.
| N | (%) | |
|---|---|---|
| Year of diagnosis | ||
| 1988 | 1295 | (1.9) |
| 1989 | 1293 | (1.9) |
| 1990 | 1294 | (1.9) |
| 1991 | 1340 | (2.0) |
| 1992 | 1820 | (2.7) |
| 1993 | 1858 | (2.8) |
| 1994 | 1745 | (2.6) |
| 1995 | 1773 | (2.6) |
| 1996 | 1758 | (2.6) |
| 1997 | 1811 | (2.7) |
| 1998 | 1833 | (2.7) |
| 1999 | 1859 | (2.8) |
| 2000 | 3871 | (5.7) |
| 2001 | 3900 | (5.8) |
| 2002 | 3730 | (5.5) |
| 2003 | 3681 | (5.5) |
| 2004 | 3636 | (5.4) |
| 2005 | 3544 | (5.3) |
| 2006 | 3663 | (5.4) |
| 2007 | 3589 | (5.3) |
| 2008 | 3645 | (5.4) |
| 2009 | 3606 | (5.4) |
| 2010 | 3643 | (5.4) |
| 2011 | 3568 | (5.3) |
| 2012 | 3630 | (5.4) |
| Age | ||
| <40 | 3991 | (5.9) |
| 40-49 | 9663 | (14.3) |
| 50-59 | 15489 | (23.0) |
| 60-69 | 16248 | (24.1) |
| 70-79 | 13934 | (20.7) |
| ≥80 | 8060 | (12.0) |
| Race | ||
| White | 57387 | (85.2) |
| Black | 4689 | (7.0) |
| Other | 5173 | (7.7) |
| Unknown | 136 | (0.2) |
| Marital status | ||
| Married | 34469 | (51.2) |
| Single | 30587 | (45.4) |
| Unknown | 2329 | (3.5) |
| Histology | ||
| Serous | 33848 | (50.2) |
| Mucinous | 5729 | (8.5) |
| Endometrioid | 8280 | (12.3) |
| Clear cell | 4166 | (6.2) |
| Transitional cell | 264 | (0.4) |
| Not otherwise specified | 15098 | (22.4) |
| Grade | ||
| Well differentiated | 5270 | (7.8) |
| Moderately differentiated | 10884 | (16.2) |
| Poorly differentiated | 30929 | (45.9) |
| Unknown | 20302 | (30.1) |
| Region | ||
| West | 34292 | (50.9) |
| Central | 16029 | (23.8) |
| East | 17064 | (25.3) |
| Stage | ||
| IA | 8100 | (12.0) |
| IB | 764 | (1.1) |
| IC | 4871 | (7.2) |
| INOS | 455 | (0.7) |
| II | 5302 | (7.9) |
| IIIA | 1205 | (1.8) |
| IIIB | 1791 | (2.7) |
| IIIC | 16275 | (24.2) |
| IIINOS | 5923 | (8.8) |
| IV | 19465 | (28.9) |
| Unknown | 3234 | (4.8) |
At last follow-up, 37.5% (95% CI, 37.1-37.9%) of the cohort was alive while 53.6% (95% CI, 53.2-54.0%) had died from ovarian cancer (Table 2). For the remainder of the cohort, 3.0% (95% CI, 2.8-3.1%) had died from cardiovascular disease, 2.0% (95% CI, 1.9-2.1%) had died from other cancers, 0.9% (95% CI, 0.8-0.9) had died from other chronic diseases and 3.0% (95% CI, 2.9-3.2%) had died from other causes.
Table 2.
Cause of death stratified by stage.
