Abstract
Over 300,000 heart valve replacements are performed annually to replace stenotic and regurgitant heart valves. Bioprosthetic heart valves (BHVs), derived from glutaraldehyde crosslinked (GLUT) porcine aortic valve leaflets or bovine pericardium are often used. However, valve failure can occur within 12–15 years due to calcification and/or progressive degeneration. In this study, we have developed a novel fabrication method that utilizes carbodiimide, neomycin trisulfate, and pentagalloyl glucose crosslinking chemistry (TRI) to better stabilize the extracellular matrix of porcine aortic valve leaflets. We demonstrate that TRI treated leaflets show similar biomechanics to GLUT crosslinked leaflets. TRI treated leaflets had better resistance to enzymatic degradation in vitro and demonstrated better tearing toughness after challenged with enzymatic degradation. When implanted subcutaneously in rats for up to 90 days, GLUT control leaflets calcified heavily while TRI treated leaflets resisted calcification, retained more ECM components, and showed better biocompatibility.
Keywords: Bioprosthesis, heart valve replacements, glycosaminoglycans, elastin, tissue fixative
INTRODUCTION
Bioprosthetic heart valves (BHVs) are used to replace stenotic and regurgitant heart valves [1–3]. BHVs do not require long-term anticoagulant therapy and also have more optimal hemodynamics, thus making it the preferred option for valve replacements. BHVs are fabricated out of two types of xenogeneic tissues: (1) Porcine aortic valve (PAV) leaflets or (2) bovine pericardium sheet. Commercially available BHVs fail due to structural degradation and/or calcification.
PAV leaflets resemble human heart valve leaflet three layered structure. The fibrosa faces the aortic side of the leaflet and contains circumferentially aligned type I collagen that provides strength to withstand high cyclic pressure environment [3–5]. Facing the opposite side towards the ventricle, the ventricularis contains radially aligned elastin along with collagen. Elastic fibers are coupled with collagen bundles and brings the collagen fiber structure back to a resting configuration between loading cycles, thus the ventricularis provides elasticity and recoil to the leaflet [6–9]. Between the fibrosa and ventricularis lies the spongiosa that primarily contains glycosaminoglycans (GAGs). This layer is assumed to mechanically function as a shearing medium between the two other layers as the GAGs absorb water and form a hydrogel-like layer [7, 10, 11].
PAV’s are crosslinked with glutaraldehyde (GLUT) to fabricate BHV implants to make them resistant against enzymatic degradation and less-immunogenic [1, 12, 13]. GLUT crosslinked BHVs tear and calcify after implantation, causing valve failure within 12–15 years of use [1–3, 12, 14]. Furthermore, calcification and structural degradation seem to accelerate in younger patients most likely due to a more competent immune system/increased metabolism and increased physical activity [1, 15]. Therefore, BHVs are less frequently used for patients under 65 years of age. It should also be noted that degeneration and calcification are not mutually exclusive and one can lead to the other. GLUT crosslinking does not stabilize components such as elastin and GAGs of the heart valve. Therefore, these major ECM components are lost from the tissue due to enzymatic degradation [10, 16–18]. We have recently shown that GAGs in BHVs are strongly connected with fiber-fiber and fiber-matrix interactions at low force levels and that they may be important in providing a damping mechanism to reduce leaflet flutter when the leaflet is not under high tensile stress [7]. Thus, the degradation of the essential ECM components leads to the compromised mechanical function of the leaflet and this could be a major contributor to degenerative tears of BHVs.
Here we have developed a novel tissue fixative method that utilizes carbodiimide, neomycin trisulfate, and pentagalloyl glucose (PGG) crosslinking chemistry to stabilize all ECM components. We name this new method TRI as three crosslinkers were used. We demonstrate that PAV leaflets treated with TRI improve tear toughness, resist in vivo calcification, structural degradation, and are more biocompatible than GLUT treated leaflets.
METHODS
Heart Valve biomaterial crosslinking
Porcine aortic heart valves (PAVs) were harvested fresh from a local slaughter house and transported on 0.9% saline and ice to the laboratory. Whole PAVs were washed in 0.9% saline for 15 minutes. The leaflets were then cut from the aortic root and washed in 0.9% saline for 3 to 5 minutes. These leaflets were then treated with two different chemical treatment techniques: (1) GLUT – control and (2) TRI – newly proposed crosslinking technique.
