Abstract
Allergic contact dermatitis (ACD) is a common condition that can significantly impact the quality of life. Contact with allergens results in delayed hypersensitivity reactions involving T-lymphocytes, with associated skin inflammation and spontaneous itch and nociceptive sensations. However, psychophysical studies of these sensations are lacking. In the present study, we sensitized eight healthy volunteers to squaric acid dibutyl ester (SADBE). Two weeks later, one volar forearm was challenged with SADBE, and the other with acetone vehicle control. Subsequently, subjects rated the maximal perceived intensity of spontaneous itch, pricking/stinging, and burning every 6–12 hours for one week, using the generalized labeled magnitude scale. In the laboratory, they judged stimulus-evoked sensations within and outside the chemically-treated area. The SADBE- but not the acetone-treated skin resulted in a) localized inflammation, with spontaneous itch and nociceptive sensations peaking at 24–48 hours post-challenge, b) alloknesis, hyperknesis, and hyperalgesia to mechanical stimuli that were reduced or eliminated by anesthetic cooling of the SADBE-treated area and restored upon re-warming, suggesting sensations and dysesthesias are dependent on ongoing peripheral neural activity, and c) enhanced itch to intradermal injection of histamine, BAM8-22, or β-alanine. This experimental model of T-cell-mediated inflammation may prove useful in evaluating potential treatments of itch from ACD.
Keywords: Allergic contact dermatitis, itch, pain, hyperknesis, hyperalgesia
INTRODUCTION
Psychophysical measurements of the sensations evoked in humans by pruritic chemicals applied to the skin have advanced our understanding of the peripheral neural mechanisms of acute itch. A chemically evoked itch is typically accompanied by less intense nociceptive sensations such as pricking/stinging and burning 19, 28, 29. The skin can become hypersensitive to mechanical stimuli and develop itch to stroking the skin (alloknesis) and/or enhance itch and pain to pricking the skin (hyperknesis and hyperalgesia, respectively). The hypersensitivities to mechanical stimulation of the skin surrounding the primary chemical application site are termed “secondary cutaneous dysesthesthias”. These psychophysical observations provide evidence that itch and pain and pruritic and algesic dysesthesias can co-exist.
Electrophysiological recordings from peripheral sensory neurons in humans, monkeys and rodents support the hypothesis that pruritic chemicals elicit itch by generating action potentials in a subpopulations of cutaneous nociceptive neurons that also respond to stimuli that can elicit pain. Histamine and non-histaminergic chemical agents such as cowhage, appear to be signaled by separate neural pathways 16, 22, 27. Alloknesis, hyperknesis and hyperalgesia surrounding a pruritic chemical application site are thought to be mediated by central sensitization of itch and pain mediating pathways in the CNS17.
The majority of studies in which pruritic chemicals have been applied in similar fashion in humans and in animal models have focused on the mechanisms of acute itch and dysesthesias lasting from minutes to less than an hour. Experimental models inducing spontaneous continuous itch and pain in humans and animals that parallel clinical chronic itch symptoms in patients are very limited 1, 5. It would therefore be useful to explore models in which a more persistent and more clinically relevant pruritic disorder could be produced in a similar fashion in both humans and animals.
One potential model is allergic contact dermatitis (ACD), ACD is a common skin disorder affecting millions of people each year, and is associated with a diminished quality of life and significant socio-economic impact on society 2. This disorder is characterized by an antigen-specific activation of the immune system, namely the effector T cells, resulting in localized, cutaneous inflammation at the allergen contact site.
Experimentally produced ACD is studied using contact sensitizers like dinitrochlorobenzene (DNCB) and squaric acid dibutyl ester (SADBE). These agents have been used in topical immunotherapy for treating alopecia areata 21, 26 and verrucae 21, 30 and in research laboratories for inducing T-cell-mediated responses 5, 20. Camouse et al. (2008) performed a dose response study to determine the ED50 of SADBE in eliciting delayed type contact hypersensitivity in healthy human subjects and measured SADBE-specific T cell proliferation in vitro following development of ACD.
Much is known about the dosing, mechanism of sensitization, and possible loss of sensitization over time for SADBE-induced ACD 5, 20. What is lacking, and prompted the present study, is quantitative measurements of spontaneous sensations produced by ACD in humans and the effects of ACD on itch and nociceptive sensations elicited by mechanical, thermal and chemical stimulation. The advantages of using SADBE to produce ACD is that it is a chemical not normally encountered in the environment and has been safely used in the clinical setting. Our goal in the present study was to provide an experimental model of ACD that could be used in both humans and animals to facilitate interspecies comparisons in the development of new pharmacologic agents to treat itch and pain.
METHODS
Subjects
All study protocols were approved by the Yale University Human Investigative Committee and were in accordance with the Declaration of Helsinki Principles involving human subjects. Four healthy females and four healthy males gave their written, informed consent to participate in the study. Subjects reporting a history of dermatological, neurological, immunological, or cardiac disorders were excluded. Subjects were instructed to refrain from taking antihistamines and/or analgesics during the course of the study beginning at least 24 hours before the start of an experiment. In addition they were instructed to refrain from scratching and moisturizing the experimental sites and to cover them with plastic wrap while bathing.