| All Patients | Stage I | Stage II | Stage III/IV | |||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| N | Rate (95% CI) | N | Rate (95% CI) | N | Rate (95% CI) | N | Rate (95% CI) | |
| Alive | 25274 | 37.5 (37.1, 37.9) | 10,567 | 74.5 (73.8, 75.2) | 2889 | 54.5 (53.1, 55.8) | 11,036 | 24.7 (24.3, 25.1) |
| Ovarian cancer | 36125 | 53.6 (53.2, 54.0) | 1928 | 13.6 (13.0, 14.2) | 1872 | 35.3 (34.0, 36.6) | 30,262 | 67.8 (67.3, 68.2) |
| Other cancer | 1373 | 2.0 (1.9, 2.1) | 372 | 2.6 (2.4, 2.9) | 97 | 1.8 (1.5, 2.2) | 827 | 1.9 (1.7, 2.0) |
| Cardiovascular disease | 1985 | 3.0 (2.8, 3.1) | 591 | 4.2 (3.8, 4.5) | 223 | 4.2 (3.7, 4.8) | 1030 | 2.3 (2.2, 2.4) |
| Other chronic disease | 580 | 0.9 (0.8, 0.9) | 225 | 1.6 (1.4, 1.8) | 49 | 0.9 (0.7, 1.2) | 274 | 0.6 (0.5, 0.7) |
| Death from other | 2048 | 3.0 (2.9, 3.2) | 507 | 3.6 (3.3, 3.9) | 172 | 3.2 (2.8, 3.7) | 1230 | 2.8 (2.6, 2.9) |
Rate expressed as percentage.
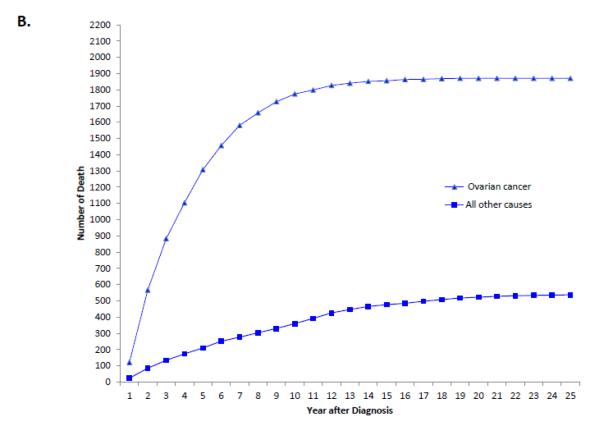
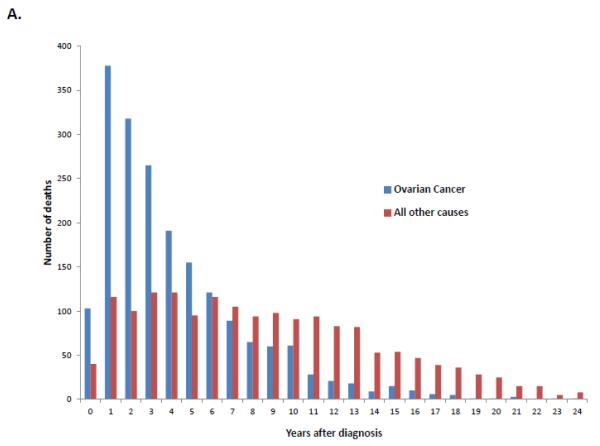
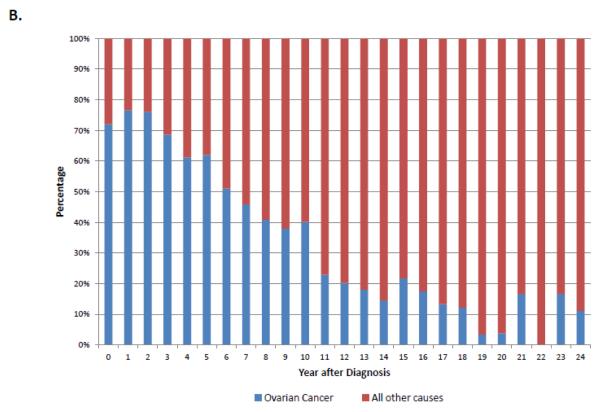
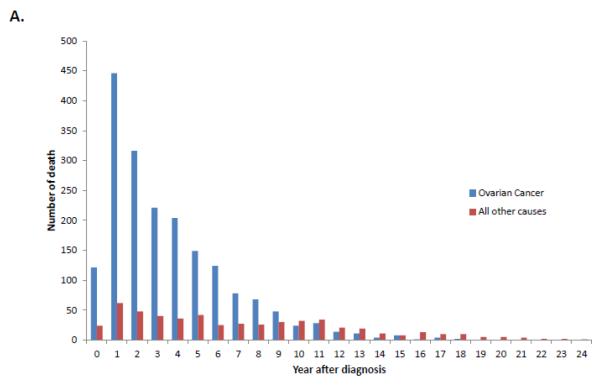
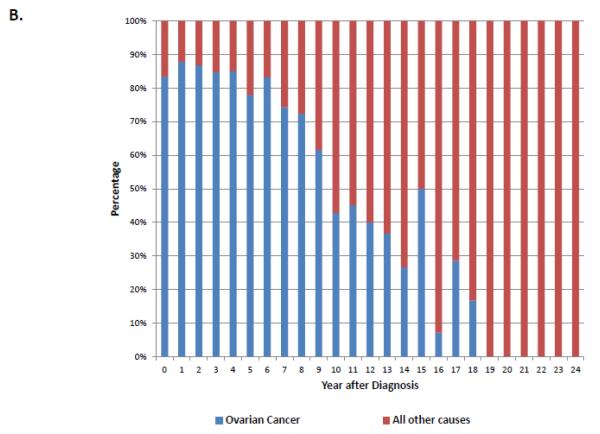
When stratified by stage, 74.5% (95% CI, 73.8-75.2%) of stage I patients, 54.5% (95% CI, 53.1-55.8%) of stage II patients and 24.7% (95% CI, 24.3-25.1%) of those with stage III-IV neoplasms were alive at last follow-up (Table 2). Overall, ovarian cancer was the most common cause of death for each of the individual stage groups. For women with stage I neoplasms, 13.6% (95% CI, 13.0-14.2%) died from ovarian