GLUT
Fresh leaflets were treated with 0.6% glutaraldehyde in 50 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffered saline (pH 7.4) at room temperature with gentle shaking for 24 hours, solution decanted, and replaced with 0.2% glutaraldehyde in 50 mM HEPES buffered saline (pH 7.4) and the crosslinking was continued for at least six days [6].
TRI
Fresh leaflets were treated with 0.5 mM neomycin trisulfate solution in 2-(N-morpholino) ethanesulfonic acid (MES) buffer for 1 hour. The solution was decanted and leaflets were then incubated in a 30 mM 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and 6 mM N-hydroxysuccinimide (NHS) / 0.05% PGG solution in 50 mM MES buffered saline (pH 5.5) for twenty-four hours. The solution was decanted and then the leaflets were crosslinked further in 30 mM EDC and 6 mM NHS solution in 50mM MES buffered saline (pH 5.5) for 24 hours. Following fixation, valves were placed in 20% isopropanol in 50 mM HEPES buffer (pH 7.4) for at least six days.
Differential Scanning Calorimetry
Differential scanning calorimetry (DSC) was used to measure the thermal denaturation temperature (Td) of collagen. Samples (3–8 mg) from each of the TRI and GLUT groups were carefully cut from generally the same region of the leaflet, blotted dry, and placed flat in hermetically sealed pans. Samples were tested using a DSC 2920 (TA Instruments, Newcastle, DE). A pilot run verified the Td range for GLUT compared to previous results, and the remainder of samples were equilibrated at 20 °C, and heated at 10 °C/min. The denaturation temperature was recorded as the maximum value of the endotherm peak.
In vitro enzymatic challenge studies
Resistance to collagenase and elastase
PAV leaflets were cut in half symmetrically, rinsed in nanofiltered water, blotted dry, frozen, lyophilized, and weighed. Half leaflets were incubated in 1.2 mL of 5 U/mL porcine pancreatic elastase (Elastin Products Co. Inc., Owensville, MO) in 100 mM Tris, 1mM CaCl2, 0.02% NaN3 (pH 7.8) for 24 hours, or 75 U/mL collagenase (Type I, Sigma, St. Louis, MO) in 50 mM Tris, 10 mM CaCl2, 0.02% NaN3 (pH 8.0) for 48 hours at 37°C while shaking at 650 RPM while other half was incubated with buffer only. Enzyme liquid was saved for further studies, digested cusps rinsed in nanofiltered water, blotted dry, frozen, lyophilized, and weighed. Percent weight loss was calculated. For storage studies, samples were stored for 21, 60, or 180 days in 0.2% glutaraldehyde solution (GLUT samples) or 20% isopropanol in HEPES buffer (TRI samples), taken out of storage, washed in saline 3x for 15 minute, and challenged with the aforementioned protocol with collagenase or elastase and percent weight loss calculated.
Resistance to GAGases
PAV leaflets were carefully excised from the aortic wall, and washed thoroughly in 100 mM ammonium acetate buffer (AAB) at pH 7.0 3x for 5 minutes. Leaflets were cut symmetrically, one half incubated in 1.2 mL GAG degrading enzyme solution (5 U/mL hyaluronidase (Sigma, St. Louis, MO), 0.1 U/mL chondroitinase (Sigma, St. Louis, MO) in 100 mM AAB, pH 7.0) while the other half incubated in AAB. Samples were shaken vigorously for 24 hours at 37°C. Following 24 hours incubation, leaflets were washed in distilled water 3x for 5 min, frozen, and lyophilized. Total hexosamine content was measured using the hexosamine assay as previously reported [6]. Elson and Morgan’s modified hexosamine assay was used to measure GAG-related hexosamines [18]. Lyophilized GAG digested samples were weighed, digested in 2 mL 6 N HCl (24 hours, 96 degrees C), and dried under nitrogen gas. Samples were resuspended in 2 mL 1M sodium chloride and reacted with 2 mL of 3% acetyl acetone in 1.25 M sodium carbonate. 4 mL ethanol and 2 ml of Ehrlich’s reagent (0.18M p-dimethylaminobenzaldehyde in 50% ethanol containing 3N HCl) were added, and solutions left for 45 min to allow the color to develop. A pinkish-red color is indicative of tissue hexosamine quantities, and the absorbance was read at 540 nm. A set of D(+)-glucosamine standards were used. For storage studies, samples were stored for 21, 60, or 180 days in 0.2% glutaraldehyde solution (GLUT samples) or 20% isopropanol in HEPES buffer (TRI samples) taken out of storage, washed in saline 3x for 15 minute, and challenged with the aforementioned protocol with collagenase or elastase and percent weight loss calculated.