SADBE Sensitization
Subjects were sensitized to SADBE (VWR, Radnor, PA) as described by Camouse et al., 20085. An aliquot of 48 μL of 250 μg of SADBE prepared in acetone was applied to a 1.2 cm diameter, filter-paper-lined allergen patch (“Finn chamber”; Allerderm, Phoenix, AZ), and taped onto the lower back. The resulting sensitizing dose for the application area was 222 ug/cm2, previously found to cause a T-cell proliferative response, as measured in vitro, and to sensitize 100% of subjects tested5. The allergen patch was removed after 48 hours. Any skin reaction (occasionally faint pink in color) was photographed to document the site of SADBE sensitization. No spontaneous sensations or dysesthesias were present in the sensitized skin.
SADBE Challenge
Two weeks after sensitization, subjects returned to the laboratory to receive 48 μL of 450 μg of SADBE applied to a 1.2 cm diameter, filter-paper-lined Finn chamber (dose of 398 μg/cm2) taped onto the designated site on the experimental volar forearm. Another Finn chamber containing 48 μl of acetone was applied to the homologous site on the control arm. Subjects were asked to return to the laboratory 6 hours later for patch removal. Changes in skin thickness following SADBE challenge were measured on three homologous sites on both the experimental and control volar forearm by a micrometer up to 96 hrs post challenge (Mitutuyo, Tokyo, Japan). The order of measuring skin thickness of the ACD or control skin was randomized between subjects.
Ratings of spontaneous sensations
Prior to the experiments, subjects were trained to use the generalized Labeled Magnitude Scale (gLMS) 3, 9, 19. The subjects were given a copy of the scale and asked to record spontaneous sensations felt at each site post-sensitization every 24 hours for two days, and post-challenge at hours 0–6, 6, 24, 30, 48, 50–55, 72, 96, 120, and 144. Spontaneous sensations, if any, felt in the two weeks between sensitization patch removal and SADBE and acetone challenge were not recorded on the scale. Subjects marked on the gLMS the maximal perceived intensity of any itch, pricking/stinging and/or burning they experienced from the challenged site since the last rating. “Itch” was defined as a sensation that evokes a desire to scratch. “Pricking/stinging” was defined as a sensation that was sharp and well-localized, either intermittent like a needle prick or continuous like an insect sting. “Burning” was defined as a sensation most often associated with sunburns or thermal burns, but also with skin abrasions, strong cold, and/or chemical irritants. Subjects were instructed that the nociceptive sensations (pricking/stinging or burning) may or may not be painful. At the end of each experimental session, subjects took home paper copies of the gLMS to rate the maximal intensity of each sensation in the 12–24 hours between lab visits, to be submitted at the next visit. The highest rating was considered. The subjects were instructed to rate only those spontaneous sensations that occurred in the absence of external thermal or mechanical stimulation such as air currents or the movement of clothing against the skin
A sample of topical steroid was supplied to aid healing of the ACD site once all testing had completed.
Dysesthesias: Areas and intensity measurements
Before each experiment, areas of mechanical hypersensitivity called dysesthesias (alloknesis, hyperknesis, and hyperalgesia) were measured on both arms as described in 28, 29. Mechanical stimuli were delivered along 8 radial paths that intersected at the center of the ACD site (or control site on the other arm) to determine the borders of any areas of itch to lightly stroking the skin with a cotton swab (alloknesis), enhanced pricking-evoked itch (hyperknesis) and pricking-evoked pain (hyperalgesia) the latter elicited, respectively, by indentations of 1-second duration with von Frey filaments with tip diameters and bending forces of 50 μm, 20 mN, and 200 μm, 80 mN. Subjects called out numbers (0 to 100 on the gLMS) to indicate the intensity of itch or pain evoked by the von Frey filaments of 50 and 200 μm diameter, respectively. The ratings were manually recorded.
The skin was then cooled at the ACD site using a hollow metallic tube (2-cm diameter) filled with ice that was applied to the site until it was numb to pricking by the 200 μm von Frey filament (approximately 5–7 minutes). The purpose was to determine whether the areas and magnitude ratings of secondary dysesthesias depended on ongoing neuronal activity that originated from the ACD site. We assumed this activity would be reduced or eliminated by numbing the site. Areas of dysesthesias were re-marked around the cooled ACD site and subjects rated the intensity of prick-induced pain and itch at each of the test sites around the application site. The cooling probe was then removed and the ACD site was slowly heated (~2–3 minutes) back to 32°C with a Peltier contact thermode (1 cm2) that maintained the stimulus temperature to within 0.1°C of the desired value 18. The areas of dysesthesias around the ACD site were again marked and their intensity measured. A picture was taken for analysis. Prior to cooling, for each subject, the ratings of itch evoked by the 50 μm filament were obtained at designated test sites within the area of hyperknesis and a grand mean obtained for all subjects. A grand mean was similarly calculated for ratings at these same sites after the ACD site was numbed by cooling and once again after it had been re-warmed to normal skin temperature. Similar means were obtained to examine the effects of cooling on the ratings of pain (evoked by the 200 μm filament) within the areas of hyperalgesia. Ratings of itch and pain were also obtained in the absence of dysesthetic areas and without cooling from homologous test sites around the control site on the other arm. The rationale for measuring the magnitude of sensation as well as the areas of hypersensitivities is that cooling the site of a capsaicin injection could reduce the magnitude of secondary hyperalgesia to pricking without a major reduction of the area18.