cancer, 4.2% (95% CI, 3.8-4.5%) from cardiovascular disease, 3.6% (95% CI, 3.3-3.9%) from other causes and 2.6% (95% CI, 2.4-2.9%) from other tumors. Figure 1A displays the cumulative frequency of death from ovarian cancer and other causes based on years from diagnosis. Figure 2 displays the annual frequency and percentage of deaths from ovarian cancer and other causes based on the number of years since diagnosis. For women with stage I tumors, ovarian cancer is the leading cause of death until 7 years after diagnosis after which time death is more frequently due to other causes than ovarian cancer. Similarly, among women with stage II tumors, ovarian cancer was the leading cause of death until 10 years after diagnosis (Figure 3).
Figure 1.
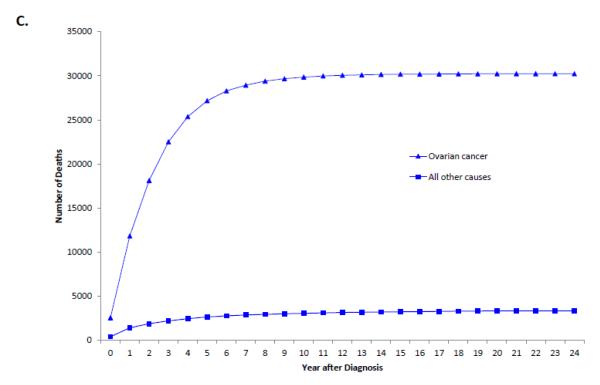
A. Cumulative frequency of death from ovarian cancer and other causes among patients with stage I tumors. 1B. Cumulative frequency of death from ovarian cancer and other causes mong patients with stage II tumors. 1C. Cumulative frequency of death from ovarian cancer and other causes among patients with stages III-IV tumors.
Figure 2.
A. Annual frequency of death from ovarian cancer and other causes among patients with stage I tumors. 2B. Annual percentage of deaths from ovarian cancer and other causes among patients with stage I tumors.
Figure 3.
A. Annual frequency of death from ovarian cancer and other causes among patients with stage II tumors. 3B. Annual percentage of deaths from ovarian cancer and other causes among patients with stage II tumors.
