Abstract
Manganese in excess promotes unstable emotional behavior. Our previous study showed that olfactory manganese uptake into the brain is altered in Hfe−/− mice, a model of iron overload hemochromatosis, suggesting that Hfe deficiency could modify the neurotoxicity of airborne manganese. We determined anxiety-related behavior and monoaminergic protein expression after repeated intranasal instillation of MnCl2 to Hfe−/− mice. Compared with manganese-instilled wild-type mice, Hfe−/− mice showed decreased manganese accumulation in the cerebellum. Hfe−/− mice also exhibited increased anxiety with decreased exploratory activity and elevated dopamine D1 receptor and norepinephrine transporter in the striatum. Moreover, Hfe deficiency attenuated manganese-associated impulsivity and modified the effect of manganese on the expression of tyrosine hydroxylase, vesicular monoamine transporter and serotonin transporter. Together, our data indicate that loss of HFE function alters manganese-associated emotional behavior and further suggest that HFE could be a potential molecular target to alleviate affective disorders induced by manganese inhalation.
Keywords: dopamine, elevated plus maze, impulsivity, intranasal instillation, norepinephrine, serotonin
1. INTRODUCTION
Manganese (Mn) is an essential metal required for proper brain function, serving as a cofactor of several critical enzymes, including superoxide dismutase, glutamine synthetase and arginase (Horning et al., 2015; Takeda, 2003). However, when over-deposited in the brain, manganese promotes neurotoxicity, which is characterized by memory loss, impaired motor coordination and psychotic behavior resembling Parkinson’s disease (Avila et al., 2013). Manganese absorption from the gastrointestinal tract is limited due to first-pass elimination via biliary excretion (Roth, 2006). Hence, the enteral route provides a protective barrier against manganese poisoning by ingestion, such as drinking water or acute intoxication of dietary manganese. However, manganese bioavailability after inhalation is much greater than that after oral exposure due to lack of presystemic (hepatic) clearance mechanism (Brenneman et al., 2000). Moreover, the close proximity of the olfactory tract to the brain enables inhalation to represent the primary route of exposure for manganese neurotoxicity (Brenneman et al., 2000; Tjalve et al., 1996). This has raised significant concerns about Mn toxicity in human health, in particular for people in occupational settings (Avila et al., 2013), such as workers employed in mining and Mn ore processing (Park et al., 2005) and agricultural workers exposed to Mn-containing pesticide (Lucchini et al., 2009).
Neurological problems resulting from Mn intoxication are associated with altered monoaminergic signaling pathways (Guilarte, 2013; Subhash and Padmashree, 1990), which are involved in controlling emotional behavior (Kern et al., 2010; Li et al., 2011). For example, the enzymatic activity of tyrosine hydroxylase (TH), a critical enzyme for catecholamine synthesis, is impaired upon Mn exposure (Zhang et al., 2011). Norepinephrine transporter (NET) is differentially regulated in different brain regions upon Mn exposure (Anderson et al., 2009). In addition, it is well-documented that Mn exposure significantly affects dopaminergic function in the striatum (Kim et al., 2012; Subhash and Padmashree, 1990). Moreover, increasing evidence indicates that abnormal emotion state and general activity in several affective disorders are associated with altered dopaminergic pathway in the striatum (Fusar-Poli et al., 2012; Krause et al., 2000). These results suggest that striatal monoamine homeostasis is impaired by airborne manganese, which could be a potential risk for the development of psychiatric disorders.
A large body of evidence has indicated that manganese absorption is enhanced in iron-deficient anemia due to iron-responsive up-regulation of metal transporters (Erikson et al., 2002; Kim et al., 2012; Thompson et al., 2007), suggesting that altered body iron status can influence manganese transport and toxicity. However, the information about the role of iron overload in Mn’s neurotoxic effects is scarce. This question is particularly important because of a high prevalence of the iron overload disorder hemochromatosis, which is one of the most common genetic diseases in the North American Caucasian population (Merryweather-Clarke et al., 2000; Pietrangelo, 2004). Mutations of the HFE (High iron or Fe) gene are the primary cause of this disease; the two most prevalent HFE missense variants are C282Y (7–17%) and H63D (10–32%) in the US population (Zhang et al., 2010). While airborne Mn exposure provides the greatest neurotoxic effects, whether or not HFE is the genetic determinant for manganese neurotoxicity has yet to be examined.
Our previous studies demonstrated that iron-loaded Hfe-deficient mice display increased olfactory uptake of Mn to the brain after a single intranasal dose of 54MnCl2, suggesting an increased vulnerability of Mn neurotoxicity in HFE-related hemochromatosis (Kim et al., 2013). However, we also found that the steady-state levels of Mn in blood are decreased in humans with HFE variants, as well as in Hfe-deficient mice (Claus Henn et al., 2011). These results suggest that HFE deficiency could increase the clearance of Mn from blood, thereby protecting the body against Mn toxicity. Therefore, it is necessary to directly determine if loss of HFE function could modify the neurotoxic effects of Mn. Since Mn exposure is associated with psychiatric and mood disorders (Avila et al., 2013; Tran et al., 2002), in the present study we tested emotional behavior after repeated intranasal instillations of manganese using the Hfe−/− mouse model that recapitulates iron overload hemochromatosis in humans (Levy et al., 2000). Our data demonstrate that olfactory exposure to manganese increases an impulsivity-like response, which is partly reversed by Hfe deficiency, suggesting that HFE could be a potential therapeutic target to alleviate emotional dysfunction induced by manganese inhalation.
2. MATERIALS AND METHODS
2.1. Ethics statement
This study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Northeastern University Animal Care and Use Committee.
2.2. Animals and manganese exposure
Breeders for Hfe-deficient (Hfe−/−) mice (Levy et al., 1999) and wild-type control (Hfe+/+) mice were kindly provided by Dr. Nancy Andrews (Duke University, NC, USA). All mice used for these studies were on the 129S6/SvEvTac background (Levy et al., 1999). One-month old male mice were fed facility chow (Prolab Isopro RMH 3000, LabDiet; 96 mg manganese and 380 mg iron per kg diet) and given water ad libitum. Neither manganese nor iron was detected in drinking water. Male mice were chosen because estrogen affects iron metabolism (Hou et al., 2012; Yang et al., 2012). For olfactory exposure to manganese, mice were intranasally-instilled daily with manganese chloride (5 mg MnCl2/kg body weight; 0.08 mL/kg) or double-distilled water as vehicle control for 22 days. This dose was chosen because we previously showed behavioral effects of intranasal Mn at 10 mg/kg of MnCl2 twice a week in rats (Kim et al., 2012).
2.3. Elevated plus maze test
After the last dose, all animals were subject to elevated plus maze (EPM) test in order to examine anxiety/impulsivity-like behavior (n = 10–13 per group). The EPM apparatus (Med Associates, St. Albans, VT, USA) was composed of two open arms (35 L × 6 W cm) and two closed arms (35 L × 6 W × 15 H cm, height of the wall), which extended from the central zone platform (6 L × 6 W cm). The maze was 70 cm above the floor. Each mouse was placed in the center zone facing an open arm and allowed to explore the maze for 5 min. The latency of the first entry into an open arm, the number of entries and time spent in the open arms, distance traveled in the open arms and total distance in the maze were recorded and analyzed by ANY-maze (Stoelting, Wood Dale, IL, USA). The maze was cleaned thoroughly with Quatricide TB (Pharmacal Research Laboratory Inc, Naugatuck, CT, USA) after each test.
