Abstract
Persons aged over 65 years account for over 75% of healthcare expenditures and deaths attributable to cardiovascular disease (CVD). Accordingly, reducing CVD risk among older adults is an important public health priority. Functional status, determined by measures of physical performance, is an important predictor of cardiovascular outcomes in older adults and declines more rapidly in seniors with hypertension. To date, physical exercise is the primary strategy for attenuating declines in functional status. Yet despite the general benefits of training, exercise alone appears to be insufficient for preventing this decline. Thus, alternative or adjuvant strategies are needed to preserve functional status among seniors with hypertension. Prior data suggest that angiotensin converting enzyme inhibitors (ACEi) may be efficacious in enhancing exercise-derived improvements in functional status yet this hypothesis has not been tested in a randomized controlled trial. The objective of this randomized, double-masked pilot trial is to gather preliminary efficacy and safety data necessary for conducting a full-scale trial to test this hypothesis. Sedentary men and women ≥ 65 years of age with functional limitations and hypertension are being recruited into this 24 week intervention study. Participants are randomly assigned to one of three conditions: (1) ACEi plus exercise training, (2) thiazide diuretic plus exercise training, or (3) AT1 receptor antagonist plus exercise training. The primary outcome is change in walking speed and secondary outcomes consist of other indices of CV risk including exercise capacity, body composition, as well as circulating indices of metabolism, inflammation and oxidative stress.
Keywords: Physical Activity, Exercise, Hypertension, Aging, Antihypertensive, ACE
1. INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of death in the United States, and persons over 65 years of age account for over 80% of deaths attributable to CVD.[1] CVD is also the second leading cause of disability among older adults,[2] an important contributor to the loss of independence and subsequent institutionalization. As a consequence, older adults account for nearly three-quarters of health care expenditures related to CVD.[3] Importantly, these expenditures are expected to increase dramatically in coming years as the number of older adults is expected to double to approximately 80 million in the next three decades.[4] Thus the clinical and economic costs related to CVD are expected to increase dramatically in coming years. Consequently, the identification of interventions capable of reducing CVD risk among older adults is an important goal with dramatic public health implications.
Functional status, determined by measures of physical performance, is an important predictor of cardiovascular outcomes in older adults. Functionally-limited older adults, i.e. those with physical limitations which limit their ability to perform daily activities (e.g. low muscle strength, impaired gait), experience more cardiovascular events, have a higher risk of undergoing cardiac surgery and higher risk of cardiovascular-related death than higher-functioning peers.[5-10] Declines in self-paced walking speed, a recommended indicator of health and well-being among seniors,[11, 12] are also associated with incident stroke,[6] adverse outcomes following cardiac surgery,[8] as well as cardiovascular and all-cause mortality.[5, 10, 13] Compared to normotensive counterparts, older persons with hypertension experience accelerated declines in functional status.[14-17] Among older persons enrolled in the Charleston Heart Study, higher systolic blood pressure was associated with greater declines in functional outcomes and seniors with hypertension were at increased risk of developing new disability.[14] Hypertension was also associated with accelerated declines in walking speed among seniors participating in the Cardiovascular Health and Three-City studies.[15, 17] Thus, older adults with hypertension represent a particularly high risk group for functional decline and associated cardiovascular events.
Accumulating evidence suggests that the choice of antihypertensive medication may play an important role in the rate of functional decline among older adults. Epidemiologic evidence from the Women's Health and Aging Study (WHAS) and the Systematic Assessment of Geriatric drug use via Epidemiology (SAGE) study indicated that, compared to non-users, seniors using angiotensin converting enzyme (ACE) inhibitors displayed attenuated declines in walking speed and limitations in Activities of Daily Living (ADL).[18, 19] However, the results of subsequent randomized controlled trials (RCTs) were mixed, with studies reporting that ACE inhibitors may [20, 21] or may not [22, 23] improve functional status. While it remains possible that the efficacy of ACE inhibitors as a therapeutic for physical function may vary by drug and/or patient population, recent evidence from our group suggests that the greatest benefit of ACEi may be observed when combined with regular physical exercise[24, 25] though a conflicting report exists.[26] This study was designed to begin to test our central hypothesis among older adults with hypertension that – compared to other first-line antihypertensive therapies – ACE inhibitors improve functional status and other cardiovascular risk factors when combined with regular exercise. The objective of this randomized pilot trial is to refine and finalize elements critical to conducting a future, fully-powered randomized, controlled trial to definitively test our central hypothesis.
