Abstract
The consensus criteria for the diagnosis and classification of primary progressive aphasia (PPA) have served as an important tool in studying this group of disorders. However, a large proportion of patients remain unclassifiable whilst others simultaneously meet criteria for multiple subtypes. We prospectively evaluated a large cohort of patients with degenerative aphasia and/or apraxia of speech using multidisciplinary clinical assessments and multimodal imaging. Blinded diagnoses were made using operational definitions with important differences compared to the consensus criteria. Of the 130 included patients, 40 were diagnosed with progressive apraxia of speech (PAOS), 12 with progressive agrammatic aphasia, 9 with semantic dementia, 52 with logopenic progressive aphasia, and 4 with progressive fluent aphasia, while 13 were unclassified. The PAOS and progressive fluent aphasia groups were least impaired. Performance on repetition and sentence comprehension was especially poor in the logopenic group. The semantic and progressive fluent aphasia groups had prominent anomia, but only semantic subjects had loss of word meaning and object knowledge. Distinct patterns of grey matter loss and white matter changes were found in all groups compared to controls. PAOS subjects had bilateral frontal grey matter loss, including the premotor and supplementary motor areas, and bilateral frontal white matter involvement. The agrammatic group had more widespread, predominantly left sided grey matter loss and white matter abnormalities. Semantic subjects had bitemporal grey matter loss and white matter changes, including the uncinate and inferior occipitofrontal fasciculi, whereas progressive fluent subjects only had left sided temporal involvement. Logopenic subjects had diffuse and bilateral grey matter loss and diffusion tensor abnormalities, maximal in the posterior temporal region. A diagnosis of logopenic aphasia was strongly associated with being amyloid positive, (46/52 positive). Our findings support consideration of an alternative way of identifying and categorizing subtypes of degenerative speech and language disorders.
Keywords: Primary progressive aphasia, Progressive apraxia of speech, Volumetric based morphometry, Diffusion tensor imaging, Amyloid PET imaging
1. Introduction
Over the last three decades, the diagnostic criteria for, and classification of, neurodegenerative speech and language disorders have been evolving. That the impairment of language may be the only or predominant feature of a degenerative neurologic disease was recognized more than a century ago by Pick and Dejerine and Serieux (Déjerine & Sérieux, 1897; Pick, 1892; Serieux, 1893). A few case reports followed, but only in the latter half of the century did our understanding of degenerative speech and language disorders begin to deepen. In the 1970s, Warrington and colleagues described a series of patients with anomia, loss of word meaning and ‘visual associative agnosia’ (Warrington, 1975). In 1977 Wechsler described a case of presenile dementia with aphasia as the most prominent feature for the first few years of illness (Wechsler, 1977). In 1982, Mesulam reported a series of patients who had a selective and insidiously progressive disruption of spoken language (Mesulam, 1982). A series of similar case reports followed and, in 1987 the term ‘primary progressive aphasia’ (PPA) was coined (Mesulam, 1987).
Around this time, frontotemporal dementia was becoming increasingly recognized as a degenerative dementia distinct from Alzheimer's disease (Neary, Snowden, Northen, & Goulding, 1988), as were other forms of ‘lobar atrophy’ (Neary, 1990). Early classifications of ‘asymmetric cortical degenerations’ included ‘progressive aphasia’, which was divided into ‘nonfluent’ and ‘fluent’ variants (Caselli & Jack, 1992; Neary, Snowden, & Mann, 1993). Early criteria for frontotemporal dementia did not include a language variant (Englund et al., 1994; Kumar & Gottlieb, 1993), but did include speech and language dysfunction as co-existing or supportive findings. In 1989, the term ‘semantic dementia’ was applied to three patients who presented with profound language impairment characterized by marked anomia and loss of word meaning, loss of person and object knowledge, and fluent and syntactically correct speech, a disorder likened to that described by Warrington and colleagues (Baxter & Warrington, 1987; McCarthy & Warrington, 1986; Snowden, Goulding, & Neary, 1989). This syndrome was further refined by Hodges et al. in 1992, as was the underlying pathology, with asymmetric, bilateral temporal pole atrophy noted in most cases (Hodges, Patterson, Oxbury, & Funnell, 1992).
It was against this backdrop that the first set of codified criteria for PPA emerged, first in the form of consensus criteria for frontotemporal dementia proposed in 1998 (Neary et al., 1998), which included ‘progressive nonfluent aphasia’ and ‘semantic aphasia and associative agnosia’ as subtypes, followed by Mesulam's criteria for PPA in 2001 (Mesulam, 2001), which included ‘PPA with agrammatism’ and ‘PPA with comprehension (verbal semantics) deficits’. Shortly thereafter, the spectrum was expanded to the three main syndromes, with the addition of logopenic aphasia (Gorno-Tempini et al., 2004).
While these criteria served the important purpose of providing diagnostic guidelines and facilitated comparisons among studies by different investigators, several areas of diagnostic uncertainty emerged. First, no clear consensus regarding the basis for the fluency-nonfluency distinction exists, with some emphasizing reduced output and telegraphic speech, and others stressing the presence of ‘hesitant, effortful production’ as markers for nonfluency (Gorno-Tempini et al., 2004; Kertesz, Davidson, McCabe, Takagi, & Munoz, 2003; Mesulam, 2001; Neary et al., 1998). Some patients with motor speech disorders, such as apraxia of speech, met the nonfluency criterion when the latter was emphasized, despite the fact that they did not meet the ‘core’ PPA criterion for aphasia (Didic, Ceccaldi, & Poncet, 1998; Mesulam, 2001; Nestor et al., 2003). In addition, some patients were designated as nonfluent by some investigators on the basis of word finding pauses and hesitancy that resulted in a reduced speaking rate (Mesulam, Wieneke, et al., 2009).
Another matter of disagreement or uncertainty was the degree of overlap between ‘semantic dementia’ and ‘PPA with comprehension (verbal semantics) deficits’ (Adlam et al., 2006; Kertesz et al., 2003; Mesulam, Grossman, Hillis, Kertesz, & Weintraub, 2003; Neary et al., 1998). The 1998 consensus criteria required the presence of an ‘associative agnosia’ as evidence for involvement beyond the language system. This seemed to contradict the requirement by Mesulam's criteria that aphasia must be the dominant and most debilitating feature of the disease, and that it was the verbal ‘access’ to semantic knowledge that was impaired rather than semantic memory itself (Mesulam, 2001). As such, two opposing views emerged (Adlam et al., 2006). One regarded semantic dementia and the semantic variant of PPA as one disease, possibly at different stages (Kertesz et al., 2003), caused by disruption of the amodal semantic memory system secondary to bilateral temporal pathology (Hodges et al., 1992; Patterson & Hodges, 2000; Rogers et al., 2004). The other hypothesized that there were two systems involved in semantic dementia – the left sided language network and the bilateral temporal network for face/object knowledge – and that semantic variant PPA represented a selective disruption of the former (Gorno-Tempini et al., 2004; Mesulam, 2001; Mesulam et al., 2003; Sonty et al., 2003). The validity of the logopenic subtype was also questioned, as it was unclear whether or not it was simply a label for patients who did not meet criteria for either of the other two subtypes (Knibb, Xuereb, Patterson, & Hodges, 2006).
Despite advances following publication of these early sets of criteria, no classification scheme was universally accepted. Several groups subsequently proposed classifications that sought to address shortcomings of the early frontotemporal dementia and PPA criteria (Josephs et al., 2010, Josephs, Duffy, et al., 2006; Kertesz et al., 2003; Knibb et al., 2006; Mesulam, Wieneke, et al., 2009; Rabinovici et al., 2008), but none were widely adopted.
In 2011, the first consensus set of criteria for the diagnosis and classification of PPA was published. It formally recognized the logopenic variant, clarified the relationship between the semantic variant of PPA and semantic dementia, and made no assumptions regarding the underlying pathology (Gorno-Tempini et al., 2011). Furthermore, strict inclusion and exclusion criteria for PPA were proposed, in order to exclude other conditions that may result in prominent language impairment without it being the first or dominant feature (Gorno-Tempini et al., 2011; Mesulam, 2001, 2003).
The consensus criteria served to guide the diagnosis of PPA and its subtypes, and represented a marked improvement over the plethora of prior diagnostic schemes that existed prior to its publication. Nonetheless, several issues persist. For example, a large proportion of patients remain unclassifiable, and some patients simultaneously meet criteria for more than one subtype (Harris et al., 2013; Mesulam, Wieneke, Thompson, Rogalski, & Weintraub, 2012; Sajjadi, Patterson, Arnold, Watson, & Nestor, 2012; Wicklund et al., 2014). Variable interpretations of ‘nonfluency’ also persist, as does the uncertainty regarding the relationship between progressive motor speech disorders and PPA. Recently, some groups have used modified versions of the consensus criteria, and have suggested changes to make the criteria more inclusive (Mesulam, 2013; Mesulam & Weintraub, 2014; Sajjadi, Patterson, & Nestor, 2014).
Over the past 4 years, we have prospectively evaluated patients with degenerative speech and language disorders with detailed, multidisciplinary clinical assessments and multimodal imaging with one goal of better characterizing and classifying the spectrum of disorders. In this paper, we present our operational definitions for the diagnosis of PPA and progressive apraxia of speech (PAOS), which have been modified somewhat from our previously published definitions (Josephs et al., 2010; Josephs, Duffy, et al., 2006). Although there is a large degree of overlap with the existing consensus criteria (Gorno-Tempini et al., 2011), there are important differences. We present several lines of evidence as justification for this classification, including speech and language data, volumetric and diffusion tensor imaging findings and data regarding the proportion of cases with probable underlying Alzheimer's pathology through amyloid imaging. As well as advancing our understanding of PPA and PAOS, our findings support consideration of an alternative classification, and highlight modifications that may be needed to resolve some of the issues that have emerged with the consensus criteria.
2. Materials and methods
2.1. Subjects
Between July 2010 and October 2013 we prospectively evaluated a large cohort of patients with aphasia and/or apraxia of speech presumed to be due to a degenerative etiology. Patients were eligible for inclusion if they were over the age of 18, had English as their primary language and had an informant. Patients were excluded if they had a coexisting or alternative degenerative diagnosis, such as Alzheimer's disease (Albert et al., 2011; Dubois et al., 2014; McKhann et al., 1984, 2011), behavioral variant frontotemporal dementia (Neary et al., 1998; Rascovsky et al., 2011), progressive supranuclear palsy (Litvan et al., 1996; Respondek et al., 2013) or corticobasal syndrome (Armstrong et al., 2013; Boeve, Lang, & Litvan, 2003). All subjects were evaluated by a neurologist (KAJ), underwent standardized speech and language assessment by a speech pathologist (JRD or EAS) and had multimodal imaging studies performed. Diagnostic classification occurred blinded to the results of the imaging studies, and was based on results and recordings of the speech and language assessment, as well as samples of general conversation, which were reviewed at a consensus meeting occurring 4–8 weeks after the assessment. Operational definitions used (Table 1) were derived from our prior criteria, and were designed before study initiation (Josephs et al., 2010). Cases that could not reliably be placed in one of the diagnostic categories were classified as PPA-Unclassified. The Mayo Clinic institutional review board approved the study and all subjects consented for enrollment into the study.