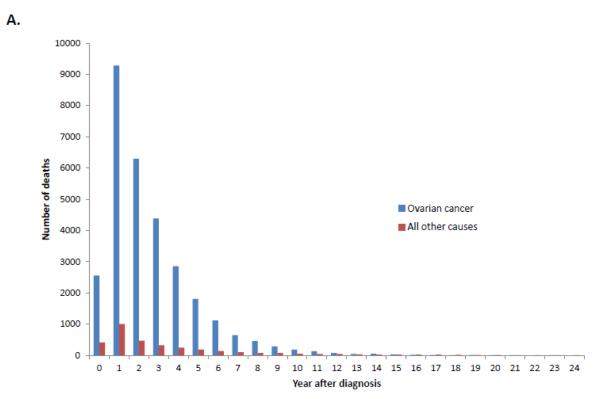
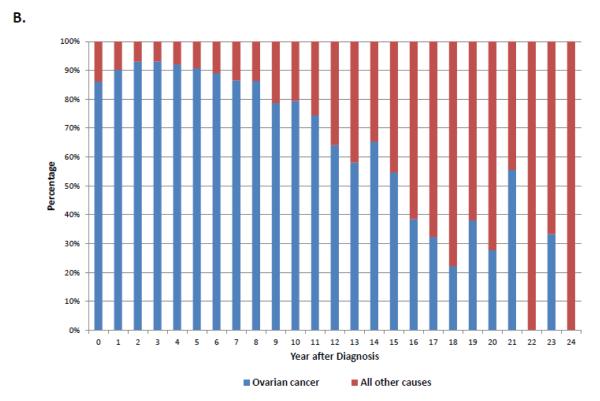
Among women with stage III-IV tumors, 67.8% (95% CI, 67.3-68.2%) had died from ovarian cancer, 2.8% (95% CI, 2.6-2.9%) from other causes, 2.3% (95% CI, 2.2-2.4%) from cardiovascular disease and 1.9% (95% CI, 1.7-2.0%) from other cancers. Compared to early-stage disease, the cumulative death rate for women with advanced stage disease shows a sharper peak (Figure 1B). When the annual percentage of deaths is stratified by cause for women with stage III-IV tumors, ovarian cancer is the most frequent cause of death in years 1 through 15 after diagnosis after which time deaths that occur are more commonly due to reasons other than ovarian cancer (Figure 4).
Figure 4.
A. Annual frequency of death from ovarian cancer and other causes among patients with stage III-IV tumors. 4B. Annual percentage of deaths from ovarian cancer and other causes among patients with stage III-IV tumors.
A sensitivity analysis in which patients were stratified by age is displayed in Table 3. For each stage group a higher number of younger compared to older women survived. Among women >60 years of age death from ovarian cancer and death from cardiovascular disease was more common compared to younger women.
Table 3.
Cause of death stratified by stage and age.
| <60 years of age | ≥60 years of age | |
|---|---|---|
|
|
||
| Rate (95% CI) | Rate (95% CI) | |
| Stage I | ||
| Alive | 85.0 (84.3, 85.7) | 56.2 (54.9, 57.5) |
| Ovarian cancer | 9.9 (9.3, 10.5) | 20.0 (18.9, 21.1) |
| Other cancer | 1.8 (1.5, 2.1) | 4.1 (3.6, 4.6) |
| Cardiovascular disease | 1.0 (0.8, 1.2) | 9.7 (8.9, 10.5) |
| Other chronic disease | 0.5 (0.4, 0.6) | 3.4 (2.9, 3.9) |
| Death from other | 1.9 (1.6, 2.2) | 6.6 (5.9, 7.3) |
| Stage II | ||
| Alive | 68.6 (66.8, 70.4) | 40.9 (39.0, 42.8) |
| Ovarian cancer | 26.4 (24.7, 28.1) | 43.9 (42.0, 45.8) |
| Other cancer | 1.4 (0.9, 1.9) | 2.3 (1.7, 2.9) |
| Cardiovascular disease | 1.2 (0.8, 1.6) | 7.1 (6.1, 8.1) |
| Other chronic disease | 0.4 (0.2, 0.6) | 1.4 (1.0, 1.8) |
| Death from other | 2.0 (1.5, 2.5) | 4.5 (3.7, 5.3) |
| Stage III/IV | ||
| Alive | 34.3 (33.6, 35.0) | 19.0 (18.5, 19.5) |
| Ovarian cancer | 60.6 (60.0, 61.3) | 72.0 (71.5, 72.5) |
| Other cancer | 1.5 (1.3, 1.7) | 2.1 (1.9, 2.3) |
| Cardiovascular disease | 0.9 (0.8, 1.0) | 3.2 (3.0, 3.4) |
| Other chronic disease | 0.3 (0.2, 0.4) | 0.8 (0.7, 0.9) |
| Death from other | 2.5 (2.3, 2.7) | 2.9 (2.7, 3.1) |
Discussion
Our findings suggest that women with ovarian cancer remain at substantial risk of cancer-related death for many years after initial diagnosis. For women with early stage ovarian cancer, the leading cause of death remains ovarian cancer for seven years after diagnosis. In patients diagnosed with advanced stage tumors, the most likely cause of death remains ovarian cancer until sixteen years after diagnosis. Cardiovascular mortality and other causes of death are responsible for only a fraction of deaths in women with ovarian cancer within a decade after diagnosis.