2.4. Tissue collection
After the EPM test, mice were euthanized by isoflurane overdose, followed by exsanguination to harvest brain and liver tissues. The brain samples were microdissected to collect different brain regions, including prefrontal cortex, striatum, hippocampus and cerebellum. All tissues were flash-frozen in liquid nitrogen and stored at −80°C until analysis.
2.5. Western blot analysis
The striatum was selected for western blot analysis because intranasal Mn alters dopamine-related proteins in the striatum (Kim et al., 2012), a brain region involved in emotional behavior (Fusar-Poli et al., 2012; Krause et al., 2000). Striatum tissues (n = 4 per group) were homogenized, electrophoresed on 10% SDS-polyacrylamide gels (30 μg proteins) and transferred to polyvinylidene difluoride membranes. After blocking with 5% non-fat milk, the membrane was incubated with different antibodies, including goat anti-DAT (1:200; Santa Cruz Biotech, Dallas, TX, USA), rabbit anti-D1R (1:500, Abcam, Cambridge, MA, USA), mouse anti-D2R (1:200, Santa Cruz), mouse anti-TH (1:200, Santa Cruz), rabbit anti-VMAT (1:200, Santa Cruz), rabbit anti-COMT (1:200, Santa Cruz), goat anti-serotonin transporter (SERT; 1:200, Santa Cruz) and rabbit anti-NET (1:200, Santa Cruz). As a loading control, the immunoblot was incubated with mouse anti-actin (1:5,000, MP Biomedicals, Solon, OH, USA). The blots were incubated with secondary antibodies conjugated with HRP, including donkey anti-goat IgG (1:1,000, Santa Cruz), goat anti-rabbit IgG (1:1,000, Santa Cruz) or sheep anti-mouse IgG (1:1,000 or 1:5,000, GE Healthcare, Piscataway, NJ, USA). Immunoreactivity was detected using ECL West Dura substrate (Thermo Scientific, Tewksbury, MA, USA). Protein bands were visualized by ChemiDoc XRS (Bio-Rad, Hercules, CA, USA) and intensities of protein bands were quantified using Image Lab (version 4.1, Bio-Rad).
2.6. Analysis of metals in the brain
Liver (n = 6–8 per group) and microdissected brain tissues (n = 6–7 per group) were digested in 0.5 mL of 20% nitric acid (Trace grade, Fisher Scientific; Pittsburgh, PA, USA) at 125°C for 1 h. After a complete digestion, the samples were diluted with metal-free double-distilled water up to a volume of 5 mL. Metal concentrations were determined by inductively coupled plasma mass spectrometry (ICP-MS) (Varian 810/820MS, Bruker, Billerica, MA, USA) and calculated as μg/g tissue.
2.7. Statistical analysis
Values reported were expressed as means ± SEM. A two-way ANOVA was performed using SigmaPlot (version 12.3; Systat Software Inc., San Jose, CA, USA) to determine individual main effects and an interaction effect between Hfe deficiency and olfactory manganese exposure. Post-hoc multiple comparisons were performed by the Holm-Sidak method. Differences were considered significant at P < 0.05.
3. RESULTS
3.1. Hfe deficiency alters the levels of iron and manganese in the brain
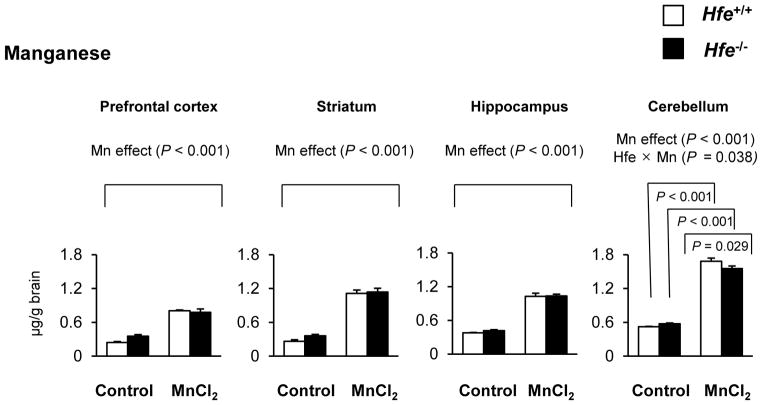
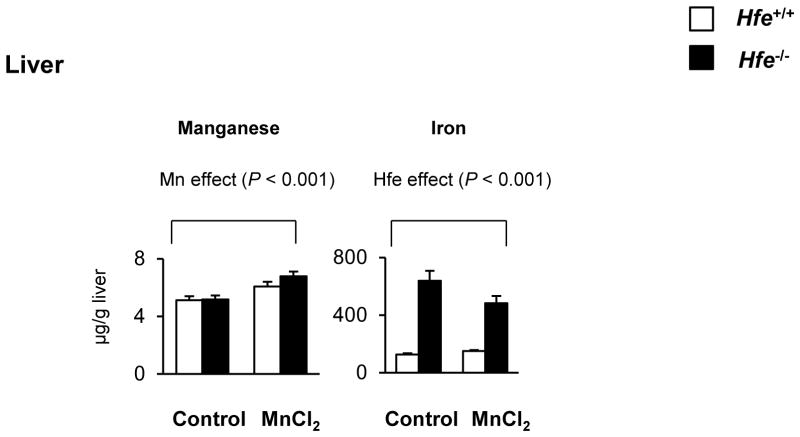
One-month-old mice received intranasal instillation of manganese chloride (5 mg/kg) or water (as vehicle control) for 3 weeks to characterize the influence of Hfe deficiency on the steady-state concentrations of manganese and iron (Figures 1 and 2). After Mn instillation, Mn concentrations increased in most brain regions, including prefrontal cortex, striatum, hippocampus and cerebellum, compared with water instillation (Figure 1, P < 0.001, Mn effect). Interestingly, Hfe−/− mice showed reduced accumulation of Mn in the cerebellum compared with Hfe+/+ mice (Figure 1, P = 0.029, Hfe effect), but not in the prefrontal cortex, striatum or hippocampus. In addition, Hfe−/− mice displayed elevated iron levels in the prefrontal cortex (Figure 2, P = 0.020), striatum (P = 0.002) and cerebellum (P = 0.012), indicating that HFE deficiency promotes iron accumulation in several brain regions. In the liver, Hfe−/− mice displayed significantly increased iron stores (Figure 3), verifying that Hfe deficiency promotes iron overload hemochromatosis. Mn exposure increased Mn levels in the liver, but did not affect hepatic iron stores regardless of Hfe expression.
Figure 1. Effect of intranasal manganese on the levels of manganese in different brain regions of Hfe-deficient mice.
Microdissected brain tissues, including prefrontal cortex, striatum, hippocampus and cerebellum, were collected from mice that were intranasally instilled with MnCl2 (5 mg/kg) or water. The steady-state concentrations of manganese were quantified by inductively coupled plasma mass spectrometry (ICP-MS; n = 7–8 per group). Empty and closed bars represent wild-type (Hfe+/+) and Hfe-deficient (Hfe−/−) mice, respectively. Data were presented as mean ± SEM and were analyzed using two-way ANOVA, followed by post-hoc comparisons. Hfe x Mn, an interaction effect between Hfe deficiency and olfactory Mn exposure.
Figure 2. Effect of intranasal manganese on the levels of iron in different brain regions of Hfe-deficient mice.