Table 1. Operational definitions.
| Progressive apraxia of speech (PAOS) |
| Apraxia of speech is the only or dominant speech or language disturbance at the time of testing. This category is a combination of what we have previously described as primary progressive apraxia of speech and dominant apraxia of speech (Josephs et al., 2013, 2012). If aphasia is present, it must be less severe than the apraxia of speech. In aphasic patients, verbal and/or written output are agrammatic or telegraphic. Difficulties with verbal comprehension, reading comprehension, writing and naming also may be present. In patients with anomia the target words should be recognized when provided on the majority of items. Cases in which apraxia of speech and aphasia are judged to be equally severe are categorized as progressive agrammatic aphasia (see below). Dysarthria may be present. |
| Progressive agrammatic aphasia (PAA) |
| Aphasia is present, and core criteria for PPA are met (Gorno-Tempini et al., 2011). Verbal and/or written output are agrammatic or telegraphic. Difficulties with verbal comprehension, reading comprehension, writing and naming may be present. In patients with anomia the target words should be recognized when provided on the majority of items. Apraxia of speech may be present, but should be equal or less severe than the aphasia. Dysarthria may be present. |
| Logopenic progressive aphasia (LPA) |
| Aphasia is present and core criteria for PPA are met (Gorno-Tempini et al., 2011). Verbal output characteristics are not agrammatic or telegraphic. Speech may be hesitant and slow, but in contrast to the motor deficits seen in apraxia of speech or dysarthria, this results from pauses for apparent word retrieval or verbal formulation efforts. There is poor retention of spoken stimuli, resulting in poor repetition that typically increases with stimulus length and complexity. Phonemic paraphasic errors are often present, but care should be taken to differentiate them from the distortions heard in apraxia of speech. Performance on tasks involving single word comprehension should be better than on those that involve complex sentence comprehension. Anomia is usually present, but target words should be recognized on the majority of items; there are no reports from subjects or informants that the subject doesn't understand the meaning of common words. |
| Semantic dementia (SD) |
| Aphasia is present and core criteria for PPA are met (Gorno-Tempini et al., 2011). The dominant features are anomia and poor single word comprehension. Verbal output is grossly normal with regards to grammar, syntax, average phrase length and prosody, excluding pauses for word retrieval. Brief, unelaborated or simply structured sentences are infrequent. Content may be lacking in terms of substantive nouns and verbs, replacing common words with ‘thing’, for example. Anomia is most striking on confrontational naming. Loss of word meaning can be supported by failure to recognize target words when provided, or by statements from the patient or informant that he/she doesn't seem to understand or recognize the meaning of certain common words; loss of single word meaning should be disproportionate to overall aphasia severity. Performance on single word comprehension tasks should be poorer than comprehension of complex sentences containing individual words that are comprehended. Repetition, especially for non-lengthy stimuli, is relatively preserved, and phonological errors are rare. Other supporting features include disproportionately poor performance on word fluency tasks, especially category fluency, more difficulty reading irregular words than regularly spelled nonsense words, poor performance on semantic association or receptive vocabulary tasks (e.g., Pyramids and Palm Trees Test), and difficulty with facial recognition. |
| Progressive fluent aphasia (PFA) |
| Aphasia is present, and core criteria for PPA are met (Gorno-Tempini et al., 2011), with anomia being the predominant feature. Speech is fluent, but does not meet criteria for logopenic progressive aphasia or semantic dementia. As such, grammar, syntax, average phrase length and prosody are normal. There is little if any loss of word meaning and repetition of complex sentences is nearly normal. Phonemic paraphasias are absent or rare. |
| Primary progressive aphasia unclassified (PPA-U) |
| Aphasia is present, with or without apraxia of speech, and core criteria for PPA are met (Gorno-Tempini et al., 2011). The patient doesn't reliably meet criteria for any of the other categories. |
2.2. Clinical assessment
Details of the speech and language assessments performed have been described elsewhere (Josephs et al., 2012). The Western Aphasia Battery-revised (WAB-R) was administered as a measure of global language ability. Additional supplementary reading and writing tasks from the WAB-R were also administered. A 22-item version of the Token Test (De Renzi & Vignolo, 1962) was used to assess verbal comprehension of complex instructions. A shortened version of the Boston Naming Test was used to further assess confrontational naming (Lansing, Ivnik, Cullum, & Randolph, 1999). In addition to the animal fluency task included in the WAB-R, both letter (FAS) (Loonstra, Tarlow, & Sellers, 2001) and action (verb) (Woods et al., 2005) word fluency were assessed. Grammar was assessed by review of conversational speech and detailed language testing, including verbal and written picture description tasks. Agrammatism was assessed in speech and writing separately, with more emphasis placed on the writing sample in cases of severe apraxia of speech, after which a consensus determination was made as to whether or not a subject was agrammatic or fluent. Additional speech tasks were used to assess motor speech disorders (dysarthria and apraxia of speech) (Duffy, 2006, 2012; Josephs et al., 2012; McNeil, Robin, & Schmidt, 2009), and apraxia of speech was graded with the Apraxia of Speech Severity Rating Scale (ASRS) (Strand, Duffy, Clark, & Josephs, 2014). Nonverbal oral apraxia (Botha et al., 2014; Duffy, 2012) was also assessed. Global severity ratings for aphasia, apraxia of speech and dysarthria were also made (0–4). Additional tests of nonverbal semantic knowledge included the Pyramids and Palm Trees Test (Howard & Patterson, 1992) and testing for prosopagnosia with a test of famous faces (Josephs et al., 2012). Additional information on published normative values and cut-offs, when available, are provided in Supplement A, although the pattern of impairment was viewed as more important than raw scores in the current study.
2.3. Image acquisition
As part of a standardized protocol all subjects had a 3.0 T MRI, which included a 3D prepared rapid acquisition gradient echo sequence and a single-shot echo-planar DTI pulse sequence with 41 diffusion encoding steps and four non-diffusion weighted images, as previously described in detail (Josephs et al., 2012). PET scans were acquired using a PET/CT scanner (GE Healthcare) operating in 3D mode. Subjects were injected with Pittsburgh compound B (PiB) (average, 614 MBq; range, 414–695 MBq) and after a 40–60-min uptake period a 20 min PiB scan was obtained consisting of four 5-min dynamic frames following a low dose CT transmission scan. Standard corrections were applied.
2.4. Image processing
After standard pre-processing steps (Josephs et al., 2012), voxel-based morphometry using SPM5 (Ashburner & Friston, 2000, 2005) was used to analyze patterns of grey matter loss among the disease groups compared to a group of 130 subjects (mean age± SD 68.1 ± 8.2, 68 females, 11 left handed, 2 ambidextrous) from a healthy control reference group that were matched to the study patients by age and gender, as well as to one another. Regarding DTI processing, each of the 41 diffusion-weighted images were affine transformed and registered to the non-diffusion weighted b0 volumes. After brain extraction, fractional anisotropy and mean diffusivity maps were created. Whole-brain, voxel-based analysis was performed on fractional anisotropy and mean diffusivity images separately, comparing disease groups to controls, as well as to one another, as previously described (Schwarz et al., 2014). Subjects were classified as amyloid-positive using a cortical-to-cerebellar (SUVR) ratio cut-point of greater or equal to 1.5 as previously defined (Jack et al., 2008).
2.5. Statistical analyses
Clinical data were analyzed using JMP (version 8.0.0; SAS Institute Inc., Cary, NC). Categorical variables were assessed using the chi-square test and, for variables with small numbers, Fisher's exact test. For continuous variables ANOVA and pairwise Student's t-tests were used for normally distributed data and the Kruskal–Wallis and Mann–Whitney U tests for non-normally distributed data. The Holm–Bonferroni method was used to account for multiple comparisons.
Two-sided t-tests with SPM5 were used to assess differences in voxel-based morphometry, fractional anisotropy and mean diffusivity results among disease groups and controls. Results were analyzed at a threshold of p < .05, corrected for multiple comparisons using family wise error for disease groups compared to controls, and at .001 (uncorrected) when disease groups were compared with each other. The Anatomy Toolbox in SPM was used to locate cluster and local maxima MNI coordinates, which were labeled via the ‘anatomy toolbox’ for grey matter structures and according to a modified Johns Hopkins University (JHU) atlas for white matter structures and tracts (Eickhoff et al., 2005; Hua et al., 2008; Mori, Wakana, & Van Zijl, 2005; Oishi et al., 2009; Wakana et al., 2007).
3. Results
3.1. Demographics
A total of 130 patients were included. Forty were diagnosed with PAOS, twelve with progressive agrammatic aphasia, nine with semantic dementia, fifty-two with logopenic progressive aphasia and four with progressive fluent aphasia. Thirteen patients did not meet criteria for any specific subtype (PPA-Unclassified). Subject demographics are shown in Table 2.
Table 2. Demographics and clinical findings for subgroups of progressive aphasia and apraxia of speech (Mean ± Standard deviation).
