Abstract
Background
Forced vital capacity (FVC) is a key measure of disease severity in patients with idiopathic pulmonary fibrosis (IPF) and is an important clinical trial endpoint. We hypothesize that reversible airflow limitation co-exists in a subgroup of patients with IPF, and that bronchodilator use will improve the performance characteristics of FVC.
Methods
IPF patients with pre and post-bronchodilator spirometry testing performed were identified from two tertiary referral cohorts. The difference between pre and post-bronchodilator FVC (intra-test difference) was calculated. The test characteristics of pre and post-bronchodilator FVC change over time (inter-test difference) were assessed in patients with sequential spirometry, and were used to generate sample size estimates for hypothetical clinical trials using change in FVC as the primary endpoint.
Results
There were 551 patients, contributing 967 unique spirometry tests. The mean intra-test increase in FVC with bronchodilator use was 0.04 liters (2.71 vs. 2.75 liters, p <0.001). Reversible airflow limitation (increase in FEV1 or FVC of ≥12% and ≥200 milliliters) occurred in 9.1% of patients. The inter-test difference in change in FVC over time were equivalent for pre and post-bronchodilator (p = 0.65), leading to similar sample size estimates in a hypothetical clinical trial using change in FVC as the primary endpoint.
Conclusion
Approximately one in ten patients with IPF has physiological evidence of reversible airflow limitation, and bronchodilator use in these patients may improve the assessment of disease progression based on FVC change over time. Bronchodilator use does not appear to meaningfully impact the precision of FVC as an endpoint in clinical trials.
Keywords: Idiopathic pulmonary fibrosis, Spirometry, Lung function, Bronchodilators, Clinical trials
INTRODUCTION
Forced vital capacity (FVC) is a standard spirometric measure that quantifies the volume of air moved during forced exhalation, defined as the difference between total lung capacity and residual volume.[1, 2] In interstitial lung diseases like idiopathic pulmonary fibrosis (IPF), the FVC is generally reduced in proportion to the total lung capacity and is used as a measure of disease severity.[3] Longitudinal decline in FVC is a key metric of disease progression in IPF and is reliably associated with reduced survival.[4–7] For these and other practical reasons, FVC has been the most common measure of disease progression in clinical practice and the primary endpoint for most recent clinical trials in IPF.[8–15]
FVC is influenced by the presence of both physiologic restriction and obstruction, and its value can fluctuate in patients, particularly in those with reversible airflow limitation.[1] The extent to which FVC is influenced by reversible airflow limitation in patients with IPF is unknown. However, common comorbidities in IPF include asthma and emphysema,[16] a history of cigarette smoking is present in the majority of patients with IPF,[3] and emphysema on chest imaging is common,[17–19] all of which suggest patients with IPF may often have comorbid obstructive airways disease with some degree of reversibility.
We hypothesize that reversible airflow limitation co-exists in a subgroup of patients with IPF, and that its presence reduces the performance characteristics of FVC as a measure of IPF disease severity and progression. The objective of this study was to determine the prevalence of physiologically-defined reversible airflow limitation in a large, well-characterized IPF population, describe the impact of inhaled bronchodilator use on the measurement of FVC (and other spirometric measures) and clinical trial design.
MATERIALS AND METHODS
Patients were retrospectively identified from two ongoing longitudinal cohorts of IPF patients - one at the University of California San Francisco (San Francisco, CA) and the other at the Mayo Clinic, Rochester (Rochester, MN). Patients were enrolled between the years 2001 and 2013, and all patients provided informed consent to use their de-identified data for future research. Institutional review board at both institutions approved this study. As part of the parent cohort studies, all patients were prospectively evaluated with multidisciplinary review, and a diagnosis of IPF was made in accordance with consensus guidelines.[3, 20] Patients with IPF and at least one spirometry test with pre and post-bronchodilator values were included in the current study.
Patient demographics, clinical characteristics, self-reported comorbidities, inhaled medication use, and spirometry results (FVC, forced expiratory volume in 1 second (FEV1), and FEV1/FVC ratio) were extracted. All spirometry testing was performed as part of the patients’ clinical care in certified pulmonary function laboratories according to American Thoracic Society / European Respiratory Society standards.[21] The best spirometric maneuver was selected according to guidelines. All available pre and post-bronchodilator spirometry tests were included (i.e. patients could contribute more than one spirometry test over time). A 12% and at least 200 milliliter increase in FEV1 or FVC following bronchodilator was considered physiological evidence of clinically significant reversible airflow limitation. An obstructive pattern on spirometry was defined as an FEV1/FVC ratio < 0.7.[22]
Conventional statistics (mean, standard deviation, median, range) were used to describe the study population. The difference between pre and post-bronchodilator values within the same spirometry test (defined as the intra-test difference) was calculated with statistical significance determined by the paired t-test. Mixed effects regression models were used to determine if baseline demographics and clinical characteristics were predictive of the observed intra-test difference.
