Abstract
The use of placebo to reduce pain is well documented; however, knowledge of the neural mechanisms underlying placebo analgesia (PA) remains incomplete. This study used fMRI data from 30 healthy subjects, and dynamic causal modeling (DCM) to investigate changes in effective connectivity associated with the placebo analgesic response. Before scanning, subjects were conditioned to expect less thermal pain at 2-of-4 sites on their feet. VAS pain ratings revealed a significant but small difference between the baseline and placebo sites [mean difference = 6.63, t (29) = 3.91, p≤0.001, d =0.97], confirming an analgesic effect. However, no significant differences in magnitude of brain activation between conditions were observed via traditional random effects general linear modeling. DCM was then used to investigate changes in effective connectivity during PA. The results indicate that during the PA but not baseline condition, the couplings between brain regions including those involved in cognitive processes (e.g., attention, expectation, and evaluation) were significantly enhanced. Specifically, a significantly consistent decrease in the DLPFC→PAG coupling was found. These findings highlight the differences between pain processing and modulation at the network level. Moreover, our results suggest that small placebo effects may be better characterized via changes in the temporal dynamics among pain modulatory regions rather than only changes in the magnitude of BOLD activation. Further application of nuanced analytical approaches that are sensitive to temporal dynamics of pain-related processes such as DCM are necessary to better understand the neural mechanisms underlying pain processing in patient populations.
Keywords: Placebo, fMRI, effective connectivity, dynamic causal modeling, pain modulation
1. Introduction
Chronic pain is a significant health concern, affecting over 100 million Americans and resulting in over $600 billion in lost income and healthcare costs9, 19 however, long-term, powerful treatments for chronic pain remain elusive. One way to mitigate this problem is through the enhancement of currently available treatments. Placebo analgesia (PA) is an endogenous process that can effectively reduce an individual’s pain31. Furthermore, PA is seen as an acceptable treatment by many patients who have learned that they have received a placebo7. PA, however, is a complex and multifaceted phenomenon that is influenced by multiple psychological constructs and mediated by multidimensional neuronal systems20, 24, 26, 29, 37, 39, 40. Given this complexity, the neural mechanisms that underlie PA and the factors that predict an individual’s placebo response are only partially understood. Early investigations of PA that used functional MRI (fMRI) associated PA with the modulation of neural activity among pain-related brain regions. Nuanced analytical methods that investigate the temporal development of PA’s are necessary to better understand the dynamic changes among brain regions involved in endogenous pain modulation.
Placebo analgesia has been linked to the pain-modulatory processes of classical conditioning 40, expectation 41, anxiety 27, 30, and optimism 20. This complexity is reflected in the results of neuroimaging studies of PA, which have shown effects at regional and network levels. Multiple studies have associated PA with reductions in BOLD activity in pain-related brain areas such as the thalamus, somatosensory cortices, insula, and anterior cingulate cortex 14, 31, 42. Increased activity in regions responsible for cognitive control and evaluative processes, such as the dorsolateral prefrontal cortex (DLPFC), orbitofrontal cortex (OFC), and rostral anterior cingulate cortex (rACC), has also been observed in anticipation of and during PA 14, 31, 42. Afferent inhibition and the activation of pathways involving the release endogenous opioids, noradrenaline and serotonin 4, 6, 29, 33 have been implicated in these activation differences. In an SEM analysis of placebo analgesia in chronic pain patients, Craggs and colleagues11 demonstrated that, compared to a baseline painful condition, the inter-regional relationships among pain-related brain regions were drastically altered during the experience of placebo analgesia. However, the data in this study for the baseline painful and PA conditions were collected on separate visits. Thus, whether these same changes occur among healthy individuals, and whether the BOLD response to rapidly presented thermal stimuli could distinguish pain and PA processes from a single scanning session remains unclear.
fMRI studies of PA have used experimental paradigms in which the stimulation of baseline pain-related and PA sites were either temporally separated by several seconds or performed during separate scanning sessions 11, 41, preventing a more robust understanding of PA neural processes. The present study examined effective connectivity during PA using dynamic causal modeling (DCM). In critical distinction from past studies 11, 41, rapid succession of experimental conditions (baseline painful vs. PA) allowed for a robust understanding of PA-related modulation. Based upon our previous work investigating the placebo analgesic response 10, 11, 30, we hypothesized that: 1) comparisons between BOLD activation during PA versus baseline pain would show decreased activation in regions commonly associated with pain experience (thalamus, insula, primary and secondary somatosensory cortices, ACC) and increased activation in regions associated with descending pain modulation (DLPFC and ACC) and 2) PA but not baseline stimuli would be associated with the modulation of descending pain-related, inter-regional connectivity parameters among regions such as the DLPFC, and dACC.
2. Methods
The data used in the present study represent a portion of a larger study designed to investigate the mechanisms of placebo analgesia. This study aimed to identify the temporal characteristics and psychological processes associated with brain networks involved in afferent pain processing and pain modulation. The study received approval by the University of Florida Institutional Review Board and all participants provided written informed consent.
During a screening visit, “pain” and “placebo” temperatures were identified for each subject. Subjects then completed three fMRI scanning visits designed to establish baseline neural response to thermal quantitative sensory testing (QST), identify the neural correlates of placebo analgesia (placebo-imaging visit) and assess the durability of the placebo response over time. Subjects completed an initial demographics questionnaire and during each visit, completed two self-report questionnaires, the State-Trait Anxiety Inventory (STAI) and the Pennebaker Inventory of Limbic Languidness (PILL) and provided electronic VAS ratings of their pain during QST. Only fMRI data and VAS ratings from subjects’ placebo imaging visit were analyzed in the present study, which utilized a within subjects design to assess differences in brain activation and effective connectivity during painful and placebo analgesic stimulation.