For women with ovarian cancer diagnosed at any stage, the probability of dying from ovarian cancer decreases with time and the probability of dying from other causes increases. The concept of conditional survival, or the chance of survival after achieving a set amount of time without recurrence or death from cancer, has been examined for a number of solid tumors including ovarian cancer.23,24 An analysis of the Hormones and Ovarian Cancer Prediction case-control study noted that the probability of surviving an 3 additional years without recurrence in women who had already survived to 1, 3, and 5 years after remission was 64%, 90% and 98%.24 Similar findings of improved conditional survival over time were noted in a population-based cohort in the U.S.23 Our data is in accord with prior studies; although the absolute number of women with ovarian cancer who die decreases with time since diagnosis, ovarian cancer remains the leading cause of mortality for those women who do die for many years after treatment.
Unlike other gynecologic cancers that are associated with higher cure rates, we noted that women with ovarian cancer remained at substantial risk of cancer-related death for more than a decade after diagnosis. Ward and colleagues analyzed cause of death in a cohort of over 44,000 endometrial cancer patients derived from the SEER database. The investigators noted that women with low grade, localized disease were 6 times more likely to die from cardiovascular disease than endometrial cancer. In this report endometrial cancer was the most common cause of death in the first 5-year interval after diagnosis but thereafter cardiovascular mortality predominated.25 The high mortality and risk of ovarian cancer specific mortality we noted reflects the aggressive nature of these neoplasms. Like endometrial cancer, we noted a higher rate of cardiovascular-related death among older women.
These findings have important implications for ovarian cancer patients and clinicians. First, these data help inform discussions surrounding survival. Survival estimates are often conveyed in the form of five year survival and long-term cure rates. Our findings allow clinicians to address the dynamic aspects of prognosis and individualize discussions about outcomes and how they change over time. Particularly for women with early-stage tumors these data provide reassurance that after six years mortality risks from other causes outweigh the risk from ovarian cancer. These data can help promote age appropriate screening and utilization of preventive health services.
Second, and more controversial, these data can be used to help guide surveillance for women with ovarian cancer receiving follow-up care. Surveillance guidelines for patients with ovarian cancer typically rely on periodic physical examination, serum tumor marker testing (CA125) and often imaging.26,27 To date early intervention based on CA125 and imaging has not been shown to impact survival, however these tests are commonly used in clinical practice.28 Our data suggest that it may be reasonable to prospectively evaluate surveillance regimens that become appropriately less intensive further out from the time of diagnosis, particularly for women with early stage neoplasms.
We recognize a number of important limitations. First, utilization of death certificate data and algorithms to determine cause of death is subject to some degree of misclassification.29-31 Prior work examining cancer specific mortality using registry data has reported acceptable validity.31 In addition to misclassification of cancer-related death there may be misclassification of other causes of death. To limit this bias our primary analysis compared ovarian cancer specific mortality to all other causes of death. Second, SEER lacks a number of important factors that likely were associated with mortality including comorbidity, performance status and cardiovascular and other risk factors. Further work examining specific patterns of survival in women with specific comorbidities would be of great interest. Lastly, because the analysis spans a twenty-four year time period, changing treatment practices over time likely influenced the allocation of treatments and outcomes.
In summary, our findings show that women diagnosed with early stage epithelial ovarian cancer have a less prolonged risk of death from ovarian cancer than women diagnosed with stage III or IV ovarian cancer. These data suggest that it may be appropriate to utilize stratified surveillance strategies for early stage versus advanced stage ovarian cancer patients. Additionally, we hope that this information may benefit practitioners in counseling and expectation setting among ovarian cancer survivors.
Research Highlights.
-The probability of dying from ovarian cancer decreases with time.
-Ovarian cancer remains the most common cause of death for 15 years after diagnosis in women with stage III-IV tumors.