| PAOS (40) | PAA (12) | SD (9) | PFA (4) | LPA (52) | p-value | |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Agea | 70.73 ± 9.3 | 67.67 ± 7.0 | 66.33 ± 6.4 | 70.0 ± 7.3 | 66.25 ± 8.5 | .1517 |
| Female (%) | 20 (50) | 8 (67) | 4 (44) | 3 (75) | 26 (50) | .6802 |
| Handedness (R/L/A) | 34/5/1 | 11/1/0 | 7/2/0 | 4/0/0 | 48/3/1 | .8150 |
| Duration (in years)b | 3.64 ± 1.8 | 2.5 ± 1.2 | 5.39 ± 3.7 | 2.75 ± 1.3 | 3.44 ± 1.4 | .0725 |
| Age of Onset (in years)a | 67.18 ± 9.6 | 65.25 ± 7.9 | 60.94 ± 6.7 | 67.25 ± 6.8 | 62.87 ± 8.3 | .1075 |
| Education (in years)b | 15.31 ± 2.7 | 14.92 ± 2.8 | 16.78 ± 2.8 | 16.38 ± 2.9 | 15.32 ± 2.6 | .4856 |
| Speech/Language Assessmentc | ||||||
| Western Aphasia Battery | ||||||
| Aphasia Quotient (/100)b | 91.68 ± 10.8e,h,j | 80.31 ± 14.0d | 80.54 ± 17.5 | 92.48 ± 5.2 | 77.22 ± 15.8d | <.001* |
| Spontaneous Speech (/20)b | 18.00 ± 2.7e,h | 14.5 ± 3.7d | 16.89 ± 3.0 | 18.75 ± 1.0 | 15.46 ± 3.3d | <.001* |
| Info content (/10)b | 9.60 ± 0.7e,h | 8.5 ± 1.9d | 9.25 ± 1.2 | 9.75 ± 0.5 | 7.77 ± 2.1d | <.001* |
| Fluency (/10)b | 8.40 ± 2.2e,h | 6.0 ± 2.2d | 8.25 ± 1.4 | 9.00 ± 0.8 | 7.65 ± 1.6d | .0002* |
| AV Comp (/10)b | 9.81 ± 0.3e,h | 9.40 ± 0.7d | 8.56 ± 2.1 | 9.86 ± 0.2 | 8.70 ± 1.3d | <.001* |
| Repetition (/10)b | 8.88 ± 1.9h | 7.85 ± 2.5 | 8.78 ± 1.3 | 9.60 ± 0.7h | 7.39 ± 1.8g | <.001* |
| Naming (/10)b | 9.16 ± 1.0e,f,h | 8.41 ± 1.3d | 6.04 ± 3.2d | 8.03 ± 1.2 | 7.07 ± 2.3d | <.001* |
| Animal Fluencya | 14.15 ± 4.6e,f,h | 9.5 ± 5.1d | 7.33 ± 5.5d | 12.25 ± 4.6 | 9.00 ± 5.4d | <.001* |
| Writing Output (/34)b | 29.41 ± 7.0h | 23.71 ± 10.3 | 27.22 ± 10.9 | 30.75 ± 3.9 | 20.57 ± 11.4d | .0009* |
| Western Aphasia Battery Supplemental Tests | ||||||
| Reading Irregular words (/10)b | 9.46 ± 1.4e,f,h | 8.25 ± 3.0d | 7.22 ± 3.1d | 10.00 ± 0.0 | 7.71 ± 2.6d | <.001* |
| Reading non-words (/10)b | 8.32 ± 2.2e,h | 5.0 ± 3.3d | 7.56 ± 2.1 | 9.25 ± 1.0 | 5.77 ± 3.2d | <.001* |
| Reading Comprehension (/40)b | 37.80 ± 4.1e,h | 30.5 ± 9.9d | 30.22 ± 12.8 | 38.00 ± 4.0 | 31.19 ± 7.8d | .0002* |
| Reading Commands (/20)b | 19.11 ± 2.3h | 16.25 ± 4.1 | 18.22 ± 5.0 | 20.0 ± 0.0 | 16.31 ± 5.3d | .0004* |
| Writing irregular words (/10)b | 7.85 ± 2.5h | 6.0 ± 2.9 | 6.11 ± 3.7 | 7.00 ± 2.2 | 3.81 ± 3.2d | <.001* |
| Writing non-words (/10)b | 6.60 ± 2.5h | 4.17 ± 3.0 | 5.78 ± 2.8 | 6.75 ± 1.3 | 3.88 ± 2.9d | .0003* |
| Token Test (/22)b | 18.68 ± 3.3e,h | 11.75 ± 6.5 | 15.00 ± 6.9 | 18.75 ± 3.0h | 9.75 ± 5.5d,g,j | <.001* |
| Boston Naming Test (/15)b | 13.40 ± 1.8f,g,h | 11.42 ± 4.7f,h | 3.78 ± 4.2d,e | 3.75 ± 2.9d | 6.75 ± 4.5d,e | <.001* |
| Failure to recognize target words on unnamed items | 33 ± 0 7f,g,h | .42 ± 0.9f,k,l | 5.22 ± 6.1d,e,k | 1.75 ± 1.0d | 1.58 ± 2.0d | <.001* |
| Action Fluencya | 11.42 ± 4.0h,l | 7.92 ± 5.6 | 8.56 ± 5.3g | 17.75 ± 6.0f,h | 8.42 ± 4.6d,g | .0002* |
| Letter Fluencyb | 20.87 ± 10.4 | 12.92 ± 8.2 | 18.78 ± 11.5 | 27.75 ± 12.1 | 17.86 ± 11.5 | .0705 |
| Phonologic errors present (%) | 2 (5)e,h | 7 (58)d,h | 2 (22)h | 0 (0)h | 50 (96)i | <.001* |
| Agrammatism present (%) | 16 (40)e,f,h | 12 (100)i | 0 (0)d,e | 0 (0)e | 0 (0)d,e | <.001* |
| In speech onlym | 8 (20)h | 4 (33)h | 0 (0) | 0 (0) | 0 (0)d,e | <.001* |
| In writing onlyn | 4 (10) | 0 (0) | 0 (0) | 0 (0) | 6 (8) | .2760 |
| In writing and speechm,n | 4 (10)e | 8 (67)d,f,h | 0 (0)e | 0 (0) | 0 (0)e | <.001* |
| Aphasia Severity (/4)b | 56 ± 0.8e,f,h | 1.67 ± 0.9d | 1.78 ± 1.0d | 1.13 ± 0.3 | 2.08 ± 1.0d | <.001* |
| Dysarthria present (%) | 17 (43)f,h | 1 (8) | 0 (0)d | 0 (0) | 0 (0)d | <.001* |
| Dysarthria Severity (/4)b | .63 ± 1.0 | .08 ± 0.3 | N/A | N/A | N/A | .6459 |
| AOS present (%) | 40 (100)i | 8 (67)i | 0 (0)d,e | 0 (0)d,e | 0 (0)d,e | <.001* |
| AOS Severity (/4)b | 1.99 ± 1.0 | 1.29 ± 1.4 | N/A | N/A | N/A | .5294 |
| ASRS total (/64)b | 21.7 ± 10.0i | 13.5 ± 10.3i | .22 ± 0.4d,e,h | .00 ± 0.0d,e,h | 2.15 ± 1.8i | <.001* |
| Number of abnormal features (/16)b | 11.77 ± 3.88f,g,h | 8.33 ± 4.3f,g,h | .22 ± 0.4d,e,h | .00 ± 0.0d,e,h | 1.88 ± 1.5i | <.001* |
| NVOA score (/32)b | 22.65 ± 9.6 | 23.92 ± 9.8 | 26.56 ± 6.2 | 31.50 ± 1.0 | 27.25 ± 6.0 | .0603 |
| Supplemental semantic tests | ||||||
| Pyramids and palm trees (/52)b | 49.73 ± 1.6f,h,l | 49.25 ± 1.5f,k | 39.78 ± 9.2d,e | 47.50 ± 1.9 | 45.38 ± 6.4d | <.001* |
| Famous faces (/10)b | 9.53 ± 1.4f | 9.67 ± 0.7j | 6.67 ± 3.6d,h | 10.0 ± 0 | 9.53 ± 1.4f | .0105* |
Abbreviations: A = ambidextrous; AOS = apraxia of speech; ASRS = apraxia of speech severity rating scale; L = left; LPA = logopenic progressive aphasia; NVOA = nonverbal oral apraxia; PAA = progressive agrammatic aphasia; PAOS = progressive apraxia of speech; SD = semantic dementia; PFA = progressive fluent aphasia; PPA-U = primary progressive aphasia unclassified; R = right.
Parametric.
Non-parametric.
See supplemental data for information regarding normative values.
Significant compared to PAOS.
Significant compared to PAA.
Significant compared to SD.
Significant compared to PFA.
Significant compared to LPA.
Significant compared to all other groups.
Trend towards significance compared to SD.
Trend towards significance compared to LPA.
Trend towards significance compared to PFA.
20 subjects (1 PAOS, 1 SD, 15 LPA, 3 PPA-U) did not have adequate writing output to be assessed.
4 subjects (3 PAOS, 1 PPA-U) did not have enough speech output to be assessed.
The majority of patients were right handed, but there were eleven left-handed patients and two ambidextrous patients. The distribution of non-right-handed patients did not differ significantly among groups. The majority of patients reported further education after high school.
3.2. Speech and language assessment results
Results of the speech and language assessments for classifiable subjects are shown in Table 2 (See Supplementary Table B1 for PPA-Unclassified subjects). Thirty patients were deemed agrammatic, sixteen of which were classified as having PAOS, twelve as progressive agrammatic aphasia and the remaining two were unclassified. Six subjects ultimately classified as logopenic progressive aphasia had some evidence of agrammatism in their writing samples alone, but had good grammar and syntax in spoken language and were deemed fluent by consensus. Both in terms of the WAB-R Aphasia Quotient, as well as consensus aphasia severity rating, the PAOS and progressive fluent aphasia groups were least impaired. Mean scores for most of the tests were higher in the PAOS and progressive fluent aphasia groups, except for those involving motor speech in the former and those involving naming in the latter.
Mean scores for the logopenic progressive aphasia and progressive agrammatic aphasia groups reflected severe impairment in several domains. Performance on repetition and the Token Test was especially poor in the logopenic progressive aphasia group. Both the semantic dementia and progressive fluent aphasia groups performed poorly on the Boston Naming Test, but only the semantic dementia subjects often failed to identify target words when were provided for unnamed items. Scores for the Pyramids and Palm Trees test as well as the Famous Faces test were also substantially lower in the semantic dementia group compared with other subtypes.
3.3. Imaging findings
Compared to controls, PAOS subjects had grey matter loss in bilateral premotor and supplementary motor areas, middle cingulate gyri, inferior frontal areas including Broca's area and insular grey matter (Fig. 1, Supplementary Table C1). Reduced fractional anisotropy was found in the body of the corpus callosum, bilateral precentral and superior frontal white matter, left superior temporal white matter, and left inferior occipitofrontal fasciculus (Fig. 1, Supplementary Table C8). Increased mean diffusivity was more widespread including the areas already noted, plus the bilateral middle and inferior frontal white matter, and bilateral postcentral and supra-marginal white matter (Fig. 1, Supplementary Table C9). On both modalities the left side was more involved than the right. DTI abnormalities also included regions associated with the left uncinate fasciculus and bilateral superior longitudinal fasciculi.
Fig. 1.
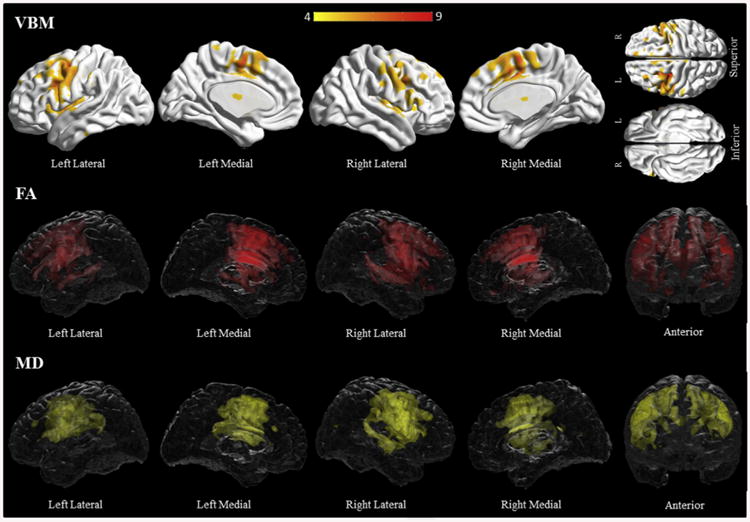
Results of the voxel-based morphometry and diffusion tensor imaging analysis in the PAOS group. Patterns of grey matter loss on voxel-based morphometry (top), and reduced fractional anisotropy (middle) and increased mean diffusivity (bottom) on diffusion tensor imaging analysis, in the PAOS group compared with controls. All results shown at p < .05 (corrected for multiple comparisons using family-wise error). Results are shown in 3D renderings of the brain.
Subjects with progressive agrammatic aphasia had more widespread grey matter loss compared to controls, with premotor areas, superior, middle and inferior frontal gyri, fusiform gyri and caudate nuclei involved bilaterally (Fig. 2, Supplementary Table C2). Additionally, the left supplementary motor area, cingulate, middle and inferior temporal gyri, precuneus and superior parietal lobule were involved. On both fractional anisotropy and mean diffusivity analyses, there was bilateral involvement of the precentral, middle and superior frontal white matter, as well as the body of the corpus callosum. Additionally, there was increased mean diffusivity in bilateral inferior frontal white matter areas, and then widespread on the left involving temporal, post–central, and lingual white matter (Fig. 2, Supplementary Table C10–11). DTI abnormalities included regions associated with the left cingulum and uncinate fasciculus, and the superior longitudinal fasciculi bilaterally.
Fig. 2.
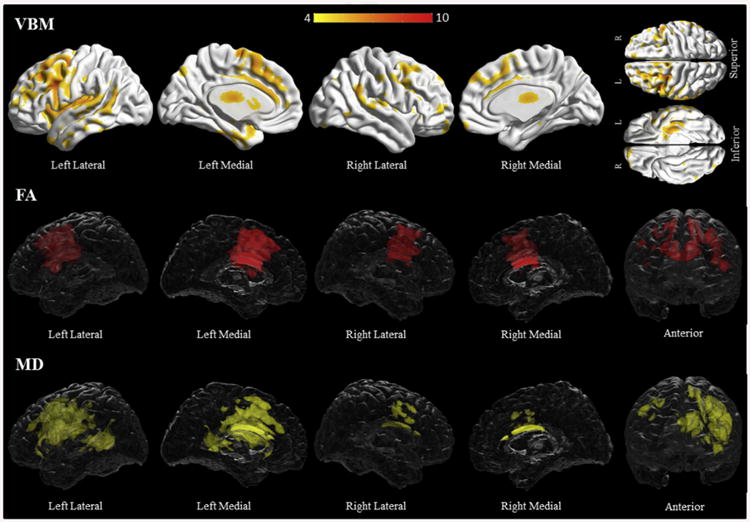
Results of the voxel-based morphometry and diffusion tensor imaging analysis in the progressive agrammatic aphasia group. Patterns of grey matter loss on voxel-based morphometry (top), and reduced fractional anisotropy (middle) and increased mean diffusivity (bottom) on diffusion tensor imaging analysis, in the progressive agrammatic aphasia group compared with controls. All results shown at p < .05 (corrected for multiple comparisons using family-wise error). Results are shown in 3D renderings of the brain.