2. STUDY DESIGN/METHODS
2.1 Overview
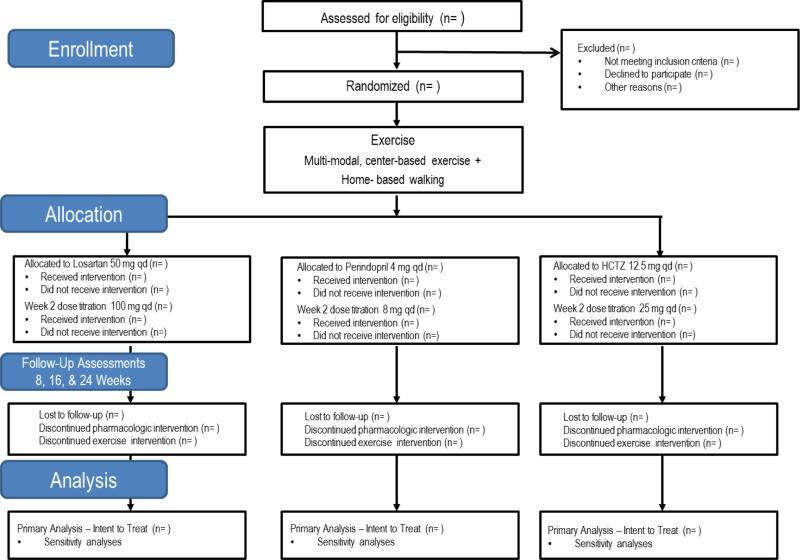
The study is a three-arm randomized, double-masked pilot trial among older, hypertensive men and women with functional limitations. All participants are assigned to participate in a structured exercise intervention while also being randomly assigned to one of three first-line antihypertensive medications for blood pressure management at target pressures of < 140 mm Hg (systolic) and < 90 mm Hg (diastolic). Study medications are ACE inhibitor perindopril, the AT1 receptor antagonist losartan, and the thiazide diuretic hydrochlorothiazide. Participants are followed for a period of 24 weeks to evaluate changes in study outcomes indicative of functional status and cardiovascular risk. Study assessments are conducted by blinded research staff during clinic visits at baseline, as well as 2-, 8-, 16-, and 24-weeks post-randomization (Figure 1). Randomization and dispensing of study medications are conducted by an academic health center investigational pharmacy. Participant safety is overseen by a comprehensive study team – including the principal investigator, study cardiologist, study geriatrician, study staff, and an appointed Data and Safety Monitoring Board. The study is registered at www.clinicaltrials.gov prior to participant recruitment (NCT01891513) and all participants provide written informed consent based on documents approved by a university Institutional Review Board.
Figure 1.
Overview of study design according to CONSORT format.
2.2 Participants
The study team is recruiting up to 72 (n = 24/group) functionally-limited older men and women with hypertension. Eligible participants are community-dwelling persons ≥ 65 years of age with a sedentary lifestyle, objective signs of functional limitations, as well as untreated hypertension or known uncomplicated hypertension. Persons with a primary indication for ACE inhibitor use, congestive heart failure (CHF), or coronary heart disease (CAD) are excluded. Persons with a known hypersensitivity to ACE inhibitors, with absolute contraindication(s) to exercise training according to American College of Sports Medicine guidelines[27] (e.g. unstable angina, uncontrolled cardiac dysrhythmias causing symptoms or hemodynamic compromise, symptomatic severe aortic stenosis, uncontrolled symptomatic heart failure) or with other medical conditions precluding safe participation are excluded. Participants are being recruited from Alachua County, FL and surrounding areas using multiple recruitment strategies including mass mailings (i.e. postcards), flyers and local media (i.e. newspaper) advertisements, clinic referrals, and existing research registries. Incentive for participation is provided at the completion of each assessment visit in the form of gift cards (value = 30 USD) for the time and effort required for participation.
2.3 Screening and randomization
Interested individuals initially complete a telephone pre-screening prior to an in-person screening visit. Following the provision of informed consent, initial screening procedures include a review of medical history and medication use, a physical exam including blood pressure and measurement and performance of an electrocardiogram (ECG), the Community Health Activities Model Program for Seniors (CHAMPS) physical activity questionnaire[28] to ensure participants are sedentary (≤ 150 minutes moderate-intensity physical activity/week), the Mini-mental State Exam[29] to ensure participants have normal cognitive function (MMSE ≥ 24), as well as the 400 m walk test[30] and Short Physical Performance Battery[31] (walking speed < 1.2 m/sec or SPPB ≤ 10) as evidence of functional limitations. Participants also provide a urine specimen and a fasted blood sample to evaluate study entry criteria related to kidney and liver function.
If all study entry criteria are met, participants return to the clinic for baseline assessments prior to randomization. Block randomization, stratified by gender, is used to assign subjects to intervention arms (1:1:1 ratio) to ensure approximately equal accrual to each intervention group throughout the study. The randomization scheme was performed by an independent biostatistician using a random number generator program specifically designed for this purpose (Mersenne-Twister) and sent directly to the study pharmacy. Treatment is assigned by the study pharmacy according to the randomization list in order that prescription requests are received by pharmacy. The executed randomization scheme is maintained in the study pharmacy concealed to investigators and study staff until the completion of the study. At the end of the study, the study team will be provided with a full executed copy of the randomization schedule. Each study drug is provided in identical capsules and packaging. Because study medications may have opposing effects on circulating mineral concentrations, any potentially abnormal clinical chemistry result is communicated directly from the study coordinator to the study physician(s) to prevent unmasking of the PI.
