Abstract
Omega-3 and omega-6 fatty acids are biosynthetic precursors to endocannabinoids with antinociceptive, anxiolytic, and neurogenic properties. We recently reported that targeted dietary manipulation—increasing omega-3 fatty acids while reducing omega-6 linoleic acid (the H3-L6 intervention)—reduced headache pain and psychological distress among chronic headache patients. It is not yet known whether these clinical improvements were due to changes in endocannabinoids and related mediators derived from omega-3 and omega-6 fatty acids. We therefore used data from this trial (n=55) to investigate (1) whether the H3-L6 intervention altered omega-3 and omega-6 derived endocannabinoids in plasma, and (2) whether diet-induced changes in these bioactive lipids were associated with clinical improvements. The H3-L6 intervention significantly increased the omega-3 docosahexaenoic acid derivatives 2-docosahexaenoylglycerol (+65%, p<0.001) and docosahexaenoylethanolamine (+99%, p<0.001), and reduced the omega-6 arachidonic acid derivative 2-arachidonoylglycerol (-25%, p=0.001). Diet-induced changes in these endocannabinoid derivatives of omega-3 docosahexaenoic acid, but not omega-6 arachidonic acid, correlated with reductions in physical pain and psychological distress. These findings demonstrate that targeted dietary manipulation can alter endocannabinoids derived from omega-3 and omega-6 fatty acids in humans, and suggest that 2-docosahexaenoylglycerol and docosahexaenoylethanolamine could have physical and/or psychological pain modulating properties. Trial Registration: ClinicalTrials.gov (NCT01157208)
Perspective
This article demonstrates that targeted dietary manipulation can alter endocannabinoids derived from omega-3 and omega-6 fatty acids, and that these changes are related to reductions in headache pain and psychological distress. These findings suggest that dietary interventions could provide an effective, complementary approach for managing chronic pain and related conditions.
Keywords: endocannabinoids, psychological distress, headache, 2-docosahexaenoylglycerol, docosahexaenoylethanolamine, 2-arachidonoylglycerol
Introduction
Omega-3 (n-3) and omega-6 (n-6) fatty acids are major components of immune and neuronal cell membranes35 and the biosynthetic precursors to several families of lipid autacoids posited to modulate physical pain and psychological distress (e.g. eicosanoids, endovanilloids, endocannabinoids)8-10, 20, 32, 38, 42. Humans lack the enzymatic machinery needed for de novo biosynthesis of n-3 and n-6 fatty acids. Therefore, targeted dietary manipulation is a promising strategy for altering bioactive lipid autacoids in a manner that could reduce pain and comorbid conditions. We recently tested this hypothesis in a population with chronic headaches.
The Chronic Daily Headache (CDH) trial
The CDH trial was a randomized, 12-week trial designed to test the clinical and biochemical effects of a diet high in n-3 and low in n-6 fatty acids (the H3-L6 intervention) compared to a diet low in n-6 fatty acids (the L6 intervention) in a population with CDH. We previously reported that the H3-L6 intervention produced marked reductions in headache frequency and severity 30, reduced psychological distress 29, and enhanced quality of life and function 29 compared to the L6 intervention, while reducing the use of acute pain medications 30 . Diet-induced changes in one or more families of n-3 or n-6 derived lipid autacoids likely contributed to these clinical benefits; however the specific lipid autacoids responsible for these effects are unknown. In the present manuscript, we investigate whether changes in one such family of lipid autacoids—endocannabinoids and related mediators derived from n-6 and n-3 fatty acids—could help explain the beneficial effects of the H3-L6 intervention.
n-6 and n-3 fatty acids as precursors to endocannabinoids and related mediators
The n-6 arachidonic acid (n-6 AA) derivatives 2-arachidonoylglycerol (2-AG) and N-arachidonoylethanolamine (AEA) (Figure 1) have complex relationships with chronic pain and psychological distress. 2-AG and AEA act as endogenous ligands for cannabinoid receptors (i.e., endocannabinoids) to produce analgesic and anxiolytic effects 1, 8, 12, 33. However, in addition to activating cannabinoid receptors, 2-AG also serves as a major source for production free AA and prostanoids 4, 28, which have been implicated in headache pathogenesis 3. AEA is also the best-characterized endogenous ligand for the TRPV1 receptor channel (i.e., endovanilloid), which is involved in pain signaling 10.
Figure 1. Model depicting diet-induced alterations in n-3 and n-6 derived endocannabinoids.
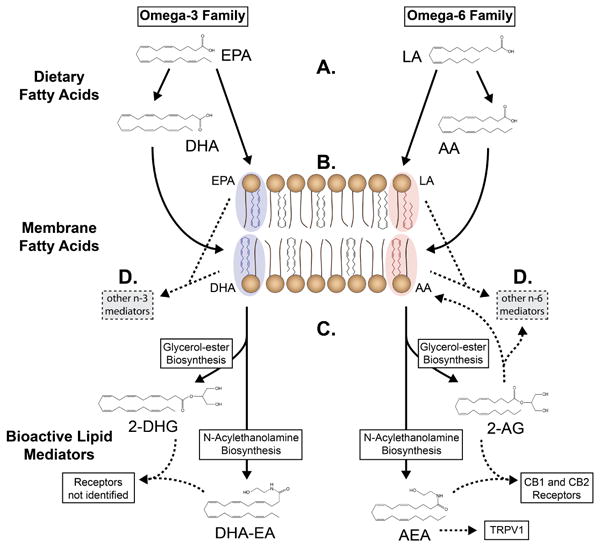
(A) Dietary n-3 and n-6 polyunsaturated fatty acids compete for enzymatic conversion into their respective elongated and desaturated products, notably n-3 DHA and n-6 AA.
(B) n-3 DHA and EPA and n-6 AA and LA compete for esterification into cell membranes.
(C) Membrane n-3 DHA and n-6 AA are converted to their respective glycerol-ester and N-acylethanolamine endocannabinoids in proportion to available substrate.
(D) n-3 and n-6 fatty acids can be converted to numerous other lipid autacoids with pro- or anti-nociceptive properties (eg, prostaglandins, EETs, HODEs, lipoxins, resolvins, protectins).
DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; LA, linoleic acid; AA, arachidonic acid; DHG, docosahexaenoylglycerol; DHA-EA, docosahexaenoylethanolamine; AG, arachidonoylglycerol; AEA, arachidonoylethanolamine; EET, epoxyeicosatrienoic acid; HODE, hydroxyoctadecadienoic acid; CB, cannabinoid; TRPV1, transient receptor potential cation channel, subfamily V, member 1.
The n-3 DHA derivatives 2-docosahexaenoylglycerol (2-DHG) and docosahexaenoylethanolamine (DHA-EA) have low affinity for cannabinoid receptors 38 but could potentially affect pain and psychological distress via other mechanisms. 2-DHG is abundant in nervous system tissues 42, but its specific biological actions have not yet been identified. DHA-EA has anti-inflammatory 25, and neurogenic 9, 20, 32 properties, and has been linked to improved functional recovery and reduced sensitivity to noxious heat after experimental spinal cord injury 15. It is not yet known whether 2-DHG or DHA-EA plays a role in modulating physical or psychological dimensions of the pain experience.
In the present study, we used data and samples from this completed trial to test whether plasma levels of 2-AG, AEA, 2-DHG and DHA-EA can be modified by diet, and whether such changes could help explain the beneficial effects of the H3-L6 intervention. The specific objectives are to: (1) determine whether the dietary interventions altered these n-3 and n-6 derived endocannabinoids in plasma; (2) assess the relations between these endocannabinoids and their precursor fatty acids; and (3) examine how changes in these endocannabinoids related to improvements in physical, psychological, and functional aspects of the pain experience.
Methods
Trial overview
The trial protocol, dietary compositions, and the primary clinical and biochemical findings were previously described 24, 30, 31. Briefly, adults meeting the Chronic Daily Headache (CDH) criteria of headaches >4 hours per day and >15 days per month for at least 3 months and a headache history of >2 years were recruited to participate. Eligibility criteria are shown in Supplementary Table S1. After the nature and possible consequences of the trial were explained, all participants provided written informed consent. During the 4-week pre-intervention phase, participants continued usual care and habitual diets and recorded headache characteristics in a web-based daily headache diary. Upon completion of the run-in phase, participants were randomized to either the H3-L6 intervention or L6 intervention, which lasted 12 weeks. Participants were masked to the nature and content of the other group's intervention. The dietitian was unmasked by necessity at randomization in order to assign patients to their group and to administer the interventions. All other investigators, study personnel, and each participant's personal physician were masked to group assignment for the full duration of the trial. The trial was conducted at the University of North Carolina at Chapel Hill (UNC) from April 2009 to November 2011. Trial procedures were approved by the UNC Institutional Review Board. This trial is registered under ClinicalTrials.gov (NCT01157208).
Dietary interventions
The nutrient intakes of the H3-L6 and L6 interventions have previously been reported 24 and are summarized in Supplementary Table S2. The H3-L6 diet reduced dietary n-6 linoleic acid (LA), and concurrently increased n-3 eicosapentaenoic acid (EPA) and DHA. The L6 diet reduced n-6 LA, and maintained low n-3 EPA and DHA intakes typical of U.S. diets 24. EPA and DHA were increased in the H3-L6 diet by providing fatty fish (e.g. canned fatty tuna). To maximize credibility, the L6 diet provided foods that were low in n-3 fatty acids but similar in appearance to H3-L6 foods (e.g. very low fat tuna with added oil). LA was reduced in both diets by restricting consumption of vegetable oils and other rich sources of LA. The interventions were designed to produce weight maintenance, and to be equally credible and equivalent with respect to macronutrient and caloric intake, interactions with the dietitian and investigators, and intensity and breadth of the dietary advice and web-based intervention materials 24. Expectation of benefit was moderate in both diet groups, with no between-group differences in the Borkovec and Nau credibility questionnaire 30. There were no significant weight changes in either group.
Sample collection and laboratory analysis of endocannabinoids and precursor fatty acids
Sample preparation and analyses were performed by investigators who were blinded to study protocol and clinical data. Fasting blood was drawn at baseline and after 12-weeks of diet exposure. Plasma aliquots were immediately frozen at -80°C. Endocannabinoids were analyzed via LC-MS/MS at Northeastern University's Center for Drug Discovery (Boston, MA) 40-42. Plasma fatty acids and the n-6 in HUFA score were previously reported [10] and are summarized in Supplementary Table 3.
Pain, quality of life, and psychological measures
Measures of headache pain, headache-specific quality of life (HIT-6) [16], psychological distress (BSI-18) 22, and health-related quality of life (SF-12) 23, 39 were collected before and after the intervention, and a daily headache diary was used to record hourly headache characteristics (rated as mild, moderate, or severe) throughout the trial, as previously described 19, 30 and summarized in Table 1. For each of these outcomes, the H3-L6 intervention group experienced statistically significant improvements compared to the L6 group 30.
Table 1. Description of clinical outcomes in the chronic daily headache trial.
| Description | Time points | Baseline mean (SD) | |
|---|---|---|---|
|
|
|||
| Pain frequency and intensity | |||
| Headache days/month | Number of headache days experienced during the previous 4 weeks | Baseline and Intervention Week 12 | 23.2 (7.5) |
| Headache hours/day | Calculated from web-based daily Headache Diary recording hourly headache characteristics (rated as mild, moderate, or severe). Mean Headache Hours per day and Severe Headache Hours per day were calculated by averaging the last 4 weeks before randomization and during intervention weeks eight through 12. | Recorded daily throughout the run-in and intervention phases (112 days total) | 10.2 (6.4) |
| Severe headache hours/day | 4.3 (4.8) | ||
| Functional dimensions of pain | |||
| Headache Impact on quality of life (HIT-6) | Measure of headache-related disability based on self-reported pain, social functioning, role functioning, vitality, cognitive functioning, and psychological distress. | Baseline and Intervention Week 12 | 60.8 (5.0) |
| Physical Health Composite Score (SF-12) | Summary measure for quality of life, function and well-being in physical dimensions of life | Baseline and Intervention Week 12 | 43.6 (9.4) |
| Psychological dimensions of pain | |||
| Mental Health Composite Score (SF-12) | Summary measure for quality of life, function and well-being in psychological dimensions of life | Baseline and Intervention Week 12 | 44.3 (9.8) |
| Psychological distress (BSI-18) | Measure of general psychological distress over the last 7 days by each of 18 symptoms, including separate scores for anxiety, depression, somatization, and a global symptom severity index | Baseline and Intervention Week 12 | 52.3 (9.3) |
Data Analysis
All analyses were conducted using Stata version 13 (College Station, Texas). We examined descriptive statistics for all variables, and employed a combination of non-parametric and parametric approaches (normalizing variables when necessary) using all available data without imputations. To determine whether the dietary interventions altered plasma endocannabinoids, we used a Wilcoxon matched-pairs signed-ranks test to assess differences in pre-to-post intervention values within each diet group. To determine whether group assignment had an effect on the post-intervention value of each analyte, we used an analysis of covariance including the baseline value of each respective analyte. To determine associations between post-intervention plasma fatty acids and endocannabinoids, we used linear regressions adjusted for the baseline values of the respective fatty acid and endocannabinoid. To examine whether changes in endocannabinoids were related to clinical improvements, we used regression models adjusted for the baseline values of each outcome and endocannabinoid.