2.1 Participants
Contacts were made with 367 subjects, who were recruited from the University of Florida campus area. 126 subjects were initially screened, and 101 were enrolled. Of these, 52 completed the study. As the aims of the primary study proposed validation of results with a second sample 30 subjects, data from the first 30 subjects (mean age = 22.27 years, SD = 2.90 years) with complete behavioral and imaging data (excluding subjects with excessive in-scanner motion) were used in the present study. 11 participants identified as Caucasian, eight as Asian, five as Hispanic, six as African American, and one as Native Hawaiian or other Pacific Islander (one identified as both African American and Hispanic). Exclusion criteria included: 1) current participation in another research protocol that could interfere with or influence the present study (i.e. other studies of pain) 2) use of prescription or non-prescription drugs that might impact pain-processing that could not be stopped seven days prior to testing (e.g. NSAIDs, antihistamines, antidepressants, anti-convulsants, migraine medications, and cough suppressants) 3) history of psychiatric, psychological, neurologic, or other disorders (e.g. diabetes, thyroid disease, gastrointestinal/liver disease (other than IBS), collagen vascular disease, focal or systemic neurological disease, malignancy, seropositive for HIV, or documented psychiatric disorders) 4) current chronic pain condition 5) positive pregnancy test result 6) possession of metal in the head, neck or abdominal cavity 7) current medical condition that would contraindicate participation in this study 8) inability to provide informed consent.
2.2 Experimental Materials
Thermal stimuli were delivered to two locations on the surface of each foot with an MR-compatible, computer-controlled Medoc Thermal Sensory Analyzer (TSA-2001, Ramat Yishai, Israel). This is a peltier-element-based stimulator, capable of producing stimuli across a range of temperatures (33–51°C). A Visual Analog Scale (VAS) was used in the acquisition of pain ratings. The VAS was anchored on the left with “No pain” and on the right with “The most imaginable pain.”
2.3 Experimental Procedures
To account for individual differences in pain perception, each subject underwent a series of QST calibration trials during the screening visit to determine pain and placebo temperatures. In these trails, subjects received a series of thermal pulses on the dorsal aspect of the foot starting at 43°C and increasing by 1°C until a subject’s tolerance or 51°C was reached. Subjects rated their pain intensity after each pulse. The highest temperature with a VAS score ≤20 was used as the placebo temperature (PA), and the lowest temperature with a score ≥40 and ≤60 was used as the baseline, painful temperature.
During the first part of the placebo-imaging visit, subjects were conditioned to expect less pain from thermal stimuli applied to two randomly selected sites of their feet where an inert cream had been applied. Specifically, an inert cream was applied on two of four sites (PA) of the dorsal aspects of the subjects’ feet and subjects were then told: “The agent you have just been given is known to significantly reduce pain in some patients.” The subjects then completed a series of “conditioning trials” during which, the previously identified “placebo” temperature was surreptitiously used at the placebo sites and the “painful” temperature was used at the two non-placebo, baseline sites. Immediately after this conditioning phase, subjects completed a neuroimaging scanning session to acquire structural and functional MRI data. Following the acquisition of a 3-D anatomical scan, subjects completed three fMRI scans. During these fMRI scans, subjects completed an experimental pain protocol in which only the baseline temperature was used for of the all stimuli, regardless of site. During each scan, subjects received 16 thermal stimuli, delivered in random order and lasting 4sec seconds, with an average interstimulus interval of approximately 12s. Following each stimulus, subjects rated their pain using an electronic VAS. Thus, during each fMRI scan, the same stimulus temperature was applied at all four sites, which included two sites that had been subjected to lower intensity conditioning and two sites that had been subjected to the baseline painful temperature.
2.4 Data Acquisition and Image Processing
All MRI images were acquired with a 3.0T Phillips Achieva scanner using an 8-channel head coil. The parameters for the T1-weighted structural MRI included: saggital orientation (XYZ dimension= 240*240*180; FOV [mm] =240, 240, 180; slice thickness [mm] =1; gap thickness = 0; voxel dimension [mm]= 1.0*1.0*1.0; repetition time [ms] =8.1, FA=8). Parameters for the subsequent fMRI scans were: trans-axial orientation, echo planar acquisition (XYZ dimension = 80*80*39; FOV [mm]=240, 114, 240; slice thickness [mm] =3; gap thickness = 0; voxel dimension [mm]= 3*3*3; repetition time [ms] =2000, FA=80). Each scan lasted 5:40, and resulted in 486 volumes per subject.
The MRI data were analyzed using SPM12 (Wellcome Trust Centre for Neuroimaging, London, UK) with MATLAB 2011b (MathWorks, Sherbon, MA, USA). Preprocessing of the fMRI data included: slice-scan-time correction, and volume registration/motion correction. The structural data were then coregistered to the functional data prior to warping both sets into the common MNI stereotaxic space. Finally, the fMRI data were spatially smoothed with an isotropic 6-mm Gaussian kernel (FWHM).
A mass univariate general linear model (GLM) was used to identify cortical regions wherein baseline site stimulation (baseline stimuli) and placebo site stimulation (PA stimuli) onset were significantly convolved with the hemodynamic response function (HRF). The first-level analyses included the canonical HRF, and also temporal and dispersion derivatives, which model small differences in peak response latency and peak response duration, respectively. The inclusion of these informed basis functions allowed for inter-subject and inter–voxel response variation The planned contrasts for the first-level analysis involved the main effect for the baseline and PA conditions, and the difference between them. At the second-level, a random effects GLM (RFX-GLM) was used to analyze individual contrast images using one-sample t-tests (p ≤ 0.05), and adjusted for multiple comparisons with the family-wise error correction (FWE).