The test characteristics of the change in FVC measurement over time (defined as the inter-test difference) were assessed in the subgroup of patients with at least two spirometry tests separated by 6 (+/− 3) months. Mixed effects regression models were used to determine if baseline demographics and clinical characteristics were predictive of the observed inter-test difference. The mean and standard deviation of the 6-month change in FVC (both pre and post-bronchodilator) were calculated. Confidence intervals and p-values for the inter-test difference in FVC were obtained using bootstrapping. To demonstrate the impact of bronchodilator use on sample size requirements for a hypothetical clinical trial in IPF using FVC as a primary endpoint, we calculated the sample size needed to provide 90% power with a 2-sided type-I error rate of 5% at varying effect sizes. The standard deviation from the inter-test difference in FVC for both pre and post-bronchodilator measurements were used for the sample size calculations. Statistical analyses were performed in Stata (Version 13.1 StataCorp, College Station, Texas) and R (Version 3.0.2, R Foundation for Statistical Computing, Vienna).
RESULTS
Patient Characteristics
There were 551 patients who met inclusion criteria, contributing 967 unique spirometry tests with pre and post-bronchodilator values. Baseline demographics and clinical characteristics are detailed in Table 1. There were no significant differences in patient characteristics between the two parent cohorts (data not shown). The mean age was 69.3 years (SD ± 8.7 years) and 71% of patients were male. The majority of patients (71%) had a history of smoking (either current or former). A self-reported diagnosis of asthma was present in 9% of patients, and 14% reported a history of chronic obstructive pulmonary disease (COPD). Regular bronchodilator use at time of entry into the parent cohorts was reported in 30% of patients.
Table 1.
Baseline patient characteristics
| Mean or N (Total N = 551) | Standard deviation or % | |
|---|---|---|
| Age (years) | 69.3 | 8.7 |
| Male sex | 390 | 70.9% |
| Smoker (former or current) | 387 | 70.5% |
| Self reported co-morbid obstructive lung disease | ||
| Asthma | 48 | 8.7% |
| Chronic obstructive pulmonary disease | 78 | 14.2% |
| Bronchodilator medications | ||
| Bronchodilator* | 165 | 30% |
| Inhaled corticosteroid** | 101 | 18.3% |
| Long-term oxygen therapy | 182 | 33.6% |
| Pulmonary symptoms and signs | ||
| Cough | 460 | 83.8% |
| Wheezing on exam | 21 | 3.9% |
| Crackles on exam | 519 | 95.9% |
Bronchodilators include albuterol (n= 101), ipratropium (n= 46), salmeterol (n= 59), formoterol (n=8), tiotropium (n=29), levalbuterol (n=1), pirbuterol (n=1). Some patients reported multiple bronchodilators.
Inhaled corticosteroids include fluticasone (n=66), budesonide (n=20), mometasone (n=3), beclomethasone (n=4), flunisolide (n=2). Some patients reported multiple inhaled corticosteroids.
Intra-test difference
The mean pre-bronchodilator FVC was 2.71 liters; the mean post-bronchodilator FVC was 2.75 liters (Table 2). The mean intra-test difference in FVC with bronchodilator use was 0.043 liters, or 1.6% of the mean pre-bronchodilator FVC value. Similar findings were noted for FEV1. There were no clinical variables that significantly predicted intra-test change in FEV1 after bronchodilator use, including a diagnosis of concomitant obstructive lung disease (asthma and COPD) and inhaled bronchodilator/corticosteroid use (Supplementary Table 1). Male gender and presence of wheezing were predictive of intra-test change in FVC on unadjusted analysis. Clinically significant reversible airflow limitation was seen in 50 (9.1%) patients and in 58 (6%) spirometry tests (Figure 1). An obstructive pattern on spirometry was seen in 31 (5.6%) patients and in 46 (4.8%) spirometry tests and was correlated with the presence of reversible airflow limitation (odds ratio 2.51, 95% CI 1.02 to 6.18). Patients with reversible airflow limitation did not differ significantly from IPF patients without airflow limitation in terms of their demographics, smoking history, or self-reported comorbid obstructive airways disease.