Results
Demographic and baseline characteristics
Sixty seven participants were randomized to the H3-L6 or the L6 diet. The groups had comparable baseline characteristics (Supplementary Table S5). Fifty-five participants completed the 12-week intervention phase and provided baseline and 12-week plasma measures. Endocannabinoids were measured for this sample. We compared baseline characteristics for these 55 completers vs. the 12 non-completers using the Wilcoxon rank-sum test. No statistically significant differences were detected for demographic or pain-related characteristics, however, non-completers had lower mental health scores (SF-12 Mental Health composite score; p=0.03). At baseline, participants had high levels of physical pain (mean headache days per month=23 (SD=7.5), mean headache hours per day=10 (SD=6.4), mean severe headache hours per day=4.3 (SD=4.8), and reported taking an average of 6 different headache-related medications.
Abundance of endocannabinoids and their precursor fatty acids in plasma
Mean plasma endocannabinoid concentrations at baseline were as follows: 2-DHG 166 ng/mL (SD=74.7); DHA-EA 0.47 ng/mL (SD=0.22); 2-AG 793.0 ng/mL (SD=324.1); AEA 0.49 ng/mL (SD=0.18), with no significant between-group differences in any endocannabinoid (p>0.05). 2-DHG, 2-AG, DHA-EA and AEA were 0.57, 0.55, 0.0017 and 0.00038% as abundant as their precursors DHA and AA in plasma phospholipids.
Diet-induced changes in endocannabinoids
Compared to baseline and to the L6 intervention, the H3-L6 intervention significantly increased 2-DHG and DHA-EA, and significantly reduced 2-AG (Table 2). The H3-L6 intervention had no effect on AEA, oleoylethanolamine (OEA), palmitoylethanolamine (PEA), or oleoylglycerol (OG). The L6 intervention did not significantly alter 2-DHG, DHA-EA, 2-AG, AEA, OEA, PEA or OG.
Table 2. Plasma endocannabinoids at baseline and after 12-week dietary interventions1.
| H3-L6 group 2 | L6 group 2 | Between-group difference p-value4 | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Baseline | 12-weeks | % change | p value3 | Baseline | 12-weeks | % change | p value3 | ||
| Endocannabinoids (ng/mL) | |||||||||
| n-3 family | |||||||||
| 2-DHG | 181 (119, 242) | 264 (187, 400) | +65 | <0.001 | 143 (120, 178) | 172 (126, 226) | +17 | 0.14 | 0.001 |
| DHA-EA | 0.43 (0.29, 0.62) | 0.80 (0.55, 1.20) | +99 | <0.001 | 0.43 (0.29, 0.56) | 0.45 (0.37, 0.56) | +14 | 0.43 | <0.001 |
| n-6 family | |||||||||
| 2-AG | 908 (628, 1068) | 557 (444, 760) | −25 | 0.001 | 637 (492, 856) | 703 (478, 963) | +3 | 0.50 | 0.004 |
| AEA | 0.49 (0.38, 0.53) | 0.44 (0.34, 0.51) | −3 | 0.51 | 0.47 (0.35, 0.58) | 0.50 (0.43, 0.61) | +1 | 0.36 | 0.059 |
| Other | |||||||||
| OEA | 2.74 (2.07, 3.30) | 2.71 (2.03, 3.42) | +9 | 0.30 | 2.82 (2.18, 3.61) | 2.83 (2.52, 3.79) | +13 | 0.05 | 0.408 |
| PEA | 3.04 (2.72, 3.63) | 3.22 (2.87, 3.87) | +11 | 0.48 | 3.34 (2.51, 3.91) | 3.54 (2.86, 4.38) | +8 | 0.18 | 0.217 |
| OG | 2372 (1584, 3021) | 1893 (1429, 2935) | −7 | 0.20 | 2126 (1771, 2607) | 2380 (1703, 2758) | +8 | 0.55 | 0.265 |
Values are medians with inter-quartile ranges in parenthesis, unless otherwise noted.
High n-3, Low n-6 Diet n=27; Low n-6 Diet n=28. There were no differences between diet groups at baseline (p>0.05)
Wilcoxon matched-pairs signed-ranks tests used for within-group comparisons.
ANCOVA for intervention effect (i.e., effect of diet group assignment on post-intervention value of analyte, adjusted for respective baseline value).
DHG, docosahexaenoylglycerol; DHA-EA, docosahexaenoylethanolamine; AG, arachidonoylglycerol; AEA, arachidonoylethanolamine; OEA, oleoylethanolamine; PEA, palmitoylethanolamine; OG, oleoylglycerol.
Associations between plasma endocannabinoids and their precursor fatty acids
Figure 2 shows the relationships between the endocannabinoids 2-DHG, DHA-EA, 2-AG and AEA, and their precursor fatty acids in plasma after 12 weeks on the CDH trial. Increases in plasma DHA were significantly related to increases in 2-DHG and DHA-EA and decreases in 2-AG (p-values <0.05). Increases in plasma AA and %n6 in HUFA score were significantly related to decreases in 2-DHG and DHA-EA and increases in 2-AG and AEA (p-values <0.05).
