Abstract
Introduction
Our aim was to assess differences in movement measures in Attention-Deficit/Hyperactivity Disorder (ADHD) vs. typically developing (TD) controls.
Methods
We performed meta-analyses of published studies on motion measures contrasting ADHD with controls. We also conducted a case-control study with children/adolescents (n=61 TD, n=62 ADHD) and adults (n=30 TD, n=19 ADHD) using the McLean Motion Activity Test, semi-structured diagnostic interviews and the Behavior Rating Inventory of Executive Function and Conners (Parent, Teacher; Self) Rating Scales.
Results
Meta-analyses revealed medium-to-large effect sizes for actigraph (standardized mean difference [SMD]: 0.64, 95% Confidence interval (CI): 0.43, 0.85) and motion tracking systems (SDM: 0.92, 95% CI: 0.65, 1.20) measures in differentiating individuals with ADHD from controls. Effects sizes were similar in studies of children/adolescents ([SMD]:0.75, 95% CI: 0.50, 1.01) and of adults ([SMD]: 0.73, 95% CI: 0.46, 1.00). In our sample, ADHD groups differed significantly in number of Head Movements (p=0.02 in children; p=0.002 in adults), Displacement (p=0.009/p<0.001), Head Area (p=0.03/p<0.001), Spatial Complexity (p=0.06/p=0.02) and Temporal Scaling (p=0.05/p=0.04). Mean effect sizes were non-significantly larger (d=0.83, 95% CI: 0.20, 1.45) in adults vs. children/adolescents with ADHD (d=0.45, 95% CI: 0.08, 0.82). In the concurrent go/no-go task, reaction time variability was significantly greater in ADHD (p<0.05 in both age groups) than controls.
Conclusions
Locomotor hyperactivity remains core to the construct of ADHD even in adults. Our results suggest that objective locomotion measures may be particularly useful in evaluating adults with possible ADHD.
Keywords: ADHD, Diagnosis, Hyperactivity, McLean Motion and Attention test, Meta-analysis, Actigraphy
1. INTRODUCTION
Despite the substantial prevalence of Attention-Deficit/Hyperactivity Disorder (ADHD), estimated to be 5.9 to 7.1% in school aged-children and ~5% in adulthood (Willcutt, 2012) , we lack consensus on how best to ascertain the disorder. This is reflected in continuing concern regarding presumed over-diagnosis and evidence of both under- and over-diagnosis (Sciutto and Eisenberg, 2007). The diagnosis of ADHD is exclusively based on subjective descriptions of behavior, which is affected by multiple factors, including informant source, type of instruments used, and methods for combining information across measures and informants (Valo and Tannock, 2010). The perceived deficiencies of current diagnostic approaches have motivated a search for ADHD endophenotypes (Castellanos and Tannock, 2002), i.e., intermediate constructs that could help reveal the pathophysiology of the disorder and thereby increase diagnostic accuracy.
Given the centrality of hyperactivity for ADHD (Ohashi et al., 2010; Teicher et al., 2008), objective measures of locomotor activity have long been examined. An initial study found increased wrist actometer counts during free play in 16 children referred for possible hyperactivity (prior to the DSM-III codification of attention deficit disorder) when contrasted to a community comparison group (n=20) (Barkley and Ullman, 1975). Subsequently, Porrino et al. conducted the first studies in children systematically diagnosed with DSM-III attention deficit disorder with hyperactivity (ADDH) versus healthy controls, reporting that children with ADDH exhibited significantly greater locomotor activity, even during sleep (Porrino et al., 1983). Since then, increasingly sophisticated measures have been examined. This culminated in the May 2014 clearance by the USA Food and Drug Administration of the QbTest 1 an infrared motion tracking system, “to aid in the clinical assessment of ADHD and in the evaluation of treatment interventions in ADHD.” The FDA finding stated: “QBTest results should be interpreted only by qualified professionals.” However, guidelines on how such objective data should be incorporated into the diagnostic process have yet to be included in diagnostic systems.
Questions regarding diagnostic accuracy are particularly germane when assessing adults who may lack documentation regarding having met the diagnosis in childhood. Indeed, the DSM-5 (APA, 2013) requires that some symptoms be met prior to age 12, but does not specify how such retrospective history should be confirmed. The text acknowledges “adult recall of childhood symptoms tends to be unreliable, and it is beneficial to obtain ancillary information” (p. 61). Obtaining such confirmatory information, whether from parents or old report cards, is not always possible. Validation of the diagnosis through objective measures of attention and locomotion might be useful in such circumstances (Biederman et al., 2000; Brocki et al., 2008; Faraone et al., 2000; Lahey and Willcutt, 2010).
Given interest in the potential value of objective locomotor measures as biomarkers, we first performed meta-analyses contrasting individuals with ADHD to controls to assess the extent to which locomotor measures discriminate individuals with ADHD from controls. To our knowledge, this is the first meta-analysis providing standardized effect sizes and their 95% confidence intervals for these types of measures with respect to the diagnosis of ADHD in children and adults. One meta-analysis was focused on actigraphy, and another on measures that incorporate an infrared motion tracking camera with computerized attention testing, marketed under the names OPTAx, McLean Motion and Attention Test System (MMAT) or QbTest Plus. We also performed separate meta-analyses of studies in children and adolescents, on the one hand, and in adults, on the other, to address the possibility that diagnostic differences in movement vary with age. Finally, we report the results of two new studies that used MMAT, a precursor to the QbTest, in children/adolescents and in adults with ADHD, respectively, contrasted to typically developing individuals. We hypothesized that individuals with ADHD, whether children, adolescents, or adults, would exhibit significantly greater locomotor activity than TD comparisons. We also expected to observe greater activity in younger individuals, across both diagnostic groups.
2. METHODS
2.1.Meta-analysis methods
Methods for this meta-analysis have been developed according to recommendations from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement (PRISMA) (Liberati et al., 2009). For a more detailed description of methods, please see the protocol (supplementary material).
2.1.1. Inclusion criteria
All types of published peer-review controlled studies were included. As recommended by the Cochrane Group (2011) we did not search for unpublished data to avoid the inevitable bias caused by dependence on investigators agreeing to provide data from unpublished studies. To be included, ADHD groups needed a categorical diagnosis of ADHD per DSM (III,III-R, IV, IV-TR or 5) or Hyperkinetic Disorder according to ICD-10 or previous ICD versions. We did not include studies assessing only symptoms of ADHD, without a formal diagnosis of the disorder. As we wanted to assess the accuracy of locomotor measures for the diagnosis of ADHD, the inclusion of a control group was a requirement. Comparisons had to be individuals without ADHD, typically healthy controls. When studies reported two groups of controls, we chose only the healthy comparison group. No restrictions of age, sex or socioeconomic status were applied. The presence of comorbidity was not an exclusion criterion. No restriction of medication status was applied. Studies including participants recruited in any setting were retained. All types of movement measures were accepted, but not other measures assessed along with locomotor parameters, such as sleep parameters.
2.1.2 Search Strategy
The search was performed on June 1, 2014. LGM and SC blindly conducted the same search using the search terms and strategy in the same set of databases (PubMed, Ovid and Web of Knowledge) accessed via the New York University Electronic Library. Manual searches were also performed, scanning reference lists of relevant papers retrieved. No a priori limitations on language or period of publication were applied.
2.1.3 Outcome
The primary outcome was the effect sizes of objective measures of locomotion when used to contrast individuals with ADHD and controls.
2.1.4 Study selection
After removing duplicates, LGM and SC independently screened titles and abstracts and excluded papers judged not pertinent. A final list was agreed with discrepancies to be resolved by consensus between the two authors. When consensus was not reached, a third author (FXC) was available to act as arbitrator. If any doubt about inclusion existed, the article proceeded to the next stage.
2.1.5 Data extraction and statistical analysis
Data extraction was independently performed by two authors (LGM and SC). A single mean effect size was obtained per study by averaging; indices were multiplied by -1 if higher locomotor activity was associated with smaller values, e.g., for Immobility Duration. We initially performed separate analyses for actigraphy measures and tracking motion system measures because of the different characteristics of these approaches. Study information was entered into RevMan 5.3 (Collaboration, 2014). Standardized mean difference (SMD) for each study was computed using the inverse-variance method. Given the inherent heterogeneity of studies, random-effects models were used. The I2 statistic was calculated to estimate between-study SMD heterogeneity.
2.2 Original data
We designed a case-control study contrasting patients with ADHD to TD subjects. We included children and adolescents in one group and adults in another. Typically developed participants were recruited through IRB-approved advertisements. Patients with ADHD were mostly referred from the Child Study Center at NYU Langone Medical Center. Families and adult participants received US$60 for participation and a full psychodiagnostic assessment and report. Inclusion criteria were: children/adolescents between 8.0-17.9 and adults between 18.0-54.9 years with estimated full-scale or verbal IQ of at least 80. Comorbid diagnoses of anxiety or learning disorders were allowed; mood, psychotic, or pervasive developmental disorders were excluded. ADHD groups required a clinician-based diagnosis of DSM-IV-TR ADHD, any subtype, obtained through semi-structured diagnostic interview and assisted in the child/adolescent group by reviewing parent and teacher rating scales. Participants taking psychostimulants were asked to discontinue treatment for at least 24 hours before the experimental session.