Table 2.
|
Table 2a. Intra-test difference in spirometry after bronchodilator
| ||||
|---|---|---|---|---|
| N | Pre-BD mean (SD) | Post-BD mean (SD) | p-value | |
|
Intra-test difference
| ||||
| Baseline FVC (L) | 967 | 2.71 (0.79) | 2.75 (0.80) | <0.001 |
| Baseline FEV1 (L) | 967 | 2.22 (0.85) | 2.27 (0.63) | 0.03 |
| Baseline FEV1/FVC ratio | 967 | 0.83 (0.27) | 0.83 (0.07) | 0.71 |
| Table 2b. Inter-test difference in spirometry after bronchodilator | ||||
|---|---|---|---|---|
| N | Pre-BD | Post-BD | p-value | |
|
Inter-test difference
| ||||
| Mean 6-month change in FVC (L) | 322 | −0.077 | −0.080 | 0.65 |
| SD of change (L) | 322 | 0.267 | 0.260 | 0.40 |
| 95% CI for SD (L) | 322 | 0.241 to 0.293 | 0.236 to 0.283 | -- |
Abbreviations: BD = bronchodilator; FVC = forced vital capacity; FEV1 = forced expiratory volume in one second; L = liters; SD = standard deviation; CI, confidence interval.
Figure 1.
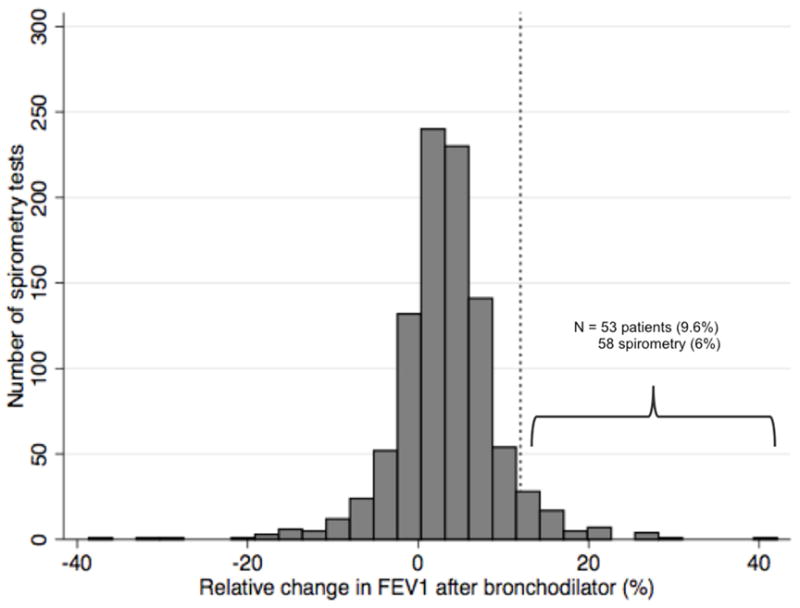
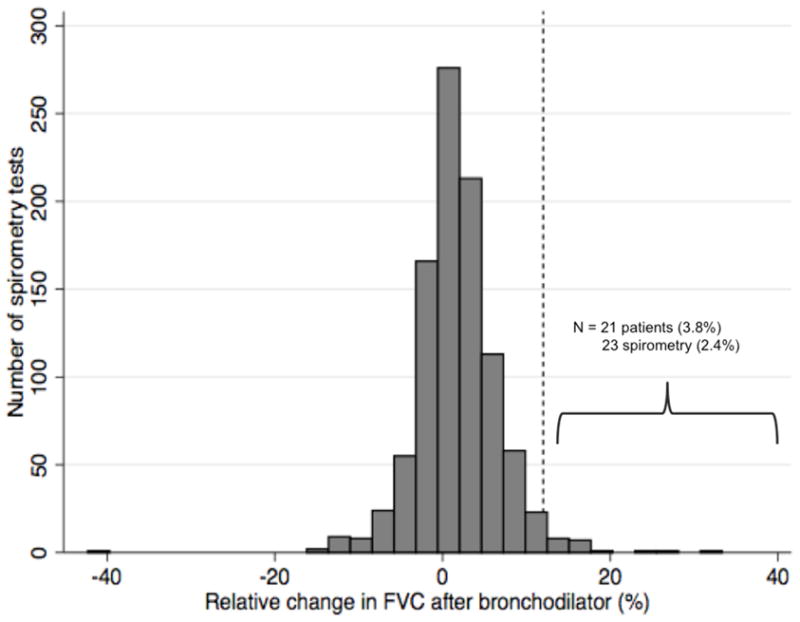
Distribution of change in FEV1 and FVC with bronchodilator use
N = 967 unique spirometry tests with pre and post-bronchodilator measurements of FEV1 (a) and FVC (b). The dashed vertical line represents a 12% improvement in FEV1 or FVC following bronchodilator, corresponding to physiologic airflow limitation.