The effective connectivity among brain regions involved in processing pain-related information, as well as the changes in EC that correspond with the PA response was estimated with dynamic causal modeling (DCM), as implemented in SPM12 (DCM12, Wellcome Trust Centre for Neuroimaging, London, UK). DCM 18 provides a number of advantages in the estimation of EC compared to previously used methods such as SEM 25. For example, unlike other approaches, DCM models the influence of experimental manipulations on a network of brain regions is modeled at the neuronal level. A plausible biophysical model is then used to translate modeled neuronal activity into hemodynamic responses17, that can be compared to the observed regional BOLD responses acquired in fMRI. Thus, DCM is less sensitive to HRF variability across brain regions and has been found to yield more accurate results than other methods EC analysis 13. This process allows for the comparison of evidence for competing models of neural dynamics and produces mechanistically interpretable effective connectivity parameter estimates. The present study estimated bilinear, deterministic DCMs with centered inputs, which provide estimates of three classes of effective connectivity parameters: 1) experimental inputs, which estimate the effect of experimental conditions on regional activity 2) endogenous connections, which estimate of inter- and intra-regional effective connectivity 3) modulatory parameters, which estimate the effects of experimental conditions on inter-regional connectivity. Furthermore, DCM offers interpretational ease in the sense that it readily allows the estimation of the effects of multiple experimental stimuli or cognitive, contextual variables on inter-regional dynamics. Compared to other methods, these advantages make DCM ideal for studying the neural response to rapidly presented stimuli, and the differential influence in EC that accompanies the rapid cycling between the experience of pain and placebo analgesia as used in the present study.
Neural pathways identified in functional and anatomical studies were used to inform the creation of a theoretically informed model of how painful stimuli are processed 2, 3, 5, 6, 21, 28, 29. The areas of activation identified by RFX-GLM and previous functional studies of pain 6, 10, 30, 41, 42 were used to guide region of interest (ROI) selection subsequent analysis of effective connectivity via DCM (e.g. thalamus, anterior cingulate cortex, prefrontal cortex, insula). To account for individual variability in the BOLD response, for each subject, data were extracted for each ROI and DCMs were inverted on a per scan basis. Time series were extracted from supra-threshold (p≤0.05, uncorrected) voxels within 9 mm of the group peak in all ROIs. In the event that suprathreshold activation was not observed in a given ROI, the local maximum within a 9 mm sphere around the group peak was used. Time series (eigenvariates) were extracted from a 6mm sphere around the peak voxel identified by a contrast of combined activation in response to stimulation of baseline and PA sites for each ROI.
DCM model comparison proceeded in two steps. 1) Bayesian omnibus risk (BOR) was calculated to ensure that differences in evidence exist between models. The BOR statistic represents the probability that all models being tested are equally likely to represent the observed data34. 2) To identify the optimal model in each hemisphere, random effects Bayesian model selection (BMS) 34, 36 was used to compare hypothesized models. The optimal model demonstrated the highest protected exceedance probability (PXP; certainty that a given model is more likely than any other of those tested, given the data). PXP calculation was implemented to correct overconfidence bias inherent in exceedance probability as calculated in previous versions of SPM 34. Parameter estimates of the winning model, averaged across sessions, were extracted for each subject. To determine parameter consistency across subjects, one-sample t-tests on each parameter class (experimental inputs, endogenous connections, and modulatory parameters) were conducted to determine parameter consistency at the subject-level, and Bonferroni corrected for multiple comparisons separately for each class 35.
3.0 Results
3.1 Whole Brain Random Effects General Linear
During fMRI scanning, mean VAS pain ratings at baseline and PA sites of the feet were 48.49 (SD=18.49) and 41.87 (SD=16.90), respectively. A significant main effect was observed when comparing mean VAS ratings between baseline and PA conditions [mean difference = 6.63, t (29) = 3.91, p≤0.001, d =0.97]. Whole brain RFX-GLM did not identify significant differences in brain activation between the baseline and PA conditions (pFWE < 0.05; t (29); ns; see Table 1 for results in regions included in DCM). However, at a more liberal threshold (p<0.05, uncorrected) within a mask of pain-related brain regions (bilateral thalamus, insula, primary and secondary somatosensory cortex, anterior cingulate cortex, nucleus accumbens, amygdala, dorsolateral prefrontal cortex, and periaqueductal gray) decreased right thalamic and insular activity during stimulation of placebo conditioned vs. unconditioned sites was observed.
Table 1.
Comparison of RFX-GLM Activations at Painful and Placebo Sites in Select Regions
| Right Hemisphere | Left Hemisphere | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | t | d | x | y | z | t | d | |
| PAG | 6 | −28 | −19 | 1.94 | 0.35 | 6 | −28 | −19 | 1.94 | 0.35 |
| THAL | 15 | −13 | 5 | 3.40 | 0.62 | −15 | −19 | −1 | 0.77 | 0.14 |
| pINS | 45 | −19 | 17 | 1.16 | 0.21 | −45 | −25 | 14 | −1.52 | −0.28 |
| dACC | 9 | 11 | 41 | −0.23 | −0.04 | −9 | 8 | 44 | −0.61 | −0.11 |
| dlPFC | 42 | 35 | 26 | 1.40 | 0.26 | −39 | 29 | 26 | 1.77 | 0.32 |
Note: All regional activations for painful vs. placebo sites are nonsignificant at p<0.05, FWE. Abbreviations: PAG, periaqueductal gray; THAL, Thalamus; pINS, posterior insula; dACC, dorsal anterior cingulate cortex, dlPFC, dorsolateral prefrontal cortex.