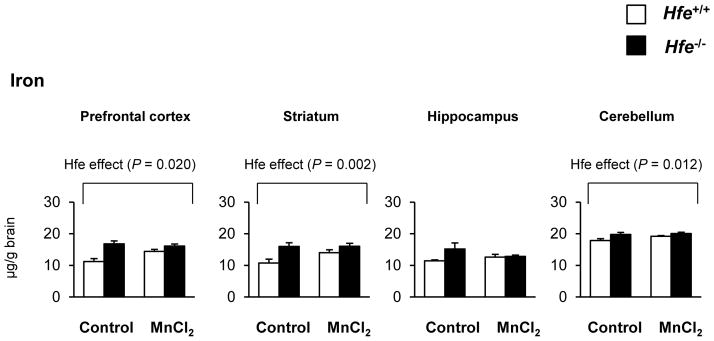
The steady-state concentrations of iron in microdissected brain tissues were quantified by ICP-MS (n = 6–7 per group). Empty and closed bars represent wild-type (Hfe+/+) and Hfe-deficient (Hfe−/−) mice, respectively. Data were presented as mean ± SEM and were analyzed using two-way ANOVA, followed by post-hoc comparisons.
Figure 3. Effect of intranasal manganese on the levels of manganese and iron in the liver of Hfe-deficient mice.
Metal levels in the liver collected from mice intranasally instilled with MnCl2 or water were determined by ICP-MS (n = 6–8 per group). Empty and closed bars represent wild-type (Hfe+/+) and Hfe-deficient (Hfe−/−) mice, respectively. Data were presented as mean ± SEM and were analyzed using two-way ANOVA, followed by post-hoc comparisons.
3.2. Levels of anxiety and impulsivity are altered upon olfactory Mn exposure and Hfe deficiency
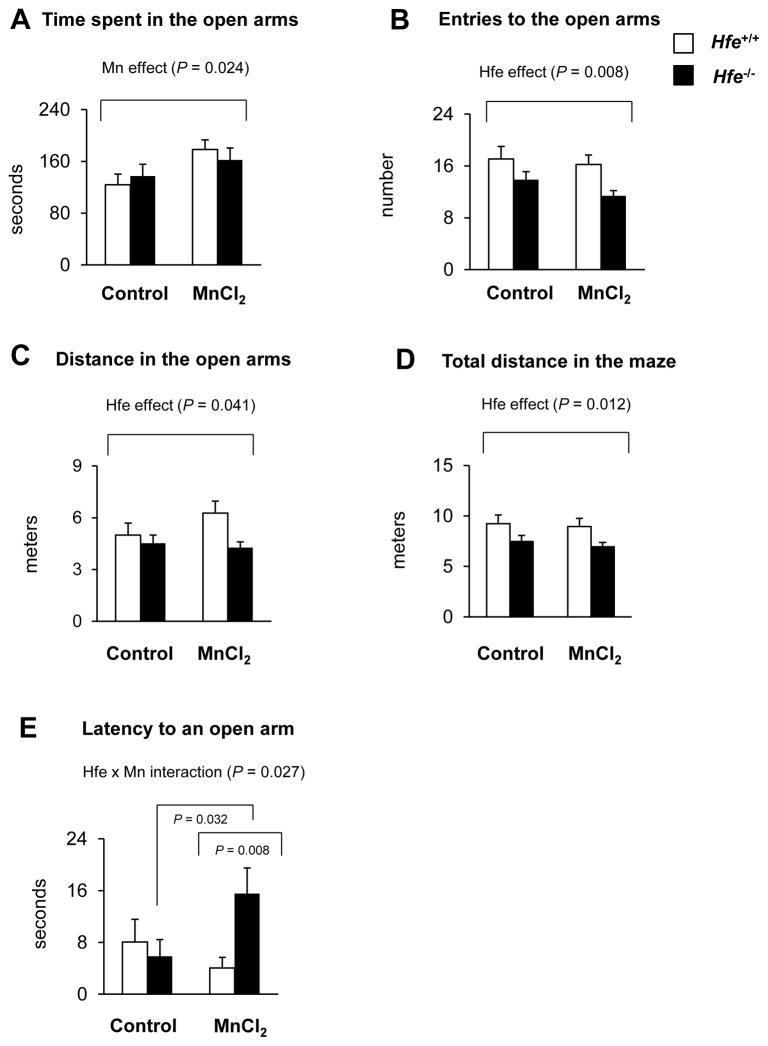
Hfe−/− and Hfe+/+ mice were intranasally-instilled with water or MnCl2 and tested for anxiety- and impulsivity-related responses using the elevated plus maze paradigm (Figure 4). We found differential effects of Mn exposure and Hfe deficiency on emotional behavior. First, Mn-instilled mice spent more time in the open arms compared with water-treated mice regardless of Hfe expression (Figure 4A; P = 0.024, Mn effect), indicating increased impulsivity-like behavior and/or reduced anxiety-like behavior. Second, Hfe-deficient mice entered into the open arms less frequently (Figure 4B; P = 0.008, Hfe effect) and traveled less distance in the open arms (Figure 4C; P = 0.041, Hfe effect). Total distance traveled in the whole maze was also significantly decreased in Hfe−/− mice (Figure 4D; P = 0.012, Hfe effect). These data suggest that Hfe deficiency could increase anxiety and/or decrease exploratory/locomotor activity. Mn exposure did not alter the distance traveled either in the open arms (Figure 4C) or in the whole maze (Figure 4D). Interestingly, latency of the first entry to an open arm was unchanged in Mn-instilled Hfe+/+ mice (P = 0.334), but significantly prolonged in Mn-instilled Hfe−/− mice (P = 0.032), compared with water-treated control mice of the respective genotype (Figure 4E; P = 0.027, Hfe x Mn interaction effect).
Figure 4. Effect of intranasal manganese on emotional behavior in Hfe-deficient mice.
Mice intranasally instilled with MnCl2 (5 mg/kg, daily) or water for 3 weeks were tested on the elevated plus maze in order to determine anxiety- and impulsivity-related behavior, including time in the open arms (A), entries to the open arms (B), distance in the open arms (C), total distance in the whole maze (D) and latency of the first entry to an open arm (E). Empty and closed bars represent wild-type (Hfe+/+) and Hfe-deficient (Hfe−/−) mice, respectively. Data were presented as mean ± SEM (n = 10–13 per group) and were analyzed using two-way ANOVA, followed by post-hoc comparisons.