Mechanical Testing
Tissue specimens (n = 7 per group) were tested under biaxial tension using a custom-built biaxial device [19, 20]. Roughly 1 cm by 1 cm sections were taken from the central belly region of the leaflets and mounted to the device with the circumferential and radial directions along the device axes. The specimens were then loaded to a maximum membrane tension of 60 N/m over a period of 15s following a preconditioning step [21, 22]. The strain was determined via four fiducial markers were glued to the central region of the specimen [22]. All mechanical testing were performed in PBS at room temperature.
Suture Pull-out Test
PAV leaflets were evaluated for structural integrity and its ability to resist tearing. Fresh, GLUT, and TRI, leaflets were cut with a die in rectangular 40mm × 4.0mm sections and were then treated with GAGase, porcine pancreatic elastases, or no enzyme treatment (n = 10, 9 groups total). For suture pull-out test, half of the samples were oriented circumferentially (with collagen alignment) and half samples were aligned radially (perpendicular collagen alignment). One side of tissue samples was attached to a bottom clamp in a tensile tester (MTS, Minneapolis, MN). A single suture (Polypropylene blue monofilament 4-0 P-3 Ethicon™, San Lorenzo, Puerto Rico) was pierced through the sample a third of the way down the sample and was clamped to the top clamp. Samples were tensile tested at 12 mm/min to determine the amount force (N) it took to pull the suture out of the sample. The peak load, thickness of the sample, and width were used to calculate cross sectional area to determine stress. Tested samples were embedded in paraffin blocks and sectioned for alcian blue staining and movat’s pentachrome staining to visualize ECM components.
In Vivo Calcification Model
Following fixation, all tissues were rinsed thoroughly three times in 30 minute washes of sterile saline, and remained in sterile saline until implantation. Male juvenile Sprague-Dawley rats were anesthetized by inhalation of 3% isoflurane gas. A dorsal surgical incision was made, and two subdermal pockets created on either side of the sagittal plane. Cusps were blotted and positioned to lie as flat as possible in the pocket. One cusp was placed per pocket. Incisions were closed with surgical staples and samples with fibrous capsule still intact were retrieved after 30 or 90 days implantation. Blood (2 mL) was drawn from the rats before euthanasia with CO2 asphyxiation. The leaflets with surrounding capsule were removed from the subdermal sites. The middle section was saved for histological analysis. The remaining sections were placed immediately on dry ice, and frozen at −80°C as soon as possible.
Mineral analyses
Half cusps (without the capsule) from subdermal implantation studies were frozen, lyophilized, weighed, and acid hydrolyzed in 2 mL 6N Ultrex II HCl for 20 hours at 96°C. Samples were dried under nitrogen gas, resuspended in 1 mL 6N Ultrex II HCl, and centrifuged at high speeds to separate any remaining particles. Samples were diluted 1:50 in nanofiltered water, Calcium and phosphorous content in samples were analyzed using the Spectro Acros ICP Spectrometer (SPECTRO Analytical Instruments, Kleve, Germany) at Clemson University Agricultural Service Laboratory. Dilution ratios were used to calculate element content of the sample, and values were normalized to dry sample weight.
Histology
Radial cross sections were taken from the center of cusps, stored in formaldehyde, processed, embedded in paraffin, and sectioned at 6 µm for light microscopy analysis. Movat’s pentachrome and alcian blue stains were utilized to visualize and assess ECM content. Dahl’s alizarin red stain with light green counterstain was used to visualize calcium deposition in implanted samples; calcium deposits appear red. Hematoxylin and eosin staining was used to visualize and assess the cellular response to implants.