After a phone screen, we conducted a diagnostic evaluation session, lasting approximately four hours. We recruited 264 children and adolescents of whom 65 did not complete the MMAT and 76 did not meet inclusion criteria. We recruited a total of 74 adult subjects, of whom six did not complete the MMAT, 12 were excluded because of comorbidity and seven had lost data. Analyses were based on 62 ADHD and 61 TD child/adolescent subjects and 19 adults with ADHD and 30 TD adults. In terms of pharmacological history, in the child/adolescent ADHD group, 18 of 62 patients (29%) reported current psychostimulant treatment (methylphenidate, dexmethylphenidate, or amphetamines), eight (12.9%) reported past history of treatment but no current medication use and 36 (58%) were medication-naïve. Among adults with ADHD, three of 19 patients (15.8%) reported current treatment with medication (methylphenidate or amphetamines) and seven (36.8%) reported a history of pharmacological treatment but denied current treatment. The rest (47.4%) were medication-naïve. The study was approved by the NYU Langone Medical Center Institutional Review Board (IRB). All participants provided IRB-approved written informed consent (and assent, for children/adolescents), prior to participating.
In the child and adolescent group, diagnosis was determined using the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (KSADS-PL) (Kaufman et al., 1997) in separate meetings with a parent and the child. For adults, the ADHD Clinical Diagnostic Scale (ACDS) (Adler and Spencer, 2004) was obtained, along with the Structured Clinical Interview for DSM-IV-TR, Non-patient Edition (First et al., 2002) to assess ADHD and Axis-I comorbidity, respectively. In both cases, diagnosis was made by a licensed clinician or a psychology graduate student under the supervision of a licensed clinician. Each participant was invited for a diagnostic and a cognitive assessment, consisting of the Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler, 1999) and the Wechsler Individual Achievement Test (WIAT-II) (Wechsler, 2001). Demographic information was collected with a standardized questionnaire used at the Child Study Center at NYU Langone Medical Center. Socioeconomic status was characterized using the Hollingshead Index (Hollingshead, 1975). To measure symptom severity, the Conners Parent Rating Scale-Revised: Long Version and Conners Teacher Rating Scale-Revised: Long Version (Conners, 1997) and Conners Adult ADHD Rating Scales (CAARS) (Conners et al., 1999) were utilized. Finally, to measure the impact of executive functioning in daily life, we used the Behavior Rating Inventory of Executive Function (BRIEF) (Gioia et al., 2000) for children and the Behavior Rating Inventory of Executive Function-Adult Version (BRIEF-A) Self- and Informant-reports for adults (Roth et al., 2005).
We used the MMAT (Teicher, 1996b) an infrared motion tracking system that quantifies micromovements of participants during a go/no-go task lasting 15 minutes for children or adolescents and 20 minutes for adults. The target stimuli (8-pointed stars) and non-targets (5-pointed stars) appeared at random screen position in a random sequence for 200ms each, at 2000ms intervals. For children/adolescents, targets and non/targets stimuli were presented in a 1:1 ratio, while adults were presented a 3:1 ratio. During the task, we measured head movements by measuring displacement of a small spherical reflector secured via elastic bands on the forehead. The variables recorded for the go/no-go task were “Percent Accuracy,” “Percent Omission Errors,” “Percent Commission Errors,” “Reaction Time Latency” (in ms; RT Latency), “Reaction Time Variability” (intraindividual standard deviation (SD) of the response times; RT Variability) and “Coefficient of Variation” (response time SD/mean latency; RT COV). RT latency, RT Variability and RT COV were based only on correct responses. Movement variables were “Head Immobility Duration” averaged over each 5 minute period, “Head Movements,” measuring the average number of position changes greater than 1mm averaged over each 5 minute interval, “Head Displacement,” the total distance traveled, in meters, averaged over each 5 minute period; “Head Area,” the two-dimensional space in which the reflector moved; “Head Spatial Complexity,” describing the fractal dimensionality or complexity of the movement path, with values ranging from 1 to 2; and “Head Temporal Scaling,” indexing the frequency of movement (Teicher, 1996a).
Analyses were performed using SPSS version 21 (IBM-Corporation, 2012). For nominal group characteristics, chi-square tests were used. One-way ANOVAs were used to compare cognitive measures, BRIEF and Conners scores. ANCOVAs adjusting for age and sex were performed to compare micromovement measures across diagnostic groups for each age range (children and adolescents; adults). We performed Pearson correlations or partial correlations adjusting for age (when age was significantly correlated with motion measures) between MMAT motion measures and the following: BRIEF, Conners ratings, and with the Go/no-go measures. To balance rates of type 1 and type 2 errors, the significance threshold for correlations was set at p < 0.01. Otherwise, we used the standard threshold of p < 0.05, two-tailed.
3 RESULTS
3.1 Meta-analysis results
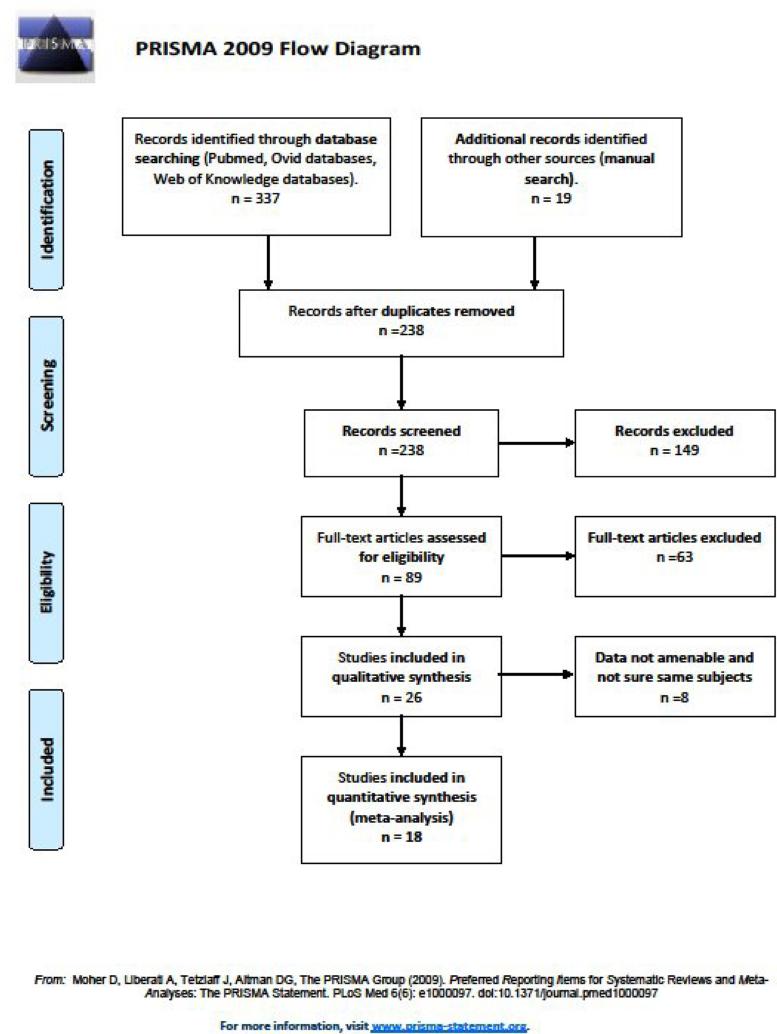
Twenty-six studies met our criteria. We were unable to extract SMD values from seven studies (Edebol et al., 2012; Inoue et al., 1998; Kam et al., 2011; Martin-Martinez et al., 2012; O'Mahony et al., 2014; Ohashi et al., 2010; Wood et al., 2009). Two reports (Tsujii et al., 2007; Tsujii et al., 2009), appeared to be based on the same data, so we only included the former. For each of these eight exclusions, we attempted to contact authors to ask for additional data, without satisfactory results. Final analyses were performed with 18 studies. Search results are shown in Figure 1 according to the PRISMA flowchart (Liberati et al., 2009).
Figure 1.
Flowchart showing the selection of studies.
In two studies, a subgroup of ADHD subjects was receiving pharmacological treatment: 23.1% in Baird et al. (2012) and 25% in Glass et al.(2014). In the remaining studies, treatment was discontinued at least 24 hours before locomotion assessment. Characteristics of included papers are summarized in Table 1.
TABLE 1.