Significant activations due to thermal stimulation were observed when viewing the combination response due to both conditions (p ≤ 0.05, FWE). Activation was observed in regions including the bilateral thalamus, posterior insula, primary and secondary somatosensory cortices, dorsal anterior cingulate cortex, and supplementary motor area (Figure 1). Activation was also seen in the brainstem, including the PAG, and right anterior insula.
Figure 1.
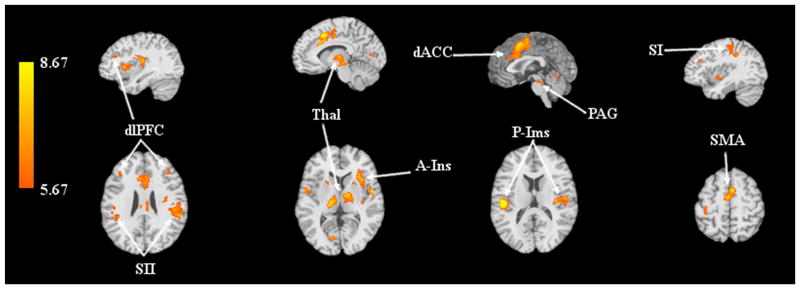
Significant activation (p≤0.05, FWE) in response to combined baseline painful and PA stimuli. Abbreviations: Abbreviations: PAG, periaqueductal gray; Thal, thalamus; P-Ins, posterior insula; SI, primary somatosensory cortex; SII, secondary somatosensory cortex; dACC, dorsal anterior cingulate cortex, dlPFC, dorsolateral prefrontal cortex; A-Ins, anterior insula
3.2 Dynamic Causal Modeling
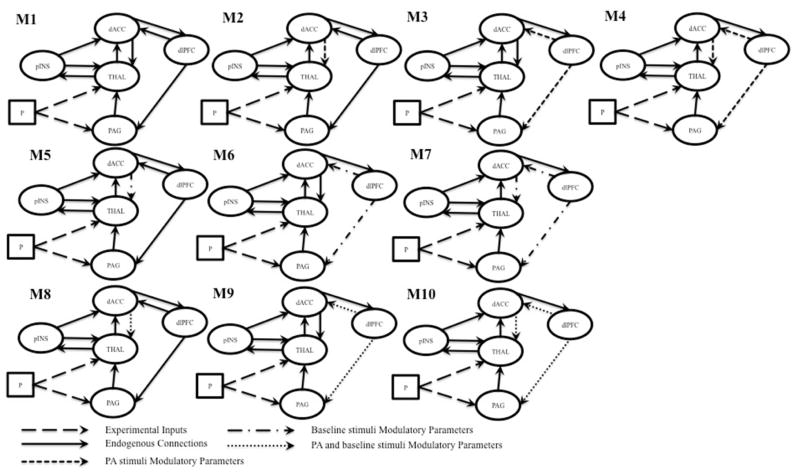
The regions chosen for DCM included the PAG, thalamus, posterior insula, dACC, and DLPFC. These regions were selected for their frequent implication in studies of pain processing and endogenous pain modulation due to PA 11, 24, 28, 30, 41. Regional coordinates based upon group maxima identified by RFX-GLM are listed in Table 2. Ten bilinear, deterministic DCMs with centered inputs were specified for comparison in BMS (Figure 2). All models contained the same underlying structure of endogenous connections. Pain was assumed to act as an experimental input to the thalamus and PAG. Specified endogenous connections functioned to explain how nociceptive stimuli are processed by this set of regions first via ascending projections from the thalamus and PAG to the posterior insula, cingulate and prefrontal cortices, and descending pathways from the DLPFC and dACC, from which inhibitory input to the spinal cord originates.3, 5, 6.
Table 2.
MNI Coordinates of ROI’s used in DCM.
| Right Hemisphere | Left Hemisphere | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | t | d | x | y | z | t | d | |
| PAG | 6 | −28 | −19 | 6.59 | 1.20 | 6 | −28 | −19 | 6.59 | 1.20 |
| THAL | 15 | −13 | 5 | 6.91 | 1.26 | −15 | −19 | −1 | 6.99 | 1.28 |
| pINS | 45 | −19 | 17 | 6.21 | 1.13 | −45 | −25 | 14 | 10.08 | 1.84 |
| dACC | 9 | 11 | 41 | 5.86 | 1.07 | −9 | 8 | 44 | 8.54 | 1.56 |
| dlPFC | 42 | 35 | 26 | 5.96 | 1.09 | −39 | 29 | 26 | 6.08 | 1.11 |
Note: All regional activations are significant at p≤0.05 FWE in response to combined stimulation at baseline and PA sites. Abbreviations: PAG, periaqueductal gray; THAL, Thalamus; pINS, posterior insula; dACC, dorsal anterior cingulate cortex, dlPFC, dorsolateral prefrontal cortex.
Figure 2.
Models of pain processing and placebo-related pain modulation compared in BMS. Abbreviations: P, baseline stimuli; PAG, periaqueductal gray; THAL, thalamus; pINS, posterior insula; dACC, dorsal anterior cingulate cortex; dlPFC, dorsolateral prefrontal cortex
The models compared differed in their estimation of the modulation of pain-related effective connectivity during PA. Models were specified to compare the unique influence of pain and placebo site stimulation on effective connectivity parameters implicated in pain modulation (dACC→thalamus, and DLPFC→dACC and DLPFC→PAG). Model one (M1) was a baseline model of pain processing model, and proposed no modulatory effects of PA. The same endogenous structure was used in all subsequent models. Models two through four estimated modulatory parameters only during PA stimuli, models five through seven estimated modulatory parameters during baseline stimuli, and models eight through ten estimated modulatory effects during both baseline and PA stimuli (Figure 2). Models were estimated separately for each hemisphere. This model space allowed us to determine the unique influences of pain and placebo site stimulation on regional connectivity.