Mechanical stimulation of ACD and control sites
Von Frey filaments each exerting a different bending force (5 to 180 mN) but having the same tip diameter of 200 μm were applied alternately to the ACD and control sites. Each filament, randomly selected, was indented into the skin for 1 second at least three times at each site and an average of the ratings of six subjects was obtained.
Heat stimulation of ACD and control sites
Subjects were presented with a series of heat stimuli delivered by a Peltier thermode (1 cm2)18. Every 30–60 seconds, an incremental heat stimulus with a trapezoidal temperature waveform, a 6-second duration, and an onset ramp of 19°C/s was delivered from the base temperature in order of ascending plateau temperature in 2°C increments of 35 – 38°C (from a base of 33°C) and 41 to 51°C (from a base of 38°C). Before heating began, subjects reported the intensity of any spontaneous sensation from the ACD and control sites. The skin was heated in ascending order. After each heat stimulus, subjects used the gLMS to rate the maximal perceived intensity of any itch, pricking/stinging, and burning they experienced during the stimulus application.
Measurement of intradermal injections of pruritogens
Histamine dihydrochoride (Sigma-Aldrich; 10 μg/10μl), BAM8-22 (Tocris Bioscience; 10 μg/10μl), and β-alanine (Sigma Aldrich; 90 μg/10μl) were each prepared in sterile extracellular fluid 29. Intradermal injection of a given chemical was administered in the ACD or control site 24–72 hours post-challenge. Subjects reported the intensity of any spontaneous itch, pricking/stinging or burning they were experiencing from the ACD and control sites prior to injection. They were instructed to disregard the initial prick of the needle entering the skin, and to report the highest magnitude of each sensation they felt every 30 seconds by moving a cursor along the gLMS on a computer screen 28, 29. Numerical values (not presented to the subject) of 1 to 100 were derived from the cursor position. In each testing period, only one injection was given in each site. The three chemicals were administered in no specific order over 24–72 hours post-challenge.
Statistical Analyses
Repeated measures analyses of variance (RMANOVAs), followed by Bonferroni’s or Tukey’s posthoc tests, were performed to test the significance of experimental treatment (SADBE in acetone or acetone alone) on ratings of the magnitude of pricking pain evoked by different forces of von Frey stimulation at the ACD site and different temperatures of heat stimuli applied to the ACD site.
Sensory time-profile data were analyzed following a logarithmic (Log) transformation. A value of 1.0 was added to all sensory ratings to avoid the presence of zeros before logarithmic execution. For each sensation, the perceived intensity versus time after injection curves were analyzed for the AUC, the peak magnitude of sensation, and the total duration of the sensation from the first nonzero value to the first of the three consecutive zeros or until 20 min had elapsed.
Statistical calculations were performed using GraphPad Prism 6 (GraphPad Software). The data are presented as mean ± SEM and the probability value for statistical significance was p<0.05.
RESULTS
SADBE challenge induced skin reactions indicative of ACD
There were no skin reactions or significant changes in skin-fold thickness with time after acetone treatment on the control arm (Figure 1a). In contrast, the SADBE-challenged skin showed erythema (a faint area of redness) at 6 hours. The skin reaction became more intense with time, developing localized edema and vesiculations representing ACD. A significant increase in the skin-fold thickness of the SADBE-challenged skin was measured 24 hours post-challenge (Figure 1b, p< 0.0001) and each time point beyond, up to 96 hours (p<0.0001, paired t-tests).
Figure 1.
Challenge with SADBE but not vehicle produced inflammation characteristic of ACD. (a) SADBE but not acetone produced erythema, edema, and vesiculation, as shown for a typical subject (b) Mean skin-fold thickness for all subjects increased within the SADBE- but not the vehicle-treated skin.
ACD was accompanied by spontaneous itch
The skin reaction was accompanied by spontaneous sensations, predominantly itch (Figure 2a), and areas of mechanical hypersensitivity (Figure 2b). A predominant spontaneous itch sensation was reported (in 6 of 8 subjects) in the experimental arm after a 6 hour SADBE challenge. No subject reported any sensation from the acetone-challenged arm at or beyond 6 hours. Two subjects reported no spontaneous itch while 6 reported barely detectable to moderate itch during the 6 hour SADBE challenge. All subjects experienced weak to strong spontaneous itch 24 hours after SADBE challenge. The intensity of spontaneous itch increased with time and peaked 48 hours post-challenge. Thereafter, it slowly decreased over time and was still present in 6 of 8 subjects at the end of 144 hours post-challenge. In 3 subjects, spontaneous itch was occasionally accompanied by transient, weak sensations of pricking/stinging and burning. The mean peak magnitude of pricking/stinging and the peak burning occurred at 24 hours following SADBE challenge.