Characteristics of included studies
| N | Age | % Boys | % Comorbidity | Setting | Method | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| ADHD | TD | ADHD | TD | ADHD | TD | ADHD | TD | |||
| Porrino et. al (1983) | 12 | 12 | 8.6±2.1 | 8.6±1.9 | 100 | 100 | 58 | 0.16 | NC | Act waist |
| Halperin et al. (1992) | 31 | 18 | 9.6±1.9 | 9.17±1.78 | 80.6 | 100 | 71 | 0 | C | Act waist |
| Teicher et al. (1996a) | 18 | 11 | 9.3±2.4 | 8.6 ±1.8 | 100 | 100 | 83.3 | 0 | C | MMAT |
| Dane et al. (2000) | 42 | 22 | 9.19±1.46 | 9.14±1.38 | 83.43 | 67 | 50 | 41 | C | Act wrist |
| Boonstra et al. (2007) | 33 | 39 | 37.9±10.3 | 37.8 ± 9.5 | 48.4 | 46.15 | NR | 0 | NC | Act wrist |
| Tsujii et al. (2007) | 16 | 20 | 9±1.26 | 9.1 ± 1.21 | 81.25 | 75 | 25 | 0 | NC | Act wrist |
| Halperin et al. (2008) | 98 | 85 | 18.3±1.6 | 18.51±1.66 | * | * | NR | NR | C | Act ankles/waist |
| Licht & Tryon (2009) | 9 | 9 | 9.33±1 | 9.11±1.17 | 88.9 | 77.8 | 0 | 0 | NC | Act waist |
| Rapport et al. (2009) | 12 | 11 | 8.75±1.29 | 9.36±1.43 | NR | NR | 50 | 0 | C | Act ankles/ wrist |
| Lis et al. (2010) | 20 | 20 | 37.3±8.3 | 37.5±9.2 | 65 | 65 | 0 | 0 | C | QbTest Plus |
| Baird et al. (2012) | 13 | 19 | 31.3±11.7 | 32.3±13.3 | 61.5 | 63.1 | 46.2 | 0 | NC | Act wrist |
| Alderson et al. (2012) | 11 | 11 | 8.64±1.29 | 9.45±1.44 | 100 | 100 | 54,5 | 0 | C | Act ankles/ wrist |
| Glass et al.(2014) | 16 | 22 | 12.2±3.43 | 11.9±2.71 | 75 | 50 | 0 | 0 | C | Act wrist |
| Teicher et al. (2012) | 40 | 60 | 35±10 | 29±9 | 57.5 | 46.67 | 0 | 0 | C | MMAT |
| Hudec et al. (2014) | 20 | 15 | 19.65±1.93 | 19.33±1.05 | 50 | 60 | 0 | 0 | C | Act ankles/wrist |
| Miyahara et al. (2014) | 93 | 76 | 3.77±0.45 | 3.7±0.46 | 74.19 | 67.1 | 7.5 | 0 | C | Act ankle/ waist |
| Reh et al. (2014) | 45 | 45 | 9.2±1.7 | 8.9±1.2 | 77.8 | 44.4 | 46 | NR | C | QbTest Plus |
| Soderstrom et al. (2014) | 41 | 20 | 32.46±8.99 | 30±9.76 | 43.9 | 40 | 56.1 | 80 | C | QbTest Plus |
Age is expressed in mean ± standard deviation. TD= Typically developed.
87.8% across all subjects in the study. Setting: NC= Non Clinic, C= Clinic. Method: Act=Actigraph. NR=not reported
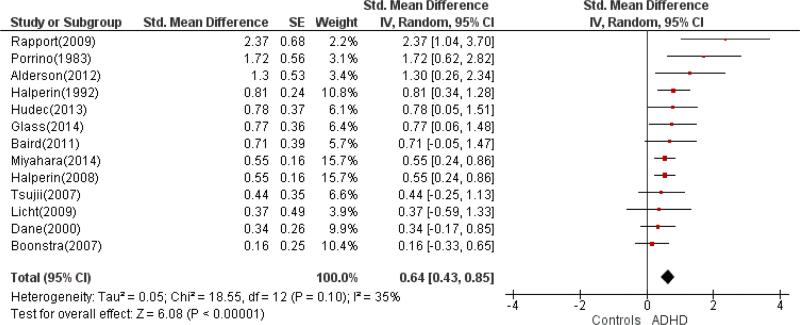
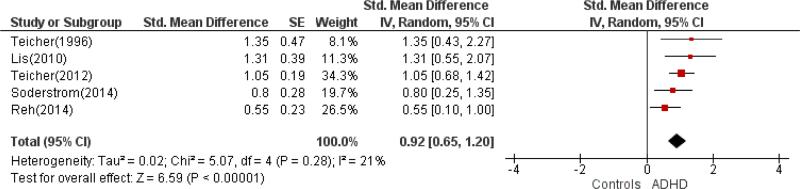
The meta-analyses focused on differences in locomotion measures between groups of individuals diagnosed with ADHD and healthy controls. The combined sample sizes were 406 patients with ADHD versus 359 controls with actigraphy data and 164 patients with ADHD versus 156 controls with motion tracking system data. As shown in Figures 2 and 3, actigraphic measures were associated with a medium effect size (SMD: 0.64, 95% CI: 0.43, 0.85) indicating greater activity in ADHD. The SMD for motion tracking studies was large (SMD: 0.92, 95%CI: 0.65, 1.20) in the same direction of greater activity in ADHD, although the confidence intervals overlapped for the two types of measures.
Figure 2.
Forest plot for actigraphy studies
Figure 3.
Forest plot for Motion Tracking Systems.
Given the overlapping effect sizes across methods (actigraphy and motion tracking systems), we reexamined the data stratified by mean sample age. The combined studies of children and adolescent included 305 patients with ADHD and 257 healthy controls. The studies of adults (defined on the basis of mean age > 18.0 years) included 265 patients with ADHD and 258 healthy controls. Locomotor measures were associated with a medium-to-large effect size in both groups (in children/adolescents, SMD=0.75, 95% CI: 0.50, 1.01; in adults, SMD=0.73, 95% CI: 0.46, 1.00).
3.2 Original data
As shown in Tables 2 and 3, the two diagnostic groups did not differ significantly with regard to age, parent- or self-reported race, socioeconomic status or IQ. In the child and adolescent group, we found significant differences between ADHD and TD with regard to sex distribution (79% male ADHD vs. 39% male TD) and in subscales related to lower academic achievement in the ADHD group (WIAT, p<0.01).
Table 2.
Demographic data in children/adolescents
| TD | ADHD | χ2/F | df | p | ||
|---|---|---|---|---|---|---|
| Sex | Female | 37 | 13 | 20.07 | 1 | <0.001 |
| Male | 24 | 49 | ||||
| Total | 61 | 62 | ||||
| Race | Caucasian | 27 | 32 | 10.45 | 6 | 0.107 |
| Black/African | 16 | 12 | ||||
| Asian | 6 | 3 | ||||
| Hispanic | 5 | 12 | ||||
| American | 1 | 0 | ||||
| Other | 6 | 1 | ||||
| Mixed | 0 | 1 | ||||
| SES | 1 | 1 | 0 | 4.66 | 4 | 0.324 |
| 2 | 3 | 4 | ||||
| 3 | 8 | 15 | ||||
| 4 | 12 | 7 | ||||
| 5 | 31 | 29 | ||||
| Total | 55 | 55 | ||||
| Age | 11.79±3.06 | 11.05±2.63 | 2.06 | 1,121 | 0.154 | |
| CGAS/GAF | 85.50±6.39 | 67.94±11 | 109.30 | 1,116 | <0.001 | |
| WASI Full_IQ | 111.97±14.20 | 108.27±13.65 | 2.15 | 1,120 | 0.146 | |
| WIAT Total | 117.00±14.44 | 105.13±16.81 | 17.63 | 1, 121 | <0.001 | |
TD= typically developing. SES= Socioeconomic Status (Hollingshead Index). CGAS= Children's Global Assessment Scale. WASI= Wechsler Abbreviated Scale of Intelligence. WIAT= Wechsler Individual Achievement Test. df = Degrees of freedom. Results are expressed as mean ± standard deviation.
Table 3.
Demographic data in adults
| TD | ADHD | χ2/F | df | p | ||
|---|---|---|---|---|---|---|
| Sex | Female | 16 | 7 | 1.27 | 1 | 0.203 |
| Male | 14 | 12 | ||||
| Total | 30 | 19 | ||||
| Race | Black/African | 16 | 12 | 3.81 | 4 | 0.432 |
| Asian/Pacific | 6 | 2 | ||||
| Hispanic/Latino | 2 | 2 | ||||
| Other | 4 | 0 | ||||
| Mixed | 2 | 2 | ||||
| Total | 30 | 18 | ||||
| SES | 1 | 6 | 2 | 10.86 | 6 | 0.93 |
| 2 | 5 | 6 | ||||
| 4 | 5 | 2 | ||||
| 5 | 8 | 0 | ||||
| 6 | 3 | 1 | ||||
| 7 | 1 | 2 | ||||
| 8 | 1 | 3 | ||||
| Age | 29.67±8.93 | 33.84±10.94 | 2.13 | 1,47 | 0.15 | |
| CGAS/ GAF | 82.87±7.41 | 69.69±7.22 | 33.61 | 1,44 | <0.001 | |
| WASI_Full_IQ | 108.53±11.14 | 112.26±13.60 | 1.10 | 1,47 | 0.30 | |
| WIAT_Total | 106.80±13.63 | 105.00±15.50 | 0.18 | 1,47 | 0.67 | |
TD= Typically developed. SES= Socioeconomic Status (Hollingshead Index). CGAS= Children's Global Assessment Scale. WASI= Wechsler Abbreviated Scale of Intelligence. WIAT= Wechsler Individual Achievement Test. df= Degrees of freedom. Results are expressed as mean ± standard deviation.