Figure 2. Precursor fatty acids and their n-3 and n-6 endocannabinoid products in plasma after 12 weeks.
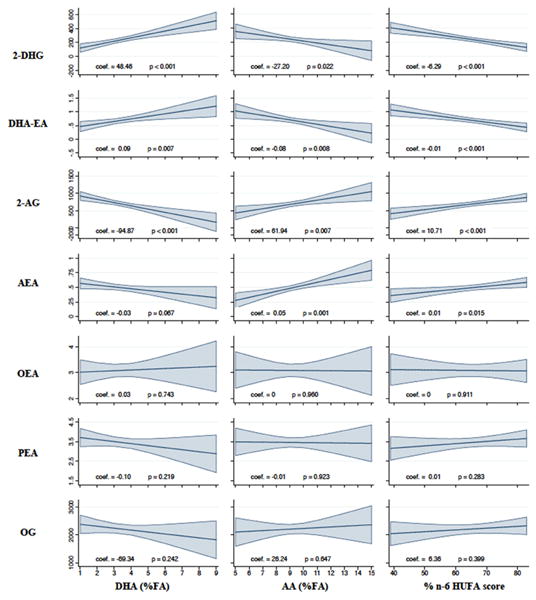
Plots are post-estimations of linear regression models for the effect plasma fatty acids on each endocannabinoid after 12 weeks on the Chronic Daily Headache Study (n=55), adjusting for the baseline value of each respective endocannabinoid and fatty acid. Endocannabinoids are measured in ng/mL.
The shaded areas around regression lines are 95% confidence intervals. DHG, docosahexaenoylglycerol; DHA-EA, docosahexaenoylethanolamine; 2-AG, 2-arachidonoylglycerol; AEA, arachidonoylethanolamine; DHA, docosahexaenoic acid; n-6, omega-6; HUFA, highly unsaturated fatty acids.
Association between 12-week changes in plasma endocannabinoids and clinical measures
The relationships between changes in plasma endocannabinoids and clinical measures are shown in Table 3. Since there was no significant effect modification by diet group, these associations are reported for the combined groups (Stratified results shown in Supplementary Table 4). For each SD increase in plasma 2-DHG, we observed a 10% reduction in the number of headache days per month (p=0.006) and a 40% reduction in the number of severe headache hours per day (p<0.001)(Table 3.a.). Increases in 2-DHG were also related to improvements in psychological distress (p=0.002) and the SF-12 Mental Health composite score (p=0.006), and tended to correlate with lower headache impact (p=0.051) and higher physical health (0.080)(Table 3.b.). For each SD increase in plasma DHA-EA, we observed a 7% reduction in the number of headache days per month (p=0.027) and a 30% reduction in the number of severe headache hours per day (p<0.001). However, unlike 2-DHG, changes in DHA-EA were not significantly related to psychological distress. Decreases in 2-AG were related to decreased headache severity (p=0.011), but were not related to any other clinical outcomes. Changes in n-3 and n-6 derived endocannabinoids were not significantly related to functional dimensions of pain.
Table 3. a. Associations between 12-week changes in endocannabinoids and physical pain (n=55).
| n-3 DHA-derived1 (ng/mL) | n-6 AA derived1 (ng/mL) | |||
|---|---|---|---|---|
|
|
|
|||
| 2-DHG | DHA-EA | 2-AG | AEA | |
| Headache days/month (n=55) | -10% (0.006) | -7% (0.027) | -6% (0.191) | -2% (0.533) |
| Headache hours/day (n=51) | -12% (0.031) | -3% (0.642) | 13% (0.068) | 8% (0.175) |
| Severe headache hours/day (n=51) | -40% (<0.001) | -30% (<0.001) | 39% (0.011) | 34% (0.001) |
|
| ||||
| 1Standardized coefficients represent the % change in count (i.e., days or hours) for each standard deviation change in the respective endocannabinoid (p-values in parenthesis) and are derived from Poisson regression models relating endocannabinoids and headache outcomes at week 12 and adjusting for the respective baseline values. | ||||
| b. Associations between 12-week changes in endocannabinoids and pain-related quality of life measures and psychological stress – both groups (n=55) | ||||
|
| ||||
| n-3 DHA-derived1 | n-6 AA derived1 | |||
|
|
|
|||
| 2-DHG | DHA-EA | 2-AG | AEA | |
|
| ||||
| Functional dimensions of pain | ||||
| Headache Impact on quality of life (HIT-6)2 | -0.23 (0.051) | -0.19 (0.089) | 0.21 (0.124) | 0.12 (0.332) |
| Physical Health Composite Score (SF-12)3 | 0.21 (0.080) | 0.16 (0.171) | -0.17 (0.231) | -0.19 (0.125) |
| Psychological dimensions of pain | ||||
| Mental Health Composite Score (SF-12)3 | 0.34 (0.006) | 0.11 (0.346) | -0.002 (0.986) | 0.01 (0.912) |
| Psychological distress (BSI)4 | -0.36 (0.002) | -0.16 (0.138) | 0.12 (0.351) | 0.15 (0.205) |
Standardized coefficients represent a standard deviation change in the outcome variable for each one standard deviation increase in the respective endocannabinoid (p-values in parenthesis) and are derived from linear regression models relating endocannabinoids and outcomes at week 12 and adjusting for the respective baseline values.
Higher score = more headache impact on quality of life.
Higher score = better quality of life.
Higher score = more psychological distress.
Discussion
Here we report for the first time in humans that targeted alterations in dietary n-3 and n-6 fatty acids significantly increased circulating 2-DHG and DHA-EA, and decreased 2-AG. In light of research demonstrating that endocannabinoids regulate many critical biochemical and behavioral processes in animals, the present demonstration in humans that these lipid mediators are modifiable by diet could have important implications for a wide range of pathological conditions.
Since DHA and AA are intermediates in the proposed causal pathway linking dietary fatty acids to their endocannabinoid products, the finding that changes in plasma 2-DHG, DHA-EA and 2-AG were correlated with changes in their respective precursor fatty acids was not unexpected. In the present trial, changes in DHA seemed to be the best predictor of changes in both the n-3 and n-6 endocannabinoid families. The finding that changes in plasma DHA were positively related to DHA-derived endocannabinoids and inversely related to 2-AG is consistent with the model shown in Figure 1, in which DHA and AA compete for the same endocannabinoid biosynthetic enzymes. In this model, increased bioavailability of DHA produced by the H3-L6 intervention displaces AA as a substrate for endocannabinoid biosynthesis.