2.4 Assessments
The primary outcome of interest for the study will be the change in usual-pace walking speed, measured over a 4 m course. Secondary outcomes include exercise capacity, total body fat mass and fat-free mass, as well as circulating indices of cardiovascular risk. Extended details on the definition and measurement of primary and secondary outcomes are provided below. A timetable of events is also provided (Table 1) to indicate study visits at which each outcome is assessed.
Table 1.
Data collection summary by study visit
| Study Phase | Pre-randomization | Randomization | ||||
|---|---|---|---|---|---|---|
| Visit description (FU=follow-up, CO= close-out) | Screen | Baseline | Dose | FU | FU | CO |
| Visit number | 1 | 2 | 3 | 4 | 5 | 6 |
| Week number | −2 | 0 | 2 | 8 | 16 | 24 |
| Month number | 0 | 1 | 2 | 4 | 6 | |
| Prepare for visit, schedule, track | x | x | x | x | x | x |
| Informed consent, review inclusion/exclusion criteria | x | |||||
| Personal interview, medical history, medication use | x | |||||
| Office blood pressure (sitting + standing), vital signs | x | x | x | x | x | x |
| Physical exam, height/weight, ECG | x | |||||
| 400 m walk, Short Physical Performance Battery | x | |||||
| Blood and urine for clinical labs | x | x | x | x | x | x |
| Randomization | x | |||||
| 4 m walk, 6 minute walk | x | x | x | x | ||
| Blood for study assays | x | x | x | x | ||
| DEXA | x | x | ||||
| Dispense study medications | x | x | x | x | x | |
| Collect home BP monitor data | x | x | x | x | ||
| Collect home physical activity data | x | x | x | x | ||
| Assess adverse experiences | x | x | x | x | ||
| Assess compliance pill count | x | x | x | x | ||
2.4.1Walking speed
A decline in one's usual-pace walking speed is associated with increased risk of numerous cardiovascular outcomes among older adults[5, 6, 8, 10] and has been proposed as a simple, cost-effective cardiovascular screening tool.[32] Participants are instructed to stand with both feet touching the starting line and to start walking after a specific verbal command. Timing begins when the command is given, and the time needed to complete the entire distance is recorded. The test is performed twice and the faster of two walks is used. The reliability of the 4 m walk test is excellent – with an intraclass correlation coefficient (ICC) above 0.9.[33]
2.4.2 Exercise Capacity
Exercise capacity is assessed using 6-min walk test, a safe and reliable test of aerobic endurance in older persons and those with cardiovascular conditions.[34, 35] This test has strong reproducibility, with intra-subject coefficients of variation averaging < 10%, and has a modest correlation with peak VO2.[36] Participants are asked to walk as far and fast as possible for 6-min on a 100 ft course, and the total distance walked is recorded. Participants are allowed to use their customary walking aids during the test and to rest if required.
2.4.3 Body composition
Body composition is measured using dual x-ray absorptiometry (DEXA). Fat mass and fat-free mass (FFM) are assessed using a fan-bean densitometer (Hologic; Bedford, MA). Body composition analysis is performed in lower and upper body compartments using Lunar software. Values of fat-free mass are calculated after removing mass due to bone mineral content (BMC) using the equation, (FFM+BMC)-BMC=FFM.
2.4.4 Circulating indices of cardiovascular risk
In addition to measurement of clinical safety parameters, fasting blood samples (serum or plasma as appropriate) are evaluated for blood lipids, glucose, and hemoglobin A(1c) levels. Samples will also be assayed for prominent markers of inflammation (TNF-α, IL-6, VCAM-1, E-selectin) and oxidative stress, including oxidized LDL and myeloperoxidase (MPO) using previously-utilized, commercially-available ELISA kits.
2.5 Pharmacologic intervention
Eligible participants are randomly assigned to one of three study antihypertensive medications (perindopril, losartan, or HCTZ). Participants assigned to perindopril are given an initial dose of 4 mg/day for two weeks after which, no adverse reactions are observed, the dose is increased to 8 mg/day. If the 4 mg/day dose is not tolerated due to issues such as hypotension, cough, or hyperkalemia, the participant is given the lower tolerated dose. The same general scheme will be utilized for the losartan group (dose titrated from 50 mg/day to 100 mg/day) and the HCTZ group (12.5 mg/day to 25 mg/day). Per protocol, doses may also be adjusted by the study cardiologist to safely control blood pressure in the target range. Study drugs are prepared by the study investigational pharmacy and dispensed upon provision of the requisite information including a copy of the signed informed consent form. The tablets of active drugs are loaded into identical opaque gelatin capsules with methylcellulose and participants are instructed not to tamper with or disassemble the capsules. All unused study drug is returned to the investigational pharmacy for tracking purposes.