-For stage I tumors, ovarian cancer remains the most common cause of death for 6 years after diagnosis.
Acknowledgements
Dr. Wright (NCI R01CA169121-01A1) and Dr. Hershman (NCI R01 CA166084) are recipients of grants and Dr. Tergas is the recipient of a fellowship (NCI R25 CA094061-11) from the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest or disclosures.
References
- 1.Howlader NNA, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA. SEER Cancer Statistics Review. National Cancer Institute; Bethesda, MD: pp. 1975–2012. based on November 2014 SEER data submission, posted to the SEER web site, April 2015 ed. [Google Scholar]
- 2.Goff BA, Mandel LS, Drescher CW, et al. Development of an ovarian cancer symptom index: possibilities for earlier detection. Cancer. 2007;109:221–7. doi: 10.1002/cncr.22371. [DOI] [PubMed] [Google Scholar]
- 3.Lim AW, Mesher D, Gentry-Maharaj A, et al. Predictive value of symptoms for ovarian cancer: comparison of symptoms reported by questionnaire, interview, and general practitioner notes. J Natl Cancer Inst. 2012;104:114–24. doi: 10.1093/jnci/djr486. [DOI] [PubMed] [Google Scholar]
- 4.Fleming GFSJ, Lengyel E. Epithelial Ovarian Cancer. In: Barakat RR, Markman M, Randall ME, editors. Principles and Practice of Gynecologic Oncology. 6th ed Lippincott Williams & Wilkins; Philadelphia: 2013. pp. 757–847. [Google Scholar]
- 5.Prat J. Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet. 2014;124:1–5. doi: 10.1016/j.ijgo.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Heintz AP, Odicino F, Maisonneuve P, et al. Carcinoma of the ovary. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. Ireland. 2006:S161–92. doi: 10.1016/S0020-7292(06)60033-7. [DOI] [PubMed] [Google Scholar]
- 7.Mertens AC, Yong J, Dietz AC, et al. Conditional survival in pediatric malignancies: Analysis of data from the Childhood Cancer Survivor Study and the Surveillance, Epidemiology, and End Results Program. Cancer. 2014 doi: 10.1002/cncr.29170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baxi SS, Pinheiro LC, Patil SM, Pfister DG, Oeffinger KC, Elkin EB. Causes of death in long-term survivors of head and neck cancer. Cancer. 2014;120:1507–13. doi: 10.1002/cncr.28588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Youn P, Milano MT, Constine LS, Travis LB. Long-term cause-specific mortality in survivors of adolescent and young adult bone and soft tissue sarcoma: a population-based study of 28,844 patients. Cancer. 2014;120:2334–42. doi: 10.1002/cncr.28733. [DOI] [PubMed] [Google Scholar]
- 10.Van Hemelrijck M, Folkvaljon Y, Adolfsson J, et al. Causes of death in men with localised prostate cancer: a nationwide, population-based study. BJU Int. 2015 doi: 10.1111/bju.13059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kato I, Severson RK, Schwartz AG. Conditional median survival of patients with advanced carcinoma: surveillance, epidemiology, and end results data. Cancer. United States: 2001 American Cancer Society. 2001:2211–9. doi: 10.1002/1097-0142(20011015)92:8<2211::aid-cncr1565>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 12.Davis FG, McCarthy BJ, Freels S, Kupelian V, Bondy ML. The conditional probability of survival of patients with primary malignant brain tumors: surveillance, epidemiology, and end results (SEER) data. Cancer. United States. 1999:485–91. [PubMed] [Google Scholar]
- 13.Hwang SL, Yang YH, Lieu AS, et al. The conditional survival statistics for survivors with primary supratentorial astrocytic tumors. J Neurooncol. 2000;50:257–64. doi: 10.1023/a:1006484220764. [DOI] [PubMed] [Google Scholar]
- 14.de Blank PM, Ostrom QT, Rouse C, et al. Years of life lived with disease and years of potential life lost in children who die of cancer in the United States, 2009. Cancer Med. 2015 doi: 10.1002/cam4.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin CL, Lieu AS, Lee KS, et al. The conditional probabilities of survival in patients with anaplastic astrocytoma or glioblastoma multiforme. Surg Neurol. United States. 2003:402–6. doi: 10.1016/s0090-3019(03)00322-7. discussion 6. [DOI] [PubMed] [Google Scholar]