In the group of children/adolescents, 35 of the participants with ADHD were diagnosed with Combined Subtype, 24 with Inattentive Subtype, two Not-otherwise Specified (NOS) and one Hyperactive/Impulsive. In adults with ADHD, 13 were diagnosed with the Combined Subtype, four Inattentive Subtype, one Hyperactive/Impulsive Subtype and one NOS.
In the child and adolescent group with ADHD, 26 children met criteria for another disorder (41.9%): eight had a learning disorder (12.9%), six oppositional defiant disorder (9.7%), four an adjustment disorder (6.5%), three had enuresis (4.8%), two had tics disorders (3.2%), two had specific phobias (3.2%), one had encopresis (1.6%), one dysthymia (1.6%) and one an anxiety disorder (1.6%). Among adults, one of the participants with ADHD had a prior history of substance abuse disorder and one a history of major depressive disorder. As expected, in both age groups, BRIEF and Conners scores were significantly higher in patients with ADHD (p<0.01) on all measures (See Tables 4 and 5).
Table 4.
Summary measures of Behavioral Rating Inventory of Executive Function (BRIEF) for children/adolescents and adults
| BRIEF measure | TD | ADHD | ||||||
|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | F | df | |
| BRIEF Children/Adolescents | ||||||||
| BRI | 57 | 43.16 | 6.52 | 62 | 61.48 | 11.71 | 108.64 | 1, 118 |
| MI | 57 | 43.58 | 8.98 | 62 | 71.18 | 9.74 | 256.97 | 1, 118 |
| GEC | 57 | 42.86 | 8.12 | 62 | 68.45 | 9.53 | 246.28 | 1, 118 |
| BRIEF Adults Self-Report | ||||||||
| BRI | 30 | 39.73 | 6.29 | 18 | 59.00 | 10.11 | 66.61 | 1, 46 |
| MI | 30 | 41.57 | 6.40 | 18 | 65.61 | 11.46 | 87.39 | 1, 46 |
| GEC | 30 | 39.93 | 6.72 | 18 | 63.94 | 10.64 | 92.23 | 1, 46 |
| BRIEF Adults Informant-Report | ||||||||
| BRI | 21 | 44.71 | 7.95 | 13 | 56.92 | 12.10 | 12.67 | 1,32 |
| MI | 21 | 43.00 | 7.72 | 13 | 62.77 | 10.30 | 40.75 | 1,32 |
| GEC | 21 | 42.48 | 7.28 | 13 | 61.08 | 10.77 | 36.22 | 1,32 |
BRI= Behavior Regulation Index, MI= Metacognition Index, GEC= Global Executive Composite p < 0.01 for all measures.
Table 5.
Conners questionnaire T-scores for children/adolescents and adults
| TD | ADHD | |||||||
|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | F | df | |
| Conners' Parent Rating Scale | ||||||||
| Global Impairment | 60 | 45.17 | 5.80 | 62 | 64.53 | 9.99 | 170.05 | 1, 121 |
| DSM-IV Inattentive | 60 | 45.23 | 4.58 | 62 | 71.71 | 9.14 | 405.24 | 1, 121 |
| DSM-IV Hyperactive/ Impulsive | 60 | 46.38 | 5.90 | 62 | 67.13 | 12.51 | 135.70 | 1, 121 |
| DSM-IV Total | 60 | 45.27 | 5.20 | 62 | 71.58 | 9.73 | 343.83 | 1, 121 |
| Conners' Teacher Rating Scale | ||||||||
| Global Impairment | 37 | 47.59 | 8.07 | 57 | 66.49 | 11.87 | 71.96 | 1, 93 |
| DSM- IV Inattentive | 37 | 46.30 | 5.54 | 57 | 66.02 | 11.60 | 92.88 | 1, 93 |
| DSM- IV Hyperactive/Impulsive | 37 | 49.62 | 10.10 | 57 | 62.77 | 13.67 | 25.24 | 1, 93 |
| DSM- IV Total | 37 | 47.68 | 8.09 | 57 | 65.98 | 11.52 | 70.68 | 1, 93 |
| Conners' Adult ADHD Rating Scales | ||||||||
| ADHD Index | 30 | 38.97 | 7.59 | 17 | 69.76 | 15.86 | 81.31 | 1, 45 |
| DSM-IV Inattentive Symptoms | 30 | 36.27 | 6.28 | 17 | 63.35 | 15.27 | 73.48 | 1, 45 |
| DSM-IV Hyperactive Impulsive | 30 | 35.87 | 7.36 | 17 | 68.94 | 14.72 | 105.98 | 1, 45 |
| DSM-IV Total ADHD Symptoms | 30 | 36.73 | 5.10 | 17 | 61.71 | 13.43 | 83.67 | 1, 45 |
p < 0.01 for all measures.
Age was significantly correlated with locomotor indices across all participants (e.g., with Head Movements, r = -0.431, n=172, p<0.001). This relationship with age was also significant within the group of children and adolescents (e.g., Head Movements, r= -0.430, n=123, p<0.01) but not within the group of adults (e.g., Head Movements, r= -0.065, n=49, p=0.66).
As shown in Tables 6 and 7, ADHD groups differed significantly from controls on all motion tracking parameters except Head Immobility Duration and Head Spatial Complexity in children/adolescents. Specifically, Cohen's d for number of Head Movements was 0.51 for children and 0.99 for adults; Head Displacement also differed, with d=0.59 in children/adolescents and d=1.11 in adults; Head Area produced d=0.50 in children/adolescents and d=1.21 in adults. Head Spatial Complexity differed significantly in adults (p=0.02, d=0.65). Finally, Head Temporal Scaling resulted in d=0.44 in children/adolescents and d=0.63 in adults. Interestingly, number of Head Movements was lower in ADHD adults than in TD children (2434 vs. 2833), despite nearly double the effect size in adults vs. children/adolescents. In the concurrent go/no-go task, reaction time (RT) variability was significantly greater in ADHD (p<0.05; RT coefficient of variability (COV) in adults, RT variance in children).
Table 6.
Go/no-go task and motion tracking results in children/adolescents.
| TD | ADHD | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | N | Mean | SD | N | F | Sig | df | Eta Squared | |
| Go/no-go task | ||||||||||
| Accuracy (%) | 88.96 | 9.48 | 61 | 84.16 | 10.50 | 62 | 3.591 | .061 | 119 | .029 |
| Omission Errors (%) | 5.65 | 8.37 | 61 | 8.87 | 10.74 | 62 | 2.567 | .112 | 119 | .021 |
| Commission Errors (%) | 25.06 | 21.08 | 61 | 28.06 | 19.96 | 62 | 1.436 | .233 | 119 | .012 |
| Latency (ms) | 475.72 | 94.43 | 61 | 509.73 | 98.06 | 62 | 2.589 | .110 | 119 | .021 |
| Reaction Time Variance | 140.00 | 68.61 | 61 | 176.02 | 71.60 | 62 | 4.726 | .032 | 119 | .038 |
| Reaction Time COV | 29.16 | 13.57 | 61 | 34.11 | 10.87 | 62 | 2.256 | .136 | 119 | .019 |
| MMAT | ||||||||||
| Head Immobility Duration | 0.21 | 0.18 | 61 | 0.15 | 0.23 | 62 | 1.975 | .163 | 119 | .016 |
| Head Movements | 2833.18 | 1836.97 | 61 | 3755.44 | 1752.04 | 62 | 5.476 | .021 | 119 | .044 |
| Head Displacement | 4.58 | 3.52 | 61 | 6.85 | 4.13 | 62 | 7.077 | .009 | 119 | .056 |
| Head Area | 146.64 | 129.40 | 61 | 216.05 | 148.51 | 62 | 4.642 | .033 | 119 | .038 |
| Head Spatial Complexity | 1.16 | 0.13 | 61 | 1.11 | 0.14 | 62 | 3.634 | .059 | 119 | .030 |
| Head Temporal Scaling | 0.67 | 0.34 | 61 | 0.81 | 0.29 | 62 | 4.030 | .047 | 119 | .033 |
TD: Typically developing controls; COV: Coefficient of variation; MMAT: McLean Motion and Attention Test. Significant p-values (<.05) are in bold.
Table 7.