RESULTS
We determined the thermal denaturation temperatures (Td) of the tissue with DSC. Td has been shown to effectively correlate with tissue collagen stabilization [6]. Both GLUT and TRI treated leaflets showed adequate increase in Td (88 ± 1.2°C or 89 ± 0.9°C, respectively compared to fresh tissue 56± 3.2 °C [6]), suggesting complete collagen stabilization (Figure 1A).
Figure 1.
Initial ECM stability of crosslinked porcine aortic valve leaflets. (A) DSC analysis determining denaturing temperature of GLUT or TRI™ treated leaflets. Fresh, GLUT, or TRI samples were challenged with (B) collagenase or (C) elastase and percent mass loss was determined for ECM stability (n = 6). (D) GLUT or TRI treated leaflets were challenged with GAGase or buffer solution and hexosamine content of the leaflet was quantified to determine GAG stability. *Indicates significant difference (p<0.05) from GLUT.
Enzyme Stability Studies
When GLUT or TRI treated leaflets were challenged with type I collagenase, 1.52% ± 1.07 or 0.42% ± 0.42 mass loss, respectively, was exhibited while fresh leaflet, with no stabilization by crosslinking lost almost 70% of its weight as expected (Figure 1B). No significant difference was detected between GLUT and TRI treated leaflets indicating that collagen within the PAV leaflets was adequately protected from simulated enzymatic degradation in both crosslinking methods.
When GLUT or TRI treated leaflets were challenged with porcine pancreatic elastase, elastin within the PAV leaflets was not protected in GLUT treated leaflets but was protected in TRI treated leaflets from simulated enzymatic degradation (16.89% ± 2.14 vs. 4.60% ± 1.87 mass loss, respectively, p < 0.0001) Figure 1C). When GLUT treated leaflets were challenged with GAGase enzyme cocktail, 54% loss of GAGs was found as compared to control buffer treatment (110.50 ± 11.14 vs. 241.71± 10.22 µg sulfated GAGs/10 mg tissue respectively, p < 0.0001, Figure 1D). However, TRI treated leaflets did not lose significant amounts of GAGs when challenged with GAGase (265.19 ± 16.91 vs. 286.32± 19.05 µg sulfated GAGs/10 mg tissue respectively, p > 0.05, Figure 1D). This clearly suggested that TRI treatment stabilizes GAGs within the leaflets.
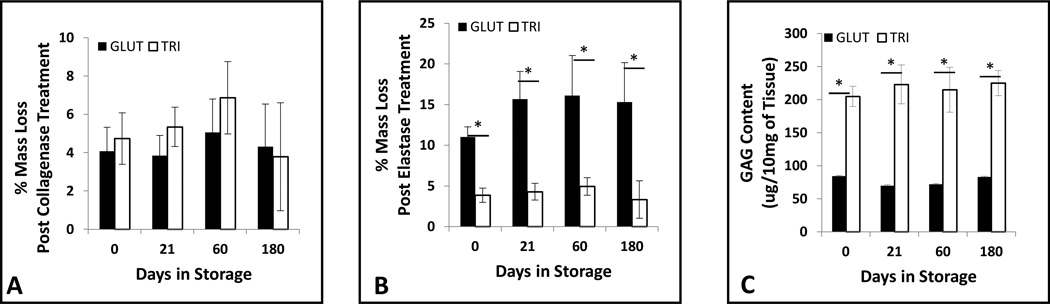
We next determined the storage stability of the leaflet stored in 0.2% GLUT or 20% ispropanol for 21, 60, or 180 days. Weight retention after challenging with collagenase or elastase after storage illustrated the biochemical stability of GLUT or TRI treated leaflets (Figure 2A and 2B, respectively). No significant change in weight was found with reference to t = 0 days in GLUT and TRI samples when challenged with collagenase (Figure 2A); however, GLUT treated tissues exhibited a significant decrease in weight after elastase treatment at each time point (Figure 2B) and lost GAGs (Figure 2C). Significantly lower weight loss after elastase was found in TRI treated samples (Figure 2B). No decrease in GAG content was detected at any time point as compared to t = 0 in TRI tissues (Figure 2C). This data demonstrates the efficacy of our storage solution to maintain ECM stability during storage of TRI treated BHVs.