2.6 Exercise intervention
In addition to taking the study drug, all participants participate in structured, exercise intervention involving both center-based and home-based components. During the first 12 weeks, participants are asked to participate in center-based, multi-modal exercise intervention on three days/week and also to engage in 30 minutes of home-based walking on two additional days. During the latter 12 weeks, center-based sessions are reduced to twice-weekly and home-based walking increased to three days/week. This intervention was designed to meet the exercise and physical activity guidelines for older adults established by the American College of Sports Medicine (ACSM) and American Heart Association[37-39] as well as the Physical Activity Guidelines for Americans established by the Department of Health and Human Services[40] and ACSM's exercise guidelines for persons with hypertension.[41]
Each center-based session begins with a brief warm-up followed by 30 minutes of moderate-intensity walking. Coupled with home-based walking, this program was designed to achieve a total of 150 min/week of endurance activity according to the established guidelines. Flexibility and balance exercises are performed at the end of the session to promote cool-down. After 12 weeks, upper- and lower-body resistance exercises are added to the center-based sessions. Initiation of resistance training during the second half of the trial will provide an opportunity to investigate any potential differences in the influences of aerobic and anaerobic (i.e. resistance training) exercise on study outcomes.
According to ACSM/AHA guidelines,[37] exercise intensity is monitored using a subjective 0-10 scale for physical exertion (Borg CR10 scale).[42] For endurance activity, participants are initially instructed to walk at a moderate intensity, equivalent to a 5-6 on the CR10 scale. They are encouraged to, as possible, incorporate brief periods of vigorous walking (7-8 on CR10 scale) with a target goal of achieving at least 10 minutes of vigorous walking per session. Participants wear a hear rate monitor (Polar FT2, Lake Success, NY) to measure pulse during center-based walking sessions to promote safety and to help to guide participants with gauging the accuracy of their subjective ratings. Participants are encouraged to perform home-based walking at a moderate intensity throughout the duration of the study based on the CR10 scale.
Resistance training is performed using standard isotonic resistance training equipment (Life Fitness, Schiller Park, IL). Resistance exercise is intended to be performed between moderate (5-6) and vigorous (7-8) intensity throughout the intervention, according to guidelines.[37] Participants perform both lower- and upper-body exercises with the primary emphasis being on the lower extremity. Exercises vary by session and include leg press, leg extension, leg curl, chest press, overhead press, arm curls, and calf flexion. Participants perform two sets of each exercise and are encouraged to perform between 8-10 repetitions per set. Participants initially perform exercises at 75% of their 1 repetition maximum (1RM). The load for a given exercise is increased by 10% for the next session when participants perform ≥ 12 repetitions on both sets. Study staff track participant exercise volume to promote goal-setting and encourage progression.
2.7 Adherence to interventions
To aid adherence to the pharmacologic intervention, study medication is provided in blister packs labeled with the day of the week. These packs serve as a “pillbox” equivalent and aid the participant in remembering if they took their dose. Participants are asked to bring their blister packs to study visits, from which a pill count is made. This method of pill counting is a validated method of compliance tracking.[43] Participants with poor adherence (e.g. < 70%) are provided behavioral counseling on improved adherence strategies. Collection of this data will also allow for inclusion of adherence data in analysis models.
Attendance to the exercise intervention sessions is carefully documented and missed sessions are monitored. Bi-weekly study meetings enable staff and investigators to discuss potential problems and solutions to participation barriers. Adherence to the home-based intervention is tracked weekly by the use of written logs to record home-based exercise activity. Interventionists provide frequent instruction regarding the importance of proper completion of the logs. Home log completion rates are reviewed frequently to determine if behavioral counseling strategies are warranted to improve adherence. Home-based physical activity will also be measured objectively using wearable physical activity monitors (Body Media, Pittsburgh, PA) for one week following each assessment visit.
2.7 Safety
Numerous safety procedures are in place to ensure participant safety. For instance, interest individuals are advised during the telephone pre-screening to consult with their primary care physician prior to participation. Potential adverse events for study related activities and interventions are explained to each participant by trained study personnel during the informed consent process. Participants are encouraged to notify study staff immediately if they have any adverse experiences that could be related to the study drug or their blood pressure management. The dose titration and physician monitoring of blood pressure and clinical chemistries are in place to minimize adverse responses to the study drugs. Abnormal symptoms or vital signs detected during intervention visits are discussed with the study physician(s) in real time, who dictate the appropriate course of action. If study treatments according to the scheduled protocol do not manage hypertension safely, the study cardiologist has full discretion to manage the antihypertensive regimen to ensure safety. Notably, communication with the participant's physician is an important component of maintaining safety for the trial. Upon enrollment, participants are provided with a laminated “wallet-card” which provides details about the study for communication with their primary care physician. The study teams also provides written communication to the participant's primary care physician upon initial enrollment in the trial, as well as of all changes in medication prescription during their participation in the trial (e.g. drug initiation, dose escalation, study withdrawal/close-out). For per protocol changes, physicians are informed that participants are being moved to “Perindopril (dose), Losartan (dose), or HCTZ (dose)” to maintain blinding. For off-protocol changes, they are informed of the actual drug and dose utilized.