On direct comparison, subjects with progressive agrammatic aphasia had grey matter loss in the left temporal region, including the temporal pole, the superior, middle and inferior temporal gyri, the hippocampus and the fusiform gyrus, compared to subjects with PAOS (Fig. 3, Supplementary Table C7). There were also left frontal, opercular and parietal areas of increased involvement. Increased mean diffusivity was noted in the left superior, middle and inferior frontal white matter, including the area of the superior longitudinal fasciculus, as well as the left superior and middle temporal white matter and body of the corpus callosum among subjects with progressive agrammatic aphasia compared to those with PAOS (Fig. 3, Supplementary Table C20).
Fig. 3.
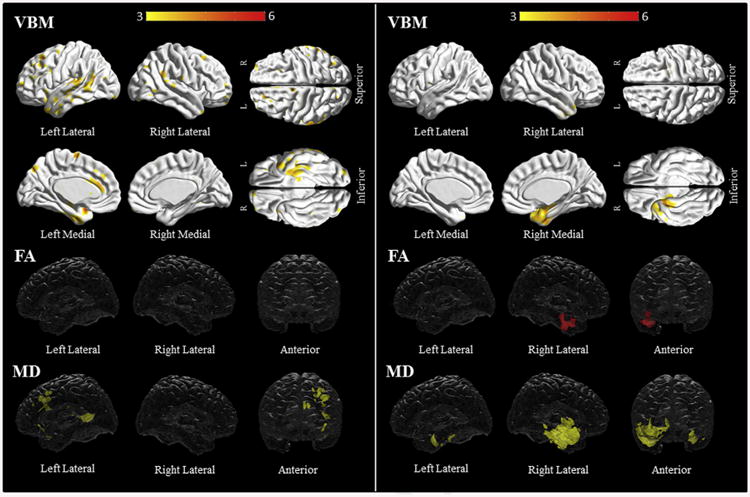
Title: Voxel-based morphometry and diffusion tensor imaging analysis in progressive agrammatic aphasia compared to PAOS (left) and semantic dementia compared to progressive fluent aphasia (right). Patterns of grey matter loss on voxel-based morphometry (top), and reduced fractional anisotropy (middle) and increased mean diffusivity (bottom) on diffusion tensor imaging analysis, for progressive agrammatic aphasia compared to PAOS (left panel) and semantic dementia compared to progressive fluent aphasia (right panel). All results shown at p < .001 (uncorrected for multiple comparisons). Results are shown in 3D renderings of the brain.
Subjects with semantic dementia had bilateral temporal grey matter loss, including the fusiform gyri, temporal poles, hippocampi and inferior temporal gyri; the left side was more involved than the right (Fig. 4, Supplementary Table C3). Both fractional anisotropy and mean diffusivity analyses also revealed bilateral temporal involvement, left more than right, including the anterior temporal and parahippocampal white matter (Fig. 4, Supplementary Table C12–13). There was bilateral involvement of the fronto-orbital white matter, the uncinate, and the inferior longitudinal and inferior occipitofrontal fasciculi. Additional left temporal involvement included the superior, middle and inferior temporal white and the left cingulum.
Fig. 4.
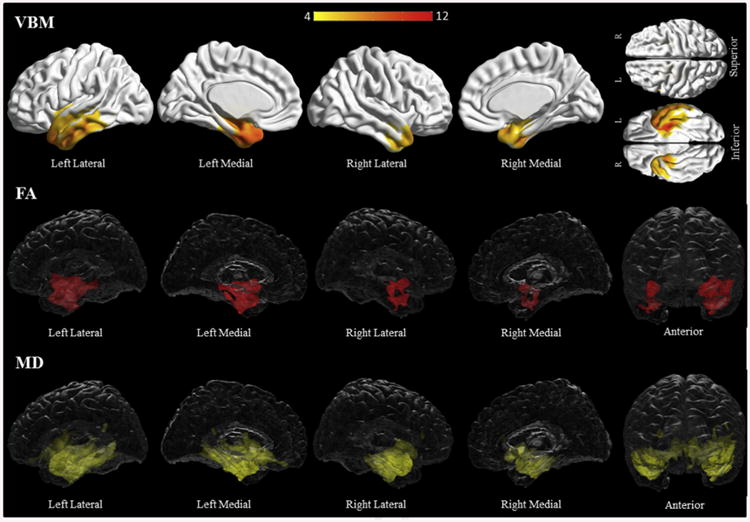
Results of the voxel-based morphometry and diffusion tensor imaging analysis in the semantic dementia group. Patterns of grey matter loss on voxel-based morphometry (top), and reduced fractional anisotropy (middle) and increased mean diffusivity (bottom) on diffusion tensor imaging analysis, in the semantic dementia group compared with controls. All results shown at p < .05 (corrected for multiple comparisons using family-wise error). Results are shown in 3D renderings of the brain.
Progressive fluent aphasia subjects only had left sided grey matter loss involving the middle and inferior temporal gyri, the parahippocampal gyrus and the fusiform gyrus (Fig. 5, Supplementary Table C4). White matter involvement was also limited to the left side, with the anterior temporal white matter the most involved (Fig. 5, Supplementary Table C14–15). Additional areas of involvement include the left parahippocampal and inferior temporal white matter, and the uncinate, inferior longitudinal and inferior occipitofrontal fasciculi.
Fig. 5.
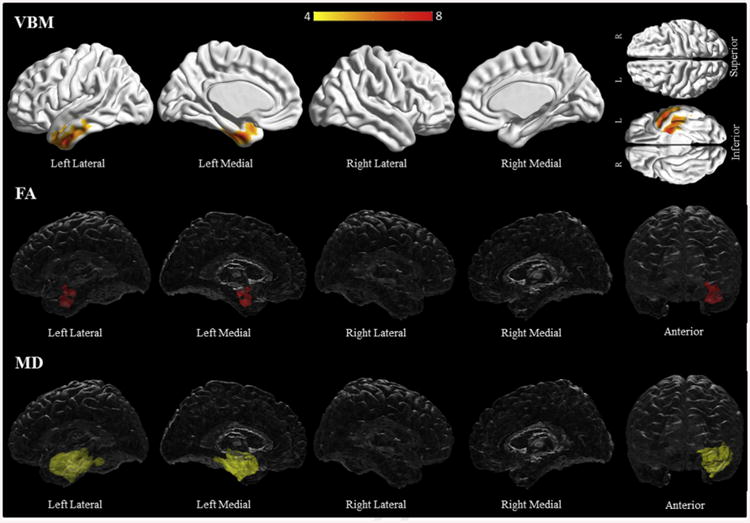
Results of the voxel-based morphometry and diffusion tensor imaging analysis in the progressive fluent aphasia group. Patterns of grey matter loss on voxel-based morphometry (top), and reduced fractional anisotropy (middle) and increased mean diffusivity (bottom) on diffusion tensor imaging analysis, in the progressive fluent aphasia group compared with controls. All results shown at p < .05 (corrected for multiple comparisons using family-wise error). Results are shown in 3D renderings of the brain.
On direct comparison to progressive fluent aphasia subjects, semantic dementia subjects showed right-sided temporal grey matter loss involving the temporal pole, the hippocampus and parahippocampal gyrus, the fusiform and the inferior temporal gyrus (Fig. 3, Supplementary Table C6). There was reduced fractional anisotropy in the right anterior, inferior, middle and superior white matter, and the parahippocampal white matter in comparison to progressive fluent aphasia. In addition, there was increased mean diffusivity in the left temporal white matter, and the right uncinate, and the inferior longitudinal and inferior occipitofrontal fasciculi in the semantic dementia group (Fig. 3, Supplementary Table C21–22).
Logopenic progressive aphasia subjects had diffuse and bilateral grey matter loss, maximal in the posterior temporal areas, extending to several frontal, temporal and parietal regions, with the left side more involved (Fig. 6, Supplementary Table C5). Both fractional anisotropy and mean diffusivity analyses revealed left greater than right diffuse and bilateral white matter involvement (Fig. 6, Supplementary Table C16–17). Involvement was again maximal in the posterior aspects of the left temporal white matter, but extended into the anterior temporal, frontal, parietal and occipital white matter and involved the superior and inferior longitudinal fasciculi and inferior occipitofrontal fasciculi bilaterally.
Fig. 6.
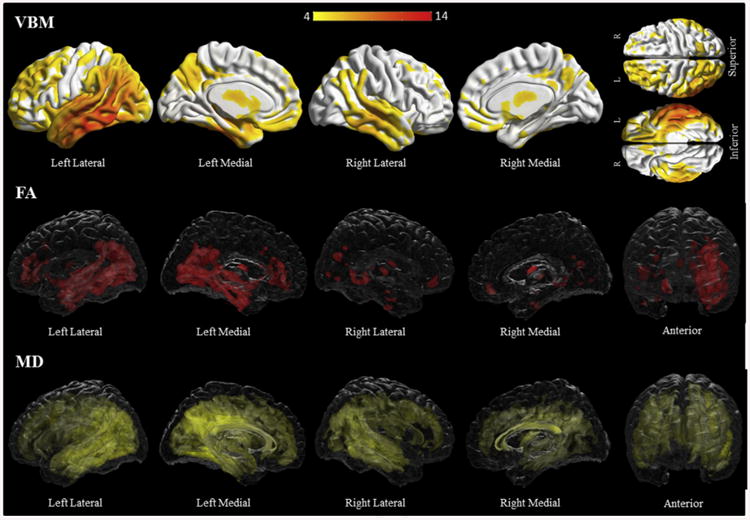
Results of the voxel-based morphometry and diffusion tensor imaging analysis in the logopenic progressive aphasia group. Patterns of grey matter loss on voxel-based morphometry (top), and reduced fractional anisotropy (middle) and increased mean diffusivity (bottom) on diffusion tensor imaging analysis, in the logopenic progressive aphasia group compared with controls. All results shown at p < .05 (corrected for multiple comparisons using family-wise error). Results are shown in 3D renderings of the brain.
PPA-Unclassified subjects only had several small, scattered areas of left sided grey matter loss and white matter changes compared to controls, mostly involving the frontal and temporal lobes (Supplementary Tables C18–19).
With regards to amyloid imaging, 59/130 subjects were deemed amyloid-positive. The amyloid-positivity rate by group was 6/40 for PAOS, 1/12 for progressive agrammatic aphasia, 3/9 for semantic dementia, 0/4 for progressive fluent aphasia, 46/52 for logopenic progressive aphasia and 3/13 for PPA-Unclassified.
4. Discussion
Based on a large cohort of prospectively recruited patients, our data show that the syndromes as we have classified for this study show specific patterns of grey and white matter loss. Using amyloid imaging, the data also show the proportion of cases in each syndrome with probable underlying Alzheimer's pathology. The operational definitions presented here evolved from our prior definitions, used over the course of several years of studying a large, prospectively recruited group of patients with PPA and PAOS. Whilst not specifically designed to address the issues that have become apparent with the current consensus criteria, they offer potential modifications for classifying and diagnosing PPA and PAOS that address some of them. We will discuss our definitions with reference to the consensus criteria for each subtype, and use the clinical and imaging findings as justification for our classification.
4.1. PAOS and progressive agrammatic aphasia
Consensus criteria for the nonfluent/agrammatic variant require the presence of agrammatism or apraxia of speech, as well as two of the following: impaired comprehension of syntactically complex sentences; spared single-word comprehension; spared object knowledge. Whilst the consensus statement recognized that apraxia of speech may be the initial manifestation of the nonfluent/agrammatic, it also states that such cases usually develop aphasia and as such could reasonably be classified as nonfluent/agrammatic (Gorno-Tempini et al., 2011). However, it has been shown previously that apraxia of speech may be the only manifestation of a degenerative disease for more than 5 years, during which time the root criteria for PPA (i.e., aphasia) is not met (Josephs et al., 2012). As others have pointed out, the structure of the consensus criteria for nonfluent/agrammatic PPA inherently result in three subgroups of patients based on the fact that only one core criterion is required: a group with apraxia of speech alone, a group with agrammatism but no apraxia of speech and a group with agrammatism and apraxia of speech (Harris et al., 2013). However, this does not account for the fact that patients may present with apraxia of speech alone, given that these patients would not meet criteria for PPA, nor does it recognize the differences among the possible subgroups in terms of the presence and relative severity of apraxia of speech. We recently proposed recognizing PAOS, consisting of cases that have apraxia of speech alone or as a dominant feature in the setting of aphasia, as a separate group from PPA altogether based on clinical, radiological and pathological grounds (Harris et al., 2013; Josephs et al., 2010, 2013, 2012; Josephs, Duffy, et al., 2006; Mesulam, 2013). Data driven approaches have also supported viewing patients with apraxia of speech as a distinct group (Leyton, Ballard, Piguet, & Hodges, 2014; Silveri et al., 2014). Recent longitudinal data showed that many subjects with primary progressive AOS develop a devastating progressive supranuclear palsy-like syndrome approximately 5 years after onset, further highlighting the importance of recognizing this group as distinct from the agrammatic group (Josephs, Duffy, Strand, Machulda, Senjem, et al., 2014). One of the strengths of our classification is that it recognizes PAOS and progressive agrammatic aphasia as distinct, albeit related disorders.