Figure 2.
Long-term ECM stability of crosslinked porcine aortic valve leaflets. GLUT or TRI treated leaflets were stored for upto 180 days in storage solution and then challenged with (A) collagenase or (B) elastase and percent mass lost was utilized to determine ECM stability (n = 6). (C) hexosamine content of leaflets was quatified to determine GAG stability after GAGase treatment (n = 8). *Indicates significant difference (p<0.05) from GLUT.
Planar biaxial Biomechanics
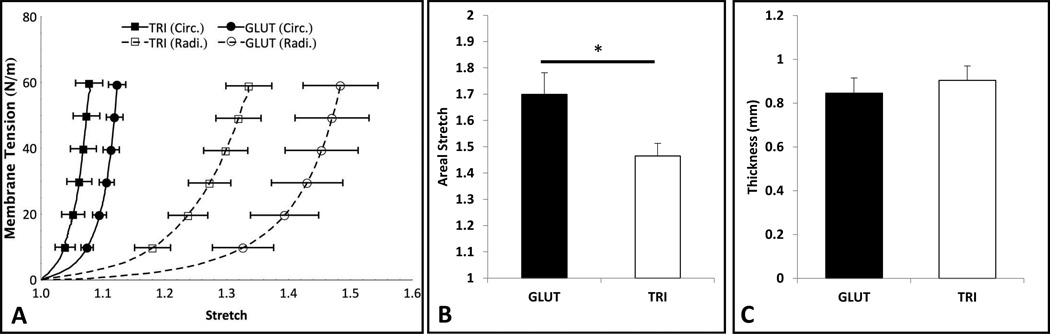
Both GLUT and TRI exhibited a nonlinear anisotropic response under equibiaxial tension typical of valvular tissue (Figure 3A). We found a significant difference in the overall compliance between the two groups (areal stretch of 1.699 ± 0.082 and 1.465 ± 0.048 for GLUT and TRI respectively (p < 0.036), Figure 3B). The difference in radial stretch itself was not statistically significant (p < 0.678). There was no statistical difference in thickness measured, 0.846 ± 0.069 and 0.904 ± 0.066 respectively (Figure 3C), and general differences in leaflet shape and geometry was not observed.
Figure 3.
Equibiaxial mechanical testing on GLUT and TRI treated leaflets (n = 7). (A) The mean equibiaxial mechanical response is shown with the standard error of the mean. (B) The areal stretch at 60 N/m show compliance in GLUT and TRI treated leaflets. (C) Thickness of the specimens after crosslinking.
Resistance to tearing
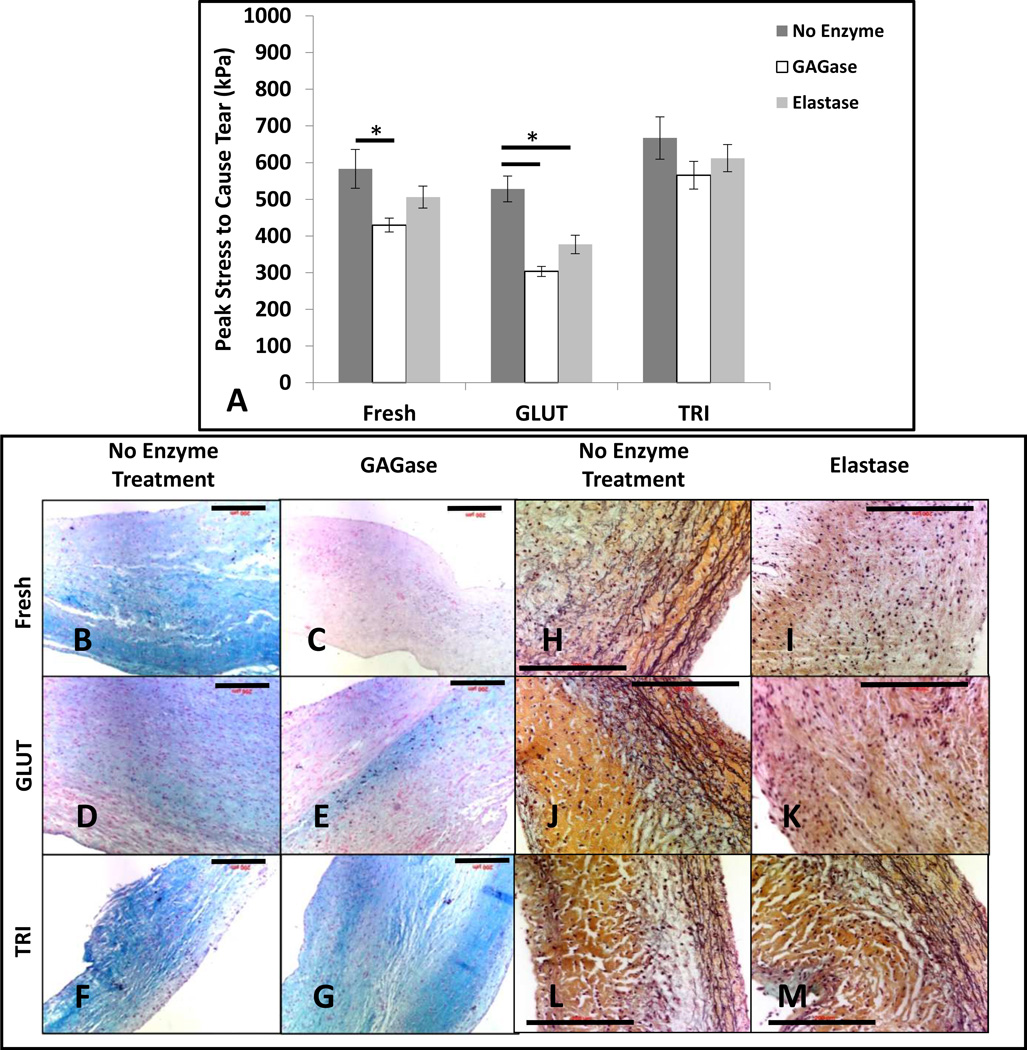
A suture pull-out test was used to evaluate the biomaterial’s ability to resist tearing. Before enzymatic treatment with GAGase or elastase, Fresh samples required 583.0 ± 59.1 MPa of peak stress to cause the suture to pull out of the tissue (Figure 4A). After treatment with GAGase, the peak stress required to cause suture pull out was