Diet-induced increases in 2-docosahexaenoylglycerol (2-DHG)
To our knowledge, the finding that plasma 2-DHG can be modified by diet is the first such demonstration in humans. Previous animal studies examining diet-induced alterations in 2-DHG were inconclusive. Berger et al.7 showed that addition of DHA to DHA-free piglet formula increased total brain lipid 2-DHG levels by 53%, however the relevance of these pig findings for human adults consuming omnivorous diets (containing some preformed DHA) is unclear. By contrast, Wood et al.42 found that DHA supplementation for 2 weeks in mice did not significantly alter plasma or brain 2-DHG, despite significant increases in DHA and DHA-EA. This discrepancy may be due to the longer duration of feeding (12 weeks vs. 2 weeks) or to the concurrent lowering of dietary n-6 LA in the present trial. LA lowering may promote increased 2-DHG synthesis by increasing hepatic synthesis-secretion and tissue incorporation of the 2-DHG precursor DHA (Figure 1).
Although 2-DHG is a structural analog of the endocannabinoid 2-AG, it has low affinity for cannabinoid receptors 38 and specific function(s) of 2-DHG have not yet been elucidated. However, the relatively high concentration of 2-DHG in brain tissue 7, 42, indicates that 2-DHG could have important functions in the brain. In the present trial, increases in 2-DHG correlated with reductions in headache frequency and severity, as well as psychological distress. Notably, these diet-induced improvements in psychological distress were selective for 2-DHG and were not associated with changes in 2-AG or DHA-EA. These findings are consistent with the hypothesis that 2-DHG might play a role in modulating physical pain and psychological distress. Future research into specific mechanisms of action of 2-DHG is needed to test this hypothesis.
Diet-induced increases in docosahexaenoylethanolamine (DHA-EA)
The demonstration in humans that DHA-EA can be modified by diet in the present trial is consistent with previous reports in animal models. Berger et al.7 reported that piglets fed formula containing n-3 DHA had higher DHA-EA levels in brain lipid extracts than piglets consuming DHA-free formula. Wood et al.42 showed in mice that DHA supplementation produced significant increases in plasma and brain DHA-EA. Figueroa et al.15 showed in rats that DHA supplementation increased 2-docosahexaenoyl-glycerophospoethanolamine, the precursor to DHA-EA, in experimental spinal cord injury (SCI). This increase was associated with improved functional recovery and reduced sensitivity to noxious heat. This link between DHA-EA and reduced pain sensitivity in experimental SCI is consistent with the association between increases in DHA-EA and reductions in headache frequency in the present trial. Importantly, however, specific mechanisms linking DHA-EA to anti-nociception have not been identified.
In addition to these putative anti-nociceptive actions, DHA-EA has well-known anti-inflammatory 25 and neurogenic properties. Kim et al. demonstrated that DHA-EA is a potent stimulator of neuritogenesis, synaptogenesis, and synaptic protein expression in developing hippocampal neurons 32, 34, 39. These neurogenic properties could help explain the finding of improved functional recovery with DHA supplementation after experimental SCI reported by Figueroa et al 37. In addition, these properties highlight the potential of the H3-L6 intervention as a therapeutic strategy that should be tested for enhancing recovery after traumatic brain or spinal cord injury.
Diet-induced reduction in 2-arachidonoylglycerol (2-AG)
The finding that plasma 2-AG was reduced by the H3-L6 intervention is consistent with a previous report that supplementation with krill oil (a source of n-3 EPA and DHA) reduced plasma 2-AG in obese humans.5 However, the same group of investigators recently found that supplementation with krill oil powder reduced plasma AEA, but it had no effect on 2-AG.6 Our finding of reduced 2-AG with the H3-L6 intervention is consistent with findings in mouse plasma 42 and brain 2 with supplemental DHA. Hibbeln et al. demonstrated significant reductions in liver and brain n-6 AA and 2-AG with dietary n-6 LA lowering alone in mice. 2 By contrast, we found in humans that n-6 lowering alone (the L6 intervention) for 12 weeks had no effect on plasma AA or 2-AG. This discrepancy may be due to differences between mouse diets and omnivorous human diets which contain preformed AA and DHA. AA and DHA inhibit hepatic conversion of LA to AA,14 the precursor for 2-AG biosynthesis. Hence, dietary n-6 LA intake may be less relevant for 2-AG biosynthesis in humans. Alternatively, 12 weeks of dietary LA-lowering may not be long enough to reduce circulating AA and 2-AG due to the high LA content of adipose tissue in the U.S. 16, 21,18
The relationship between 2-AG and physical pain and psychological distress is complex and incompletely understood. Acute stimulation of CB-1 and CB-2 cannabinoid receptors by 2-AG elicits anti-nociceptive22, 33 and anxiolytic 1 effects. However, 2-AG also serves as a major source for production free AA and pronociceptive eicosanoids 4, 28, which have been implicated in the pathogenesis of multiple chronic pain syndromes including headaches 3. Moreover, 2-AG also serves as a substrate for cyclooxygenase 2 mediated conversion into pro-nociceptive mediators with actions that largely opposite to known anti-nociceptive and anti-inflammatory actions of 2-AG 17. These observations, which highlight the complex and interactive nature of lipid signaling pathways, indicate that higher levels of 2-AG might promote nociceptive signaling in certain circumstances. Consistent with this, in a recent study in patients with end-stage osteoarthritis undergoing total knee arthroplasty, central (cerebrospinal fluid) and peripheral (synovial fluid) levels of 2-AG were significantly elevated in patients with higher postoperative pain, and synovial fluid 2-AG levels positively correlated with postoperative opioid use 4. In the present study, the findings that the reduction in pain in the H3-L6 group was accompanied by a reduction in plasma 2-AG, and that the decrease in 2-AG was associated with fewer severe headache hours, are consistent with the idea that 2-AG could contribute to nociceptive signaling. However, these relationships are complex and require further investigation.