4.2. Semantic dementia and progressive fluent aphasia
The consensus criteria for semantic variant PPA are largely consistent with previous criteria for semantic dementia in that it requires both impaired confrontational naming and impaired single word comprehension, along with three of the following: impaired object knowledge; surface dyslexia/dysgraphia; spared repetition; spared grammar and motor speech. Importantly however, it recognized that disruption of semantic memory can also be found through nonverbal testing (Adlam et al., 2006; Bozeat, LambonRalph, Patterson, Garrard, & Hodges, 2000; Hodges et al., 1992; Luzzi et al., 2007; Snowden et al., 1989), a departure from earlier sets of PPA criteria. The operational definition for semantic dementia presented here overlaps substantially with the consensus criteria. However, our classification includes progressive fluent aphasia as a distinct disorder. This progressive fluent aphasia group had minimal impairment on nearly all of the tests employed, with the exception of naming where their performance was similar to that of the semantic dementia group. In contrast to cases of semantic dementia, they were able to recognize the target word in almost all instances, had no complaints of loss of word meaning and performed in the normal range on other tests of semantic knowledge.
Similar patients have been described by other groups, again in small numbers (Mesulam et al., 2013). Some unclassified patients in other studies were also described as having anomia with spared word meaning and no agrammatism or motor speech difficulties (Mesulam et al., 2014, 2012). These patients could not be classified as having the logopenic variant because of relatively spared repetition. Some have suggested introducing an ‘anomic PPA’ variant to account for them (Mesulam, 2013; Mesulam et al., 2012). It has also been suggested that such patients do not constitute a separate group, but rather represent logopenic patients who cannot be so classified because the criteria for the logopenic variant may be too strict (Mesulam et al., 2014; Sajjadi et al., 2014). There may be merit to both suggestions. We have previously suggested that fluent cases that are unclassifiable according to the consensus criteria include two subgroups, one representing a milder form of logopenic aphasia and the other a milder form of semantic dementia (Wicklund et al., 2014), and progressive fluent aphasia may capture the latter group. Naturally, the relationship between progressive fluent aphasia and semantic dementia will be questioned. Some, but not all patients with similar presentations have gone on to develop semantic dementia (Czarnecki et al., 2008; Mesulam et al., 2012). Others may point to the tests we used to assess for breakdown of semantic knowledge, which were not as comprehensive as our assessment of language function. However, in all but the most severe cases of semantic dementia a clear pattern exists, where performance on tasks of object knowledge exceeds that of single word comprehension, which in turn exceeds performance on naming tasks (Adlam et al., 2006; Mayberry, Sage, & Ralph, 2011; Mesulam et al., 2013). As such, as the anomia worsens, the first evidence of semantic breakdown should manifest as deficits in single word comprehension (Mesulam, Rogalski, et al., 2009). Our progressive fluent aphasia subjects were profoundly anomic, but did not show any evidence of word comprehension deficits (Kashibayashi et al., 2010; Kertesz, Jesso, Harciarek, Blair, & McMonagle, 2010). Furthermore, these cases did not meet consensus criteria for the semantic variant or logopenic variant of PPA either (see Supplementary Table B2 for subject level data for this group, including follow up data on three subjects). It should be borne in mind that subjects had to be aphasic to be considered for the diagnosis of semantic dementia, and as such our criteria and findings may not generalize well to semantic dementia subjects with predominantly right-sided temporal pathology (Chan et al., 2009; Josephs et al., 2009).
4.3. Logopenic progressive aphasia
Some have questioned the validity of logopenic variant PPA as a distinct linguistic syndrome on the basis of cluster analyses (Sajjadi et al., 2012). But other data driven approaches suggest at least a subset of patients within logopenic variant PPA have a recognizable syndrome that helps predict the pattern of atrophy as well as the underlying pathology (Leyton et al., 2014; Leyton, Hsieh, Mioshi, & Hodges, 2013). In addition, a recent autopsy confirmed series retrospectively applied the consensus criteria and found that 55% of cases previously classified as progressive nonfluent aphasia, according to the 1998 criteria, would now be reclassified as logopenic variant PPA, with most having underlying Alzheimer's pathology (Chare et al., 2014). The consensus criteria for the logopenic variant are not without fault however, as they are largely to blame for the large proportion of unclassifiable patients as well as the fact that some patients meet criteria for more than one subtype (Harris et al., 2013; Mesulam et al., 2012; Sajjadi et al., 2012; Wicklund et al., 2014). The consensus criteria require impaired single word retrieval in spontaneous speech and naming as well as impaired repetition of sentences/phrases, together with three of the following: phonological errors; spared single word comprehension and object knowledge; spared motor speech; absence of frank agrammatism. As already mentioned, the criteria may be too strict to capture milder cases (Leyton et al., 2014; Machulda et al., 2013; Sajjadi et al., 2014; Wicklund et al., 2014). Additionally, some agrammatic patients without apraxia of speech may also meet criteria for the logopenic variant. This led other groups to the suggest that “absence of definite grammar and comprehension impairment” replace repetition as a core feature of the logopenic variant, with impaired repetition serving as an ancillary criterion (Mesulam & Weintraub, 2014; Mesulam et al., 2014, 2012). Our operational definition requires that verbal output be fluent, i.e., not agrammatic or telegraphic, and also clarifies that the apparent nonfluency that may be present on account of word finding difficulty does not preclude a diagnosis of logopenic progressive aphasia. Furthermore, our requirement regarding single word comprehension is less strict, allowing for impairment as long as it doesn't exceed that of complex sentence comprehension. Like the consensus criteria, we emphasize the importance of phonological errors, a feature that has been shown to independently predict amyloid positivity (Leyton et al., 2014), although we do not view it as a required feature (Petroi, Duffy, Strand, & Josephs, 2014). Finally, by placing patients in the category that best fits their presentation, rather than relying on a strict list of core and supplementary features, we avoid dual designation. In contrast to the large number of unclassifiable patients in prior studies, only 10% of our subjects were unclassifiable. The fact that six of the LPA subjects had some evidence for agrammatism in their writing samples was surprising. However, others have noted mild grammatical errors in logopenic subjects (Leyton et al., 2011). Furthermore, the fact that their verbal output remained grammatically intact together with an overall pattern of impairment consistent with LPA allowed for confident classification.
4.4. Structural changes
The finding of bilateral frontal atrophy in both PAOS and progressive agrammatic aphasia, with the left side being more involved, is consistent with prior studies (Josephs et al., 2010, 2013, 2012; Lam, Halliday, Irish, Hodges, & Piguet, 2014; Leyton et al., 2014). We have previously shown that when apraxia of speech was the dominant feature there was more superior frontal involvement, including the premotor and supplementary motor areas; when aphasia was the dominant feature, inferior and posterior frontal areas were involved early after which more widespread frontal involvement followed (Josephs et al., 2010, 2013). Involvement of the supplementary and premotor areas in the present study further extends the important role they play in planning and sequencing articulatory movements (Graff-Radford et al., 2014; Josephs et al., 2012; Whitwell et al., 2013). The pattern of frontal involvement, including the supplementary motor area, body of the corpus callosum and superior longitudinal fasciculi, is often seen in cases with underlying progressive supranuclear palsy pathology (Whitwell, Jack, et al., 2010). Others have argued that the aslant tract, connecting the inferior frontal gyrus to the anterior cingulate and the pre-supplementary motor area superior frontal gyrus, is important for verbal fluency, measured in words per minute (Catani et al., 2013). Although we did not perform tractography, our findings are consistent with this suggestion. On direct comparison of white matter involvement between PAOS and progressive agrammatic aphasia, the latter group showed more widespread involvement extending into the temporal cortex, including the temporal pole and fusiform gyrus, and inferior frontal and temporal white matter, in keeping with their more pronounced language deficits. Specifically, involvement of the left anterior-superior temporal lobe, the body of the corpus callosum and the inferior occipitofrontal fasciculus has been linked to grammatical comprehension deficits in PPA (Charles et al., 2014), suggesting that the more severe temporal and corpus callosum involvement we found may be implicated in the more marked aphasia noted in the progressive agrammatic aphasia group.
Our progressive fluent aphasia subjects all had unilateral anterior temporal atrophy, as well as unilateral temporal white matter abnormalities, whereas the semantic dementia group showed bilateral temporal changes with more left sided involvement. This is consistent with prior studies that show profound temporal abnormalities in semantic dementia patients, as well as prior work that demonstrated unilateral temporal involvement in progressive fluent aphasia (Agosta et al., 2010; Brambati et al., 2009; Josephs et al., 2010; Seeley et al., 2005; Snowden, Neary, & Mann, 2007; Whitwell, Avula, et al., 2010). Left sided cortical atrophy included regions associated with naming deficits, but also semantic deficits, in both groups. The same was true of white matter abnormalities, as the uncinate fasciculus, inferior orbitofrontal fasciculus and inferior longitudinal fasciculus have been linked to semantic and naming deficits (Catani, Jones, Donato, & Ffytche, 2003; Catani & Mesulam, 2008; Catani et al., 2013; Duffau, Moritz-Gasser, & Mandonnet, 2014; Grossman et al., 2004; Kantarci et al., 2011; Lu et al., 2002; Mandonnet, Nouet, Gatignol, Capelle, & Duffau, 2007; Mummery et al., 1999; Whitwell, Avula, et al., 2010). Although the degree of overlap is interesting given the stark clinical differences between the two subtypes, the abnormalities in the semantic dementia group were far more severe. Atrophy of the left ventral temporal lobe been shown to correlate with performance on tasks of semantic knowledge (Adlam et al., 2006) and right temporal involvement is most often implicated in nonverbal deficits seen in semantic dementia (Gefen et al., 2013; Mesulam et al., 2013), most notably involvement of the right fusiform which we have shown to underlie prosopagnosia (Josephs, Whitwell, Vemuri, et al., 2008). We found increased involvement of the left anterior fusiform gyrus together with right temporal atrophy in the semantic dementia group compared to the progressive fluent aphasia group, which likely underlies the fact that semantic deficits were only found in the semantic dementia group (Mion et al., 2010). It maybe that, as the disease progresses, pathology spreads more posteriorly and/or to the opposite temporal lobe (Brambati et al., 2009; Whitwell, Anderson, Scahill, Rossor, & Fox, 2004) in the progressive fluent aphasia group. Ultimately, while these subjects did not meet criteria for logopenic progressive aphasia or semantic dementia at time of our evaluation, it remains unclear whether or not they will progress to one of these phenotypes or whether they will remain distinct.
Despite our definition of logopenic progressive aphasia being less strict, the atrophy pattern on volumetric imaging was similar to that of the logopenic variant group in prior studies, which was maximal in the left posterior temporal region (Gorno-Tempini et al., 2008, 2004; Madhavan et al., 2013; Migliaccio et al., 2012; Rabinovici et al., 2008; Rohrer et al., 2013). In keeping with this, there were diffuse white matter abnormalities that were also maximal in the posterior temporal and inferior parietal white matter (Mandelli et al., 2014). The posterior temporal and inferior parietal regions involved in the present study have been linked to phonological loop functions (Gorno-Tempini et al., 2008), and the prominent involvement of the underlying white matter tracts, including the superior and inferior longitudinal fasciculi, likely underlie the poor repetition, naming and comprehension observed in this group (Powers et al., 2013).