Interestingly, the presence of reversible airflow limitation on one test did not predict the presence of reversible airflow limitation on a second test. Figure 2 illustrates the intra-test difference in FEV1 and FVC after bronchodilator in patients with two or more tests over time and at least one test with significant reversible airflow limitation. Of these 33 patients, only 5 (15%) had two or more tests with a 12% or greater increase in FEV1 following bronchodilator.
Figure 2.
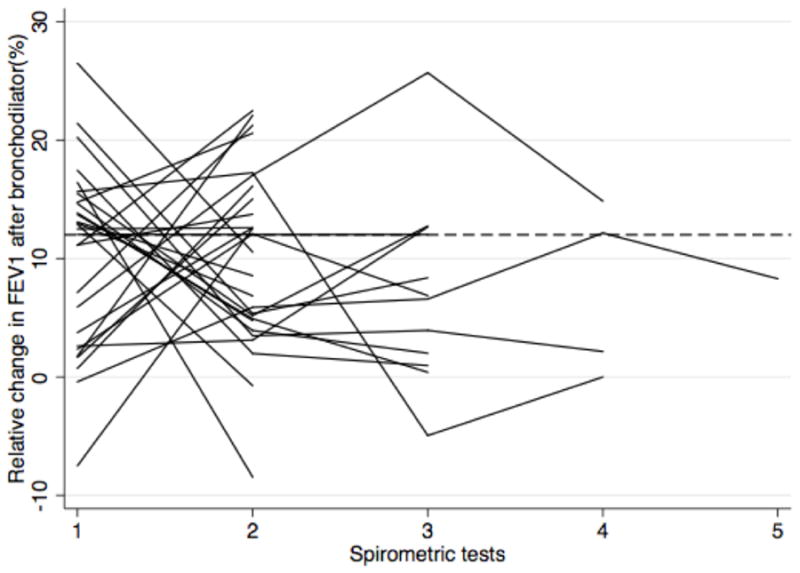
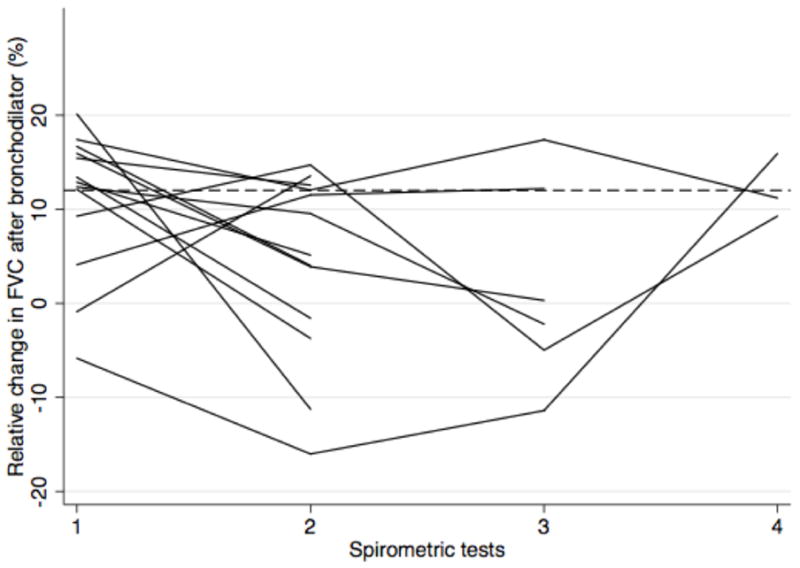
Persistence of reversible airflow limitation across multiple spirometric tests
Of the patients with multiple spirometry tests over time, 33 demonstrated reversible airflow limitation in at least one test by FEV1 criteria (a) and 13 by FVC criteria (b). Of those, only 5 patients had consistent evidence of reversible airflow limitation across all tests.
Inter-test difference
There were 194 patients who contributed 322 unique pairs of serial spirometry tests to the inter-test analysis. There were no significant differences in demographics or clinical characteristics between this subgroup and the overall cohort (data not shown). The inter-test difference over 6 months was −0.077 liters (2.8% decline, standard deviation 0.267 liters) using the pre-bronchodilator FVC values, and −0.080 liters (2.9% decline, standard deviation 0.260 liters) using the post-bronchodilator FVC values (Table 2). These point estimates and standard deviations were not significantly different. The presence of cough was negatively associated with, and the use of long-term oxygen therapy was positively associated with, the inter-test change in FVC, with or without bronchodilator use (Supplementary Table 2).