Could the H3-L6 intervention reduce addictive risk in chronic pain patients?
Patients with chronic pain have high rates of alcohol 13 and tobacco misuse 26. Abuse of prescription opioids and other analgesic medications is a major public health challenge 36. In the present trial, the H3-L6 group experienced a major reduction in headache pain while reducing their use of acute pain medications 30. This group also had a reduction in plasma concentrations of 2-AG. Given the links between n-6 endocannabinoid hyperactivity, impaired satiety, craving and addiction 11, 27, the prospect of reducing 2-AG in the context of pain reduction as a strategy for reducing addictive tendency is appealing. These preliminary data suggest that the H3-L6 intervention could be investigated in future trials as a complementary approach for managing pain and reducing relapse in patients with chronic pain and comorbid opioid misuse.
Limitations
The present trial was relatively small and requires replication in a larger trial with nutrients altered as controlled variables. While the results clearly demonstrate that diet can alter circulating DHA and AA derived endocannabinoids, they cannot establish whether any of these endocannabinoids are causally related to improvements in pain-related clinical endpoints. Future studies are needed to: (1) establish whether comparable diet-induced biochemical alterations are possible in tissues more directly associated with physical and psychological dimensions of pain; (2) determine whether the magnitude of diet induced changes in endocannabinoid levels are physiologically relevant; (3) examine whether and how these lipid mediators modulate physical and psychological pain responses; (4) assess the relative contributions of the n-3 and n-6 derived endocannabinoids in relation to other lipid mediators posited to modulate inflammation and pain; (5) examine the effects of age and gender on the biochemical and clinical effects of the interventions; and (6) to examine the potential interplay between dietary n-3 and n-6 fatty acids and pain medications that alter fatty acid metabolism (e.g. non-steroidal anti-inflammmatory medications).
Conclusion
In summary, we found that targeted dietary changes can alter circulating 2-DHG, DHA-EA and 2-AG in accordance with changes in their biosynthetic precursor pools. Physical pain relief correlated with increases in the 2-DHG and DHA-EA, while improvements in psychological aspects of pain were correlated with increases in 2-DHG alone. These findings establish that DHA-derived endocannabinoid-like mediators are modifiable by diet in humans, and highlight 2-DHG and DHA-EA as lipid autacoids worthy of further investigation as mediators or biomarkers of physical pain and related conditions.
Supplementary Material
Supplementary Table S1. Inclusion and exclusion criteria for trial participation
Supplementary Table S2: Baseline and intra-intervention nutrient intakes the H3-L6 and L6 groups a
Supplementary Table S3.a. Plasma phospholipid fatty acid (%FA) at baseline and after 12-week dietary interventions 1
Supplementary Table S3.b. Plasma phospholipid fatty acid concentrations at baseline and after 12-week dietary interventions 1
Supplementary Table S4.a. Associations between 12-week changes in endocannabinoids and physical pain – by diet group
Supplementary Table S4.b. Associations between 12-week changes in endocannabinoids and pain-related quality of life measures and psychological stress – by diet group
Supplementary Table S5. Baseline characteristics of 67 patients with chronic headaches a
Highlights.
Endocannabinoids have analgesic, anxiolytic, and neurogenic properties.
Endocannabinoids derived from n-3 and n-6 fatty acids can be modified by diet in humans.
Diet induced changes in n-3 derived endocannabinoids correlated with clinical improvement.
Acknowledgments
The authors thank the patients who participated in the trial, and acknowledge the following individuals for their contributions: Chanee Lynch, Susan Gaylord, Chirayath Suchindran, Oli Palsson, David Barrow, Angela Johnston, Rebecca Coble, Meg Mangan, Beth Fowler, Carol Carr, Regina McCoy, Tim McCaskill, Gus Swenson, Marjorie Busby, Ameer Taha, Stanley Rapoport and Mark Horowitz. This project was supported by the Mayday Fund (primary funding source); the UNC Research Fellowship in Complementary and Alternative Medicine (grant T32-AT003378, NCCAM, NIH); the North Carolina Clinical and Translational Sciences Institute (grant UL1RR025747, NCRR, NIH); the UNC Nutrition Obesity Research Center, CHAI Core (grant DK056350, NIDDK, NIH); the National Institute on Drug Abuse (DA07215 and DA09158); and the Intramural Program of the National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures: Research Funding: This project was supported by the Mayday Fund (primary funding source); the UNC Research Fellowship in Complementary and Alternative Medicine (grant T32-AT003378, NCCAM, NIH); the North Carolina Clinical and Translational Sciences Institute (grant UL1RR025747, NCRR, NIH); the UNC Nutrition Obesity Research Center, CHAI Core (grant DK056350, NIDDK, NIH); the National Institute on Drug Abuse (DA07215 and DA09158); and the Intramural Program of National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health.