Participants are also provided a home blood pressure monitor and encouraged to conduct daily blood pressure monitoring. The provided home BP monitor meets the standards of the British Society of Hypertension and the Association for the Advancement of the Medical Instrumentation.[44] Participants are instructed to take their home BP twice daily, on rising from bed and before retiring and to report any SBP > 180 or < 90 mmHg or DBP > 100 or < 60 mmHg to the study coordinator immediately. Blood pressure is also monitored at each assessment visit in the clinic. Participants are withdrawn from the pharmacologic intervention at the discretion of the study cardiologist for any DBP > 100 mmHg or SBP > 180 mmHg, and will be automatically removed from intervention for any weekly average home BP above these thresholds. The cardiologist may decide to withdraw treatment from subjects with slightly lower BP levels than these cut points, but for whom there is any medically-relevant concern that would preclude continued participation. Withdrawn or completed participants are returned to their standard antihypertensive therapy under the supervision of their physician or referred for care.
2.8 Statistical analyses
The primary statistical analyses will estimate an intention to treat effect, the causal effect of being randomized to the treatment arm. Pattern mixture models will be used to determine the relative effect of the intervention on study outcomes in the presence of attrition.[45] Differences in mean outcome measures between intervention groups will be estimated with visit number and intervention group in the model. Hypothesis tests for intervention effects at assessment visits will be performed using contrasts of the 8, 16, and 24 week intervention means. Overall comparisons between groups for the outcome measure across follow-up visits will be obtained using a contrast to compare average effects. Sensitivity analyses will be performed to account for potentially non-ignorable missing data due to dropout[46] as well as the possible impact of other potentially influential factors. Caution will be taken in the interpretation of hypothesis tests as power is limited and the relatively small sample size may create an imbalance in pre-randomization covariates.[47] This sample size will, however, provide for nominal estimation (using a 95% confidence interval) of the mean changes in dependent variables within each arms of the study which will facilitate planning of a larger, fully-powered trial.
DISCUSSION
Functional status is a key clinical indicator of cardiovascular risk in the elderly as seniors with compromised function (e.g. impaired gait) experience more cardiovascular events, have a higher risk of undergoing cardiac surgery and higher risk of cardiovascular-related death than higher-functioning peers.[5-10] Compared to normotensive counterparts, older persons with hypertension experience accelerated functional declines.[14-17] Physical exercise is currently the standard intervention for improving functional status among older adults. However, despite the global benefits of exercise, many individuals do not experience significant improvements in functional status[25, 48] – potentially limiting the cardiovascular benefits of exercise among older persons.[49] Consequently, alternative or adjuvant strategies are needed optimize the functional benefits of exercise training for older adults at highest risk of functional decline, including those with hypertension.
Our long-term goal is to develop interventions that optimize the efficacy of exercise as a strategy to reduce cardiovascular risk among older adults with hypertension. The present report provides a summary of an ongoing pilot study designed to provide preliminary evidence necessary to design a trial to test the hypothesis that when combined with regular exercise, ACE inhibitors are superior to other first-line antihypertensive therapies for improving functional status and other cardiovascular risk factors among older adults with hypertension.
We previously reported – using retrospective analyses of a multi-site trial of exercise for older adults – that older adults who took ACE inhibitors had greater exercise-derived improvements in functional status than non-users.[25] Improvements in functional measures observed in response to exercise were driven largely by persons using ACE inhibitors – despite the fact that this group accounted for approximately one quarter of the study population. Indeed, persons not using ACE inhibitors displayed relatively poor functional adaptations to exercise. These findings were in line with evidence from aged rats indicating that combining exercise with systemic ACE inhibition improved exercise tolerance significantly more than exercise alone.[24, 50] Biologic rationale for such as potential interaction stems from evidence that some therapeutic effects of ACE inhibitors may be mediated by mechanisms that are independent of blood pressure regulation. For instance, prior work from members of our group has demonstrated effects of ACE inhibitors in endocrine signaling, regulation to tissue-specific oxidative stress and inflammation, and in the regulation of body composition.[51-53] Clinically, several large-scale trials have reported – compared to other antihypertensive drug classes – beneficial effects of ACE inhibitors on cardiovascular outcomes that are unrelated to differences in blood pressure.[54-58] This evidence suggests that, compared to other antihypertensive medications, ACE inhibitors may have beneficial effects on the functional status and overall cardiovascular risk of older adults with hypertension when utilized in conjunction with exercise. Such a finding, if confirmed in a fully-powered randomized trial, would have critical implications for the prescription of antihypertensive medications for managing the blood pressure of older adults.
In addition to gathering preliminary data, a key objective of the trial is also to refine the study protocol and procedures prior to conducting a fully-powered trial. For instance, a key aspect of the pilot study is to identify the most appropriate type of exercise to utilize (i.e. aerobic vs. resistance) for a fully-powered trial. Our prior findings utilized multimodal (i.e. aerobic and resistance combined) exercise, but evidence exists to suggest that the biological pathways influenced by and physiologic effects of ACE inhibitors and AT1 receptor antagonists are not identical (reviewed in[59]) – making it important to evaluate the influence of exercise modalities which stress differing energetic systems. Additionally it should be noted that the study protocol may be modified during the trial to optimize study procedures (recruitment, safety, etc) based upon information obtained during the course of the trial. Due to these factors and other limitations (e.g. relatively small sample size; single site design), it will be important not to over-interpret the results of this pilot study.