3.3. Both intranasal Mn and Hfe deficiency modulate dopaminergic pathway in the striatum
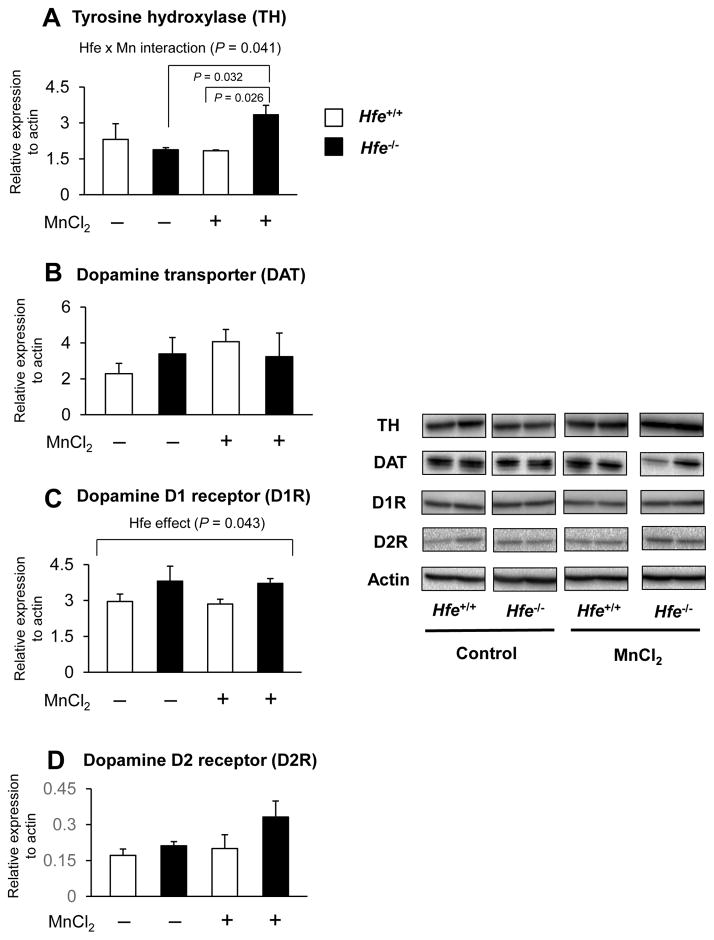
Since Mn’s neurotoxic effects are associated with impaired dopaminergic function, we determined the expression levels of dopamine-related proteins in the striatum by western blotting analysis (Figure 5). First, Hfe deficiency did not alter the expression of striatal TH, the rate-limiting enzyme for dopamine production. However, after repeated Mn instillation the TH levels were significantly increased in Hfe-deficient mice, but not in wild-type mice (Figure 5A; P = 0.041, Hfe x Mn interaction effect). In contrast, DAT, which influences synaptic dopamine levels, was unchanged among all four groups (Figure 5B). Second, since dopamine D1 and D2 receptors are two most abundant dopamine receptors expressed in the striatum (Jaber et al., 1996), we examined the effects of Mn and Hfe deficiency on the expression of these receptors. D1R was up-regulated in Hfe deficiency (P = 0.043, Hfe effect), whereas olfactory Mn exposure showed no effect on D1R expression (Figure 5C) in both genotypes. There was no statistical difference in D2R expression among the four groups (Figure 5D).
Figure 5. Effect of intranasal manganese on the expression of key proteins involved in dopaminergic pathway in the striatum from Hfe-deficient mice.
Striatum collected from mice was analyzed by western blot to determine the expression levels of tyrosine hydroxylase (A), dopamine transporter (B) and dopamine D1 (C) and D2 (D) receptors. Relative intensities of protein bands normalized to actin were determined using Image Lab (version 4.1). Empty and closed bars represent wild-type (Hfe+/+) and Hfe-deficient (Hfe−/−) mice, respectively. Data were presented as mean ± SEM (n = 4 per group) and were analyzed using two-way ANOVA, followed by post-hoc comparisons.
3.4. Other monoaminergic pathways are also affected by Mn instillation and Hfe deficiency
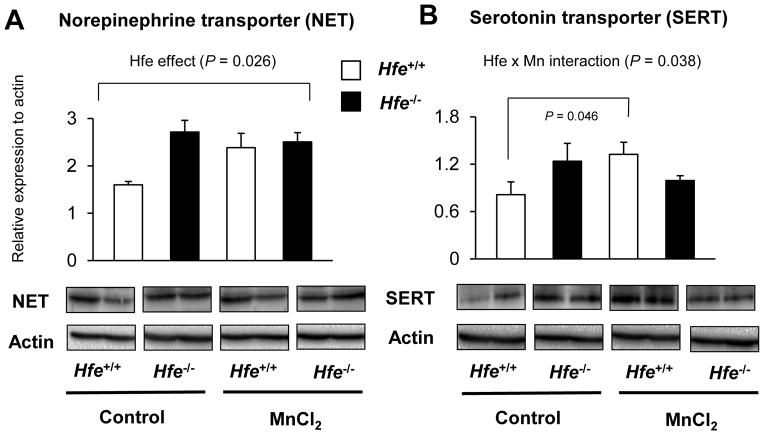
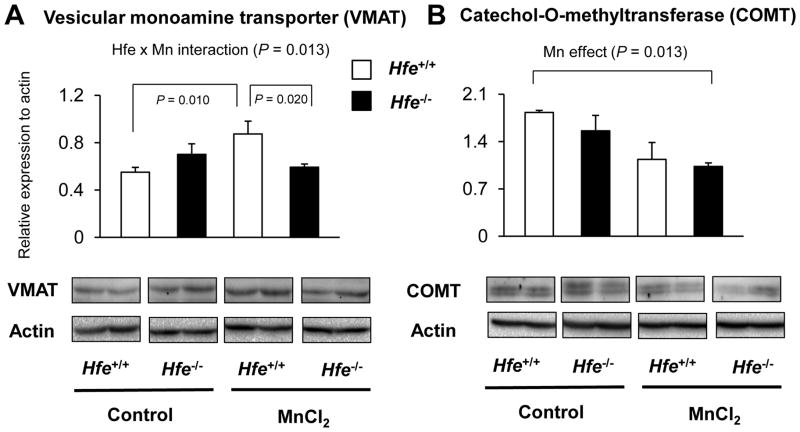
Since norepinephrine and serotonin also play an important role in emotional behavior and share several transporters and/or enzymes with dopamine, we characterized key proteins for norepinephrine and serotonin turnover in the striatum from Mn-instilled Hfe+/+ and Hfe−/− mice (Figure 6). First, we found that Hfe deficiency increased NET levels (P = 0.026). Second, Mn instillation increased SERT levels in Hfe+/+ mice (P = 0.046), but not in Hfe−/− mice (Figure 6B; P = 0.038, Hfe x Mn interaction effect). Third, we examined the expression levels of VMAT, a principal vesicular transporter for dopamine and other monoamines (Figure 7A); Mn exposure increased VMAT levels in wild-type mice (P = 0.010, Mn effect), but not in Hfe-deficient mice (P = 0.013, Hfe x Mn interaction effect). Moreover, Mn-instilled Hfe−/− mice displayed significantly lower VMAT levels compared with Mn-instilled Hfe+/+ mice (P = 0.020, Hfe effect). In addition, COMT, an enzyme involved in monoamine degradation, was significantly reduced by Mn exposure (P = 0.013, Mn effect), whereas Hfe deficiency did not alter COMT levels in the striatum (Figure 7B).
Figure 6. Effect of intranasal manganese on the expression of norepinephrine and serotonin transporters in the striatum from Hfe-deficient mice.
Striatum collected from mice was analyzed by western blot to determine the expression levels of norepinephrine transporter (A) and serotonin transporter (B). Relative intensities of protein bands were normalized to actin. Empty and closed bars represent wild-type (Hfe+/+) and Hfe-deficient (Hfe−/−) mice, respectively. Data were presented as mean ± SEM (n = 4 per group) and were analyzed using two-way ANOVA, followed by post-hoc comparisons.
Figure 7. Effect of intranasal manganese on the expression of proteins involved in monoaminergic turnover in the striatum from Hfe-deficient mice.
Striatum collected from mice was analyzed by western blot to determine the expression levels of vesicular monoamine transporter (A) and catechol-O-methyltransferase (B). Relative intensities of protein bands were normalized to actin. Empty and closed bars represent wild-type (Hfe+/+) and Hfe-deficient (Hfe−/−) mice, respectively. Data were presented as mean ± SEM (n = 4 per group) and were analyzed using two-way ANOVA, followed by post-hoc comparisons.