Figure 2.
ACD produced spontaneous itch and itchy skin. (a)The time course of the mean perceived intensity of spontaneous itch, pricking/stinging and/or burning sensations after SADBE challenge. No spontaneous sensations were reported on the opposite arm treated with acetone (control). (b) The dysesthetic areas of alloknesis, hyperknesis and hyperalgesia of a typical subject. These areas of enhanced itch and pain to mechanical stimuli were within and surrounding the area of allergic contact dermatitis (ACD).
Mechanical, heat or chemical stimuli elicited sensations that were perceived and rated as separate from (e.g. not additive to) any ongoing background sensation.
ACD produced dysesthesias to mechanical stimuli
Weak or no areas of mechanical hypersensitivity were observed within and surrounding the area challenged by SADBE at the end of the 6-hour challenge period. Areas of spontaneous dysesthesias were first reported 24 hours following the development of a localized ACD reaction at the SADBE-challenged site. These included areas of alloknesis (itch to stroking) and enhanced pricking-evoked itch and pain (hyperknesis and hyperalgesia; Figure 2b). After reaching maximal size by 24 hours, the areas of dysesthesias, which included and extended beyond the area of ACD, persisted through the entire course of the study.
Mechanically evoked secondary dysesthesias were reduced by cooling the ACD
Subjects used the generalized labeled magnitude scale to rate the intensity of itch and pain, respectively, evoked by von Frey filaments differing in tip diameter (50 vs. 200 μm). The intensity of alloknesis was not measured. Following initial mapping of the dysesthesias, the ACD site was directly cooled using an ice-cold probe (~2°C) for 5–7 minutes to ensure that the skin was unresponsive (numb) to the pricking probes. The areas of dysesthesias decreased during cooling, as illustrated for a typical subject (Figure 3a) but returned to normal size upon re-warming using a Peltier thermode maintained at 32°C (see methods section for details). The mean areas of alloknesis, hyperknesis and hyperalgesia after numbness by cooling were significantly smaller than before cooling (p<0.05, paired t-test; Figure 3b). The mean perceived intensity ratings of pricking-evoked itch and pain were significantly decreased within the former areas of dysesthesias surrounding the ACD site relative to pre-cooling ratings (p<0.05, paired t-tests; Figure 3c). Upon rewarming, the mean areas and intensity ratings of hyperknesis and hyperalgesia and the area of alloknesis all returned to pre-cooling values (p>0.2, paired t-test).
Figure 3.
Cutaneous dysesthesias were reduced or eliminated by numbing the ACD site with prolonged application of cold (4°C). (a) The dysesthetic areas of alloknesis, hyperknesis and hyperalgesia of a typical subject before and during the application of cold, and after rewarming the ACD site. (b) The mean areas of alloknesis, hyperknesis and hyperalgesia and mean ratings of pricking pain or itch to von Frey filaments. Ratings of pricking pain vs. itch were evoked, respectively, by a von Frey filament of 200 μm tip diameter (80 mN bending force) and a filament of 50 μm diameter (20 mN). The “control” was skin surrounding an application of acetone vehicle.
Mechanically-evoked pricking pain was enhanced at the ACD site
Control and ACD sites were indented with von Frey filaments of 200 μm diameters and differing bending force of 5 to 180 mN. The ACD site was significantly hyperalgesic (Fig 4). The mean perceived intensity of the pricking pain in the ACD vs. control site was significantly increased for forces of 40 mN and greater (p<0.001, RMANOVA, and Bonferroni’s multiple comparisons tests).
Figure 4.
Punctate mechanical stimulation of the ACD site evoked enhanced pricking pain. The mean ratings of the perceived intensity of pricking pain evoked by von Frey filaments of the same tip diameter (200 μm) but differing bending forces were significantly greater for indentations of the SADBE-treated (ACD) site than for the acetone-treated control site. The locations of 3 of the verbal descriptors are shown on the right vertical axis in correspondence with the ratings of perceived intensity indicated on the left axis.
Noxious heat evoked enhanced pricking pain and itch at the ACD site
In six subjects, the control and ACD sites were heated by a thermode using a series of steps from a base of 33°C for increments reaching 35 to 38°C and a base of 38°C for increments to 41–51°C. The baseline itch repo rted at 33°C was weak to moderate at the ACD site, and absent at the control site (Figure 5a). All subjects reported experiencing itch when the ACD site, but rarely the control site, was heated. For example, at 45°C the itch ind uced by heating the ACD site was 20.6 ± 9.3, vs. 6.7 ± 3.4 at the control site (Figure 5a, p<0.0001). The intensity of pricking/stinging increased with heating at both sites, but was significantly higher in the ACD skin than the control skin at 45°C and every temperature above (Figure 5b, p<0.05). The ratings of burning evoked by the ascending heat protocol were comparable in the control and ACD skin (Figure 5c).
Figure 5.