Go/no-go task and motion tracking results in adults
| TD | ADHD | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | N | Mean | SD | N | F | Sig | dF | Eta Squared | |
| Go/no-go task | ||||||||||
| Accuracy (%) | 96.52 | 3.39 | 30 | 95.61 | 2.92 | 19 | .460 | .501 | 45 | .010 |
| Omission Errors (%) | 0.70 | 1.87 | 30 | 1.01 | 1.35 | 19 | .211 | .648 | 45 | .005 |
| Commission Errors (%) | 28.64 | 23.43 | 30 | 35.03 | 25.08 | 19 | .420 | .520 | 45 | .009 |
| Latency (ms) | 430.53 | 74.81 | 30 | 421.74 | 83.54 | 19 | .159 | .692 | 45 | .004 |
| Reaction Time Variance | 98.70 | 33.47 | 30 | 116.21 | 31.19 | 19 | 3.516 | .067 | 45 | .072 |
| Reaction Time COV | 22.53 | 4.58 | 30 | 28.11 | 8.14 | 19 | 10.282 | .002 | 45 | .186 |
| MMAT | ||||||||||
| Head Immobility Duration | 0.48 | 0.37 | 30 | 0.34 | 0.40 | 19 | 1.524 | .223 | 45 | .033 |
| Head Movements | 1069.77 | 739.46 | 30 | 2434.26 | 2028.62 | 19 | 11.433 | .002 | 45 | .203 |
| Head Displacement | 1.41 | 1.03 | 30 | 4.13 | 3.73 | 19 | 14.173 | .000 | 45 | .240 |
| Head Area | 38.33 | 23.17 | 30 | 127.42 | 115.18 | 19 | 16.683 | .000 | 45 | .270 |
| Head Spatial Complexity | 1.28 | 0.17 | 30 | 1.17 | 0.17 | 19 | 5.511 | .023 | 45 | .109 |
| Head Temporal Scaling | 0.34 | 0.24 | 30 | 0.53 | 0.38 | 19 | 4.609 | .037 | 45 | .093 |
TD: Typically developed controls; COV: coefficient of variation; MMAT: McLean Motion and Attention Test. Significant p-values (<.05) are in bold.
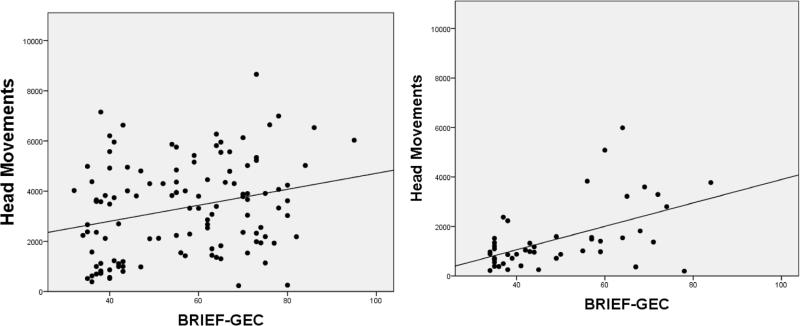
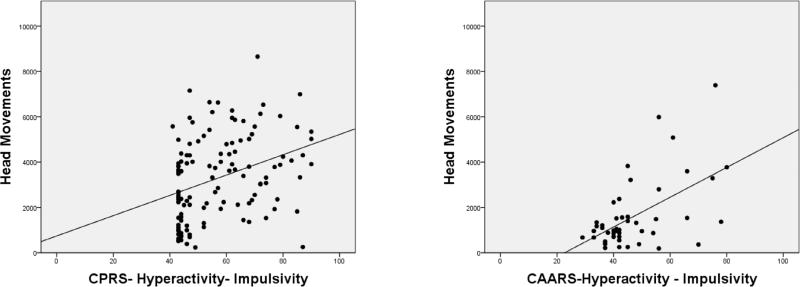
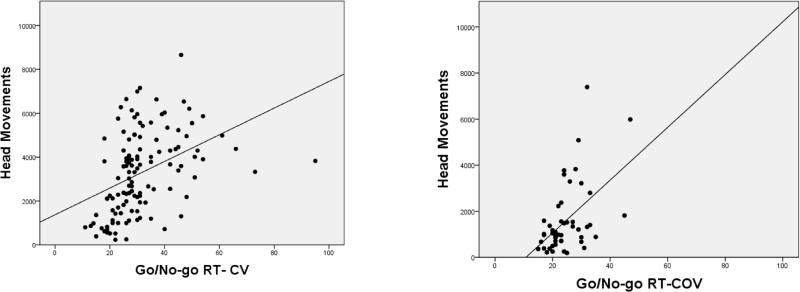
We examined correlations between locomotion measures, and summary indices from the BRIEF and Conners ratings. Results are shown in Tables 8-9. Figures 4-6 illustrate the significant positive relationships between number of Head Movements, on one hand, and BRIEF GEC, go/no-go RT COV, and DSM-IV Hyperactivity Impulsivity ratings, on the other, for children/adolescents and adults. Head Movements and Head Displacement were most robustly positively related to ratings, with up to 31% of the variance explained (e.g., for Head Displacement and DSM-IV Hyperactivity-Impulsivity in adults). Within children and adolescents, go/no-go measures (except for response latency) correlated significantly (p<0.01) with all motion tracking measures, even after adjusting for age (see Table 10). Among adults, only the reaction time variability measures (RT Variance and RT COV) correlated significantly with motion tracking measures.
Table 8.
Correlations between BRIEF summary measures and motion tracking measures.
| BRIEF measure | Head Movements | Head Displacement | Head Area | Head Spatial Complexity | Head Temporal Scaling |
|---|---|---|---|---|---|
| Children (n=119) | |||||
| BRI | .199 | .185 | .169 | −.117 | .134 |
| .031 | .044 | .068 | .208 | .148 | |
| MI | .257 | .263 | .225 | −.198 | .197 |
| .005 | .004 | .014 | .032 | .032 | |
| GEC | .255 | .260 | .232 | −.180 | .180 |
| .005 | .004 | .012 | .051 | .051 | |
| Adults BRIEF-Self (n=48) | |||||
| BRI | .505 | .510 | .433 | −.251 | .337 |
| .000 | .000 | .002 | .085 | .019 | |
| MI | .520 | .525 | .450 | −.241 | .386 |
| .000 | .000 | .001 | .099 | .007 | |
| GEC | .531 | .537 | .459 | −.254 | .380 |
| .000 | .000 | .001 | .082 | .008 | |
| Adults BRIEF-Informant (n=34) | |||||
| BRI | .253 | .211 | .159 | −.325 | .320 |
| .149 | .232 | .369 | .061 | .065 | |
| MI | .413 | .376 | .264 | −.293 | .408 |
| .015 | .028 | .131 | .093 | .016 | |
| GEC | .373 | .341 | .249 | −.279 | .377 |
| .030 | .048 | .155 | .110 | .028 | |
BRI= Behavior Regulation Index, MI= Metacognition Index, GEC= Global Executive Composite. Results are Pearson correlations (first row) and 2-tailed p-values (second row). In children, partial correlations were adjusted for age.
Table 9.
Correlations between Conners summary measures and motion tracking measures
| Conners measures | Head Movements | Head Displacement | Head Area | Head Spatial Complexity | Head Temporal Scaling |
|---|---|---|---|---|---|
| Conners Parent Rating Scale (n=122) | |||||
| Global Impairment | .249 | .260 | .241 | −.136 | .165 |
| .006 | .004 | .008 | .136 | .071 | |
| DSM-Inattentive | .221 | .227 | .198 | −.189 | .160 |
| .015 | .012 | .029 | .038 | .080 | |
| DSM-Hyperactive-Impulsive | .283 | .298 | .306 | −.164 | .158 |
| .002 | .001 | .001 | .072 | .084 | |
| DSM-Total | .265 | .274 | .262 | −.193 | .171 |
| .003 | .002 | .004 | .034 | .060 | |
| Conners Teacher Rating Scale (n=94) | |||||
| Global Impairment | .263 | .301 | .351 | −.180 | .206 |
| .011 | .003 | .001 | .084 | .047 | |
| DSM-Inattentive | .243 | .282 | .306 | −.129 | .221 |
| .019 | .006 | .003 | .218 | .033 | |
| DSM-Hyperactive-Impulsive | .216 | .244 | .296 | −.125 | .129 |
| .037 | .018 | .004 | .232 | .218 | |
| DSM-Total | .251 | .289 | .328 | −.131 | .193 |
| .015 | .005 | .001 | .209 | .064 | |
| Conners Adults ADHD Rating Scale (n=47) | |||||
| ADHD-Index | .512 | .523 | .413 | −.199 | .400 |
| .000 | .000 | .004 | .180 | .005 | |
| DSM-IV Inattentive Symptoms | .551 | .565 | .454 | −.246 | .393 |
| .000 | .000 | .001 | .095 | .006 | |
| DSM-IV Hyperactive Impulsive | .541 | .557 | .464 | −.233 | .395 |
| .000 | .000 | .001 | .115 | .006 | |
| DSM-IV Total ADHD Symptoms | .488 | .482 | .315 | −.160 | .369 |
| .000 | .001 | .031 | .284 | .011 | |
Results are Pearson correlations (first row) and 2-tailed p-values (second row). In children, partial correlations adjusted for age. Statistically significant results (p<.01) are in bold.