4. DISCUSSION
Airborne manganese exposure has raised huge global health concerns due to high systemic bioavailability of the metal and increased uptake into the brain. Manganese neurotoxicity has been best characterized by motor disturbance and psychotic disorders (Cotzias, 1958; Eriksson et al., 1987; Tran et al., 2002). It has been well-recognized that the absorption of manganese is mediated by several iron transporters, including the divalent metal transporter 1 (DMT1) and ferroportin (Chua and Morgan, 1997; Yin et al., 2010). In particular, DMT1 protein is predominantly found in normal airway and alveolar epithelium (Brain et al., 2006). Emerging evidence has suggested that pulmonary DMT1 functions to provide a protective barrier from toxic effects caused by inhaled metals and inflammatory responses (Ghio et al., 2007; Kim et al., 2011). Likewise, DMT1 is expressed in the olfactory epithelium where it transports Mn into the brain by an iron-responsive manner (Thompson et al., 2007). These results suggest that the altered expression of iron transporters in the airborne routes could modify the neurotoxicity of environmental metals.
Since defective Hfe function increases iron uptake by up-regulating iron transporters (Griffiths et al., 2001), it is important to explore the influence of Hfe-related hemochromatosis on olfactory Mn uptake and metal-associated neurotoxicity. In the present study we investigated the effect of intranasal Mn instillation in Hfe deficiency on emotional behavior using Hfe-knockout mice, a mouse model of Hfe-related hemochromatosis (Levy et al., 2000). Our data showed that Mn instillation increases impulsivity-like behavior, whereas Hfe deficiency decreases exploratory activity, which possibly results from elevated anxiety. Furthermore, Mn-instilled Hfe−/− mice exhibited decreased impulsivity-like behavior, compared with Mn-instilled wild-type mice, indicating that loss of Hfe function partially reverses or normalizes Mn-associated impulsivity. Combined, our results suggest that individuals with hemochromatosis may be less susceptible to Mn-induced emotional problems and further provides a therapeutic basis for psychiatric disorders associated with Mn exposure.
4.1. Effect of Hfe deficiency on brain Mn and Fe distribution after olfactory Mn exposure
In the present study, Mn levels in the brain were either decreased (cerebellum) or unchanged (the rest) in Hfe deficiency. This is different from our previous finding that olfactory uptake of manganese into the brain in Hfe−/− mice is increased compared with Hfe+/+ mice when determined 1 h post-instillation of 54MnCl2 (Kim et al., 2013). This suggests a possibility that Mn clearance out of the brain could be enhanced in Hfe deficiency after repeated (or sub-acute) exposures to olfactory manganese. Pharmacokinetic characterizations of Mn after different routes of exposure will help to understand the underlying mechanism: e.g., intranasal instillation to track the olfactory uptake and intracerebral injection to directly characterize Mn efflux out of the brain. Future studies should differentiate these kinetic processes along with dose-response relationships. In addition, we observed that iron status was elevated in certain brain regions of Hfe−/− mice. However, others reported that Hfe deficiency does not significantly increase levels of non-heme iron in the whole brain (Golub et al., 2005; Johnstone et al., 2012). We offer two explanations. First, we evaluated iron levels in different brain regions rather than the whole brain, and therefore it is possible that Hfe deficiency could contribute to brain region-specific distribution and accumulation of iron. Second, Golub et al. (Golub et al., 2005) measured ferric iron that is loosely bound to hemosiderin in the striatum, whereas we measured total iron (i.e. both non-heme and heme iron). Thus, it is possible that heme-associated iron is elevated in the striatum from Hfe-deficient mice.
4.2. Effect of Hfe deficiency and Mn instillation on anxiety/impulsivity-related behavior
The elevated plus maze test provides various parameters for emotional behavior and general locomotor activity of the animal (Rodgers and Dalvi, 1997; Weiss et al., 1998). The behavioral parameters we obtained in the present study reveal that both Mn exposure and Hfe deficiency can alter animal behavior and further indicate interaction effects between the two conditions. Although entries to the open arms and the distance traveled in the open arms are related to anxiety or disinhibition-related behavior, these parameters also reflect the exploratory/locomotor activity of animals (Rodgers and Dalvi, 1997). Since Hfe-deficient mice display iron overload, these altered behaviors could result from either iron overload condition or loss of Hfe function. While it is generally accepted that iron deficiency increases the levels of anxiety (Beard et al., 2002; Li et al., 2011), the effect of iron overload on anxiety/impulsivity-related behavior is controversial. Sobotka et al. (Sobotka et al., 1996) demonstrated that dietary iron overload reduces exploratory activity in 13-week-old rats using a photoactometer chamber. In contrast, Archer and Fredriksson (Archer and Fredriksson, 2007) showed that mice administrated orally with iron succinate during postnatal days 10–12 have elevated iron in the basal ganglia and display hyperactive behavior after a short period of hypoactivity. We recently reported that rats fed iron overload diet after weaning did not exhibit abnormal emotional behavior examined by the EPM test (Han and Kim, 2015). These mixed results could be due to multiple factors, including species difference, age and different treatment schedules and test paradigms. Further study is warranted to separate the influence of iron overload and Hfe deficiency by examining emotional behaviors in Hfe deficiency under normal iron status.
In contrast to open arm entries, the “time” spent in the open arms is more related to anxiety/impulsivity than to exploratory/locomotor activity (Rodgers and Dalvi, 1997; Ueno et al., 2002). Since the natural tendency of mice is to hide in the closed arms of the EPM apparatus due to anxiety triggered by a new environment, both time spent in the open arms and latency to an open arm are considered a sign of impulsivity (Almeida et al., 1996). Hence, the prolonged time spent in the open arms in Mn-instilled mice indicates that olfactory Mn exposure decreases anxiety and/or increases impulsivity, likely by deficits in disinhibition control. These results are in line with our previous findings that Mn-exposed rat pups reduce anxiety-related behavior (Molina et al., 2011). We note that our results are disparate from those by Kern et al. (Kern et al., 2010), who reported no behavioral changes upon Mn exposure by the EPM test, likely due to different exposure protocols and routes of administration (i.e., oral administration during the preweaning stage (Kern et al., 2010) vs. intranasal instillation to mice after weaning in the present study).
4.3. Effect of Hfe deficiency and olfactory Mn exposure on the monoaminergic system
Dopaminergic pathway plays a key role in regulating emotional behavior (Kern et al., 2010; Li et al., 2011). For example, both dopamine D1 and D2 receptors are involved in anxiety-like behavior (de la Mora et al., 2010). Likewise, Karlsson et al. (Karlsson et al., 2008) showed that D1R-deficient mice display hyperactivity and less anxious behavior in the open field test. Consistent with this idea, we observed that Hfe−/− mice were more anxious with reduced exploratory activity and showed elevated D1R density in the striatum, which could contribute to increased dopaminergic activity. This possibility is supported in part by the findings that D1R antagonist exhibits anxiolytic effect (de la Mora et al., 2005) and that cocaine and methamphetamine that activate dopamine transmission produce anxiogenic effects (Hayase et al., 2005; Rogerio and Takahashi, 1992). We further investigated other monoamine transporters that affect extracellular monoamine levels. While SERT is involved in regulating emotional behavior (Canli and Lesch, 2007; Williams et al., 2013), we observed that Mn-induced impulsivity could be associated with increased expression of SERT in the striatum, which appears parallel to a finding that SERT deficiency exhibits anxiogenic effect in mice (Holmes et al., 2003). While iron deficiency has been shown to down-regulate brain NET (Beard et al., 2006; Burhans et al., 2005), our study demonstrates that loss of Hfe function increases NET, which would decrease extracellular concentration of norepinephrine. Taken together, these data may hint at underlying mechanisms of the metal-monoamine relationship since the distribution of neurotransmitters between intracellular and extracellular pools impacts neurobehavioral response (Kim and Wessling-Resnick, 2014). Further study on the characterization of synaptic neurotransmitters would help to illustrate the effects of Hfe deficiency and Mn exposure on adrenergic and serotoninergic signaling pathways.