4.5. Amyloid imaging
The overwhelming majority of logopenic progressive aphasia patients were amyloid positive, in keeping with what is known about this disorder. One study prior to the release of consensus criteria found that 3 out of 4 logopenic patients were amyloid positive (Rabinovici et al., 2008), whilst two studies subsequent to their publication found that 12 of 13 and 11 of 15 were positive (Leyton et al., 2014, 2011). This is consistent with pathologic confirmed series of logopenic variant PPA, showing Alzheimer's pathology is the most common underlying abnormality (Alladi et al., 2007; Chare et al., 2014; Mesulam et al., 2014).
Based on the imaging findings and previous autopsy series, we expect that the majority of our PAOS subjects have an underlying tauopathy, most likely of the progressive supranuclear palsy form (Deramecourt et al., 2010; Josephs et al., 2005; Josephs, Petersen, et al., 2006). As for the progressive agrammatic aphasia cases, previous series have suggested that cases of prominent agrammatism have either TAR DNA-binding protein 43 (TDP-43) or tau, with the latter being more common (Grossman, 2012; Harris et al., 2013; Mesulam et al., 2014). Amyloid positive rates among subjects with progressive nonfluent aphasia reported prior to the publication of the consensus criteria range from 16% (using PET imaging) to 46% (based on pathology) (Alladi et al., 2007; Rabinovici et al., 2008), but there is a high likelihood that some of these cases would more accurately be categorized as having logopenic variant PPA (Leyton et al., 2011). Following the publication of the consensus criteria, one study reported 2 out of 8 agrammatic/nonfluent subjects were amyloid positive while another found rates of 0/3 (agrammatism alone) and 1/14 (with apraxia of speech) (Leyton et al., 2014, 2011). In light of these studies, our finding of one amyloid positive patient with progressive agrammatic aphasia is not surprising, but the number of PAOS patients who were amyloid positive (15%) remains higher than expected. Similarly, our finding of 33% amyloid positivity among semantic dementia cases was also slightly higher than that of prior studies based on imaging (11–17%) (Leyton et al., 2011; Rabinovici et al., 2008) or pathology (10–33%) (Alladi et al., 2007; Chare et al., 2014; Hodges et al., 2010; Knibb et al., 2006; Mesulam et al., 2014), as it is well known that the majority of semantic cases have an underlying TDP-43 proteinopathy. It is possible that these patients had focal cortical presentations of Alzheimer's disease (Blennerhassett, Lillo, Halliday, Hodges, & Kril, 2014; Leyton et al., 2011; Warren, Fletcher, & Golden, 2012), as retrospective data has shown that a proportion of cases diagnosed with behavioral frontotemporal dementia, semantic variant PPA or agrammatic/nonfluent PPA according to the consensus criteria have Alzheimer's pathology at autopsy (Chare et al., 2014; Mesulam et al., 2014). However, it is likely that a large proportion of cases had codeposition of amyloid together with tau or TDP-43 (Josephs, Duffy, Strand, Machulda, Senjem, et al., 2014). It is well known that cognitively normal elderly subjects have PiB positivity rates of up to 18% in their 60s, upwards of 30% in their 70s and climbing to above 50% in those over the age of 80 (Hedden et al., 2009; Jack et al., 2014, 2009; Lowe et al., 2009; Rowe et al., 2010). The fact that none of the four progressive fluent aphasia patients were amyloid positive is not surprising, as we have previously argued that the underlying pathology is more than likely TDP-43 (Josephs et al., 2010; Josephs, Duffy, et al., 2006; Josephs, Whitwell, Duffy, et al., 2008).
4.6. Unclassified cases
In the present study, we left patients not meeting any of the specific subtypes as a single group of ‘unclassified’ patients. It has been suggested that a subset of these patients may have another unrecognized subtype, labeled ‘mixed PPA’, where patients present with impaired grammatical structure and word comprehension deficits, even at the early stages of disease (Mesulam, 2013; Mesulam, Wieneke, et al., 2009; Mesulam et al., 2012). Whilst this may be true, none of the patients in our study would fit that description, although some of our patients did have different combinations of features from separate subtypes, as described in the supplemental data.
5. Conclusions
We have presented an alternative way of identifying and categorizing subtypes of degenerative speech and language disorders, and showed that this classification results in distinct clinical and radiologic syndromes. Our findings may help guide modifications to existing criteria. The strengths of the study include the large group of prospectively recruited patients, standardized evaluation by a multidisciplinary team, multimodal imaging and the blinded consensus categorization of patients. The relatively small number of semantic dementia cases compared to other groups is an obvious limitation. To what extent the operational definitions will hold outside of our group also remains to be determined, given this was a single center study. Finally, the lack of pathological data in our cohort is a limitation, as is the fact that we could not make use of newer biomarkers such as tau imaging.
Supplementary Material
Acknowledgments
The authors would like to thank Drs. Ahlskog, Boeve, Bower, Drubach, Knopman and Petersen for subject referral and Mrs. Sarah Boland, Mayo Clinic Rochester, MN, for performing the neuropsychometric testing and organizing all subjects test schedules.
Funding: This study was funded by R01 DC010367 (PI KAJ) from the National Institute on Deafness and Communication Disorders.
Abbreviations
- DTI
diffusion tensor imaging
- PAOS
progressive apraxia of speech
- PPA
primary progressive aphasia
- SPM
Statistical Parametric Mapping
- WAB-R
Western Aphasia Battery-revised
Footnotes
Supplementary data: Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.cortex.2015.05.013.
References
- Adlam AL, Patterson K, Rogers TT, Nestor PJ, Salmond CH, Acosta-Cabronero J, et al. Semantic dementia and fluent primary progressive aphasia: two sides of the same coin? Brain. 2006;129:3066–3080. doi: 10.1093/brain/awl285. [DOI] [PubMed] [Google Scholar]
- Agosta F, Henry RG, Migliaccio R, Neuhaus J, Miller BL, Dronkers NF, et al. Language networks in semantic dementia. Brain. 2010;133:286–299. doi: 10.1093/brain/awp233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alladi S, Xuereb J, Bak T, Nestor P, Knibb J, Patterson K, et al. Focal cortical presentations of Alzheimer's disease. Brain. 2007;130:2636–2645. doi: 10.1093/brain/awm213. [DOI] [PubMed] [Google Scholar]
- Armstrong MJ, Litvan I, Lang AE, Bak TH, Bhatia KP, Borroni B, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology. 2013;80:496–503. doi: 10.1212/WNL.0b013e31827f0fd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry–the methods. NeuroImage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Baxter DM, Warrington EK. Transcoding sound to spelling: single or multiple sound unit correspondence? Cortex. 1987;23:11–28. doi: 10.1016/s0010-9452(87)80016-3. [DOI] [PubMed] [Google Scholar]
- Blennerhassett R, Lillo P, Halliday GM, Hodges JR, Kril JJ. Distribution of pathology in frontal variant Alzheimer's disease. Journal of Alzheimer's Disease. 2014;39:63–70. doi: 10.3233/JAD-131241. [DOI] [PubMed] [Google Scholar]
- Boeve BF, Lang AE, Litvan I. Corticobasal degeneration and its relationship to progressive supranuclear palsy and frontotemporal dementia. Annals of Neurology. 2003;54(Suppl. 5):S15–S19. doi: 10.1002/ana.10570. [DOI] [PubMed] [Google Scholar]
- Botha H, Duffy JR, Strand EA, Machulda MM, Whitwell JL, Josephs KA. Nonverbal oral apraxia in primary progressive aphasia and apraxia of speech. Neurology. 2014;82:1729–1735. doi: 10.1212/WNL.0000000000000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozeat S, Lambon Ralph MA, Patterson K, Garrard P, Hodges JR. Non-verbal semantic impairment in semantic dementia. Neuropsychologia. 2000;38:1207–1215. doi: 10.1016/s0028-3932(00)00034-8. [DOI] [PubMed] [Google Scholar]
- Brambati SM, Rankin KP, Narvid J, Seeley WW, Dean D, Rosen HJ, et al. Atrophy progression in semantic dementia with asymmetric temporal involvement: a tensor-based morphometry study. Neurobiology of Aging. 2009;30:103–111. doi: 10.1016/j.neurobiolaging.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caselli RJ, Jack CR., Jr Asymmetric cortical degeneration syndromes. A proposed clinical classification. Archives of Neurology. 1992;49:770–780. doi: 10.1001/archneur.1992.00530310118022. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Donato R, Ffytche DH. Occipito-temporal connections in the human brain. Brain. 2003;126:2093–2107. doi: 10.1093/brain/awg203. [DOI] [PubMed] [Google Scholar]
- Catani M, Mesulam M. The arcuate fasciculus and the disconnection theme in language and aphasia: history and current state. Cortex. 2008;44:953–961. doi: 10.1016/j.cortex.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Mesulam MM, Jakobsen E, Malik F, Martersteck A, Wieneke C, et al. A novel frontal pathway underlies verbal fluency in primary progressive aphasia. Brain. 2013;136:2619–2628. doi: 10.1093/brain/awt163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan D, Anderson V, Pijnenburg Y, Whitwell J, Barnes J, Scahill R, et al. The clinical profile of right temporal lobe atrophy. Brain. 2009;132:1287–1298. doi: 10.1093/brain/awp037. [DOI] [PubMed] [Google Scholar]
- Chare L, Hodges JR, Leyton CE, McGinley C, Tan RH, Kril JJ, et al. New criteria for frontotemporal dementia syndromes: clinical and pathological diagnostic implications. Journal of Neurology, Neurosurgery, and Psychiatry. 2014;85:865–870. doi: 10.1136/jnnp-2013-306948. [DOI] [PubMed] [Google Scholar]
- Charles D, Olm C, Powers J, Ash S, Irwin DJ, McMillan CT, et al. Grammatical comprehension deficits in non-fluent/agrammatic primary progressive aphasia. Journal of Neurology, Neurosurgery, and Psychiatry. 2014;85:249–256. doi: 10.1136/jnnp-2013-305749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarnecki K, Duffy JR, Nehl CR, Cross SA, Molano JR, Jack CR, Jr, et al. Very early semantic dementia with progressive temporal lobe atrophy: an 8-year longitudinal study. Archives of Neurology. 2008;65:1659–1663. doi: 10.1001/archneurol.2008.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Renzi E, Vignolo LA. The token test: a sensitive test to detect receptive disturbances in aphasics. Brain. 1962;85:665–678. doi: 10.1093/brain/85.4.665. [DOI] [PubMed] [Google Scholar]
- Déjerine JJ, Sérieux P. Un cas de surdité verbale pure, terminée par aphasie sensorielle, suivi d'autopsie. Comptes Rendues des Seances de la Societe de Biologie (Paris) 1897;49:3. [Google Scholar]
- Deramecourt V, Lebert F, Debachy B, Mackowiak-Cordoliani MA, Bombois S, Kerdraon O, et al. Prediction of pathology in primary progressive language and speech disorders. Neurology. 2010;74:42–49. doi: 10.1212/WNL.0b013e3181c7198e. [DOI] [PubMed] [Google Scholar]
- Didic M, Ceccaldi M, Poncet M. Progressive loss of speech: a neuropsychological profile of premotor dysfunction. European Neurology. 1998;39:90–96. doi: 10.1159/000007914. [DOI] [PubMed] [Google Scholar]
- Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, et al. Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet Neurology. 2014;13:614–629. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- Duffau H, Moritz-Gasser S, Mandonnet E. A reexamination of neural basis of language processing: proposal of a dynamic hodotopical model from data provided by brain stimulation mapping during picture naming. Brain Lang. 2014;131:1–10. doi: 10.1016/j.bandl.2013.05.011. [DOI] [PubMed] [Google Scholar]