Study design implications
Figure 3 shows estimated sample sizes at varying effect sizes for hypothetical clinical trials in IPF using change in FVC over time as the primary endpoint, for pre and post bronchodilator FVC. There were no significant differences in sample size requirements noted with the use of pre or post bronchodilator FVC values. For example, a hypothetical clinical trial with a 30% between group difference in longitudinal change in FVC and a two-sided alpha of 0.05 would require 1972 subjects (95% CI 1608 to 2378) based on pre-bronchodilator FVC values versus 1868 subjects (95% CI 1542 to 2222) based on post-bronchodilator FVC values.
Figure 3.
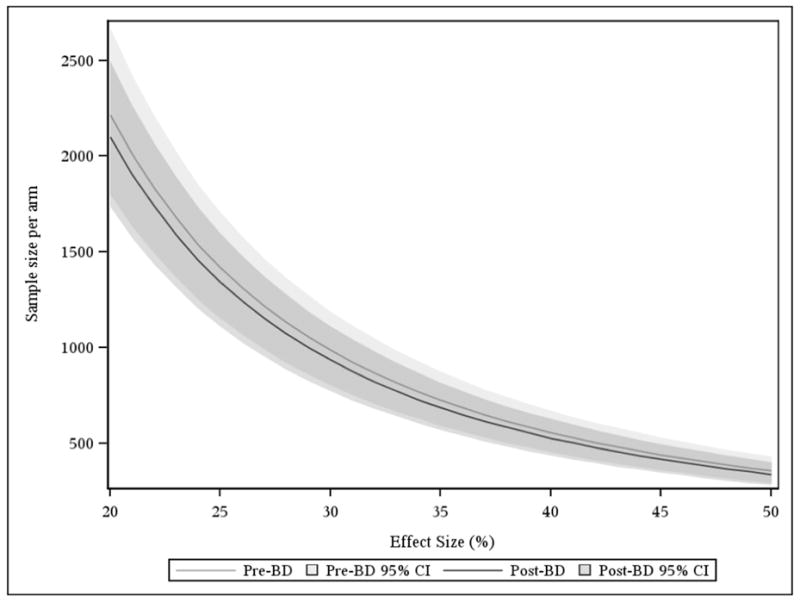
Sample size requirements using pre or post-bronchodilator FVC as the primary endpoint in a clinical trial.
The sample size in each study arm required for a hypothetical trial in IPF is slightly smaller when using the post-bronchodilator FVC measurement, however the 95% confidence intervals have substantial overlap. For purposes of demonstration, two-sided alpha is fixed at 0.05 and power is fixed at 0.9.
DISCUSSION
In a large, well-characterized cohort of patients with IPF, we found that approximately one in ten IPF subjects demonstrated spirometric evidence of clinically significant reversible airflow limitation, although this was true for less than 4% of subjects if using FVC change alone. Reversibility correlated with the presences of an obstructive pattern on spirometry, but did not correlate with a self-reported diagnosis of asthma or COPD. In patients with multiple spirometric measurements, past reversibility did not predict future reversibility. These findings suggest that reversible airflow limitation is present in a subgroup of patients with IPF, but that screening populations of IPF patients cross-sectionally by history of obstructive lung disease or previous spirometry is unlikely to reliably identify them.
In clinical practice, baseline FVC is used as the primary measure of disease severity and is a central contributor to risk prediction.[23, 24] Importantly, patients with measured reductions of 10% or greater in FVC over time are classified as having disease progression, and decisions about medical management and timing of transplantation are made based on this distinction. Our data suggests that forgoing bronchodilator use prior to FVC measurement introduces a small but real risk of misclassification due to the potential for episodic reversible airflow limitation. For example, a patient with non-progressing IPF could have an FVC of 2.00 liters that declines 10% to 1.80 liters due to the presence of reversible airflow limitation at the time of follow up spirometry (a false-positive for disease progression). Alternatively, a patient with progressive IPF could have an FVC of 2.00 liters that remains stable due to the absence of reversible airflow limitation at follow-up that was present at baseline (a false negative for disease progression).
An alternative explanation for our findings is the inherent variability of spirometric measurements. Perhaps the patients we classify as having “clinically significant reversible airflow limitation” simply represent the extreme range of the intrinsic inter-test variability of FEV1 and FVC measurement. Aside from the correlation of reversibility with evidence of obstructive physiology, there is little correlation with clinical features suggesting concomitant obstructive lung disease (e.g. a history of asthma or COPD, the use of bronchodilators). Arguing against this is the statistically significant increase in both FVC and FEV1 after bronchodilator use demonstrated in our cohort; if observed reversibility was completely explained by technical issues, we would expect to see the mean values unaffected. Future research into the true prevalence and clinical significance of concomitant obstructive airways disease and reversible airflow limitation in the IPF population is needed. If our findings are confirmed, we believe the risk of misclassifying patients as progressors or non-progressors using pre-bronchodilator spirometry is clinically significant, and that the use of post-bronchodilator FVC values might help mitigate this risk.