We have attempted to characterize the influence of olfactory Mn exposure in Hfe deficiency on other proteins involved in monoamine turnover. First, COMT, a key enzyme in deactivation of monoamine neurotransmitters, including dopamine and norepinephrine, was significantly down-regulated upon Mn instillation. Interestingly, this was associated with increased impulsivity, which is consistent with previous findings by Papaleo et al. (Papaleo et al., 2012), who showed that COMT-knockout mice are more impulsive than wild-type mice. Second, VMAT, a vital transporter for packaging neurotransmitters into vesicles for release, has been shown to regulate emotional behavior (Toren et al., 2005; Zubieta et al., 2001; Zucker et al., 2002). Recently, Lohr et al. (Lohr et al., 2014) demonstrated that mice with VMAT overexpression display less anxious behavior, supporting our results that increased impulsive behavior (or reduced anxiety) in Mn-treated mice is associated with increased striatal VMAT levels. To our knowledge, the current study is the first to characterize the neurotoxic effect of olfactory manganese with loss of Hfe function, and our results revealed that both olfactory manganese exposure and Hfe deficiency modulate not only monoamine transporters, but also proteins involved in intracellular turnover of monoamines in the striatum. While we have characterized the dopaminergic pathway in the striatum, a more comprehensive study is warranted to explore the exact relationship between Mn and Hfe/iron in different brain regions along with other neurotransmitters in the context of emotional behavior.
5. CONCLUSIONS
In conclusion, Hfe-deficient mice were less susceptible to impulsivity-like behavior induced by airborne Mn, possibly due to an effect of increased anxiety and/or reduced explorative drive associated with Hfe deficiency. One possible molecular mechanism could be altered neurotransmitter turnover in several key signaling pathways, including dopaminergic, adrenergic and serotoninergic pathways by the interaction effect between olfactory Mn exposure and Hfe deficiency. Future studies on total tissue and synaptic neurotransmitter levels in different brain regions are required to better understand Mn toxicity in the context of HFE gene polymorphism and iron overload hemochromatosis. Our study further provides a therapeutic basis for improving psychiatric disorders associated with airborne manganese.
Acknowledgments
Funding Sources and Ethics Statement
This work was supported by the NIH R00 ES017781 (J.K.). This study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Northeastern University Animal Care and Use Committee.
This work was supported by the NIH R00 ES017781 (J.K.). The authors are grateful to Dr. Abitha Sukumaran, Ms. JuOae Chang and Ms. Murui Han for help during animal experiments, to Dr. Phillip Larese-Casanova at Northeastern University (College of Engineering) for help in ICP-MS and to Dr. Timothy Maher at MCPHS University (Boston, MA, USA) for helpful comments on neurobehavioral tests.
Abbreviations
- COMT
catechol-O-methyltransferase
- D1R
dopamine D1 receptor
- D2R
dopamine D2 receptor
- DAT
dopamine transporter
- DMT1
divalent metal transporter 1
- EPM
elevated plus maze
- ICP-MS
inductively coupled plasma mass spectrometry
- Mn
manganese
- NET
norepinephrine transporter
- SERT
serotonin transporter
- TH
tyrosine hydroxylase
- VMAT
vesicular monoamine transporter
Footnotes
The authors have no conflicting financial interests.
Author contributions: QY and JK designed research; QY performed research; QY and JK analyzed data; QY and JK wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almeida SS, Tonkiss J, Galler JR. Prenatal protein malnutrition affects exploratory behavior of female rats in the elevated plus-maze test. Physiol Behav. 1996;60:675–680. doi: 10.1016/s0031-9384(96)80047-3. [DOI] [PubMed] [Google Scholar]
- Anderson JG, Fordahl SC, Cooney PT, Weaver TL, Colyer CL, Erikson KM. Extracellular norepinephrine, norepinephrine receptor and transporter protein and mRNA levels are differentially altered in the developing rat brain due to dietary iron deficiency and manganese exposure. Brain research. 2009;1281:1–14. doi: 10.1016/j.brainres.2009.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer T, Fredriksson A. Functional consequences of iron overload in catecholaminergic interactions: the Youdim factor. Neurochemical research. 2007;32:1625–1639. doi: 10.1007/s11064-007-9358-1. [DOI] [PubMed] [Google Scholar]
- Avila DS, Puntel RL, Aschner M. Manganese in health and disease. Metal ions in life sciences. 2013;13:199–227. doi: 10.1007/978-94-007-7500-8_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard JL, Erikson KM, Jones BC. Neurobehavioral analysis of developmental iron deficiency in rats. Behav Brain Res. 2002;134:517–524. doi: 10.1016/s0166-4328(02)00092-x. [DOI] [PubMed] [Google Scholar]
- Beard JL, Wiesinger JA, Jones BC. Cellular iron concentrations directly affect the expression levels of norepinephrine transporter in PC12 cells and rat brain tissue. Brain research. 2006;1092:47–58. doi: 10.1016/j.brainres.2006.03.071. [DOI] [PubMed] [Google Scholar]
- Brain JD, Heilig E, Donaghey TC, Knutson MD, Wessling-Resnick M, Molina RM. Effects of iron status on transpulmonary transport and tissue distribution of Mn and Fe. Am J Respir Cell Mol Biol. 2006;34:330–337. doi: 10.1165/rcmb.2005-0101OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenneman KA, Wong BA, Buccellato MA, Costa ER, Gross EA, Dorman DC. Direct olfactory transport of inhaled manganese ((54)MnCl(2)) to the rat brain: toxicokinetic investigations in a unilateral nasal occlusion model. Toxicol Appl Pharmacol. 2000;169:238–248. doi: 10.1006/taap.2000.9073. [DOI] [PubMed] [Google Scholar]
- Burhans MS, Dailey C, Beard Z, Wiesinger J, Murray-Kolb L, Jones BC, Beard JL. Iron deficiency: differential effects on monoamine transporters. Nutr Neurosci. 2005;8:31–38. doi: 10.1080/10284150500047070. [DOI] [PubMed] [Google Scholar]
- Canli T, Lesch KP. Long story short: the serotonin transporter in emotion regulation and social cognition. Nature neuroscience. 2007;10:1103–1109. doi: 10.1038/nn1964. [DOI] [PubMed] [Google Scholar]
- Chua AC, Morgan EH. Manganese metabolism is impaired in the Belgrade laboratory rat. J Comp Physiol B. 1997;167:361–369. doi: 10.1007/s003600050085. [DOI] [PubMed] [Google Scholar]
- Claus Henn B, Kim J, Wessling-Resnick M, Tellez-Rojo MM, Jayawardene I, Ettinger AS, Hernandez-Avila M, Schwartz J, Christiani DC, Hu H, Wright RO. Associations of iron metabolism genes with blood manganese levels: a population-based study with validation data from animal models. Environmental health : a global access science source. 2011;10:97. doi: 10.1186/1476-069X-10-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotzias GC. Manganese in health and disease. Physiol Rev. 1958;38:503–532. doi: 10.1152/physrev.1958.38.3.503. [DOI] [PubMed] [Google Scholar]