Still, the study will provide critical data necessary for designing a fully-powered trial which could have important health implications for a prevalent and clinically-relevant population. The study described here will provide important feasibility and early efficacy data regarding the benefits of physical exercise, in combination with each of three first-line antihypertensive medications, on clinically-relevant cardiovascular risk factors among older adults with hypertension. These data will inform the efficient and definitive full-scale trial to determine if, compared to other first-line therapies, ACE inhibitors potentiate the beneficial effects of exercise on these risk factors. If our hypothesis is correct, the findings could provide evidence suggesting the need to augment standard practice for prescribing both antihypertensive medication and physical exercise among hypertensive older adults.
ACKNOWLEDGMENTS
We would like to thank all study participants and staff devoting their time and effort to the study. The study is funded by a grant from the American Heart Association (13SDG17080033) with support from the University of Florida Claude D. Pepper Older Americans Independence Center (2P30AG028740).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yazdanyar A, Newman AB. The burden of cardiovascular disease in the elderly: morbidity, mortality, and costs. Clin Geriatr Med. 2009;25:563, 77, vii. doi: 10.1016/j.cger.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hodgson TA, Cohen AJ. Medical care expenditures for selected circulatory diseases: opportunities for reducing national health expenditures. Med Care. 1999;37:994–1012. doi: 10.1097/00005650-199910000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Federal Interagency Forum on Aging-Related Statistics, editor. Statistical Data of Older Americans 2009. Oct 14, 2009. Accessed.
- 5.Newman AB, Simonsick EM, Naydeck BL, Boudreau RM, Kritchevsky SB, Nevitt MC, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295:2018–26. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 6.McGinn AP, Kaplan RC, Verghese J, Rosenbaum DM, Psaty BM, Baird AE, et al. Walking speed and risk of incident ischemic stroke among postmenopausal women. Stroke. 2008;39:1233–9. doi: 10.1161/STROKEAHA.107.500850. [DOI] [PubMed] [Google Scholar]
- 7.Castaneda-Sceppa C, Price LL, Noel SE, Bassett Midle J, Falcon LM, Tucker KL. Physical function and health status in aging puerto rican adults: the Boston puerto rican health study. J Aging Health. 2010;22:653–72. doi: 10.1177/0898264310366738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Afilalo J, Eisenberg MJ, Morin JF, Bergman H, Monette J, Noiseux N, et al. Gait speed as an incremental predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. J Am Coll Cardiol. 2010;56:1668–76. doi: 10.1016/j.jacc.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 9.Shaw LJ, Olson MB, Kip K, Kelsey SF, Johnson BD, Mark DB, et al. The value of estimated functional capacity in estimating outcome: results from the NHBLI-Sponsored Women's Ischemia Syndrome Evaluation (WISE) Study. J Am Coll Cardiol. 2006;47:S36–43. doi: 10.1016/j.jacc.2005.03.080. [DOI] [PubMed] [Google Scholar]
- 10.Dumurgier J, Elbaz A, Ducimetiere P, Tavernier B, Alperovitch A, Tzourio C. Slow walking speed and cardiovascular death in well functioning older adults: prospective cohort study. BMJ. 2009;339:b4460. doi: 10.1136/bmj.b4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abellan van Kan G, Rolland Y, Andrieu S, Bauer J, Beauchet O, Bonnefoy M, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13:881–9. doi: 10.1007/s12603-009-0246-z. [DOI] [PubMed] [Google Scholar]
- 12.Hall WJ. Update in geriatrics. Ann Intern Med. 2006;145:538–43. doi: 10.7326/0003-4819-145-7-200610030-00012. [DOI] [PubMed] [Google Scholar]
- 13.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–8. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hajjar I, Lackland DT, Cupples LA, Lipsitz LA. Association between concurrent and remote blood pressure and disability in older adults. Hypertension. 2007;50:1026–32. doi: 10.1161/HYPERTENSIONAHA.107.097667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosano C, Longstreth WT, Jr, Boudreau R, Taylor CA, Du Y, Kuller LH, et al. High Blood Pressure Accelerates Gait Slowing in Well-Functioning Older Adults over 18-Years of Follow-Up. J Am Geriatr Soc. 2011;59:390–7. doi: 10.1111/j.1532-5415.2010.03282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balzi D, Lauretani F, Barchielli A, Ferrucci L, Bandinelli S, Buiatti E, et al. Risk factors for disability in older persons over 3-year follow-up. Age Ageing. 2010;39:92–8. doi: 10.1093/ageing/afp209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dumurgier J, Elbaz A, Dufouil C, Tavernier B, Tzourio C. Hypertension and lower walking speed in the elderly: the Three-City Study. J Hypertens. 2010;28:1506–14. doi: 10.1097/HJH.0b013e328338bbec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cranney A. Is there a new role for angiotensin-converting-enzyme inhibitors in elderly patients?. CMAJ. 2007;177:891–2. doi: 10.1503/cmaj.071062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sumukadas D, Struthers AD, McMurdo ME. Sarcopenia--a potential target for Angiotensin-converting enzyme inhibition?. Gerontology. 2006;52:237–42. doi: 10.1159/000093656. [DOI] [PubMed] [Google Scholar]