Change in FVC over time is an important endpoint in IPF; indeed, it has been the primary endpoint in multiple clinical trials.[8–15] Clinical trials have approached the issue of pre-spirometric bronchodilator use differently, and guidance on this issue is lacking. We found no significant impact of bronchodilator use before FVC measurement on sample size requirements in clinical trials using change in FVC over time as the primary endpoint. This is likely because the number of patients who have significant reversible airflow limitation is small and the risk of misclassification two-sided. This is not to say there isn’t risk of misclassification from reversible airflow limitation in individual patients (as discussed in the above paragraphs); simply that the risk does not impact the precision (i.e. standard deviation) of the mean change in FVC in groups of patients over time. Our results suggest that using post-bronchodilator FVC offers limited benefits to study design considerations in clinical trials with FVC as a primary endpoint. Because of the added complexity, cost, and risk of including bronchodilator use pre-spirometry in clinical trial protocols, clinical trialists should carefully consider their use.
These results should be interpreted in the context of several limitations. First, all spirometry test results were analyzed retrospectively, and were performed at different clinical centers. There are likely local differences in technique that could have affected the results. In addition, only the numerical values of the best test per spirometry testing session were available for review. Flow-volume loops and other measures of test quality were not assessed. Secondly, although it is standard practice to withhold regular daily bronchodilator use before spirometry testing, we do not know that this was done in all cases. Failure to withhold regular bronchodilator use would likely result in fewer patients with a significant intra-test bronchodilator response, meaning we may have underestimated the prevalence of reversible airflow limitation. For these reasons and for validation of these findings, this analysis should be repeated in a prospectively collected, quality controlled cohort of patients such as those enrolled in clinical trials.
CONCLUSIONS
In conclusion, we believe that the use of bronchodilator prior to FVC measurement in patients with IPF may reduce the risk of misclassification of disease progression due to the variable presence of reversible airflow limitation in a minority of IPF patients. If confirmed, this suggests post-bronchodilator spirometry may be preferred in clinical practice. Conversely, in the context of clinical trials, using change in FVC as a primary endpoint, post-bronchodilator FVC appears to offer no clear study design benefit and should be avoided. These conclusions should be validated using data from prospective trials in IPF.
Supplementary Material
Table A1.
Association with intra-test difference in pulmonary function after bronchodilator
| Difference in FVC with BD | Difference in FEV1 with BD | |||||
|---|---|---|---|---|---|---|
| Predictors | Coefficient | 95% CI | p-value | Coefficient | 95% CI | p-value |
| Age | −0.001 | −0.002 to 0 | 0.06 | −0.003 | −0.007 to 0.002 | 0.27 |
| Male gender | 0.024 | 0.005 to 0.043 | 0.01 | −0.005 | −0.092 to 0.081 | 0.90 |
| Smoking | 0.016 | −0.03 to 0.035 | 0.09 | −0.027 | −0.112 to 0.059 | 0.54 |
| Asthma | −0.008 | −0.039 to 0.023 | 0.61 | −0.005 | −0.15 to 0.14 | 0.94 |
| COPD | −0.001 | −.025 to 0.024 | 0.94 | −0.014 | −0.125 to 0.097 | 0.80 |
| Bronchodilator | 0.003 | −0.016 to 0.022 | 0.74 | 0.012 | −0.073 to 0.098 | 0.77 |
| ICS | −0.005 | −0.028 to 0.017 | 0.63 | 0.009 | −0.093 to 0.111 | 0.86 |
| LTOT | 0.014 | −0.04 to 0.032 | 0.13 | −0.043 | −0.126 to 0.039 | 0.30 |
| Cough | −0.012 | −0.048 to 0.024 | 0.51 | −0.032 | −0.138 to 0.074 | 0.55 |
| Crackles | 0.019 | −0.027 to 0.065 | 0.83 | 0.017 | −0.197 to 0.23 | 0.87 |
| Wheezing | 0.061 | 0.014 to 0.107 | 0.01 | 0.023 | −0.194 to 0.024 | 0.83 |
Abbreviations: BD, bronchodilators; COPD, chronic obstructive pulmonary disease; FVC, forced vital capacity; FEV1, forced expiratory volume in one second; ICS, inhaled corticosteroids; LTOT, long-term oxygen therapy.
Table A2.