- de la Mora MP, Cardenas-Cachon L, Vazquez-Garcia M, Crespo-Ramirez M, Jacobsen K, Hoistad M, Agnati L, Fuxe K. Anxiolytic effects of intra-amygdaloid injection of the D1 antagonist SCH23390 in the rat. Neurosci Lett. 2005;377:101–105. doi: 10.1016/j.neulet.2004.11.079. [DOI] [PubMed] [Google Scholar]
- de la Mora MP, Gallegos-Cari A, Arizmendi-Garcia Y, Marcellino D, Fuxe K. Role of dopamine receptor mechanisms in the amygdaloid modulation of fear and anxiety: Structural and functional analysis. Progress in neurobiology. 2010;90:198–216. doi: 10.1016/j.pneurobio.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Shihabi ZK, Aschner JL, Aschner M. Manganese accumulates in iron-deficient rat brain regions in a heterogeneous fashion and is associated with neurochemical alterations. Biol Trace Elem Res. 2002;87:143–156. doi: 10.1385/BTER:87:1-3:143. [DOI] [PubMed] [Google Scholar]
- Eriksson H, Magiste K, Plantin LO, Fonnum F, Hedstrom KG, Theodorsson-Norheim E, Kristensson K, Stalberg E, Heilbronn E. Effects of manganese oxide on monkeys as revealed by a combined neurochemical, histological and neurophysiological evaluation. Arch Toxicol. 1987;61:46–52. doi: 10.1007/BF00324547. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Rubia K, Rossi G, Sartori G, Balottin U. Striatal dopamine transporter alterations in ADHD: pathophysiology or adaptation to psychostimulants? A meta-analysis. The American journal of psychiatry. 2012;169:264–272. doi: 10.1176/appi.ajp.2011.11060940. [DOI] [PubMed] [Google Scholar]
- Ghio AJ, Turi JL, Madden MC, Dailey LA, Richards JD, Stonehuerner JG, Morgan DL, Singleton S, Garrick LM, Garrick MD. Lung injury after ozone exposure is iron dependent. Am J Physiol Lung Cell Mol Physiol. 2007;292:L134–143. doi: 10.1152/ajplung.00534.2005. [DOI] [PubMed] [Google Scholar]
- Golub MS, Germann SL, Araiza RS, Reader JR, Griffey SM, Lloyd KC. Movement disorders in the Hfe knockout mouse. Nutritional neuroscience. 2005;8:239–244. doi: 10.1080/10284150500277685. [DOI] [PubMed] [Google Scholar]
- Griffiths WJ, Sly WS, Cox TM. Intestinal iron uptake determined by divalent metal transporter is enhanced in HFE-deficient mice with hemochromatosis. Gastroenterology. 2001;120:1420–1429. doi: 10.1053/gast.2001.24050. [DOI] [PubMed] [Google Scholar]
- Guilarte TR. Manganese neurotoxicity: new perspectives from behavioral, neuroimaging, and neuropathological studies in humans and non-human primates. Frontiers in aging neuroscience. 2013;5:23. doi: 10.3389/fnagi.2013.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M, Kim J. Effect of dietary iron loading on recognition memory in growing rats. PloS one. 2015;10:e0120609. doi: 10.1371/journal.pone.0120609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayase T, Yamamoto Y, Yamamoto K. Persistent anxiogenic effects of a single or repeated doses of cocaine and methamphetamine: interactions with endogenous cannabinoid receptor ligands. Behavioural pharmacology. 2005;16:395–404. doi: 10.1097/00008877-200509000-00012. [DOI] [PubMed] [Google Scholar]
- Holmes A, Murphy DL, Crawley JN. Abnormal behavioral phenotypes of serotonin transporter knockout mice: parallels with human anxiety and depression. Biological psychiatry. 2003;54:953–959. doi: 10.1016/j.biopsych.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Horning KJ, Caito SW, Tipps KG, Bowman AB, Aschner M. Manganese Is Essential for Neuronal Health. Annual review of nutrition. 2015 doi: 10.1146/annurev-nutr-071714-034419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y, Zhang S, Wang L, Li J, Qu G, He J, Rong H, Ji H, Liu S. Estrogen regulates iron homeostasis through governing hepatic hepcidin expression via an estrogen response element. Gene. 2012;511:398–403. doi: 10.1016/j.gene.2012.09.060. [DOI] [PubMed] [Google Scholar]
- Jaber M, Robinson SW, Missale C, Caron MG. Dopamine receptors and brain function. Neuropharmacology. 1996;35:1503–1519. doi: 10.1016/s0028-3908(96)00100-1. [DOI] [PubMed] [Google Scholar]
- Johnstone D, Graham RM, Trinder D, Delima RD, Riveros C, Olynyk JK, Scott RJ, Moscato P, Milward EA. Brain transcriptome perturbations in the Hfe(−/−) mouse model of genetic iron loading. Brain Res. 2012;1448:144–152. doi: 10.1016/j.brainres.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Karlsson RM, Hefner KR, Sibley DR, Holmes A. Comparison of dopamine D1 and D5 receptor knockout mice for cocaine locomotor sensitization. Psychopharmacology. 2008;200:117–127. doi: 10.1007/s00213-008-1165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern CH, Stanwood GD, Smith DR. Preweaning manganese exposure causes hyperactivity, disinhibition, and spatial learning and memory deficits associated with altered dopamine receptor and transporter levels. Synapse. 2010;64:363–378. doi: 10.1002/syn.20736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Buckett PD, Wessling-Resnick M. Absorption of manganese and iron in a mouse model of hemochromatosis. PloS one. 2013;8:e64944. doi: 10.1371/journal.pone.0064944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Li Y, Buckett PD, Bohlke M, Thompson KJ, Takahashi M, Maher TJ, Wessling-Resnick M. Iron-responsive olfactory uptake of manganese improves motor function deficits associated with iron deficiency. PLoS One. 2012;7:e33533. doi: 10.1371/journal.pone.0033533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Molina RM, Donaghey TC, Buckett PD, Brain JD, Wessling-Resnick M. Influence of DMT1 and iron status on inflammatory responses in the lung. Am J Physiol Lung Cell Mol Physiol. 2011;300:L659–665. doi: 10.1152/ajplung.00343.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Wessling-Resnick M. Iron and mechanisms of emotional behavior. The Journal of nutritional biochemistry. 2014;25:1101–1107. doi: 10.1016/j.jnutbio.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause KH, Dresel SH, Krause J, Kung HF, Tatsch K. Increased striatal dopamine transporter in adult patients with attention deficit hyperactivity disorder: effects of methylphenidate as measured by single photon emission computed tomography. Neuroscience letters. 2000;285:107–110. doi: 10.1016/s0304-3940(00)01040-5. [DOI] [PubMed] [Google Scholar]
- Levy JE, Montross LK, Andrews NC. Genes that modify the hemochromatosis phenotype in mice. J Clin Invest. 2000;105:1209–1216. doi: 10.1172/JCI9635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy JE, Montross LK, Cohen DE, Fleming MD, Andrews NC. The C282Y mutation causing hereditary hemochromatosis does not produce a null allele. Blood. 1999;94:9–11. [PubMed] [Google Scholar]