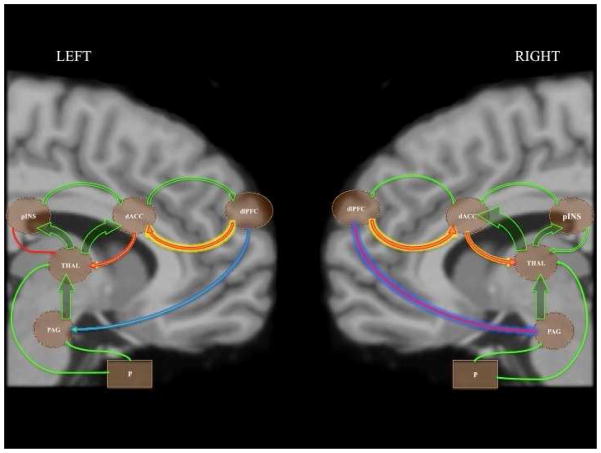
BMS was used to estimate model fit separately for each hemisphere. The BOR test indicated that the null hypothesis of equal evidence for all models could be rejected for both the left and right hemisphere (right hemisphere BOR = 0.00, left hemisphere BOR = 0.00). In both hemispheres, BMS clearly identified model four (Figure 3) as optimal (right hemisphere PXP = 0.92, left hemisphere PXP = 0.99).
Figure 3.
Model 4 was identified by BMS as the best fitting model in both hemispheres. This model suggests that descending connectivity from the dACC and dlPFC are modulated during the PA condition. Arrow and glow widths are weighted to represent parameter strengths: green arrows represent positive endogenous couplings and experimental inputs; red arrows indicate negative endogenous couplings; yellow glow indicates positive PA-related modulatory effects; blue glow indicates negative PA-related modulatory effects. Abbreviates: P, baseline stimuli; PAG, periaqueductal gray; THAL, thalamus; pINS, posterior insula; dACC, dorsal anterior cingulate cortex; dlPFC dorsolateral prefrontal cortex.
Consistency of parameter estimates across subjects was assessed with post-hoc one-sample t-tests independently for each parameter class (Tables 4, 5, and 6). Experimental inputs estimated from stimulation at pain sites to the PAG were significantly consistent in both hemispheres. The thalamic input was also significant in the right hemisphere. Significantly consistent endogenous connections were seen bilaterally in the PAG→thalamus, thalamus→posterior insula, and thalamus→dACC, connections. In the right hemisphere, the dACC→thalamus endogenous parameter was also significantly consistent. Among the estimated modulatory parameters, the PA-related modulation of the right hemisphere dlPFC→PAG coupling was significantly consistent across subjects.
Table 4.
Experimental Input Parameter Estimate Means and Standard Deviations
| Right Hemisphere | Left Hemisphere | |||
|---|---|---|---|---|
| Input Region | Mean (SD) | t | Mean (SD) | t |
| PAG | 0.06(0.08) | 4.07* | 0.07(0.09) | 4.31* |
| THAL | 0.07(0.13) | 2.78* | 0.01(0.10) | 0.62 |
Significant at p≤0.05, Bonferroni corrected.
Abbreviations: PAG, periaqueductal gray; THAL, Thalamus.
Table 5.
Endogenous Connection Parameter Estimate Means and Standard Deviations
| Parameter | Right Hemisphere | Left Hemisphere | ||
|---|---|---|---|---|
| Mean (SD) | t | Mean (SD) | t | |
| PAG→THAL | 1.21(1.60) | 4.14* | 1.14(1.43) | 4.38* |
| THAL→PINS | 0.64(0.74) | 4.70* | 0.81(0.99) | 4.46* |
| THAL→dACC | 1.33(1.36) | 5.36* | 0.97(1.08) | 4.88* |
| pINS→THAL | −0.24(0.81) | −1.62 | −0.02(0.72) | −0.20 |
| pINS→dACC | 0.19(1.25) | 0.82 | 0.27(0.90) | 1.62 |
| dACC→THAL | −0.27(0.48) | −3.08* | −0.23(0.44) | −2.88† |
| dACC→dlPFC | 0.14(0.48) | 1.60 | 0.28(0.69) | 0.26† |
| dlPFC→PAG | −0.03(0.27) | −0.68 | 0.00(0.24) | 0.02 |
| dlPFC→dACC | −0.31(0.75) | −2.23† | −0.25(0.74) | −1.85 |
Significant at p≤0.05, Bonferroni corrected.
Significant at p≤0.05, uncorrected.
Abbreviations: PAG, periaqueductal gray; THAL, Thalamus; pINS, posterior insula; dACC, dorsal anterior cingulate cortex, dlPFC, dorsolateral prefrontal cortex.
Table 6.
Modulatory Parameter Estimate Means and Standard Deviations
| Right Hemisphere | Left Hemisphere | |||
|---|---|---|---|---|
| Modulated Parameter | Mean (SD) | t | Mean (SD) | t |
| dACC→THAL | 0.23(0.76) | 1.68 | 0.17(1.06) | 0.87 |
| dlPFC→PAG | −0.77(1.16) | −3.64* | −0.23(1.46) | −0.86 |
| dlPFC→dACC | 0.45(1.81) | 1.36 | 0.53(1.24) | 2.34† |
p≤0.05, Bonferroni corrected.
Significant at p≤0.05, uncorrected.
Abbreviations: PAG, periaqueductal gray; THAL, Thalamus; dACC, dorsal anterior cingulate cortex, dlPFC, dorsolateral prefrontal cortex.