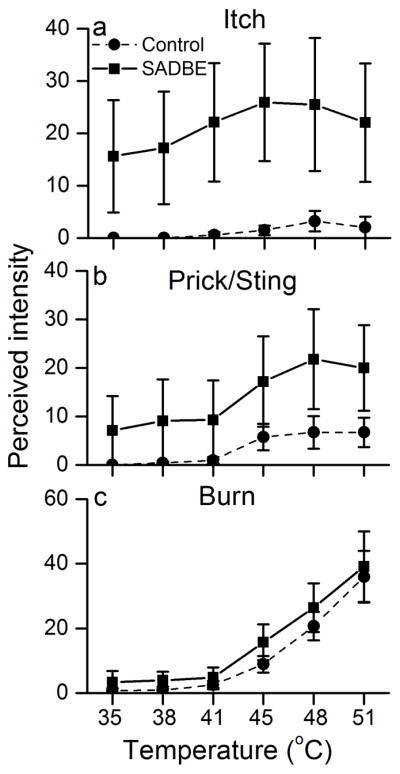
Noxious heating of the ACD site evoked enhanced pricking pain and also elicited itch. Heat stimuli evoked significantly greater ratings of the perceived intensity of (a) itch and (b) pricking/stinging pain but not greater (c) burning pain when applied to the ACD vs. control (acetone-treated) site.
Chemical pruritogens elicited enhanced itch but normal nociceptive sensations at the ACD site
Histamine produced a predominant itch followed by weaker sensations of pricking/stinging and burning in both the ACD and control sites (Figure 6a,b; n = 7 subjects). The mean log values of the area under the curve, peak magnitude and duration of itch were significantly greater in the ACD than in the control site (paired t-tests). No significant differences were observed in the log values of intensity (peak magnitude, AUC) or duration of pricking/stinging and burning induced by histamine at the two sites.
Figure 6.
Chemical pruritogens evoked enhanced itch but normal nociceptive sensations when injected into the ACD site. Time profile curves for the perceived intensity of itch, pricking/stinging and burning for intradermal histamine (10μg), BAM8-22 (20 μg) and b-alanine (90 μg) injected in the acetone-treated control skin (a, c, e) and in the SADBE-pretreated skin (ACD site; b, d, f). There was no significant difference in these measurements for pricking/stinging and burning between the two groups.
Intradermal injection of BAM8-22 at 24–72 hours post challenging produce a predominant sensation of itch at both the control and ACD site (Figure 6c,d, n = 8). The ACD site had significantly greater mean Log AUC, peak magnitude and duration of itch than the control site. The pricking/stinging and burning sensations produced by BAM8-22 were comparable in intensity and duration for both sites. Similar to histamine and BAM, intradermal injection of β-alanine in the ACD site produced an intense itch with significantly greater mean log AUC, peak magnitude and duration than in the control site (Figure 6 e, f, n = 6). No significant difference was observed in the intensity of pricking/stinging and burning evoked by β-alanine in the two sites.
DISCUSSION
An advantage of the SADBE model of ACD is that it can be experimentally used to produce inflammatory itch and pain in humans as well as in animals. This should facilitate the process of validating and translating findings of sensory neural mechanisms of ACD from animals to human. For example, the onset of spontaneous sensory behaviors appears similar for humans and mice during the development of ACD. Both the spontaneous itch- and pain-like behaviors of mice25 and the ratings of spontaneous itch and nociceptive sensations of our human subjects were directed toward the site of SADBE challenge. Electrophysiologically, enhanced spontaneous activity was observed in a subset of pruriceptive MrgprA3- and MrgprD-expressing neurons innervating the SADBE-challenged mouse skin25. Increased sodium currents in these subsets of pruriceptive neurons likely contributed to spontaneous itch and pain responses following ACD25. Thus, similar mechanisms may be responsible for mediating the spontaneous sensations reported in sensitized human subjects after SADBE challenge.
The spontaneous sensations referred to the site of ACD were accompanied by enhanced mechanically-evoked pricking pain and itch in the surrounding skin. The enhanced pricking-evoked pain and itch are commonly attributed to a central sensitization of pain- and itch-mediating dorsal horn neurons, although the neural mechanisms are still uncertain 17. We hypothesize that the reduction in areas and magnitude of mechanically-induced hyperknesis and hyperalgesia produced by numbing the ACD site with cold, and similar in effect to anesthetizing a site of painful or itchy chemical injection 18, 31, was due to a decrease in ongoing activity in pruriceptive neurons that otherwise would act to maintain the sensitization of itch-mediating neurons in the spinal dorsal horn. An analogous hypothesis was proposed as an interpretation of the effects of numbing the site of an intradermal capsaicin injection in reducing mechanically evoked secondary dysesthesias18. Cooling reduced or abolished the allodynia to stroking similar to the effects of cooling the ACD site on the area of alloknesis in the present study. But cooling was less effective in reducing the area or the magnitude ratings of pricking pain surrounding the capsaicin injection site than it was for pricking the skin surrounding the ACD site. This suggests that the secondary hyperalgesia generated from capsaicin injection is less dependent on ongoing sensory input than it is from the site of ACD. Whether this discrepancy is due, for example, to differences in the effectiveness of pruriceptive vs. non-pruriceptive primary afferents in producing central sensitization to pricking or other factors awaits further investigation
Another interpretation of the ACD cooling experiment is that the sensation of cooling itself might act to reduce local itch and accompanying dysesthesias in surrounding skin. Though the effects of locally cooling the skin in reducing 4, 6, 34 or enhancing chemically-evoked itch 23, 24, 32 have ambiguous implications, future studies could be designed to tease out possible effects of the sensation of cold in the present experiment.