Figure 4.
Correlation between BRIEF General Executive Control & Head Movements in Children/Adolescents (left) and Adults (right)
Figure 6.
Correlation between Conners DSM-IV Hyperactivity-Impulsivity & Head Movements in Children/Adolescents (left) and Adults (right)
Table 10.
Correlations between go/no-go measures and motion tracking measures
| Go/no-go task | Head Movements | Head Displacement | Head Area | Head Spatial Complexity | Head Temporal Scaling |
|---|---|---|---|---|---|
| Children (n=123) | |||||
| Accuracy (%) | −.387 | −.417 | −.459 | .265 | −.327 |
| .000 | .000 | .000 | .003 | .000 | |
| Omission Errors (%) | .408 | .473 | .545 | −.272 | .319 |
| .000 | .000 | .000 | .002 | .000 | |
| Commission Errors (%) | .286 | .252 | .236 | −.180 | .296 |
| .001 | .005 | .009 | .047 | .001 | |
| Latency (ms) | .067 | .086 | .095 | −.178 | .053 |
| .462 | .346 | .298 | .050 | .562 | |
| Reaction Time Variance | .377 | .393 | .429 | −.355 | .353 |
| .000 | .000 | .000 | .000 | .000 | |
| Reaction Time COV | .389 | .385 | .416 | −.341 | .384 |
| .000 | .000 | .000 | .000 | .000 | |
| Adults (n=49) | |||||
| Accuracy (%) | −.143 | −.152 | −.073 | .062 | −.095 |
| .328 | .298 | .620 | .671 | .515 | |
| Omission Errors (%) | .048 | .047 | .027 | −.116 | .061 |
| .741 | .750 | .854 | .426 | .676 | |
| Commission Errors (%) | .162 | .176 | .082 | −.012 | .090 |
| .266 | .228 | .575 | .934 | .537 | |
| Latency (ms) | .025 | .031 | .172 | −.226 | .070 |
| .862 | .835 | .238 | .119 | .630 | |
| Reaction Time Variance | .415 | .438 | .532 | −.502 | .375 |
| .003 | .002 | .000 | .000 | .008 | |
| Reaction Time COV | .504 | .525 | .516 | −.461 | .426 |
| .000 | .000 | .000 | .001 | .002 | |
Results are Pearson correlations (first row) and 2-tailed p-values (second row). In children, partial correlations adjusted for age. Statistically significant results (p<.01) are in bold; COV: coefficient of variation.
When examined within diagnostic groups, few of these relationships remained significant, presumably reflecting decreased statistical power and reduced range of variation. The exceptions in which significant relationships continued to be observed were the correlations between Head Area and Go/No-go accuracy (r=-0.38, n=62 p=0.002 for children with ADHD; r=-0.52, n=61, p=0.000 for TDC); Head Area and Omission Errors (r=0.49, n=62, p=0.000 for children with ADHD; r=0.62, n=61, p=0.000 for TDC); Head Area and RT Variance (r=0.36, n=62, p=0.005 for children with ADHD; r=0.47, n=61, p=0.000 for TDC); Head Area and RT COV (r=0.34, n=62, p=0.007 for children with ADHD; r=0.46, n=61, p=0.000 for TDC) and Head Displacement and Omission Errors (r=0.38, n=62, p=0.002 for children with ADHD; r=0.60, n=61, p=0.000 for TDC). None of the correlations in the smaller adult subsamples remained significant when examined within-group.
4 DISCUSSION
Given the centrality of locomotor hyperactivity to the construct of ADHD, we performed meta-analyses of studies quantifying locomotion in ADHD, whether through actigraphy or via motion tracking systems. We also examined infrared motion tracking data from our lab contrasting children and adolescents as well as adults with ADHD to healthy comparison participants.
In the meta-analyses, we observed significantly greater locomotion in individuals with ADHD on both actigraphy and motion tracking data. Actigraphy studies yielded medium effect sizes (SMD = 0.64) in differentiating individuals with ADHD from controls. The smaller number of motion tracking system studies produced large effect sizes (SMD = 0.92). The two types of studies overlapped substantially in their confidence intervals. Accordingly, we combined across types of measures and stratified by age, yielding nearly complete overlap in the SMD (0.75 in children/adolescents, 0.73 in adults, respectively) and in their confidence intervals. This degree of overlap is not consistent with the generally accepted notion that hyperactivity decreases in adults with ADHD relative to children (Biederman et al., 2000; Das et al., 2014; Wilens et al., 2002).
Similarly, in our own original data, based on the MMAT system, we also observed significantly elevated locomotor indices in children/adolescents as well as in adults with ADHD. As expected, we found robust age-related differences in motion indices across the entire sample, which covered a broad age range, and within the subsample of children and adolescents. We also observed significant between-group differences between ADHD and controls for both age ranges, but the mean effect size across all motion measures was nearly double for adults as for children and adolescents (0.83 vs. 0.45, respectively). Despite this absolute difference in effect size, the two age ranges did not differ significantly, at least partially reflecting our relatively small subsample of adults with ADHD (n=19). We note that this diagnostic difference between adults was substantial, even though TD children moved more than adults with ADHD, because the TD adults were so much less motorically active.
In the simultaneously collected go/no-go task, only reaction time variability measures differed significantly between diagnostic groups. This is consistent with the larger literature on response time intra-subject variability (Kofler et al., 2013). Across all ages, response time variability indices correlated significantly with motion tracking measures. Within children and adolescents, error rates and accuracy also exhibited significant relationships with motion tracking measures, even when they did not differ significantly by ADHD diagnosis. This is consistent with a dimensional perspective on hyperactivity and neuropsychological performance, as advocated by the NIMH Research Domain Criteria project (Casey et al., 2014). However, most correlations did not remain significant when examined within the individual diagnostic groups. We believe this reflects decreased statistical power and decreased range of variation, at least in part.
In our study, a higher proportion of children and adolescents than adults were being recently treated with stimulants. In theory, this difference could have contributed to our findings. However, this is not consistent with the main driver of our results, which was that TD adults had the lowest levels of locomotor activity of all four subgroups, which could not have been accounted for by prior medication history. Given the pattern of medium-to-large effect sizes in adults with ADHD emerging from published results and our new data, we suggest that motion tracking tests are most likely to be clinically useful in the assessment of adults who lack other evidence of a childhood history of ADHD.
We note the limitations of our efforts. In our meta-analysis search, we reviewed papers published over a 40 year period (1975-2014). This literature comprises a wide variety of devices and techniques which presented a challenge in how to summarize the data. Accordingly, we applied rigorous selection criteria, which limited the number of studies included. The period covered in our meta-analyses encompassed changes in the diagnostic criteria for ADHD, from DSM-III, DSM-IIIR, and DSM-IVTR to DSM-5. We are unable to assess how such changes in criteria may have affected our findings, but do not believe them to be substantial, given the continuing emphasis on hyperactivity and impulsivity. Since we only included published studies, we cannot discount the risk of publication bias exaggerating positive results.
Our original data also has to be interpreted in light of limitations. Cross-sectional designs cannot provide definitive evidence of developmental effects, but longitudinal studies are exceedingly difficult to conduct, particularly with electronic devices that are subject to rapid changes. The main limitation of our study was the moderate-to-small size of the adult patient subgroup (n=19), which inevitably broadened our confidence intervals. Nevertheless, our results in both age groups were well within the confidence intervals of our meta-analyses.
Future research will need to examine indices of locomotion not only in patients with ADHD and healthy controls, but also across the spectrum of neurodevelopmental disorders. The goal in future work should be to characterize patients across a range of neurodevelopmental disorders, which is more relevant, from a clinical standpoint, than differentiating patients from typically developing individuals. Despite the challenges of longitudinal designs, it would be informative to delineate the trajectories of brain-behavior relationships with regard to locomotion using standardized tasks such as motion tracking approaches.
5 CONCLUSIONS
In the meta-analyses as well as in original data, we observed robustly significant evidence of greater locomotor activity in both children and adults with ADHD relative to controls. We found similar effect sizes in children/adolescents as in adults. Interestingly, in our data the effects sizes in adults with ADHD were non-significantly greater than in children, even though adults with ADHD moved less than TD children. This suggests hyperactivity remains an observable distinguishing trait in adults, because the overall magnitude of movement in the reference group is lower. These results suggest that objective locomotion measures may be useful for improving the process of diagnosing ADHD in difficult cases, especially in adulthood.
Supplementary Material
HIGHLIGHTS.