4. Discussion
Placebo analgesia has been shown to alter neural activity of brain regions involved in the processing and modulation of pain as well as the effective connectivity among these regions 11, 29. However, fMRI studies of PA have temporally segregated neural response during placebo and control conditions. The present study examined the effects of rapid, random succession of painful stimuli applied to unconditioned and placebo conditioned sites of the feet on: 1) overall brain activation via RFX-GLM and 2) inter-regional connectivity via DCM. Despite a significant placebo effect, RFX-GLM results indicated that no significant differences between conditions were present in our sample without the use of a more liberal statistical threshold (p < 0.05, uncorrected), which revealed decreased activation in the right thalamus and insula during conditioned site stimulation. However, DCM analyses indicated that PA significantly enhanced the effective connectivity among regions associated with the modulation of pain in the right hemisphere.
4.2 BMS Results
BMS found significant evidence for M4 as the optimal in both hemispheres, indicating that models in which PA modulates descending pain-related connectivity from both the dACC and DLPFC best explained our data. No evidence was found for models in which these connections are modulated during the experience of baseline stimuli, suggesting the unique involvement of dACC and DLPFC couplings in PA. Furthermore, while no differences in BOLD activation were found between PA and pain conditions via RFX-GLM, DCM results indicate that differences in effective connectivity underlie the placebo response in our protocol.
Although previous studies of PA 23, 32, 41 have identified differences in BOLD activity when compared to baseline painful stimuli, the placebo effect (mean = 6.63) observed in our study, while statistically significant, was considerably smaller than those observed in previous studies. For example, Price, et al., 2007 reported a mean difference of 19.2 on a 0–100 VAS between baseline and placebo stimuli, and Bingel and colleagues found a mean difference of 1.0 on a 0.0–4.0 numeric rating scale 1. Given this difference, it is conceivable that GLM-based differences in PA are dependent upon larger placebo effects, while smaller placebo effects as those observed in this study occur through more subtle changes in inter-regional dynamics.
4.3.1 Parameter-level Inference: Endogenous Connections
The results of the present study support a model of neural activity that elucidates the neural underpinnings of placebo analgesia. Consistent with current models of ascending pain pathways, the endogenous inputs of the baseline condition to the PAG and thalamus were significantly consistent 6, 27, 28. Although the best-fitting models contained both ascending and descending endogenous connections, the significant consistency of primarily ascending endogenous parameter estimates (PAG→thalamus, thalamus→posterior-insula, thalamus→dACC) in both hemispheres may reflect the relative inactivity of the descending pathways during the baseline condition compared to the PA condition.
4.3.2 Parameter-level Inference: Modulatory Parameters
BMS indicated that models including PA modulation of the dACC→thalamus, dlPFC→PAG, and dlPFC→dACC couplings best explain our data. Modulatory parameters are indicative of the additive change in endogenous connectivity between two brain regions in the presence of an experimental or contextual manipulation (e.g. PA), while endogenous parameters are suggestive of the rate of influence among regions. In both hemispheres, PA was associated with increases in the dlPFC→dACC, and dACC→thalamus couplings, and decreases in the dlPFC→PAG couplings. Parameter-level statistical tests revealed significantly consistent modulatory effect were observed in the right hemisphere dlPFC→PAG coupling (mean = −0.77, SD = 1.16). This does not suggest that the other parameter estimates which did not achieve statistical significance do not exist but rather that there is variability in their strengths 36. This variability may be indicative of differences in cognitive or affective processes underlying the placebo response across subjects. Further investigation into individual variability in placebo response, and its underlying neural and psychological factors will aid in clarifying these processes.
Across subjects though, our results indicate that PA is associated with consistent decreases in right hemisphere the DLPFC→PAG coupling, resulting in change in the rate of influence among these regions over time. The significance of only right hemisphere modulatory parameters may be consistent with evidence for a right hemisphere bias in both pain processing and modulation 8, 15, 38.
The DLPFC has shown to affect the release of endogenous opioids in the modulation of pain 24, 42. Likewise, modulation of PAG activity has been documented in previous studies of PA 16, 22. It is likely that attention- or expectation-related processes are involved in the modulation of DLPFC→PAG connectivity. This pathway has also been implicated to involved modulation of pain through the RVM to the dorsal horn 27. Uniquely, results of the present study suggest that in the absence of differences in magnitude of regional activity, PA may be affected through subtle changes in the rate of influence among pain modulatory regions. This is consistent the notion that multiple neural pathways and mechanisms may underlie the placebo analgesic response 16, 22.
4.5 Strengths and Limitations
As far as we are aware, this is the first study to examine changes in effective connectivity due to PA with DCM. Although other effective connectivity approaches have previously been used to study PA and pain modulation 11, the rapid succession of experimental conditions (PA and “pain” sites) used in study allowed insight into more subtle aspects of pain modulation. Although this design may have lead to smaller placebo effects compared our previous work 30, it allowed for the investigation of the neural mechanisms of PA when differences in GLM-based BOLD activation were absent. As such, the results of the present study provide valuable insight into PA-related neural processes in healthy individuals. As prior studies have implicated different pain modulatory functioning in individuals with chronic pain 6, 12, future studies are encouraged to examine the impact of chronic pain conditions on the processes illuminated by this study. We also suggest that future studies attempt to clarify the specific psychological processes linked with the neural mechanisms identified in this study.
Finally, although our modeling approach included many regions salient to pain processing and placebo analgesia, It is possible that unmodeled regions influenced the present results. However, to prevent exponential increases in model complexity and decreases in model interpretability, we chose to limit the number of regions and models included.