- Duffy JR. Apraxia of speech in degenerative neurologic disease. Aphasiology. 2006;20:511–527. [Google Scholar]
- Duffy JR. Motor speech disorders: Substrates, differential diagnosis, and management: Mosby 2012 [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Englund B, Brun A, Gustafson L, Passant U, Mann D, Neary D, et al. Clinical and neuropathological criteria for frontotemporal dementia. Journal of Neurology, Neurosurgery, and Psychiatry. 1994;57:416–418. doi: 10.1136/jnnp.57.4.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gefen T, Wieneke C, Martersteck A, Whitney K, Weintraub S, Mesulam MM, et al. Naming vs knowing faces in primary progressive aphasia: a tale of 2 hemispheres. Neurology. 2013;81:658–664. doi: 10.1212/WNL.0b013e3182a08f83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Brambati SM, Ginex V, Ogar J, Dronkers NF, Marcone A, et al. The logopenic/phonological variant of primary progressive aphasia. Neurology. 2008;71:1227–1234. doi: 10.1212/01.wnl.0000320506.79811.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, et al. Cognition and anatomy in three variants of primary progressive aphasia. Annals of Neurology. 2004;55:335–346. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff-Radford J, Jones DT, Strand EA, Rabinstein AA, Duffy JR, Josephs KA. The neuroanatomy of pure apraxia of speech in stroke. Brain Lang. 2014;129:43–46. doi: 10.1016/j.bandl.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M. The non-fluent/agrammatic variant of primary progressive aphasia. Lancet Neurology. 2012;11:545–555. doi: 10.1016/S1474-4422(12)70099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, McMillan C, Moore P, Ding L, Glosser G, Work M, et al. What's in a name: voxel-based morphometric analyses of MRI and naming difficulty in Alzheimer's disease, frontotemporal dementia and corticobasal degeneration. Brain. 2004;127:628–649. doi: 10.1093/brain/awh075. [DOI] [PubMed] [Google Scholar]
- Harris JM, Gall C, Thompson JC, Richardson AM, Neary D, du Plessis D, et al. Classification and pathology of primary progressive aphasia. Neurology. 2013;81:1832–1839. doi: 10.1212/01.wnl.0000436070.28137.7b. [DOI] [PubMed] [Google Scholar]
- Hedden T, Van Dijk KRA, Becker JA, Mehta A, Sperling RA, Johnson KA, et al. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. Journal of Neuroscience. 2009;29:12686–12694. doi: 10.1523/JNEUROSCI.3189-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges JR, Mitchell J, Dawson K, Spillantini MG, Xuereb JH, McMonagle P, et al. Semantic dementia: demography, familial factors and survival in a consecutive series of 100 cases. Brain. 2010;133:300–306. doi: 10.1093/brain/awp248. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic dementia. Progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115(Pt 6):1783–1806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- Howard D, Patterson KE. The pyramids and palm trees test: A test of semantic access from words and pictures. Thames Valley Test Company; 1992. [Google Scholar]
- Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, et al. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. NeuroImage. 2008;39:336–347. doi: 10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Lowe VJ, Senjem ML, Weigand SD, Kemp BJ, Shiung MM, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer's disease and amnestic mild cognitive impairment. Brain. 2008;131:665–680. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Wiste HJ, Weigand SD, Rocca WA, Knopman DS, Mielke MM, et al. Age-specific population frequencies of cerebral beta-amyloidosis and neurodegeneration among people with normal cognitive function aged 50–89 years: a cross-sectional study. Lancet Neurology. 2014;13:997–1005. doi: 10.1016/S1474-4422(14)70194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Lowe VJ, Weigand SD, Wiste HJ, Senjem ML, Knopman DS, et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer's disease: implications for sequence of pathological events in Alzheimer's disease. Brain. 2009;132:1355–1365. doi: 10.1093/brain/awp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Boeve BF, Duffy JR, Smith GE, Knopman DS, Parisi JE, et al. Atypical progressive supranuclear palsy underlying progressive apraxia of speech and nonfluent aphasia. Neurocase. 2005;11:283–296. doi: 10.1080/13554790590963004. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Fossett TR, Strand EA, Claassen DO, Whitwell JL, et al. Fluorodeoxyglucose F18 positron emission tomography in progressive apraxia of speech and primary progressive aphasia variants. Archives of Neurology. 2010;67:596–605. doi: 10.1001/archneurol.2010.78. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Gunter JL, et al. The evolution of primary progressive apraxia of speech. Brain. 2014 doi: 10.1093/brain/awu223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Lowe VJ, et al. Syndromes dominated by apraxia of speech show distinct characteristics from agrammatic PPA. Neurology. 2013;81:337–345. doi: 10.1212/WNL.0b013e31829c5ed5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Lowe VJ, et al. APOE epsilon4 influences beta-amyloid deposition in primary progressive aphasia and speech apraxia. Alzheimer's & Dementia. 2014 doi: 10.1016/j.jalz.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Master AV, et al. Characterizing a neurodegenerative syndrome: primary progressive apraxia of speech. Brain. 2012;135:1522–1536. doi: 10.1093/brain/aws032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Whitwell JL, Layton KF, Parisi JE, et al. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain. 2006;129:1385–1398. doi: 10.1093/brain/awl078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Petersen RC, Knopman DS, Boeve BF, Whitwell JL, Duffy JR, et al. Clinicopathologic analysis of frontotemporal and corticobasal degenerations and PSP. Neurology. 2006;66:41–48. doi: 10.1212/01.wnl.0000191307.69661.c3. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Duffy JR, Vanvoorst WA, Strand EA, Hu WT, et al. Progressive aphasia secondary to Alzheimer disease vs FTLD pathology. Neurology. 2008;70:25–34. doi: 10.1212/01.wnl.0000287073.12737.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Knopman DS, Boeve BF, Vemuri P, Senjem ML, et al. Two distinct subtypes of right temporal variant frontotemporal dementia. Neurology. 2009;73:1443–1450. doi: 10.1212/WNL.0b013e3181bf9945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Vemuri P, Senjem ML, Boeve BF, Knopman DS, et al. The anatomic correlate of prosopagnosia in semantic dementia. Neurology. 2008;71:1628–1633. doi: 10.1212/01.wnl.0000334756.18558.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci K, Senjem ML, Avula R, Zhang B, Samikoglu AR, Weigand SD, et al. Diffusion tensor imaging and cognitive function in older adults with no dementia. Neurology. 2011;77:26–34. doi: 10.1212/WNL.0b013e31822313dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashibayashi T, Ikeda M, Komori K, Shinagawa S, Shimizu H, Toyota Y, et al. Transition of distinctive symptoms of semantic dementia during longitudinal clinical observation. Dementia and Geriatric Cognitive Disorders. 2010;29:224–232. doi: 10.1159/000269972. [DOI] [PubMed] [Google Scholar]
- Kertesz A, Davidson W, McCabe P, Takagi K, Munoz D. Primary progressive aphasia: diagnosis, varieties, evolution. Journal of the International Neuropsychological Society. 2003;9:710–719. doi: 10.1017/S1355617703950041. [DOI] [PubMed] [Google Scholar]
- Kertesz A, Jesso S, Harciarek M, Blair M, McMonagle P. What is semantic dementia?: a cohort study of diagnostic features and clinical boundaries. Archives of Neurology. 2010;67:483–489. doi: 10.1001/archneurol.2010.55. [DOI] [PubMed] [Google Scholar]
- Knibb JA, Xuereb JH, Patterson K, Hodges JR. Clinical and pathological characterization of progressive aphasia. Annals of Neurology. 2006;59:156–165. doi: 10.1002/ana.20700. [DOI] [PubMed] [Google Scholar]
- Kumar A, Gottlieb G. Frontotemporal dementias: a new clinical syndrome? American Journal of Geriatric Psychiatry. 1993;1:95–108. doi: 10.1097/00019442-199300120-00002. [DOI] [PubMed] [Google Scholar]
- Lam BY, Halliday GM, Irish M, Hodges JR, Piguet O. Longitudinal white matter changes in frontotemporal dementia subtypes. Human Brain Mapping. 2014;35:3547–3557. doi: 10.1002/hbm.22420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansing AE, Ivnik RJ, Cullum CM, Randolph C. An empirically derived Short form of the Boston naming test. Archives of Clinical Neuropsychology. 1999;14:481–487. [PubMed] [Google Scholar]
- Leyton CE, Ballard KJ, Piguet O, Hodges JR. Phonologic errors as a clinical marker of the logopenic variant of PPA. Neurology. 2014;82:1620–1627. doi: 10.1212/WNL.0000000000000387. [DOI] [PubMed] [Google Scholar]
- Leyton CE, Hsieh S, Mioshi E, Hodges JR. Cognitive decline in logopenic aphasia: more than losing words. Neurology. 2013;80:897–903. doi: 10.1212/WNL.0b013e318285c15b. [DOI] [PubMed] [Google Scholar]
- Leyton CE, Villemagne VL, Savage S, Pike KE, Ballard KJ, Piguet O, et al. Subtypes of progressive aphasia: application of the International Consensus Criteria and validation using beta-amyloid imaging. Brain. 2011;134:3030–3043. doi: 10.1093/brain/awr216. [DOI] [PubMed] [Google Scholar]
- Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP International Workshop. Neurology. 1996;47:1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- Loonstra AS, Tarlow AR, Sellers AH. COWAT metanorms across age, education, and gender. Applied Neuropsychology. 2001;8:161–166. doi: 10.1207/S15324826AN0803_5. [DOI] [PubMed] [Google Scholar]
- Lowe VJ, Kemp BJ, Jack CR, Senjem M, Weigand S, Shiung M, et al. Comparison of 18F-FDG and PiB PET in cognitive impairment. Journal of Nuclear Medicine. 2009;50:878–886. doi: 10.2967/jnumed.108.058529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu LH, Crosson B, Nadeau SE, Heilman KM, Gonzalez-Rothi LJ, Raymer A, et al. Category-specific naming deficits for objects and actions: semantic attribute and grammatical role hypotheses. Neuropsychologia. 2002;40:1608–1621. doi: 10.1016/s0028-3932(02)00014-3. [DOI] [PubMed] [Google Scholar]
- Luzzi S, Snowden JS, Neary D, Coccia M, Provinciali L, Lambon Ralph MA. Distinct patterns of olfactory impairment in Alzheimer's disease, semantic dementia, frontotemporal dementia, and corticobasal degeneration. Neuropsychologia. 2007;45:1823–1831. doi: 10.1016/j.neuropsychologia.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Machulda MM, Whitwell JL, Duffy JR, Strand EA, Dean PM, Senjem ML, et al. Identification of an atypical variant of logopenic progressive aphasia. Brain Lang. 2013;127:139–144. doi: 10.1016/j.bandl.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhavan A, Whitwell JL, Weigand SD, Duffy JR, Strand EA, Machulda MM, et al. FDG PET and MRI in logopenic primary progressive aphasia versus dementia of the Alzheimer's type. PLoS one. 2013;8:e62471. doi: 10.1371/journal.pone.0062471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelli ML, Caverzasi E, Binney RJ, Henry ML, Lobach I, Block N, et al. Frontal white matter tracts sustaining speech production in primary progressive aphasia. Journal of Neuroscience. 2014;34:9754–9767. doi: 10.1523/JNEUROSCI.3464-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandonnet E, Nouet A, Gatignol P, Capelle L, Duffau H. Does the left inferior longitudinal fasciculus play a role in language? A brain stimulation study. Brain. 2007;130:623–629. doi: 10.1093/brain/awl361. [DOI] [PubMed] [Google Scholar]
- Mayberry EJ, Sage K, Ralph MA. At the edge of semantic space: the breakdown of coherent concepts in semantic dementia is constrained by typicality and severity but not modality. Journal of Cognitive Neuroscience. 2011;23:2240–2251. doi: 10.1162/jocn.2010.21582. [DOI] [PubMed] [Google Scholar]