- 20.Hutcheon SD, Gillespie ND, Crombie IK, Struthers AD, McMurdo ME. Perindopril improves six minute walking distance in older patients with left ventricular systolic dysfunction: a randomised double blind placebo controlled trial. Heart. 2002;88:373–7. doi: 10.1136/heart.88.4.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sumukadas D, Witham MD, Struthers AD, McMurdo ME. Effect of perindopril on physical function in elderly people with functional impairment: a randomized controlled trial. CMAJ. 2007;177:867–74. doi: 10.1503/cmaj.061339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cesari M, Pedone C, Incalzi RA, Pahor M. ACE-inhibition and physical function: results from the Trial of Angiotensin-Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors (TRAIN) study. J Am Med Dir Assoc. 2010;11:26–32. doi: 10.1016/j.jamda.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zi M, Carmichael N, Lye M. The effect of quinapril on functional status of elderly patients with diastolic heart failure. Cardiovasc Drugs Ther. 2003;17:133–9. doi: 10.1023/a:1025387702212. [DOI] [PubMed] [Google Scholar]
- 24.Carter CS, Marzetti E, Leeuwenburgh C, Manini T, Foster TC, Groban L, et al. Usefulness of preclinical models for assessing the efficacy of late-life interventions for sarcopenia. J Gerontol A Biol Sci Med Sci. 2012;67:17–27. doi: 10.1093/gerona/glr042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buford TW, Manini TM, Hsu FC, Cesari M, Anton SD, Nayfield S, et al. Angiotensin-converting enzyme inhibitor use by older adults is associated with greater functional responses to exercise. J Am Geriatr Soc. 2012;60:1244–52. doi: 10.1111/j.1532-5415.2012.04045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sumukadas D, Band M, Miller S, Cvoro V, Witham M, Struthers A, et al. Do ACE inhibitors improve the response to exercise training in functionally impaired older adults? A randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2014;69:736–43. doi: 10.1093/gerona/glt142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American College of Sports Medicine American College of Sports Medicine Guidelines for Exercise Testing and Prescription. 7th ed 2006.
- 28.Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc. 2001;33:1126–41. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 30.Chang M, Cohen-Mansfield J, Ferrucci L, Leveille S, Volpato S, de Rekeneire N, et al. Incidence of loss of ability to walk 400 meters in a functionally limited older population. J Am Geriatr Soc. 2004;52:2094–8. doi: 10.1111/j.1532-5415.2004.52570.x. [DOI] [PubMed] [Google Scholar]
- 31.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 32.Cleveland JC., Jr Frailty, aging, and cardiac surgery outcomes: the stopwatch tells the story. J Am Coll Cardiol. 2010;56:1677–8. doi: 10.1016/j.jacc.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 33.Rolland YM, Cesari M, Miller ME, Penninx BW, Atkinson HH, Pahor M. Reliability of the 400-m usual-pace walk test as an assessment of mobility limitation in older adults. J Am Geriatr Soc. 2004;52:972–6. doi: 10.1111/j.1532-5415.2004.52267.x. [DOI] [PubMed] [Google Scholar]
- 34.Enright PL, McBurnie MA, Bittner V, Tracy RP, McNamara R, Arnold A, et al. The 6-min walk test: a quick measure of functional status in elderly adults. Chest. 2003;123:387–98. doi: 10.1378/chest.123.2.387. [DOI] [PubMed] [Google Scholar]
- 35.Fleg JL, Pina IL, Balady GJ, Chaitman BR, Fletcher B, Lavie C, et al. Assessment of functional capacity in clinical and research applications: An advisory from the Committee on Exercise, Rehabilitation, and Prevention, Council on Clinical Cardiology, American Heart Association. Circulation. 2000;102:1591–7. doi: 10.1161/01.cir.102.13.1591. [DOI] [PubMed] [Google Scholar]
- 36.Steele B. Timed walking tests of exercise capacity in chronic cardiopulmonary illness. J Cardiopulm Rehabil. 1996;16:25–33. doi: 10.1097/00008483-199601000-00003. [DOI] [PubMed] [Google Scholar]
- 37.American College of Sports Medicine. Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, Minson CT, Nigg CR, et al. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41:1510–30. doi: 10.1249/MSS.0b013e3181a0c95c. [DOI] [PubMed] [Google Scholar]
- 38.Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, King AC, et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1435–45. doi: 10.1249/mss.0b013e3180616aa2. [DOI] [PubMed] [Google Scholar]
- 39.Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, King AC, et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1094–105. doi: 10.1161/CIRCULATIONAHA.107.185650. [DOI] [PubMed] [Google Scholar]
- 40.Department of Health and Human Services 2008 Physical Activity Guidelines for Americans. 2008.