4.6 Conclusion
Our results provide evidence of the involvement of afferent inhibition in pain-modulatory neural systems due to PA. Importantly, results suggest that PA may be affected by facilitation of the effective connectivity among brain regions involved in pain modulation in the context of small placebo effects, which may not be observable via differences in GLM activation traditionally associated with PA.
Table 3.
Bayesian Model Selection Results
| Model Number | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M1 | M2 | M3 | M4 | M5 | M6 | M7 | M8 | M9 | M10 | |
| RH PXP | 0.00 | 0.00 | 0.07 | 0.92 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 |
| LH PXP | 0.00 | 0.00 | 0.01 | 0.99 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
Greatest evidence was found in favor of Model 4 in both hemispheres (Shown in bold).
Abbreviations: RH, right hemisphere; LH, left hemisphere; PXP, protected exceedance probability
Perspective.
Changes in effective connectivity among pain-related brain regions may be more sensitive detectors of the neural representation of small placebo effects than changes in the magnitude of brain activation. Knowledge of these mechanisms highlights the importance of integrated neural networks in the understanding of pain modulation.
Small placebo analgesia produces changes in effective connectivity
Descending pain modulation is likely implicated
Network approaches are necessary in identifying neural mechanisms of placebo
Acknowledgments
This project received support by the National Center for Complementary and Alternative Medicine (5R01A T001424) to Dr. Michael Robinson.
Footnotes
Disclosures
The authors have no financial or other relationships that pose a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bingel U, Lorenz J, Schoell E, Weiller C, Buchel C. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain. 2006;120:8–15. doi: 10.1016/j.pain.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 2.Bingel U, Tracey I. Imaging CNS modulation of pain in humans. Physiology (Bethesda) 2008;23:371–380. doi: 10.1152/physiol.00024.2008. [DOI] [PubMed] [Google Scholar]
- 3.Bissiere S, Plachta N, Hoyer D, McAllister KH, Olpe HR, Grace AA, Cryan JF. The rostral anterior cingulate cortex modulates the efficiency of amygdala-dependent fear learning. Biological psychiatry. 2008;63:821–831. doi: 10.1016/j.biopsych.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brooks J, Tracey I. From nociception to pain perception: imaging the spinal and supraspinal pathways. Journal of anatomy. 2005;207:19–33. doi: 10.1111/j.1469-7580.2005.00428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown JE, Chatterjee N, Younger J, Mackey S. Towards a physiology-based measure of pain: patterns of human brain activity distinguish painful from non-painful thermal stimulation. PloS one. 2011;6:e24124. doi: 10.1371/journal.pone.0024124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nature reviews. Neuroscience. 2013;14:502–511. doi: 10.1038/nrn3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung SK, Price DD, Verne GN, Robinson ME. Revelation of a personal placebo response: its effects on mood, attitudes and future placebo responding. Pain. 2007;132:281–288. doi: 10.1016/j.pain.2007.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coghill RC, Gilron I, Iadarola MJ. Hemispheric lateralization of somatosensory processing. Journal of neurophysiology. 2001;85:2602–2612. doi: 10.1152/jn.2001.85.6.2602. [DOI] [PubMed] [Google Scholar]
- 9.Committee on Advancing Pain Research C, and Education; Institute of, Medicine. The National Academies Collection: Reports. National Institutes of Health; Washington (DC): 2011. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. [Google Scholar]
- 10.Craggs JG, Price DD, Perlstein WM, Verne GN, Robinson ME. The dynamic mechanisms of placebo induced analgesia: Evidence of sustained and transient regional involvement. Pain. 2008;139:660–669. doi: 10.1016/j.pain.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Craggs JG, Price DD, Verne GN, Perlstein WM, Robinson MM. Functional brain interactions that serve cognitive-affective processing during pain and placebo analgesia. NeuroImage. 2007;38:720–729. doi: 10.1016/j.neuroimage.2007.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Craggs JG, Staud R, Robinson ME, Perlstein WM, Price DD. Effective connectivity among brain regions associated with slow temporal summation of C-fiber-evoked pain in fibromyalgia patients and healthy controls. The journal of pain : official journal of the American Pain Society. 2012;13:390–400. doi: 10.1016/j.jpain.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.David O, Guillemain I, Saillet S, Reyt S, Deransart C, Segebarth C, Depaulis A. Identifying neural drivers with functional MRI: an electrophysiological validation. PLoS biology. 2008;6:2683–2697. doi: 10.1371/journal.pbio.0060315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elsenbruch S, Kotsis V, Benson S, Rosenberger C, Reidick D, Schedlowski M, Bingel U, Theysohn N, Forsting M, Gizewski ER. Neural mechanisms mediating the effects of expectation in visceral placebo analgesia: an fMRI study in healthy placebo responders and nonresponders. Pain. 2012;153:382–390. doi: 10.1016/j.pain.2011.10.036. [DOI] [PubMed] [Google Scholar]
- 15.Fardo F, Spironelli C, Angrilli A. Horizontal body position reduces cortical pain-related processing: evidence from late ERPs. PloS one. 2013;8:e81964. doi: 10.1371/journal.pone.0081964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faria V, Fredrikson M, Furmark T. Imaging the placebo response: A neurofunctional review. Eur Neuropsychopharm. 2008;18:473–485. doi: 10.1016/j.euroneuro.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Friston K. Dynamic causal modelling of brain responses. J Psychophysiol. 2006;20:322–322. [Google Scholar]