reduced to 429.5 ± 21.1 MPa (p < 0.019) (Figure 4A). After treatement with elastase, the peak stress required to cause suture pull out was 505.9 ± 33.5 MPa; however, was not significantly reduced (p < 0.141) (Figure 4A). Post treatment with GAGase and elastase resulted in loss of GAGs (Figure 4C) and elastin (Figure 4I) as evident by significant reduction in alcian blue staining for GAGs and fragmentation/absence of an extensive black fibrous network of elastin fibers in VVG stain when compared to controls (Figures 4B and 4H, respectively). GLUT samples required 528.1 ± 35.0 MPa of peak stress to cause the suture to pull out of the tissue (Figure 4A). After treatment with GAGase, the peak stress was reduced to 303.4 ± 13.7 MPa (p < 0.0002) (Figure 4A). After treatement with elastase, the peak stress was reduced to 377.0 ± 24.9 MPa (p < 0.004) (Figure 4A). Post treatment with GAGase and elastase resulted in loss of GAGs (Figure 4E) and elastin (Figure 4K) and as evident with histological stains when compared to controls (Figures 4D and 4J, respectively). Before enzymatic treatment with GAGase or elastase, TRI samples required 667.3 ± 57.7 MPa of peak stress to cause the suture to pull out of the tissue (Figure 4A). After treatment with GAGase or elastase, the peak stress was not significantly reduced (565.5 ± 37.9 MPa, p < 0.106 and 612.2 ± 36.9 MPa, p < 0.243 respectively, Figure 4A). GAGase and elastase did not digest GAGs (Figure 4G) and elastin (Figure 4M) and their presence was evident in alcian blue staining for GAGs and extensive network of elastin fibers in VVG stain when compared to controls (Figures 4F and 4L, respectively). This suggests an increased resistance to tearing was possibly due to the protection of the integrity of ECM components for the TRI crosslinking protocol.