We meta-analyzed locomotor activity in relation to ADHD diagnosis
We found comparable effects of greater activity in ADHD across methods
Effect sizes were medium-to-large for both children and adults with ADHD
We also conducted a new study in children and adults using high-resolution methods
We found similar effect sizes in both age groups in our data
The prominence of locomotor hyperactivity in adults with ADHD is underappreciated
Figure 5.
Correlation between Go/No-Go Reaction Time Variability & Head Movements in Children/Adolescents (left) and Adults (right)
ACKNOWLEDGMENTS
The first author was supported by a grant from Alicia Koplowitz Foundation, Spain. The authors wish to thank the Alicia Koplowitz Foundation for supporting the field of Child and Adolescent Psychiatry. Support for this work was also received from NIH grants R01MH081218, R01MH083246, and R01HD065282.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST:
The McLean Motion and Attention Test was made available for research without charge or restriction by Dr. Martin Teicher and colleagues at McLean Hospital. None of the authors have conflicts of interest or financial disclosures to report relative to this study.
References
- Green JPHaS., editor. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2011. [Google Scholar]
- Adler, Spencer . The Adult ADHD Clinical Diagnostic Scale (ACDS) v 1.2. New York University School of Medicine; New York: 2004. [Google Scholar]
- Alderson RM, Rapport M, Kasper L, Sarver D, Kofler M. Hyperactivity in boys with attention deficit/hyperactivity disorder (ADHD): the association between deficient behavioral inhibition, attentional processes, and objectively measured activity. Child Neuropsychol. 2012;18:487–505. doi: 10.1080/09297049.2011.631905. doi: http://dx.10.1080/09297049.2011.631905. [DOI] [PubMed] [Google Scholar]
- APA . Diagnostic And Statistical Manual of Mental Disorders, 5 th Edition: DSM-5. American Psychiatric Publishing; Arlington, VA: 2013. [Google Scholar]
- Baird AL, Coogan AN, Siddiqui A, Donev RM, Thome J. Adult attention-deficit hyperactivity disorder is associated with alterations in circadian rhythms at the behavioural, endocrine and molecular levels. Molecular Psychiatry. 2012;17:988–95. doi: 10.1038/mp.2011.149. doi: http://dx.doi.org/10.1038/mp.2011.149. [DOI] [PubMed] [Google Scholar]
- Barkley R, Ullman U. A comparison of objective measures of activity and distractibility in hyperactive and nonhyperactive children. Journal of Abnormal Child Psychology. 1975;3:231–44. doi: 10.1007/BF00916753. doi: http://doi.org/10.1007/BF00916753. [DOI] [PubMed] [Google Scholar]
- Biederman J, Mick E, Faraone S. Age-dependent decline of symptoms of attention deficit hyperactivity disorder: impact of remission definition and symptom type. Am J Psychiatry. 2000;157:816–8. doi: 10.1176/appi.ajp.157.5.816. doi: http://doi.org/10.1176/appi.ajp.157.5.816. [DOI] [PubMed] [Google Scholar]
- Boonstra A, Kooij J, Oosterlaan J, Sergeant JA, Buitelaar JK, Van Someren EJ. Hyperactive night and day? Actigraphy studies in adult ADHD: A baseline comparison and the effect of methylphenidate. Sleep: Journal of Sleep and Sleep Disorders Research. 2007;30:433–42. doi: 10.1093/sleep/30.4.433. doi. [DOI] [PubMed] [Google Scholar]
- Brocki KC, Tillman CM, Bohlin G. CPT performance, motor activity, and continuous relations to ADHD symptom domains: A developmental study. European Journal of Developmental Psychology. 2008;7:178–97. doi: http://dx.doi.org/10.1080/17405620801937764. [Google Scholar]
- Casey BJ, Oliveri ME, Insel T. A Neurodevelopmental Perspective on the Research Domain Criteria (RDoC) Framework. Biological Psychiatry. 2014;76:350–3. doi: 10.1016/j.biopsych.2014.01.006. doi: 10.1016/j.biopsych.2014.01.006. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nat Rev Neurosci. 2002;3:617–28. doi: 10.1038/nrn896. doi: http://dx.doi.org/10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- Collaboration TC. Review Manager (RevMan) [Computer program] Version 5.3. The Nordic Cochrane Center; Copenhagen: 2014. [Google Scholar]
- Conners CK. Conners' Rating Scales-Revised Technical Manual. Multi-Health Systems, Inc.; North Tonawanda, New York: 1997. [Google Scholar]
- Conners CK, Erhardt D, Sparrow E. Conners' Adult ADHD Rating Scales (CAARS) Technical Manual. Multi-Health Systems: North Tonawanda; New York: 1999. [Google Scholar]
- Dane AV, Schachar RJ, Tannock R. Does actigraphy differentiate ADHD subtypes in a clinical research setting? J Am Acad Child Adolesc Psychiatry. 2000;39:752–60. doi: 10.1097/00004583-200006000-00014. doi: http://dx.doi.org/10.1097/00004583-200006000-00014. [DOI] [PubMed] [Google Scholar]
- Das D, Cherbuin N, Easteal S, Anstey KJ. Attention deficit/hyperactivity disorder symptoms and cognitive abilities in the late-life cohort of the PATH through life study. PLoS ONE. 2014;9:e86552. doi: 10.1371/journal.pone.0086552. doi: http://dx.doi.org/10.1371/journal.pone.0086552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edebol H, Helldin L, Norlander T. Objective Measures of Behavior Manifestations in Adult ADHD and Differentiation from Participants with Bipolar II Disorder, Borderline Personality Disorder, Participants with Disconfirmed ADHD as Well as Normative Participants. Clinical practice and epidemiology in mental health : CP & EMH. 2012;8:134–43. doi: 10.2174/1745017901208010134. doi: 10.2174/1745017901208010134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Spencer T, Wilens T, Seidman LJ, Mick E, Doyle AE. Attention-deficit/hyperactivity disorder in adults: an overview. Biol Psychiatry. 2000;48:9–20. doi: 10.1016/s0006-3223(00)00889-1. doi: http://dx.doi.org/10.1016/S0006-3223(00)00889-1. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP) Biometrics Research, New York State Psychiatric Institute: New York; New York: 2002. [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, Kenworthy L. TEST REVIEW Behavior Rating Inventory of Executive Function. Child Neuropsychology. 2000;6:235–8. doi: 10.1076/chin.6.3.235.3152. doi: http://dx.doi.org/10.1076/chin.6.3.235.3152. [DOI] [PubMed] [Google Scholar]
- Glass L, Graham DM, Deweese BN, Jones KL, Riley EP, Mattson SN. Correspondence of parent report and laboratory measures of inattention and hyperactivity in children with heavy prenatal alcohol exposure. Neurotoxicol Teratol. 2014;42:43–50. doi: 10.1016/j.ntt.2014.01.007. doi: http://dx.doi.org/10.1016/j.ntt.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halperin JM, Matier K, Bedi G, Sharma V, Newcorn JH. Specificity of inattention, impulsivity, and hyperactivity to the diagnosis of attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1992;31:190–6. doi: 10.1097/00004583-199203000-00002. doi: http://dx.doi.org/10.1097/00004583-199203000-00002. [DOI] [PubMed] [Google Scholar]
- Halperin JM, Trampush JW, Miller CJ, Marks DJ, Newcorn JH. Neuropsychological outcome in adolescents/young adults with childhood ADHD: profiles of persisters, remitters and controls. Journal of Child Psychology and Psychiatry. 2008;49:958–66. doi: 10.1111/j.1469-7610.2008.01926.x. doi: http://dx.doi.org/10.1111/j.1469-7610.2008.01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. Connecticut; New Haven: 1975. (Unpublished paper) [Google Scholar]
- Hudec KL, Alderson RM, Kasper LJ, Patros CH. Working memory contributes to elevated motor activity in adults with ADHD: an examination of the role of central executive and storage/rehearsal processes. J Atten Disord. 2014;18:357–68. doi: 10.1177/1087054713497398. doi: http://dx.doi.org/10.1177/1087054713497398. [DOI] [PubMed] [Google Scholar]
- IBM-Corporation . In: IBM SPSS Statistics for Windows, Version 21.0. Corporation I, editor. IBM Corporation; Armonk, NY: 2012. [Google Scholar]
- Inoue K, Nadaoka T, Oiji A, Morioka Y, Totsuka S, Kanbayashi Y, Hukui T. Clinical evaluation of attention-deficit hyperactivity disorder by objective quantitative measures. Child Psychiatry Hum Dev. 1998;28:179–88. doi: 10.1023/a:1022885827086. doi. [DOI] [PubMed] [Google Scholar]