- 18.Friston KJ, Harrison L, Penny W. Dynamic causal modelling. NeuroImage. 2003;19:1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- 19.Gaskin DJ, Richard P. The economic costs of pain in the United States. The journal of pain : official journal of the American Pain Society. 2012;13:715–724. doi: 10.1016/j.jpain.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 20.Geers AL, Wellman JA, Fowler SL, Helfer SG, France CR. Dispositional optimism predicts placebo analgesia. The journal of pain : official journal of the American Pain Society. 2010;11:1165–1171. doi: 10.1016/j.jpain.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Groenewegen HJ. Organization of the afferent connections of the mediodorsal thalamic nucleus in the rat, related to the mediodorsal-prefrontal topography. Neuroscience. 1988;24:379–431. doi: 10.1016/0306-4522(88)90339-9. [DOI] [PubMed] [Google Scholar]
- 22.Kong J, Gollub RL, Rosman IS, Webb JM, Vangel MG, Kirsch I, Kaptchuk TJ. Brain activity associated with expectancy-enhanced placebo analgesia as measured by functional magnetic resonance Imaging. Journal of Neuroscience. 2006;26:381–388. doi: 10.1523/JNEUROSCI.3556-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lieberman MD, Jarcho JM, Berman S, Naliboff BD, Suyenobu BY, Mandelkern M, Mayer EA. The neural correlates of placebo effects: a disruption account. NeuroImage. 2004;22:447–455. doi: 10.1016/j.neuroimage.2004.01.037. [DOI] [PubMed] [Google Scholar]
- 24.Pecina M, Stohler CS, Zubieta JK. Neurobiology of placebo effects: expectations or learning? Social cognitive and affective neuroscience. 2013 doi: 10.1093/scan/nst079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Penny WD, Stephan KE, Mechelli A, Friston KJ. Modelling functional integration: a comparison of structural equation and dynamic causal models. NeuroImage. 2004;23:S264–S274. doi: 10.1016/j.neuroimage.2004.07.041. [DOI] [PubMed] [Google Scholar]
- 26.Pitman RK, van der Kolk BA, Orr SP, Greenberg MS. Naloxone-reversible analgesic response to combat-related stimuli in posttraumatic stress disorder. A pilot study. Archives of general psychiatry. 1990;47:541–544. doi: 10.1001/archpsyc.1990.01810180041007. [DOI] [PubMed] [Google Scholar]
- 27.Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–1772. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- 28.Price DD. Central neural mechanisms that interrelate sensory and affective dimensions of pain. Molecular interventions. 2002;2:392–403. 339. doi: 10.1124/mi.2.6.392. [DOI] [PubMed] [Google Scholar]
- 29.Price DD, Barrell JJ. Mechanisms of analgesia produced by hypnosis and placebo suggestions. Progress in brain research. 2000;122:255–271. doi: 10.1016/s0079-6123(08)62144-5. [DOI] [PubMed] [Google Scholar]
- 30.Price DD, Craggs J, Verne GN, Perlstein WM, Robinson ME. Placebo analgesia is accompanied by large reductions in pain-related brain activity in irritable bowel syndrome patients. Pain. 2007;127:63–72. doi: 10.1016/j.pain.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Price DD, Fillingim RB, Robinson ME. Placebo analgesia: friend or foe? Current rheumatology reports. 2006;8:418–424. doi: 10.1007/s11926-006-0035-1. [DOI] [PubMed] [Google Scholar]
- 32.Price DD, Finniss DG, Benedetti F. A comprehensive review of the placebo effect: recent advances and current thought. Annual review of psychology. 2008;59:565–590. doi: 10.1146/annurev.psych.59.113006.095941. [DOI] [PubMed] [Google Scholar]
- 33.Rainville P. Brain mechanisms of pain affect and pain modulation. Current opinion in neurobiology. 2002;12:195–204. doi: 10.1016/s0959-4388(02)00313-6. [DOI] [PubMed] [Google Scholar]
- 34.Rigoux L, Stephan KE, Friston KJ, Daunizeau J. Bayesian model selection for group studies - Revisited. NeuroImage. 2013 doi: 10.1016/j.neuroimage.2013.08.065. [DOI] [PubMed] [Google Scholar]
- 35.Stephan KE, Marshall JC, Penny WD, Friston KJ, Fink GR. Interhemispheric integration of visual processing during task-driven lateralization. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:3512–3522. doi: 10.1523/JNEUROSCI.4766-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stephan KE, Penny WD, Daunizeau J, Moran RJ, Friston KJ. Bayesian model selection for group studies. NeuroImage. 2009;46:1004–1017. doi: 10.1016/j.neuroimage.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stewart-Williams S, Podd J. The placebo effect: dissolving the expectancy versus conditioning debate. Psychological bulletin. 2004;130:324–340. doi: 10.1037/0033-2909.130.2.324. [DOI] [PubMed] [Google Scholar]
- 38.Symonds LL, Gordon NS, Bixby JC, Mande MM. Right-lateralized pain processing in the human cortex: an FMRI study. Journal of neurophysiology. 2006;95:3823–3830. doi: 10.1152/jn.01162.2005. [DOI] [PubMed] [Google Scholar]
- 39.Voudouris NJ, Peck CL, Coleman G. Conditioned placebo responses. Journal of personality and social psychology. 1985;48:47–53. doi: 10.1037//0022-3514.48.1.47. [DOI] [PubMed] [Google Scholar]
- 40.Voudouris NJ, Peck CL, Coleman G. The role of conditioning and verbal expectancy in the placebo response. Pain. 1990;43:121–128. doi: 10.1016/0304-3959(90)90057-K. [DOI] [PubMed] [Google Scholar]
- 41.Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303:1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- 42.Wiech K, Ploner M, Tracey I. Neurocognitive aspects of pain perception. Trends in cognitive sciences. 2008;12:306–313. doi: 10.1016/j.tics.2008.05.005. [DOI] [PubMed] [Google Scholar]