Association with inter-test difference in FVC over 6 month for pre and post-bronchodilator measurements
| Inter-test difference in FVC pre-BD | Inter-test difference in FVC post-BD | |||||
|---|---|---|---|---|---|---|
| Predictors | Coefficient | 95% CI | p-value | Coefficient | 95% CI | p-value |
| Age | 0 | −0.004 to 0.004 | 0.9 | −0.001 | −0.004 to 0.003 | 0.71 |
| Male gender | −0.072 | −0.145 to 0.002 | 0.05 | −0.066 | −0.134 to 0.001 | 0.05 |
| Smoking | −0.015 | −0.088 to 0.058 | 0.68 | −0.006 | −0.074 to 0.061 | 0.85 |
| Asthma | 0.045 | −0.099 to 0.189 | 0.54 | 0.061 | −0.071 to 0.197 | 0.38 |
| COPD | −0.074 | −0.171 to 0.023 | 0.13 | −0.027 | −0.117 to 0.062 | 0.55 |
| Bronchodilator | 0.018 | −0.056 to 0.092 | 0.63 | −0.002 | −0.071 to 0.067 | 0.95 |
| ICS | −0.05 | −0.144 to 0.044 | 0.30 | −0.037 | −0.124 to 0.051 | 0.41 |
| LTOT | 0.073 | 0.005 to 0.14 | 0.03 | 0.072 | 0.01 to 0.134 | 0.02 |
| Cough | −0.13 | −0.216 to −0.043 | <0.001 | −0.126 | −0.206 to −0.045 | <0.001 |
| Crackles | 0.063 | −0.154 to 0.281 | 0.56 | 0.134 | −0.075 to 0.345 | 0.21 |
| Wheezing | 0.155 | −0.024 to 0.334 | 0.09 | 0.167 | −0.001 to 0.335 | 0.05 |
Highlights.
Forced vital capacity is a key measure of disease severity in idiopathic pulmonary fibrosis
One in ten patients with IPF have reversible airflow obstruction
Bronchodilator use in IPF may improve assessment of disease progression based on FVC change over time.
Bronchodilator use does not significantly impact the precision of FVC as a primary endpoint in clinical trials
Acknowledgments
Funding information: FRQS/MSSS Resident Physician Health Research Career Program, Fonds de Recherche en Santé du Québec, Québec, Canada; NIH P01 HL108794, and the Nina Ireland Program for Lung Health.
Abbreviations list
- BD
bronchodilators
- COPD
chronic obstructive pulmonary disease
- FEV1
forced expiratory volume in one second
- FVC
forced vital capacity
- IPF
idiopathic pulmonary fibrosis
- SD
standard deviation
Footnotes
The authors report no conflict of interest pertaining to this manuscript.
Prior abstract presentation: American Thoracic Society International Conference, San Diego, California, USA; May 16 to 21, 2014.
AUTHORSHIP AND CONTRIBUTORSHIP STATEMENT
All authors have made substantial contributions to the conception and design of the study, the interpretation of the data, revised the draft critically for intellectual content, and approved the final version of this manuscript to be submitted.
DA designed the study, monitored data collection, wrote the statistical analysis plan, cleaned and analysed the data, and drafted and revised the paper. She is the guarantor. EV contributed to the design of the study, wrote the statistical analysis plan, performed data analysis, contributed to the interpretation of the data, and revised the paper for critical content. CJR, EC, RT and XH all performed data collection, contributed to the design of the study, and revised the paper for intellectual content. BME, JAG, KDJ, TEK, LLK, JSL, BL, AKS and PJW all provided substantial contributions to the design and conception of the study, the interpretation of the data and results, and revised the paper critically for important intellectual content. JHR provided access to data, contributed to the design of the study, revised the manuscript critically for key intellectual content. HRC designed the study, wrote the statistical analysis plan, drafted and revised the paper. He also is the guarantor of the study. All authors have approved the final version of the manuscript to be submitted and published.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pellegrino R, et al. Interpretative strategies for lung function tests. European Respiratory Journal. 2005;26(5):948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 2.Miller MR, et al. Standardisation of spirometry. European Respiratory Journal. 2005;26 (2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 3.Raghu G, et al. An Official ATS/ERS/JRS/ALAT Statement: Idiopathic Pulmonary Fibrosis: Evidence-based Guidelines for Diagnosis and Management. American Journal of Respiratory and Critical Care Medicine. 2011;183(6):788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flaherty KR, et al. Prognostic Implications of Physiologic and Radiographic Changes in Idiopathic Interstitial Pneumonia. American Journal of Respiratory and Critical Care Medicine. 2003;168(5):543–548. doi: 10.1164/rccm.200209-1112OC. [DOI] [PubMed] [Google Scholar]