- 16.Janssen-Heijnen ML, van Erning FN, De Ruysscher DK, Coebergh JW, Groen HJ. Variation in causes of death in patients with non-small cell lung cancer according to stage and time since diagnosis. Ann Oncol. 2015 doi: 10.1093/annonc/mdv061. [DOI] [PubMed] [Google Scholar]
- 17.Merrill RM, Henson DE, Ries LA. Conditional survival estimates in 34,963 patients with invasive carcinoma of the colon. Dis Colon Rectum. 1998;41:1097–106. doi: 10.1007/BF02239430. [DOI] [PubMed] [Google Scholar]
- 18.Meng L, Maskarinec G, Lee J. Ethnicity and conditional breast cancer survival in Hawaii. J Clin Epidemiol. England. 1997:1289–96. doi: 10.1016/s0895-4356(97)00183-2. [DOI] [PubMed] [Google Scholar]
- 19.Skuladottir H, Olsen JH. Conditional survival of patients with the four major histologic subgroups of lung cancer in Denmark. J Clin Oncol. United States. 2003:3035–40. doi: 10.1200/JCO.2003.04.521. [DOI] [PubMed] [Google Scholar]
- 20.National Cancer Institute [Accessed July 28, 2014];SEER Extent of Disease New 4-Digit Schemes Codes and Coding Instructions. at http://seer.cancer.gov/manuals/historic/EOD_1984.pdf.
- 21.Surveillance Research Program [Accessed July 28, 2014];National Cancer Institute SEER*Stat software version 8.1.5. at http://www.seer.cancer.gov/seerstat.
- 22.Schiavone MB, Herzog TJ, Lewin SN, et al. Natural history and outcome of mucinous carcinoma of the ovary. Am J Obstet Gynecol. 2011;205:480 e1–8. doi: 10.1016/j.ajog.2011.06.049. [DOI] [PubMed] [Google Scholar]
- 23.Choi M, Fuller CD, Thomas CR, Jr., Wang SJ. Conditional survival in ovarian cancer: results from the SEER dataset 1988-2001. Gynecol Oncol. 2008;109:203–9. doi: 10.1016/j.ygyno.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 24.Kurta ML, Edwards RP, Moysich KB, et al. Prognosis and conditional disease-free survival among patients with ovarian cancer. J Clin Oncol. 2014;32:4102–12. doi: 10.1200/JCO.2014.55.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ward KK, Shah NR, Saenz CC, McHale MT, Alvarez EA, Plaxe SC. Cardiovascular disease is the leading cause of death among endometrial cancer patients. Gynecol Oncol. 2012;126:176–9. doi: 10.1016/j.ygyno.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 26.Salani R, Backes FJ, Fung MF, et al. Posttreatment surveillance and diagnosis of recurrence in women with gynecologic malignancies: Society of Gynecologic Oncologists recommendations. Am J Obstet Gynecol. 2011;204:466–78. doi: 10.1016/j.ajog.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 27.NCCN Physician Clinical Practice Guidelines in Oncology: Ovarian Cancer. v.1.2015. 2015.
- 28.Rustin GJ, van der Burg ME, on behalf of MRC and EORTC collaborators A randomized trial in ovarian cancer (OC) of early treatment of relapse based on CA125 level alone versus delayed treatment based on conventional clinical indicators (MRC OV05/EORTC 55955 trials) J Clin Oncol. 27 [Google Scholar]
- 29.German RR, Fink AK, Heron M, et al. The accuracy of cancer mortality statistics based on death certificates in the United States. Cancer Epidemiol. 2011;35:126–31. doi: 10.1016/j.canep.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 30.Howlader N, Ries LA, Mariotto AB, Reichman ME, Ruhl J, Cronin KA. Improved estimates of cancer-specific survival rates from population-based data. J Natl Cancer Inst. 2010;102:1584–98. doi: 10.1093/jnci/djq366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu CY, Xing Y, Cormier JN, Chang GJ. Assessing the utility of cancer-registry-processed cause of death in calculating cancer-specific survival. Cancer. 2013;119:1900–7. doi: 10.1002/cncr.27968. [DOI] [PMC free article] [PubMed] [Google Scholar]