- Li Y, Kim J, Buckett PD, Bohlke M, Maher TJ, Wessling-Resnick M. Severe postnatal iron deficiency alters emotional behavior and dopamine levels in the prefrontal cortex of young male rats. J Nutr. 2011;141:2133–2138. doi: 10.3945/jn.111.145946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr KM, Bernstein AI, Stout KA, Dunn AR, Lazo CR, Alter SP, Wang M, Li Y, Fan X, Hess EJ, Yi H, Vecchio LM, Goldstein DS, Guillot TS, Salahpour A, Miller GW. Increased vesicular monoamine transporter enhances dopamine release and opposes Parkinson disease-related neurodegeneration in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:9977–9982. doi: 10.1073/pnas.1402134111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchini RG, Martin CJ, Doney BC. From manganism to manganese-induced parkinsonism: a conceptual model based on the evolution of exposure. Neuromolecular medicine. 2009;11:311–321. doi: 10.1007/s12017-009-8108-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merryweather-Clarke AT, Pointon JJ, Jouanolle AM, Rochette J, Robson KJ. Geography of HFE C282Y and H63D mutations. Genetic testing. 2000;4:183–198. doi: 10.1089/10906570050114902. [DOI] [PubMed] [Google Scholar]
- Molina RM, Phattanarudee S, Kim J, Thompson K, Wessling-Resnick M, Maher TJ, Brain JD. Ingestion of Mn and Pb by rats during and after pregnancy alters iron metabolism and behavior in offspring. Neurotoxicology. 2011;32:413–422. doi: 10.1016/j.neuro.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaleo F, Erickson L, Liu G, Chen J, Weinberger DR. Effects of sex and COMT genotype on environmentally modulated cognitive control in mice. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:20160–20165. doi: 10.1073/pnas.1214397109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park RM, Schulte PA, Bowman JD, Walker JT, Bondy SC, Yost MG, Touchstone JA, Dosemeci M. Potential occupational risks for neurodegenerative diseases. American journal of industrial medicine. 2005;48:63–77. doi: 10.1002/ajim.20178. [DOI] [PubMed] [Google Scholar]
- Pietrangelo A. Hereditary hemochromatosis--a new look at an old disease. N Engl J Med. 2004;350:2383–2397. doi: 10.1056/NEJMra031573. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Dalvi A. Anxiety, defence and the elevated plus-maze. Neuroscience and biobehavioral reviews. 1997;21:801–810. doi: 10.1016/s0149-7634(96)00058-9. [DOI] [PubMed] [Google Scholar]
- Rogerio R, Takahashi RN. Anxiogenic properties of cocaine in the rat evaluated with the elevated plus-maze. Pharmacol Biochem Behav. 1992;43:631–633. doi: 10.1016/0091-3057(92)90203-r. [DOI] [PubMed] [Google Scholar]
- Roth JA. Homeostatic and toxic mechanisms regulating manganese uptake, retention, and elimination. Biological research. 2006;39:45–57. doi: 10.4067/s0716-97602006000100006. [DOI] [PubMed] [Google Scholar]
- Sobotka TJ, Whittaker P, Sobotka JM, Brodie RE, Quander DY, Robl M, Bryant M, Barton CN. Neurobehavioral dysfunctions associated with dietary iron overload. Physiology & behavior. 1996;59:213–219. doi: 10.1016/0031-9384(95)02030-6. [DOI] [PubMed] [Google Scholar]
- Subhash MN, Padmashree TS. Regional distribution of dopamine beta-hydroxylase and monoamine oxidase in the brains of rats exposed to manganese. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 1990;28:567–570. doi: 10.1016/0278-6915(90)90157-i. [DOI] [PubMed] [Google Scholar]
- Takeda A. Manganese action in brain function. Brain Res Brain Res Rev. 2003;41:79–87. doi: 10.1016/s0165-0173(02)00234-5. [DOI] [PubMed] [Google Scholar]
- Thompson K, Molina RM, Donaghey T, Schwob JE, Brain JD, Wessling-Resnick M. Olfactory uptake of manganese requires DMT1 and is enhanced by anemia. FASEB J. 2007;21:223–230. doi: 10.1096/fj.06-6710com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjalve H, Henriksson J, Tallkvist J, Larsson BS, Lindquist NG. Uptake of manganese and cadmium from the nasal mucosa into the central nervous system via olfactory pathways in rats. Pharmacol Toxicol. 1996;79:347–356. doi: 10.1111/j.1600-0773.1996.tb00021.x. [DOI] [PubMed] [Google Scholar]
- Toren P, Rehavi M, Luski A, Roz N, Laor N, Lask M, Weizman A. Decreased platelet vesicular monoamine transporter density in children and adolescents with attention deficit/hyperactivity disorder. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2005;15:159–162. doi: 10.1016/j.euroneuro.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Tran TT, Chowanadisai W, Crinella FM, Chicz-DeMet A, Lonnerdal B. Effect of high dietary manganese intake of neonatal rats on tissue mineral accumulation, striatal dopamine levels, and neurodevelopmental status. Neurotoxicology. 2002;23:635–643. doi: 10.1016/s0161-813x(02)00091-8. [DOI] [PubMed] [Google Scholar]
- Ueno KI, Togashi H, Mori K, Matsumoto M, Ohashi S, Hoshino A, Fujita T, Saito H, Minami M, Yoshioka M. Behavioural and pharmacological relevance of stroke-prone spontaneously hypertensive rats as an animal model of a developmental disorder. Behavioural pharmacology. 2002;13:1–13. doi: 10.1097/00008877-200202000-00001. [DOI] [PubMed] [Google Scholar]
- Weiss SM, Wadsworth G, Fletcher A, Dourish CT. Utility of ethological analysis to overcome locomotor confounds in elevated maze models of anxiety. Neuroscience and biobehavioral reviews. 1998;23:265–271. doi: 10.1016/s0149-7634(98)00027-x. [DOI] [PubMed] [Google Scholar]
- Williams S, Vinjam MR, Ismail A, Hassan A. A parkinsonian movement disorder with brain iron deposition and a haemochromatosis mutation. Journal of neurology. 2013;260:2170–2171. doi: 10.1007/s00415-013-6995-y. [DOI] [PubMed] [Google Scholar]
- Yang Q, Jian J, Katz S, Abramson SB, Huang X. 17beta-Estradiol inhibits iron hormone hepcidin through an estrogen responsive element half-site. Endocrinology. 2012;153:3170–3178. doi: 10.1210/en.2011-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z, Jiang H, Lee ES, Ni M, Erikson KM, Milatovic D, Bowman AB, Aschner M. Ferroportin is a manganese-responsive protein that decreases manganese cytotoxicity and accumulation. J Neurochem. 2010;112:1190–1198. doi: 10.1111/j.1471-4159.2009.06534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A, Park SK, Wright RO, Weisskopf MG, Mukherjee B, Nie H, Sparrow D, Hu H. HFE H63D polymorphism as a modifier of the effect of cumulative lead exposure on pulse pressure: the Normative Aging Study. Environ Health Perspect. 2010;118:1261–1266. doi: 10.1289/ehp.1002251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Kanthasamy A, Anantharam V, Kanthasamy A. Effects of manganese on tyrosine hydroxylase (TH) activity and TH-phosphorylation in a dopaminergic neural cell line. Toxicol Appl Pharmacol. 2011;254:65–71. doi: 10.1016/j.taap.2010.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta JK, Taylor SF, Huguelet P, Koeppe RA, Kilbourn MR, Frey KA. Vesicular monoamine transporter concentrations in bipolar disorder type I, schizophrenia, and healthy subjects. Biological psychiatry. 2001;49:110–116. doi: 10.1016/s0006-3223(00)00981-1. [DOI] [PubMed] [Google Scholar]
- Zucker M, Valevski A, Weizman A, Rehavi M. Increased platelet vesicular monoamine transporter density in adult schizophrenia patients. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2002;12:343–347. doi: 10.1016/s0924-977x(02)00041-x. [DOI] [PubMed] [Google Scholar]