- 5.Collard HR, et al. Changes in Clinical and Physiologic Variables Predict Survival in Idiopathic Pulmonary Fibrosis. American Journal of Respiratory and Critical Care Medicine. 2003;168(5):538–542. doi: 10.1164/rccm.200211-1311OC. [DOI] [PubMed] [Google Scholar]
- 6.Latsi PI, et al. Fibrotic Idiopathic Interstitial Pneumonia. American Journal of Respiratory and Critical Care Medicine. 2003;168(5):531–537. doi: 10.1164/rccm.200210-1245OC. [DOI] [PubMed] [Google Scholar]
- 7.King TE, et al. Analyses of efficacy end points in a controlled trial of interferon-γ1b for idiopathic pulmonary fibrosis*. CHEST Journal. 2005;127(1):171–177. doi: 10.1378/chest.127.1.171. [DOI] [PubMed] [Google Scholar]
- 8.Raghu G, et al. A Placebo-Controlled Trial of Interferon Gamma-1b in Patients with Idiopathic Pulmonary Fibrosis. New England Journal of Medicine. 2004;350(2):125–133. doi: 10.1056/NEJMoa030511. [DOI] [PubMed] [Google Scholar]
- 9.Demedts M, et al. High-Dose Acetylcysteine in Idiopathic Pulmonary Fibrosis. New England Journal of Medicine. 2005;353(21):2229–2242. doi: 10.1056/NEJMoa042976. [DOI] [PubMed] [Google Scholar]
- 10.King TE, et al. BUILD-3: A Randomized, Controlled Trial of Bosentan in Idiopathic Pulmonary Fibrosis. American Journal of Respiratory and Critical Care Medicine. 2011;184(1):92–99. doi: 10.1164/rccm.201011-1874OC. [DOI] [PubMed] [Google Scholar]
- 11.Noble PW, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. The Lancet. 377(9779):1760–1769. doi: 10.1016/S0140-6736(11)60405-4. [DOI] [PubMed] [Google Scholar]
- 12.Prednisone, Azathioprine, and N-Acetylcysteine for Pulmonary Fibrosis. New England Journal of Medicine. 2012;366(21):1968–1977. doi: 10.1056/NEJMoa1113354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taniguchi H, et al. Pirfenidone in idiopathic pulmonary fibrosis. European Respiratory Journal. 2010;35(4):821–829. doi: 10.1183/09031936.00005209. [DOI] [PubMed] [Google Scholar]
- 14.Daniels CE, et al. Imatinib Treatment for Idiopathic Pulmonary Fibrosis. American Journal of Respiratory and Critical Care Medicine. 2010;181(6):604–610. doi: 10.1164/rccm.200906-0964OC. [DOI] [PubMed] [Google Scholar]
- 15.Richeldi L, et al. Efficacy of a Tyrosine Kinase Inhibitor in Idiopathic Pulmonary Fibrosis. New England Journal of Medicine. 2011;365(12):1079–1087. doi: 10.1056/NEJMoa1103690. [DOI] [PubMed] [Google Scholar]
- 16.Collard HR, et al. Burden of illness in idiopathic pulmonary fibrosis. Journal of Medical Economics. 2012;15(5):829–835. doi: 10.3111/13696998.2012.680553. [DOI] [PubMed] [Google Scholar]
- 17.Ryerson CJ, et al. Clinical features and outcomes in combined pulmonary fibrosis and emphysema in idiopathic pulmonary fibrosis. CHEST Journal. 2013;144(1):234–240. doi: 10.1378/chest.12-2403. [DOI] [PubMed] [Google Scholar]
- 18.Mejía M, et al. Idiopathic pulmonary fibrosis and emphysema: Decreased survival associated with severe pulmonary arterial hypertension. CHEST Journal. 2009;136(1):10–15. doi: 10.1378/chest.08-2306. [DOI] [PubMed] [Google Scholar]
- 19.Cottin V, et al. Combined pulmonary fibrosis and emphysema: a distinct underrecognised entity. European Respiratory Journal. 2005;26(4):586–593. doi: 10.1183/09031936.05.00021005. [DOI] [PubMed] [Google Scholar]
- 20.American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS) Am J Respir Crit Care Med. 2000;161(2 Pt 1):646–64. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 21.Miller MR, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 22.Global Strategy for Diagnosis, M., and Prevention of COPD. Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2015. 2015. [Google Scholar]
- 23.Ley B, et al. A Multidimensional Index and Staging System for Idiopathic Pulmonary Fibrosis. Annals of Internal Medicine. 2012;156(10):684–691. doi: 10.7326/0003-4819-156-10-201205150-00004. [DOI] [PubMed] [Google Scholar]
- 24.Ley B, et al. Unified baseline and longitudinal mortality prediction in idiopathic pulmonary fibrosis. European Respiratory Journal. 2015 doi: 10.1183/09031936.00146314. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