- McCarthy RA, Warrington EK. Visual associative agnosia: a clinico-anatomical study of a single case. Journal of Neurology, Neurosurgery, and Psychiatry. 1986;49:1233–1240. doi: 10.1136/jnnp.49.11.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil M, Robin D, Schmidt R. Apraxia of speech: definition and differential diagnosis. In: McNeil M, editor. Clinical management of sensorimotor speech disorders. New York: Thieme; 2009. [Google Scholar]
- Mesulam MM. Slowly progressive aphasia without generalized dementia. Annals of Neurology. 1982;11:592–598. doi: 10.1002/ana.410110607. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Primary progressive aphasia–differentiation from Alzheimer's disease. Annals of Neurology. 1987;22:533–534. doi: 10.1002/ana.410220414. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Primary progressive aphasia. Annals of Neurology. 2001;49:425–432. [PubMed] [Google Scholar]
- Mesulam MM. Primary progressive aphasia–a language-based dementia. New England Journal of Medicine. 2003;349:1535–1542. doi: 10.1056/NEJMra022435. [DOI] [PubMed] [Google Scholar]
- Mesulam M. Primary progressive aphasia: a dementia of the language network. Dementia & Neuropsychologia. 2013;7:2–9. doi: 10.1590/S1980-57642013DN70100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Grossman M, Hillis A, Kertesz A, Weintraub S. The core and halo of primary progressive aphasia and semantic dementia. Annals of Neurology. 2003;54(Suppl. 5):S11–S14. doi: 10.1002/ana.10569. [DOI] [PubMed] [Google Scholar]
- Mesulam M, Rogalski E, Wieneke C, Cobia D, Rademaker A, Thompson C, et al. Neurology of anomia in the semantic variant of primary progressive aphasia. Brain. 2009;132:2553–2565. doi: 10.1093/brain/awp138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Weintraub S. Is it time to revisit the classification guidelines for primary progressive aphasia? Neurology. 2014;82:1108–1109. doi: 10.1212/WNL.0000000000000272. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Weintraub S, Rogalski EJ, Wieneke C, Geula C, Bigio EH. Asymmetry and heterogeneity of Alzheimer's and frontotemporal pathology in primary progressive aphasia. Brain. 2014;137:1176–1192. doi: 10.1093/brain/awu024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Wieneke C, Hurley R, Rademaker A, Thompson CK, Weintraub S, et al. Words and objects at the tip of the left temporal lobe in primary progressive aphasia. Brain. 2013;136:601–618. doi: 10.1093/brain/aws336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M, Wieneke C, Rogalski E, Cobia D, Thompson C, Weintraub S. Quantitative template for subtyping primary progressive aphasia. Archives of Neurology. 2009;66:1545–1551. doi: 10.1001/archneurol.2009.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Wieneke C, Thompson C, Rogalski E, Weintraub S. Quantitative classification of primary progressive aphasia at early and mild impairment stages. Brain. 2012;135:1537–1553. doi: 10.1093/brain/aws080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio R, Agosta F, Possin KL, Rabinovici GD, Miller BL, Gorno-Tempini ML. White matter atrophy in Alzheimer's disease variants. Alzheimer's & Dementia. 2012;8:S78–87. e71–72. doi: 10.1016/j.jalz.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mion M, Patterson K, Acosta-Cabronero J, Pengas G, Izquierdo-Garcia D, Hong YT, et al. What the left and right anterior fusiform gyri tell us about semantic memory. Brain. 2010;133:3256–3268. doi: 10.1093/brain/awq272. [DOI] [PubMed] [Google Scholar]
- Mori S, Wakana S, Van Zijl PCM. MRI atlas of human white matter. 1st. Amsterdam; Oxford: Elsevier; 2005. [Google Scholar]
- Mummery CJ, Patterson K, Wise RJ, Vandenberghe R, Price CJ, Hodges JR. Disrupted temporal lobe connections in semantic dementia. Brain. 1999;122(Pt 1):61–73. doi: 10.1093/brain/122.1.61. [DOI] [PubMed] [Google Scholar]
- Neary D. Non Alzheimer's disease forms of cerebral atrophy. Journal of Neurology, Neurosurgery, and Psychiatry. 1990;53:929–931. doi: 10.1136/jnnp.53.11.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Mann DM. The clinical pathological correlates of lobar atrophy. Dementia. 1993;4:154–159. doi: 10.1159/000107315. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Northen B, Goulding P. Dementia of frontal lobe type. Journal of Neurology, Neurosurgery, and Psychiatry. 1988;51:353–361. doi: 10.1136/jnnp.51.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor PJ, Graham NL, Fryer TD, Williams GB, Patterson K, Hodges JR. Progressive non-fluent aphasia is associated with hypometabolism centred on the left anterior insula. Brain. 2003;126:2406–2418. doi: 10.1093/brain/awg240. [DOI] [PubMed] [Google Scholar]
- Oishi K, Faria A, Jiang H, Li X, Akhter K, Zhang J, et al. Atlas-based whole brain white matter analysis using large deformation diffeomorphic metric mapping: application to normal elderly and Alzheimer's disease participants. NeuroImage. 2009;46:486–499. doi: 10.1016/j.neuroimage.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson K, Hodges JR. Semantic dementia: one window on the structure and organisation of semantic memory. In: Cermak L, editor. Revised handbook of neuropsychology: Memory and its disorders. Amsterdam: Elsevier Science Publishers BV; 2000. pp. 313–335. [Google Scholar]
- Petroi D, Duffy JR, Strand EA, Josephs KA. Phonologic errors in the logopenic variant of primary progressive aphasia. Aphasiology. 2014;28:1223–1243. [Google Scholar]
- Pick A. U¨ber die Beziehungen der senilen Hirnatrophie zur Aphasie. Prag Med Wochenschr. 1892;17:165–167. [Google Scholar]
- Powers JP, McMillan CT, Brun CC, Yushkevich PA, Zhang H, Gee JC, et al. White matter disease correlates with lexical retrieval deficits in primary progressive aphasia. Frontiers in Neurology. 2013;4:212. doi: 10.3389/fneur.2013.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovici GD, Jagust WJ, Furst AJ, Ogar JM, Racine CA, Mormino EC, et al. Abeta amyloid and glucose metabolism in three variants of primary progressive aphasia. Annals of Neurology. 2008;64:388–401. doi: 10.1002/ana.21451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Respondek G, Roeber S, Kretzschmar H, Troakes C, Al-Sarraj S, Gelpi E, et al. Accuracy of the National Institute for Neurological Disorders and Stroke/Society for Progressive Supranuclear Palsy and neuroprotection and natural history in Parkinson plus syndromes criteria for the diagnosis of progressive supranuclear palsy. Movement Disorders. 2013;28:504–509. doi: 10.1002/mds.25327. [DOI] [PubMed] [Google Scholar]
- Rogers TT, Lambon Ralph MA, Garrard P, Bozeat S, McClelland JL, Hodges JR, et al. Structure and deterioration of semantic memory: a neuropsychological and computational investigation. Psychological Review. 2004;111:205–235. doi: 10.1037/0033-295X.111.1.205. [DOI] [PubMed] [Google Scholar]
- Rohrer JD, Caso F, Mahoney C, Henry M, Rosen HJ, Rabinovici G, et al. Patterns of longitudinal brain atrophy in the logopenic variant of primary progressive aphasia. Brain Lang. 2013;127:121–126. doi: 10.1016/j.bandl.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe CC, Ellis KA, Rimajova M, Bourgeat P, Pike KE, Jones G, et al. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiology of Aging. 2010;31:1275–1283. doi: 10.1016/j.neurobiolaging.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Sajjadi SA, Patterson K, Arnold RJ, Watson PC, Nestor PJ. Primary progressive aphasia: a tale of two syndromes and the rest. Neurology. 2012;78:1670–1677. doi: 10.1212/WNL.0b013e3182574f79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajjadi SA, Patterson K, Nestor PJ. Logopenic, mixed, or Alzheimer-related aphasia? Neurology. 2014;82:1127–1131. doi: 10.1212/WNL.0000000000000271. [DOI] [PubMed] [Google Scholar]
- Schwarz CG, Reid RI, Gunter JL, Senjem ML, Przybelski SA, Zuk SM, et al. Improved DTI registration allows voxel-based analysis that outperforms tract-based spatial statistics. NeuroImage. 2014;94:65–78. doi: 10.1016/j.neuroimage.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Bauer AM, Miller BL, Gorno-Tempini ML, Kramer JH, Weiner M, et al. The natural history of temporal variant frontotemporal dementia. Neurology. 2005;64:1384–1390. doi: 10.1212/01.WNL.0000158425.46019.5C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serieux P. Sur un cas de surdite verbale pure. Review of Medicine. 1893;13:733–750. [Google Scholar]
- Silveri MC, Pravata E, Brita AC, Improta E, Ciccarelli N, Rossi P, et al. Primary progressive aphasia: linguistic patterns and clinical variants. Brain Lang. 2014;135:57–65. doi: 10.1016/j.bandl.2014.05.004. [DOI] [PubMed] [Google Scholar]
- Snowden JS, Goulding PJ, Neary D. Semantic dementia: a form of circumscribed cerebral atrophy. Behavioural Neurology 1989 [Google Scholar]
- Snowden J, Neary D, Mann D. Frontotemporal lobar degeneration: clinical and pathological relationships. Acta Neuropathologica. 2007;114:31–38. doi: 10.1007/s00401-007-0236-3. [DOI] [PubMed] [Google Scholar]
- Sonty SP, Mesulam MM, Thompson CK, Johnson NA, Weintraub S, Parrish TB, et al. Primary progressive aphasia: PPA and the language network. Annals of Neurology. 2003;53:35–49. doi: 10.1002/ana.10390. [DOI] [PubMed] [Google Scholar]
- Strand EA, Duffy JR, Clark HM, Josephs K. The apraxia of speech rating scale: a tool for diagnosis and description of apraxia of speech. Journal of Communication Disorders. 2014;51:43–50. doi: 10.1016/j.jcomdis.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. NeuroImage. 2007;36:630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren JD, Fletcher PD, Golden HL. The paradox of syndromic diversity in Alzheimer disease. Nature Reviews Neurology. 2012;8:451–464. doi: 10.1038/nrneurol.2012.135. [DOI] [PubMed] [Google Scholar]
- Warrington EK. The selective impairment of semantic memory. Quarterly Journal of Experimental Psychology. 1975;27:635–657. doi: 10.1080/14640747508400525. [DOI] [PubMed] [Google Scholar]
- Wechsler AF. Presenile dementia presenting as aphasia. Journal of Neurology, Neurosurgery, and Psychiatry. 1977;40:303–305. doi: 10.1136/jnnp.40.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Anderson VM, Scahill RI, Rossor MN, Fox NC. Longitudinal patterns of regional change on volumetric MRI in frontotemporal lobar degeneration. Dementia and Geriatric Cognitive Disorders. 2004;17:307–310. doi: 10.1159/000077160. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Avula R, Senjem ML, Kantarci K, Weigand SD, Samikoglu A, et al. Gray and white matter water diffusion in the syndromic variants of frontotemporal dementia. Neurology. 2010;74:1279–1287. doi: 10.1212/WNL.0b013e3181d9edde. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Duffy JR, Strand EA, Xia R, Mandrekar J, Machulda MM, et al. Distinct regional anatomic and functional correlates of neurodegenerative apraxia of speech and aphasia: an MRI and FDG-PET study. Brain Lang. 2013;125:245–252. doi: 10.1016/j.bandl.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Jack CR, Jr, Boeve BF, Parisi JE, Ahlskog JE, Drubach DA, et al. Imaging correlates of pathology in corticobasal syndrome. Neurology. 2010;75:1879–1887. doi: 10.1212/WNL.0b013e3181feb2e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicklund MR, Duffy JR, Strand EA, Machulda MM, Whitwell JL, Josephs KA. Quantitative application of the primary progressive aphasia consensus criteria. Neurology. 2014;82:1119–1126. doi: 10.1212/WNL.0000000000000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Scott JC, Sires DA, Grant I, Heaton RK, Troster AI. Action (verb) fluency: test-retest reliability, normative standards, and construct validity. Journal of the International Neuropsychological Society. 2005;11:408–415. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