Figure 4.
Resistance to tearing as determined by suture pullout test for Fresh, GLUT, or TRI treated leaflets subjected to no enzymatic treatment, GAGase, or elastase (6 groups, n = 8 for each group). (A) Peak stress required to cause tearing. (B – G) alcian blue staining on Fresh, GLUT, or TRI treated leaflets subjected to no enzymatic treatment or GAGase. GAGs are stained blue and nuclei are counterstained red. (H – M) Movat’s pentachrome staining on Fresh, GLUT, or TRI treated leaflets subjected to no enzymatic treatment or elastase. Collagen and elastin are stained yellow and black, respectively. *Indicates significant difference.
Calcification and Biocompatibility of crosslinked leaflets
Leaflets were implanted subdermally in rats for 90 days to study calcification tendancy and biocompatibility. After implantation, GLUT treated leaflets presented with a thick fibrous capsule formation surrounding the implant with persistance of host cellular activity indicative of a moderate to high inflammatory response (Figure 5B). TRI treated tissues presented with minimal fibrous capsule formation (Figure 5D) and no persistance of host celluar response indicative of more biocompatible implant (Figure 5D). Unimplanted leaflet histology is depicted in Fig. 5 A and C.
Figure 5.
Histological characterization of host cellular response and ECM integrity of GLUT or TRI treated porcine aortic valve leaflets pre or post in vivo implantation into male juvenile rats for 90 days. (A – D) Hematoxylin and Eosin staining. Nuclei are stained purple and cytoplasm is stained pink. Calcification nodules are granular and stained deep purple. Black bar indicates 200 µm and 50 µm in insets, respectively. (E – H) Movat’s Pentachrome staining. Fibrous elastin is stained black or dark purple. Black bar indicates 50 µm. (I – L) Alcian blue staining. GAGs are stained blue and nuclei are counterstained red. Black bar indicates 200 µm.
Movat’s pentachrome staining and Alcian Blue staining on GLUT leaflets indicated a loss in ECM specifically in elastin and GAG content during implantation as compared to unimplanted leaflets (Figure 5F and Figure 5J, respectively). Note that the elastin fibrous network in the postimplant in GLUT leaflets demonstrate chopped and feathered rather than the long continuous networks indicative of loss of functional elastin (Figure 5F). Furthermore, note that the GLUT leaflets were so heavily calcified that the section suffered from much fretting on the microtome and did not effectively take up the Alcian Blue stain (Figure 5J). TRI treated tissues retained ECM components such as elastin and GAGs much more robustly after implantation (Figures 5H and 5L, respectively). Unimplanted samples are depicted in Figure 5 E, G, I, and K for comparison.