Within the “primary dysesthetic” site of ACD there was enhanced pricking pain both to punctate mechanical stimuli and to noxious heat. Furthermore, the moderate pain from heat was accompanied by itch, consistent with the notion that pain can coexist with itch and that itch is mediated by neurons that also respond to noxious stimuli. The possible effects in increasing the pain to a normally painful chemical stimulus or producing itch in addition to the pain remain to be tested using the ACD model in humans. But in the mouse, bradykinin, which elicits pain-like behavior when injected in healthy skin, also evokes itch-like behavior when injected into an area of SADBE induced ACD 7. Similarly, bradykinin or a citrate buffer of low pH each of which elicits only pain in normal skin evokes, in addition, sensations of itch when applied to the itchy lesional skin of patients with atopic dermatitis12, 13.
In the present study, a pruritic chemical (histamine, BAM8-22 or β alanine) evoked a greater itch when injected into the ACD vs. the control site. However the magnitude of pricking or burning was not increased. Chemical stimulation of skin may elicit one or more qualities of sensation (itch, pricking/stinging, burning) depending on the type of chemical and how it is applied 8, 10, 19. There are several mechanisms that might be explored as explanations for the dissociation between itch and nociceptive sensations. For example, ACD may alter the patterns of discharges in a given population of peripheral nociceptive neurons thereby somehow enhancing an itch- but not a pain-related signaling mechanism. For example, itchy histamine and painful capsaicin evoke the same total number of action potentials in a population of mechanically insensitive nociceptors with unmyelinated axons; but the intervals between successive action potentials are more often shorter for capsaicin resulting in a more bursting kind of discharge33. Another possibility is that chemosensitive cutaneous nociceptors may differ in their capacities to engage a particular central pathway mediating a particular sensory quality 19. Thus, those projecting strongly to a central itch-mediating pathway 11 may be more spontaneously active and more responsive to chemical pruritogens25 than those that activate non-pruriceptive projection neurons. In addition, sensitized heat- and mechanically-sensitive nociceptors that are not pruriceptive may contribute to enhanced pricking pain at the ACD site.
When injected into an area of SADBE-induced ACD (vs. into a control site) on the cheek of the mouse, BAM8-22 evoked a greater amount of itch-like, site-directed scratching but also more pain-like, wiping behavior. Histamine elicited the same behaviors as in normal, healthy skin. Similarly, intradermal injection of SLIGRL, a protease-activated peptide 2 agonist and serotonin but not histamine administration evoked greater number of scratching bouts and enhanced calcium influx in DRG neurons in a mouse model of chronic itch from dry skin1. It remains to be determined whether the latter findings translate to itchy dry skin in humans. But in the case of ACD, it appears that some of the sensory effects in mice translate to humans (i.e. the increased itch to BAM8-22) but not others. Whether the discrepant findings reflect differences in experimental protocol for the two species (for example related to allowing mice but not humans to scratch) or differences in biological mechanisms between the two species await further studies.
Enhanced itch to pruritic chemical, as in ACD, may be characteristic of other types of persistent pathological itch. For example, histamine evoked more intense itch when applied to lesional skin in patients with atopic dermatitis than when applied to non-lesional skin 14 or to the skin of healthy control subjects15.
In conclusion, this study demonstrates an effective experimental model of ACD in humans that allows for quantitative and qualitative measurements of the spontaneous sensations and dysesthesias that are characteristic of ACD. We have shown the previously unknown effects of mechanical, thermal, and chemical stimuli on nociceptive and pruriceptive sensations in the inflamed vs. normal skin. Furthermore, this model may be used in the future to evaluate potential therapies for ACD.
Perspective.
In a model of allergic contact dermatitis, experimentally applied in humans, psychophysical measurements were obtained of persistent, spontaneous itch and enhanced stimulus-evoked itch and pain sensations. These sensory measurements will be useful in the identification of the neural mechanisms underlying inflammatory itch and pain.
Highlights.
Itch and pain were characterized in experimentally produced allergic contact dermatitis in humans
Persistent, spontaneous itch peaked in two days and decreased during the ensuing week.
The inflamed skin exhibited enhanced pain and itch to heat and mechanical stimuli and increased itch to pruritic chemicals
Cooling the inflamed skin reversibly blocked surrounding areas of mechanical alloknesis, hyperknesis and hyperalgesia
These sensory measurements will be useful in studies of neural mechanisms underlying inflammatory itch and pain.
Acknowledgments
Research funding was provided by National Institutes of Neurological Disorders and Stroke P01 NS 047399 (R. LaMotte, PI).
Footnotes
Disclosures: The authors declare that there are no conflicts of interest.