- 41.Pescatello LS, Franklin BA, Fagard R, Farquhar WB, Kelley GA, Ray CA, et al. American College of Sports Medicine position stand. Exercise and hypertension. Med Sci Sports Exerc. 2004;36:533–53. doi: 10.1249/01.mss.0000115224.88514.3a. [DOI] [PubMed] [Google Scholar]
- 42.Borg G. Perceived Exertion and Pain Scales. Human Kinetics; Champaign, IL: 1988. [Google Scholar]
- 43.Choo PW, Rand CS, Inui TS, Lee ML, Cain E, Cordeiro-Breault M, et al. Validation of patient reports, automated pharmacy records, and pill counts with electronic monitoring of adherence to antihypertensive therapy. Med Care. 1999;37:846–57. doi: 10.1097/00005650-199909000-00002. [DOI] [PubMed] [Google Scholar]
- 44.O'Brien E, Waeber B, Parati G, Staessen J, Myers MG. Blood pressure measuring devices: recommendations of the European Society of Hypertension. BMJ. 2001;322:531–6. doi: 10.1136/bmj.322.7285.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Little RJA. A class of pattern-mixture models for normal incomplete data. Biometrika. 1994;81:471, 472–483. [Google Scholar]
- 46.Daniels MJ, Hogan JW, editors. Missing data in longitudinal studies: Strategies for Bayesian modeling and sensitivity analysis: Chapman & Hall. CRC Press; 2008. [Google Scholar]
- 47.Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract. 2004;10:307–12. doi: 10.1111/j..2002.384.doc.x. [DOI] [PubMed] [Google Scholar]
- 48.Manini TM, Newman AB, Fielding R, Blair SN, Perri MG, Anton SD, et al. Effects of exercise on mobility in obese and nonobese older adults. Obesity (Silver Spring) 2010;18:1168–75. doi: 10.1038/oby.2009.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Look AHEAD Research Group. Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145–54. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Habouzit E, Richard H, Sanchez H, Koulmann N, Serrurier B, Monnet R, et al. Decreased muscle ACE activity enhances functional response to endurance training in rats, without change in muscle oxidative capacity or contractile phenotype. J Appl Physiol. 2009;107:346–53. doi: 10.1152/japplphysiol.91443.2008. [DOI] [PubMed] [Google Scholar]
- 51.Carter CS, Giovannini S, Seo DO, DuPree J, Morgan D, Chung HY, et al. Differential effects of enalapril and losartan on body composition and indices of muscle quality in aged male Fischer 344 × Brown Norway rats. Age (Dordr) 2011;33:167–83. doi: 10.1007/s11357-010-9196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giovannini S, Cesari M, Marzetti E, Leeuwenburgh C, Maggio M, Pahor M. Effects of ACE-inhibition on IGF-1 and IGFBP-3 concentrations in older adults with high cardiovascular risk profile. J Nutr Health Aging. 2010;14:457–60. doi: 10.1007/s12603-010-0036-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marzetti E, Calvani R, Dupree J, Lees HA, Giovannini S, Seo DO, et al. Late-life enalapril administration induces nitric oxide-dependent and independent metabolic adaptations in the rat skeletal muscle. Age (Dordr) 2012 doi: 10.1007/s11357-012-9428-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tatti P, Pahor M, Byington RP, Di Mauro P, Guarisco R, Strollo G, et al. Outcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDM. Diabetes Care. 1998;21:597–603. doi: 10.2337/diacare.21.4.597. [DOI] [PubMed] [Google Scholar]
- 55.Estacio RO, Jeffers BW, Hiatt WR, Biggerstaff SL, Gifford N, Schrier RW. The effect of nisoldipine as compared with enalapril on cardiovascular outcomes in patients with non-insulin-dependent diabetes and hypertension. N Engl J Med. 1998;338:645–52. doi: 10.1056/NEJM199803053381003. [DOI] [PubMed] [Google Scholar]
- 56.Hansson L, Lindholm LH, Niskanen L, Lanke J, Hedner T, Niklason A, et al. Effect of angiotensin-converting-enzyme inhibition compared with conventional therapy on cardiovascular morbidity and mortality in hypertension: the Captopril Prevention Project (CAPPP) randomised trial. Lancet. 1999;353:611–6. doi: 10.1016/s0140-6736(98)05012-0. [DOI] [PubMed] [Google Scholar]
- 57.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–53. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 58.Pahor M, Psaty BM, Alderman MH, Applegate WB, Williamson JD, Furberg CD. Therapeutic benefits of ACE inhibitors and other antihypertensive drugs in patients with type 2 diabetes. Diabetes Care. 2000;23:888–92. doi: 10.2337/diacare.23.7.888. [DOI] [PubMed] [Google Scholar]
- 59.Simon CB, Lee-McMullen B, Phelan D, Gilkes J, Carter CS, Buford TW. The renin-angiotensin system and prevention of age-related functional decline: where are we now?. Age (Dordr) 2015;37:9753. doi: 10.1007/s11357-015-9753-5. 015-9753-5. Epub 2015 Feb 9. [DOI] [PMC free article] [PubMed] [Google Scholar]