- Kam HJ, Lee K, Cho SM, Shin YM, Park RW. High-Resolution Actigraphic Analysis of ADHD: A Wide Range of Movement Variability Observation in Three School Courses -A Pilot Study. Healthc Inform Res. 2011;17:29–37. doi: 10.4258/hir.2011.17.1.29. doi: http://dx.doi.org/10.4258/hir.2011.17.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–8. doi: 10.1097/00004583-199707000-00021. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kofler MJ, Rapport MD, Sarver DE, Raiker JS, Orban SA, Friedman LM, Kolomeyer EG. Reaction time variability in ADHD: A meta-analytic review of 319 studies. Clinical Psychology Review. 2013;33:795–811. doi: 10.1016/j.cpr.2013.06.001. doi: http://dx.doi.org/10.1016/j.cpr.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Willcutt EG. Predictive validity of a continuous alternative to nominal subtypes of attention-deficit/hyperactivity disorder for DSM-V. J Clin Child Adolesc Psychol. 2010;39:761–75. doi: 10.1080/15374416.2010.517173. doi: http://dx.doi.org/10.1080/15374416.2010.517173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. 2009 doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht CA, Tryon WW. Are Children Diagnosed With the Combined Form of ADHD Pervasively Hyperactive? Behavior Modification. 2009;33:655–81. doi: 10.1177/0145445509344167. doi: http://dx.doi.org/10.1177/0145445509344167. [DOI] [PubMed] [Google Scholar]
- Lis S, Baer N, Stein-En-Nosse C, Gallhofer B, Sammer G, Kirsch P. Objective measurement of motor activity during cognitive performance in adults with attention-deficit/hyperactivity disorder. Acta Psychiatrica Scandinavica. 2010;122:285–94. doi: 10.1111/j.1600-0447.2010.01549.x. doi: http://dx.doi.org/10.1111/j.1600-0447.2010.01549.x. [DOI] [PubMed] [Google Scholar]
- Martin-Martinez D, Casaseca-de-la-Higuera P, Alberola-Lopez S, Andres-de-Llano J, Lopez-Villalobos JA, Ardura-Fernandez J, Alberola-Lopez C. Nonlinear analysis of actigraphic signals for the assessment of the attention-deficit/hyperactivity disorder (ADHD). Medical Engineering & Physics. 2012;34:1317–29. doi: 10.1016/j.medengphy.2011.12.023. doi: http://dx.doi.org/10.1016/j.medengphy.2011.12.023. [DOI] [PubMed] [Google Scholar]
- Miyahara M, Healey DM, Halperin JM. One-week temporal stability of hyperactivity in preschoolers with ADHD during psychometric assessment. Psychiatry Clin Neurosci. 2014;68:120–6. doi: 10.1111/pcn.12096. doi: http://dx.doi.org/10.1111/pcn.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Mahony N, Florentino-Liano B, Carballo JJ, Baca-García E, Rodríguez AA. Objective diagnosis of ADHD using IMUs. Medical Engineering & Physics. 2014;36:922–6. doi: 10.1016/j.medengphy.2014.02.023. doi: http://dx.doi.org/10.1016/j.medengphy.2014.02.023. [DOI] [PubMed] [Google Scholar]
- Ohashi K, Vitaliano G, Polcari A, Teicher MH. Unraveling the nature of hyperactivity in children with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2010;67:388–96. doi: 10.1001/archgenpsychiatry.2010.28. doi: http://dx.doi.org/10.1001/archgenpsychiatry.2010.28. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Rapoport JL, Behar D, Sceery W, Ismond DR, Bunney WE., Jr. A naturalistic assessment of the motor activity of hyperactive boys. I. Comparison with normal controls. Arch Gen Psychiatry. 1983;40:681–7. doi: 10.1001/archpsyc.1983.04390010091012. doi: http://dx.doi.org/10.1001/archpsyc.1983.04390010091012. [DOI] [PubMed] [Google Scholar]
- Rapport MD, Bolden J, Kofler MJ, Sarver DE, Raiker JS, Alderson RM. Hyperactivity in boys with attention-deficit/hyperactivity disorder (ADHD): a ubiquitous core symptom or manifestation of working memory deficits? J Abnorm Child Psychol. 2009;37:521–34. doi: 10.1007/s10802-008-9287-8. doi: http://dx.doi.org/10.1007/s10802-008-9287-8. [DOI] [PubMed] [Google Scholar]
- Vea Reh. Preliminary evidence for altered motion tracking-based hyperactivity in ADHD siblings. Behav Brain Funct. 2014;10:7. doi: 10.1186/1744-9081-10-7. doi: http://dx.doi.org/10.1186/1744-9081-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth RM, Isquith PK, Gioia GA. Behavioral Rating Inventory of Executive Function-Adult version. Psychological Assessment Resources; Lutz, Florida: 2005. [Google Scholar]
- Sciutto MJ, Eisenberg M. Evaluating the evidence for and against the overdiagnosis of ADHD. J Atten Disord. 2007;11:106–13. doi: 10.1177/1087054707300094. doi: http://dx.doi.org/10.1177/1087054707300094. [DOI] [PubMed] [Google Scholar]
- Soderstrom S, Pettersson R, Nilsson KW. Quantitative and subjective behavioural aspects in the assessment of attention-deficit hyperactivity disorder (ADHD) in adults. Nord J Psychiatry. 2014;68:30–7. doi: 10.3109/08039488.2012.762940. doi: http://dx.doi.org/10.3109/08039488.2012.762940. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Polcari A, Fourligas N, Vitaliano G, Navalta CP. Hyperactivity persists in male and female adults with ADHD and remains a highly discriminative feature of the disorder: a case-control study. BMC Psychiatry. 2012;12:190. doi: 10.1186/1471-244X-12-190. doi: http://dx.doi.org/10.1186/1471-244x-12-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Polcari A, McGreenery CE. Utility of objective measures of activity and attention in the assessment of therapeutic response to stimulants in children with attention-deficit/hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology. 2008;18:265–70. doi: 10.1089/cap.2007.0090. doi: http://dx.doi.org/10.1089/cap.2007.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MHea. Objective Measurement of Hyperactivity and Attentional Problems in ADHD. Journal of the American Academy of Child & Adolescent Psychiatry. 1996a;35:334–42. doi: 10.1097/00004583-199603000-00015. doi: http://dx.doi.org/10.1097/00004583-199603000-00015. [DOI] [PubMed] [Google Scholar]
- Teicher MHea. Objective measurement of hyperactivity and attentional problems in ADHD. J Am Acad Child Adolesc Psychiatry. 1996b;35:334–42. doi: 10.1097/00004583-199603000-00015. doi: http://dx.doi.org/10.1097/00004583-199603000-00015. [DOI] [PubMed] [Google Scholar]
- Tsujii N, Okada A, Kaku R, Kuriki N, Hanada K, Matsuo J, Kusube T, Hitomi K. Association between activity level and situational factors in children with attention deficit/hyperactivity disorder in elementary school. Psychiatry Clin Neurosci. 2007;61:181–5. doi: 10.1111/j.1440-1819.2007.01634.x. doi: http://dx.doi.org/10.1111/j.1440-1819.2007.01634.x. [DOI] [PubMed] [Google Scholar]
- Tsujii N, Okada A, Kaku R, Kuriki N, Hanada K, Shirakawa O. Differentiation between attention-deficit/hyperactivity disorder and pervasive developmental disorders with hyperactivity on objective activity levels using actigraphs. Psychiatry and Clinical Neurosciences. 2009;63:336–43. doi: 10.1111/j.1440-1819.2009.01948.x. doi: http://dx.doi.org/10.1111/j.1440-1819.2009.01948.x. [DOI] [PubMed] [Google Scholar]
- Valo S, Tannock R. Diagnostic instability of DSM-IV ADHD subtypes: effects of informant source, instrumentation, and methods for combining symptom reports. J Clin Child Adolesc Psychol. 2010;39:749–60. doi: 10.1080/15374416.2010.517172. doi: http://dx.doi.org/10.1080/15374416.2010.517172. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) The Psychological Corporation; San Antonio, Texas: 1999. [Google Scholar]
- Wechsler D. Wechsler Individual Achievement Test. 2nd ed. (WIATT-II) The Psychological Corporation; New York: 2001. [Google Scholar]
- Wilens TE, Biederman J, Spencer TJ. Attention deficit/hyperactivity disorder across the lifespan. Annual review of medicine. 2002;53:113–31. doi: 10.1146/annurev.med.53.082901.103945. doi: http://dx.doi.org/10.1146/annurev.med.53.082901.103945. [DOI] [PubMed] [Google Scholar]
- Willcutt EG. The prevalence of DSM-IV attention-deficit/hyperactivity disorder: a meta-analytic review. Neurotherapeutics. 2012;9:490–9. doi: 10.1007/s13311-012-0135-8. doi: http://dx.doi.org/10.1007/s13311-012-0135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood AC, Rijsdijk F, Asherson P, Kuntsi J. Hyperactive-Impulsive Symptom Scores and Oppositional Behaviours Reflect Alternate Manifestations of a Single Liability. Behavior Genetics. 2009;39:447–60. doi: 10.1007/s10519-009-9290-z. doi: http://dx.doi.org/10.1007/s10519-009-9290-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.