Role of funding source: The source (NINDS) had no role in the design of the study, the collection, analysis and interpretation of data; or writing of the manuscript or its submission for publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akiyama T, Carstens MI, Carstens E. Enhanced scratching evoked by PAR-2 agonist and 5-HT but not histamine in a mouse model of chronic dry skin itch. Pain. 2010;151:378–383. doi: 10.1016/j.pain.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alikhan A, Maibach HI. Allergic contact dermatitis. Chemical immunology and allergy. 2014;100:97–100. doi: 10.1159/000358608. [DOI] [PubMed] [Google Scholar]
- 3.Bartoshuk LM, Duffy VB, Green BG, Hoffman HJ, Ko CW, Lucchina LA, Marks LE, Snyder DJ, Weiffenbach JM. Valid across-group comparisons with labeled scales: the gLMS versus magnitude matching. Physiology & behavior. 2004;82:109–114. doi: 10.1016/j.physbeh.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 4.Bromm B, Scharein E, Darsow U, Ring J. Effects of menthol and cold on histamine-induced itch and skin reactions in man. Neuroscience letters. 1995;187:157–160. doi: 10.1016/0304-3940(95)11362-z. [DOI] [PubMed] [Google Scholar]
- 5.Camouse MM, Swick AR, Ryan CA, Hulette B, Gerberick F, Tinkle SS, Nedorost ST, Cooper KD, Stevens SR, Baron ED. Determination of in vivo dose response and allergen-specific T cells in subjects contact-sensitized to squaric acid dibutyl ester. Dermatitis : contact, atopic, occupational, drug. 2008;19:95–99. [PubMed] [Google Scholar]
- 6.Fruhstorfer H, Hermanns M, Latzke L. The effects of thermal stimulation on clinical and experimental itch. Pain. 1986;24:259–269. doi: 10.1016/0304-3959(86)90048-5. [DOI] [PubMed] [Google Scholar]
- 7.Fu K, Qu L, Shimada SG, Nie H, LaMotte RH. Enhanced scratching elicited by a pruritogen and an algogen in a mouse model of contact hypersensitivity. Neuroscience letters. 2014;579:190–194. doi: 10.1016/j.neulet.2014.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green BG, Bluth J. Measuring the chemosensory irritability of human skin. J Toxicol Cut Ocular Toxicol. 1995;14:23–48. [Google Scholar]
- 9.Green BG, Dalton P, Cowart B, Shaffer G, Rankin K, Higgins J. Evaluating the ‘Labeled Magnitude Scale’ for measuring sensations of taste and smell. Chemical senses. 1996;21:323–334. doi: 10.1093/chemse/21.3.323. [DOI] [PubMed] [Google Scholar]
- 10.Green BG, Flammer LJ. Localization of chemical stimulation: capsaicin on hairy skin. Somatosensory & motor research. 1989;6:553–566. doi: 10.3109/08990228909144692. [DOI] [PubMed] [Google Scholar]
- 11.Han L, Ma C, Liu Q, Weng HJ, Cui Y, Tang Z, Kim Y, Nie H, Qu L, Patel KN, Li Z, McNeil B, He S, Guan Y, Xiao B, LaMotte RH, Dong X. A subpopulation of nociceptors specifically linked to itch. Nature neuroscience. 2013;16:174–182. doi: 10.1038/nn.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hosogi M, Schmelz M, Miyachi Y, Ikoma A. Bradykinin is a potent pruritogen in atopic dermatitis: a switch from pain to itch. Pain. 2006;126:16–23. doi: 10.1016/j.pain.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Ikoma A, Fartasch M, Heyer G, Miyachi Y, Handwerker H, Schmelz M. Painful stimuli evoke itch in patients with chronic pruritus: central sensitization for itch. Neurology. 2004;62:212–217. doi: 10.1212/wnl.62.2.212. [DOI] [PubMed] [Google Scholar]
- 14.Ikoma A, Rukwied R, Stander S, Steinhoff M, Miyachi Y, Schmelz M. Neuronal sensitization for histamine-induced itch in lesional skin of patients with atopic dermatitis. Archives of dermatology. 2003;139:1455–1458. doi: 10.1001/archderm.139.11.1455. [DOI] [PubMed] [Google Scholar]
- 15.Ishiuji Y, Coghill RC, Patel TS, Oshiro Y, Kraft RA, Yosipovitch G. Distinct patterns of brain activity evoked by histamine-induced itch reveal an association with itch intensity and disease severity in atopic dermatitis. Br J Dermatol. 2009;161:1072–1080. doi: 10.1111/j.1365-2133.2009.09308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johanek LM, Meyer RA, Friedman RM, Greenquist KW, Shim B, Borzan J, Hartke T, LaMotte RH, Ringkamp M. A role for polymodal C-fiber afferents in nonhistaminergic itch. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:7659–7669. doi: 10.1523/JNEUROSCI.1760-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LaMotte RH, Dong X, Ringkamp M. Sensory neurons and circuits mediating itch. Nature reviews. Neuroscience. 2014;15:19–31. doi: 10.1038/nrn3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LaMotte RH, Shain CN, Simone DA, Tsai EF. Neurogenic hyperalgesia: psychophysical studies of underlying mechanisms. Journal of neurophysiology. 1991;66:190–211. doi: 10.1152/jn.1991.66.1.190. [DOI] [PubMed] [Google Scholar]