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Almeida-Santos AF, Gobira PH, Rosa LC, Guimaraes FS, Moreira FA, Aguiar DC. Modulation of anxiety-like behavior by the endocannabinoid 2-arachidonoylglycerol (2-AG) in the dorsolateral periaqueductal gray. Behavioural brain research. 2013;252:10–17. doi: 10.1016/j.bbr.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 2.Alvheim AR, Malde MK, Osei-Hyiaman D, Lin YH, Pawlosky RJ, Madsen L, Kristiansen K, Froyland L, Hibbeln JR. Dietary linoleic acid elevates endogenous 2-AG and anandamide and induces obesity. Obesity. 2012;20:1984–1994. doi: 10.1038/oby.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antonova M, Wienecke T, Olesen J, Ashina M. Prostaglandin E(2) induces immediate migraine-like attack in migraine patients without aura. Cephalalgia: an international journal of headache. 2012;32:822–833. doi: 10.1177/0333102412451360. [DOI] [PubMed] [Google Scholar]
- 4.Azim S, Nicholson J, Rebecchi MJ, Galbavy W, Feng T, Reinsel R, Volkow ND, Benveniste H, Kaczocha M. Endocannabinoids and acute pain after total knee arthroplasty. Pain. 2015;156:341–347. doi: 10.1097/01.j.pain.0000460315.80981.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banni S, Carta G, Murru E, Cordeddu L, Giordano E, Sirigu AR, Berge K, Vik H, Maki KC, Di Marzo V, Griinari M. Krill oil significantly decreases 2-arachidonoylglycerol plasma levels in obese subjects. Nutrition & metabolism. 2011;8:7. doi: 10.1186/1743-7075-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berge K, Piscitelli F, Hoem N, Silvestri C, Meyer I, Banni S, Di Marzo V. Chronic treatment with krill powder reduces plasma triglyceride and anandamide levels in mildly obese men. Lipids in health and disease. 2013;12:78. doi: 10.1186/1476-511X-12-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berger A, Crozier G, Bisogno T, Cavaliere P, Innis S, Di Marzo V. Anandamide and diet: inclusion of dietary arachidonate and docosahexaenoate leads to increased brain levels of the corresponding N-acylethanolamines in piglets. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:6402–6406. doi: 10.1073/pnas.101119098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burston JJ, Woodhams SG. Endocannabinoid system and pain: an introduction. The Proceedings of the Nutrition Society. 2014;73:106–117. doi: 10.1017/S0029665113003650. [DOI] [PubMed] [Google Scholar]
- 9.Cao D, Kevala K, Kim J, Moon HS, Jun SB, Lovinger D, Kim HY. Docosahexaenoic acid promotes hippocampal neuronal development and synaptic function. Journal of neurochemistry. 2009;111:510–521. doi: 10.1111/j.1471-4159.2009.06335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Petrocellis L, Schiano Moriello A, Imperatore R, Cristino L, Starowicz K, Di Marzo V. A re-evaluation of 9-HODE activity at TRPV1 channels in comparison with anandamide: enantioselectivity and effects at other TRP channels and in sensory neurons. British journal of pharmacology. 2012;167:1643–1651. doi: 10.1111/j.1476-5381.2012.02122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Vries TJ, Schoffelmeer AN. Cannabinoid CB1 receptors control conditioned drug seeking. Trends in pharmacological sciences. 2005;26:420–426. doi: 10.1016/j.tips.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Derogatis L. BSI-18: Administration, Scoring, and Procedures Manual. Minneapolis, MN: National Computer Systems; 2000. [Google Scholar]
- 13.Egli M, Koob GF, Edwards S. Alcohol dependence as a chronic pain disorder. Neuroscience and biobehavioral reviews. 2012;36:2179–2192. doi: 10.1016/j.neubiorev.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emken EA, Adlof RO, Duval SM, Nelson GJ. Effect of dietary docosahexaenoic acid on desaturation and uptake in vivo of isotope-labeled oleic, linoleic, and linolenic acids by male subjects. Lipids. 1999;34:785–791. doi: 10.1007/s11745-999-0424-2. [DOI] [PubMed] [Google Scholar]
- 15.Figueroa JD, Cordero K, Serrano-Illan M, Almeyda A, Baldeosingh K, Almaguel FG, De Leon M. Metabolomics uncovers dietary omega-3 fatty acid-derived metabolites implicated in anti-nociceptive responses after experimental spinal cord injury. Neuroscience. 2013;255:1–18. doi: 10.1016/j.neuroscience.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garland M, Sacks FM, Colditz GA, Rimm EB, Sampson LA, Willett WC, Hunter DJ. The relation between dietary intake and adipose tissue composition of selected fatty acids in US women. The American journal of clinical nutrition. 1998;67:25–30. doi: 10.1093/ajcn/67.1.25. [DOI] [PubMed] [Google Scholar]
- 17.Guindon J, Hohmann AG. A physiological role for endocannabinoid-derived products of cyclooxygenase-2-mediated oxidative metabolism. British journal of pharmacology. 2008;153:1341–1343. doi: 10.1038/bjp.2008.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Progress in lipid research. 2008;47:348–380. doi: 10.1016/j.plipres.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Kawata AK, Coeytaux RR, Devellis RF, Finkel AG, Mann JD, Kahn K. Psychometric properties of the HIT-6 among patients in a headache-specialty practice. Headache. 2005;45:638–643. doi: 10.1111/j.1526-4610.2005.05130.x. [DOI] [PubMed] [Google Scholar]
- 20.Kim HY, Moon HS, Cao D, Lee J, Kevala K, Jun SB, Lovinger DM, Akbar M, Huang BX. N-Docosahexaenoylethanolamide promotes development of hippocampal neurons. The Biochemical journal. 2011;435:327–336. doi: 10.1042/BJ20102118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kingsbury K, Paul S, Morgan DM, Crossley A. Fatty Acid Composition of Human Depot Fat. Biochemical Journal. 1961;78:541–&. doi: 10.1042/bj0780541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LR D. BSI-18: Administration, Scoring, and Procedures Manual. Minneapolis, MN: National Computer Systems; 2000. [Google Scholar]
- 23.Luo X, George ML, Kakouras I, Edwards CL, Pietrobon R, Richardson W, Hey L. Reliability, validity, and responsiveness of the short form 12-item survey (SF-12) in patients with back pain. Spine. 2003;28:1739–1745. doi: 10.1097/01.BRS.0000083169.58671.96. [DOI] [PubMed] [Google Scholar]