- 19.LaMotte RH, Shimada SG, Green BG, Zelterman D. Pruritic and nociceptive sensations and dysesthesias from a spicule of cowhage. Journal of neurophysiology. 2009;101:1430–1443. doi: 10.1152/jn.91268.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mastrolonardo M, Diaferio A. Topical immunotherapy with squaric acid dibutylester: unusual hair pigmentary changes in two cases of alopecia areata. Journal of the European Academy of Dermatology and Venereology : JEADV. 2002;16:186. doi: 10.1046/j.1468-3083.2002.00392_12.x. [DOI] [PubMed] [Google Scholar]
- 21.Micali G, Cicero RL, Nasca MR, Sapuppo A. Treatment of alopecia areata with squaric acid dibutylester. International journal of dermatology. 1996;35:52–56. doi: 10.1111/j.1365-4362.1996.tb01618.x. [DOI] [PubMed] [Google Scholar]
- 22.Namer B, Carr R, Johanek LM, Schmelz M, Handwerker HO, Ringkamp M. Separate peripheral pathways for pruritus in man. Journal of neurophysiology. 2008;100:2062–2069. doi: 10.1152/jn.90482.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfab F, Valet M, Sprenger T, Huss-Marp J, Athanasiadis GI, Baurecht HJ, Konstantinow A, Zimmer C, Behrendt H, Ring J, Tölle TR, Darsow U. Temperature modulated histamine-itch in lesional and nonlesional skin in atopic eczema - a combined psychophysical and neuroimaging study. Allergy. 2010;65:84–94. doi: 10.1111/j.1398-9995.2009.02163.x. [DOI] [PubMed] [Google Scholar]
- 24.Pfab F, Valet M, Sprenger T, Tölle TR, Athanasiadis GI, Behrendt H, Ring J, Darsow U. Short-term alternating temperature enhances histamine-induced itch: a biphasic stimulus model. The Journal of investigative dermatology. 2006;126:2673–2678. doi: 10.1038/sj.jid.5700577. [DOI] [PubMed] [Google Scholar]
- 25.Qu L, Fan N, Ma C, Wang T, Han L, Fu K, Wang Y, Shimada SG, Dong X, LaMotte RH. Enhanced excitability of MRGPRA3- and MRGPRD-positive nociceptors in a model of inflammatory itch and pain. Brain : a journal of neurology. 2014;137:1039–1050. doi: 10.1093/brain/awu007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rokhsar CK, Shupack JL, Vafai JJ, Washenik K. Efficacy of topical sensitizers in the treatment of alopecia areata. Journal of the American Academy of Dermatology. 1998;39:751–761. doi: 10.1016/s0190-9622(98)70048-9. [DOI] [PubMed] [Google Scholar]
- 27.Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjork HE. Specific C-receptors for itch in human skin. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17:8003–8008. doi: 10.1523/JNEUROSCI.17-20-08003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sikand P, Shimada SG, Green BG, LaMotte RH. Similar itch and nociceptive sensations evoked by punctate cutaneous application of capsaicin, histamine and cowhage. Pain. 2009;144:66–75. doi: 10.1016/j.pain.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sikand P, Shimada SG, Green BG, LaMotte RH. Sensory responses to injection and punctate application of capsaicin and histamine to the skin. Pain. 2011;152:2485–2494. doi: 10.1016/j.pain.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silverberg NB, Lim JK, Paller AS, Mancini AJ. Squaric acid immunotherapy for warts in children. Journal of the American Academy of Dermatology. 2000;42:803–808. doi: 10.1067/mjd.2000.103631. [DOI] [PubMed] [Google Scholar]
- 31.Simone DA, Alreja M, LaMotte RH. Psychophysical studies of the itch sensation and itchy skin (“alloknesis”) produced by intracutaneous injection of histamine. Somatosensory & motor research. 1991;8:271–279. doi: 10.3109/08990229109144750. [DOI] [PubMed] [Google Scholar]
- 32.Valet M, Pfab F, Sprenger T, Wöller A, Zimmer C, Behrendt H, Ring J, Darsow U, Tölle TR. Cerebral processing of histamine-induced itch using short-term alternating temperature modulation--an FMRI study. The Journal of investigative dermatology. 2008;128:426–433. doi: 10.1038/sj.jid.5701002. [DOI] [PubMed] [Google Scholar]
- 33.Wooten M, Weng HJ, Hartke TV, Borzan J, Klein AH, Turnquist B, Dong X, Meyer RA, Ringkamp M. Three functionally distinct classes of C-fibre nociceptors in primates. Nature communications. 2014;5:4122. doi: 10.1038/ncomms5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yosipovitch G, Duque MI, Fast K, Dawn AG, Coghill RC. Scratching and noxious heat stimuli inhibit itch in humans: a psychophysical study. The British journal of dermatology. 2007;156:629–634. doi: 10.1111/j.1365-2133.2006.07711.x. [DOI] [PubMed] [Google Scholar]