- 24.MacIntosh BA, Ramsden CE, Faurot KR, Zamora D, Mangan M, Hibbeln JR, Mann JD. Low-n-6 and low-n-6 plus high-n-3 diets for use in clinical research. The British journal of nutrition. 2013;110:559–568. doi: 10.1017/S0007114512005181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meijerink J, Plastina P, Vincken JP, Poland M, Attya M, Balvers M, Gruppen H, Gabriele B, Witkamp RF. The ethanolamide metabolite of DHA, docosahexaenoylethanolamine, shows immunomodulating effects in mouse peritoneal and RAW264.7 macrophages: evidence for a new link between fish oil and inflammation. The British journal of nutrition. 2011;105:1798–1807. doi: 10.1017/S0007114510005635. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell MD, Mannino DM, Steinke DT, Kryscio RJ, Bush HM, Crofford LJ. Association of smoking and chronic pain syndromes in Kentucky women. The journal of pain: official journal of the American Pain Society. 2011;12:892–899. doi: 10.1016/j.jpain.2011.02.350. [DOI] [PubMed] [Google Scholar]
- 27.Mitrirattanakul S, Lopez-Valdes HE, Liang J, Matsuka Y, Mackie K, Faull KF, Spigelman I. Bidirectional alterations of hippocampal cannabinoid 1 receptors and their endogenous ligands in a rat model of alcohol withdrawal and dependence. Alcoholism, clinical and experimental research. 2007;31:855–867. doi: 10.1111/j.1530-0277.2007.00366.x. [DOI] [PubMed] [Google Scholar]
- 28.Nomura DK, Morrison BE, Blankman JL, Long JZ, Kinsey SG, Marcondes MCG, Ward AM, Hahn YK, Lichtman AH, Conti B, Cravatt BF. Endocannabinoid Hydrolysis Generates Brain Prostaglandins That Promote Neuroinflammation. Science. 2011;334:809–813. doi: 10.1126/science.1209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramsden CE, Faurot KR, Zamora D, Palsson OS, MacIntosh BA, Gaylord S, Taha AY, Rapoport SI, Hibbeln JR, Davis JM, Mann JD. Targeted alterations in dietary n-3 and n-6 fatty acids improve life functioning and reduce psychological distress among patients with chronic headache: a secondary analysis of a randomized trial. Pain. 2015;156:587–596. doi: 10.1097/01.j.pain.0000460348.84965.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramsden CE, Faurot KR, Zamora D, Suchindran CM, Macintosh BA, Gaylord S, Ringel A, Hibbeln JR, Feldstein AE, Mori TA, Barden A, Lynch C, Coble R, Mas E, Palsson O, Barrow DA, Mann JD. Targeted alteration of dietary n-3 and n-6 fatty acids for the treatment of chronic headaches: a randomized trial. Pain. 2013;154:2441–2451. doi: 10.1016/j.pain.2013.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramsden CE, Mann JD, Faurot KR, Lynch C, Imam ST, MacIntosh BA, Hibbeln JR, Loewke J, Smith S, Coble R, Suchindran C, Gaylord SA. Low omega-6 vs. low omega-6 plus high omega-3 dietary intervention for chronic daily headache: protocol for a randomized clinical trial. Trials. 2011;12:97. doi: 10.1186/1745-6215-12-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rashid MA, Katakura M, Kharebava G, Kevala K, Kim HY. N-Docosahexaenoylethanolamine is a potent neurogenic factor for neural stem cell differentiation. Journal of neurochemistry. 2013;125:869–884. doi: 10.1111/jnc.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rea K, Olango WM, Okine BN, Madasu MK, McGuire IC, Coyle K, Harhen B, Roche M, Finn DP. Impaired endocannabinoid signalling in the rostral ventromedial medulla underpins genotype-dependent hyper-responsivity to noxious stimuli. Pain. 2014;155:69–79. doi: 10.1016/j.pain.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 34.Salem N, Jr, Pawlosky R, Wegher B, Hibbeln J. In vivo conversion of linoleic acid to arachidonic acid in human adults. Prostaglandins, leukotrienes, and essential fatty acids. 1999;60:407–410. doi: 10.1016/s0952-3278(99)80021-0. [DOI] [PubMed] [Google Scholar]
- 35.Sastry PS. Lipids of nervous tissue: composition and metabolism. Progress in lipid research. 1985;24:69–176. doi: 10.1016/0163-7827(85)90011-6. [DOI] [PubMed] [Google Scholar]
- 36.Schultz D. Opioid use and abuse: a pain clinic perspective. Minnesota medicine. 2013;96:42–44. [PubMed] [Google Scholar]
- 37.Stark KD. The percentage of n-3 highly unsaturated fatty acids in total HUFA as a biomarker for omega-3 fatty acid status in tissues. Lipids. 2008;43:45–53. doi: 10.1007/s11745-007-3128-3. [DOI] [PubMed] [Google Scholar]
- 38.Sugiura T, Kodaka T, Nakane S, Miyashita T, Kondo S, Suhara Y, Takayama H, Waku K, Seki C, Baba N, Ishima Y. Evidence that the cannabinoid CB1 receptor is a 2-arachidonoylglycerol receptor. Structure-activity relationship of 2-arachidonoylglycerol, ether-linked analogues, and related compounds. The Journal of biological chemistry. 1999;274:2794–2801. doi: 10.1074/jbc.274.5.2794. [DOI] [PubMed] [Google Scholar]
- 39.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 40.Williams J, Wood J, Pandarinathan L, Karanian DA, Bahr BA, Vouros P, Makriyannis A. Quantitative method for the profiling of the endocannabinoid metabolome by LC-atmospheric pressure chemical ionization-MS. Analytical chemistry. 2007;79:5582–5593. doi: 10.1021/ac0624086. [DOI] [PubMed] [Google Scholar]
- 41.Wood JT, Williams JS, Pandarinathan L, Courville A, Keplinger MR, Janero DR, Vouros P, Makriyannis A, Lammi-Keefe CJ. Comprehensive profiling of the human circulating endocannabinoid metabolome: clinical sampling and sample storage parameters. Clinical chemistry and laboratory medicine: CCLM/FESCC. 2008;46:1289–1295. doi: 10.1515/CCLM.2008.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wood JT, Williams JS, Pandarinathan L, Janero DR, Lammi-Keefe CJ, Makriyannis A. Dietary docosahexaenoic acid supplementation alters select physiological endocannabinoid-system metabolites in brain and plasma. Journal of lipid research. 2010;51:1416–1423. doi: 10.1194/jlr.M002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1. Inclusion and exclusion criteria for trial participation
Supplementary Table S2: Baseline and intra-intervention nutrient intakes the H3-L6 and L6 groups a
Supplementary Table S3.a. Plasma phospholipid fatty acid (%FA) at baseline and after 12-week dietary interventions 1
Supplementary Table S3.b. Plasma phospholipid fatty acid concentrations at baseline and after 12-week dietary interventions 1
Supplementary Table S4.a. Associations between 12-week changes in endocannabinoids and physical pain – by diet group
Supplementary Table S4.b. Associations between 12-week changes in endocannabinoids and pain-related quality of life measures and psychological stress – by diet group
Supplementary Table S5. Baseline characteristics of 67 patients with chronic headaches a