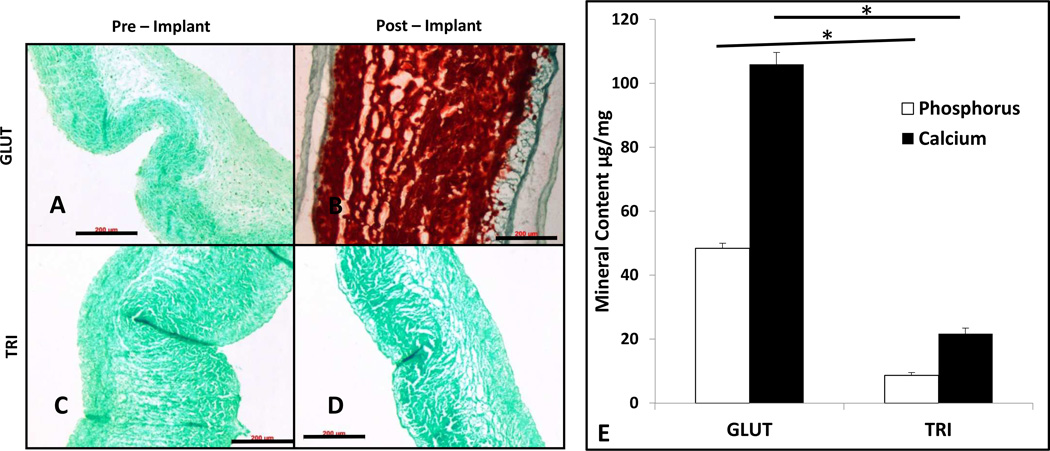
Alizarin red staining on GLUT treated leaflets showed no calcification before implantation and heavy calcification after 90 days implantation (Figure 6A and 6B, respectively). Alizarin red staining on TRI treated leaflets showed no calcification before implantation and no calcification after implantation (Figure 6C and 6D, respectively). This was further confirmed with quantitative calcium and phosphorus analyses with ICP (Figure 6E). Pre implant samples exhibited non detectable amounts of mineralization. At 90 days, GLUT samples were found to contain 106.0±3.68 µg/mg of dry tissue of calcium and 48.40±1.6 µg/mg of dry tissue of phosphorus. TRI samples were found to contain 9.00±1.71 µg/mg of dry tissue of calcium and 2.48±0.83 µg/mg of dry tissue of phosphorus. Significant differences between explanted GLUT and TRI leaflets in calcium and phosphorus content (p < 0.0001 and p < 0.0001, respectively) were found after 90 days implantation.
Figure 6.
Characterization of calcification of crosslinked porcine aortic valve leaflets pre or post subdermal implantation into male juvenile rats for 90 days. (A – D) Alizarin red staining for calcification deposits (red) counterstained with light green. Black bar indicates 200 µm. (A) Preimplantation GLUT. (B) Preimplantation TRI. (C) Postimplantation GLUT. (D) Postimplantation TRI. (E) ICP quantification of calcium and phosphorus content in GLUT or TRI treated PAV leaflets (n = 6). *Unimplanted leaflets had no detectable amount of calcification. *Indicates significant difference.
DISCUSSION
Pitfalls of GLUT crosslinking of BHVs
For the past forty years, GLUT has been used to crosslink tissues to render them stable and less immunognic for implantation into the human body. By crosslinking neighboring amine groups through a Schiff-base reaction, highly immunogenic epipitotes are masked from the immune system [23, 24]. GLUT crosslinked tissues are shown to calcify and degenerate and fail within 12 – 15 years after implantation [1–3, 12]. BHV failure related to calcification and tissue tearing can occur simultenously or separately. For example, more than fifty percent of failed valves show degeneration and tears but no calcification while fifty percent failed valves show heavy calcification making valves stiff and incompetent but without tissue tears [5, 25].
Thus, to improve durability of BHVs, both calcification and structural degeneration needs to be prevented. Several anti-calcification strategies such as treatments with surfactants, chelation reagents, and alcohols have shown prevention of calcification of BHVs in animal models and few of them are now used commercially in clinical settings [26–28]. Due to short time span of valve implants with anti-calcification treatments in humans (5–7 years), it is still awaited if such treatments make valves more durable. Even if anti-calcification treatments work to prevent calcification, structural degeneration also needs to be prevented as many valves fail clinically due to degenerative tissue tears without calcification.
Previous research has indicated that glutaraldehyde crosslinking itself may increase propensity of tissue calcification [29, 30]. Schiff-base crosslinking [24, 31] is shown to be reversible and susceptible to hydrolysis [31, 32]. Though the reaction can be made more irreversible by using reducing agents such as sodium tetraborate [33]; such treatments are not used for clinical BHVs. Inability of GLUT crosslinking to stop degradation of the integrity of the collagen triple helix during cyclic fatigue loading was shown by shifts in amine peaks in FT-IR experiments [34]. Same studies also showed that in vitro cyclic fatigue casues change in bending stiffness GLUT treated PAVs indicating a degradation of the ECM structure [34]. Glutaraldehyde only actively crosslinks collagen and leaves other key ECM components such as GAGs and elastin unstabilized in the overall tissue composite [24]. Elastin and GAGs that play crucial roles in the biomechanics of the native heart valve tissue are left open to proteolytic degradation by both host and endogenous enzymes as well as constant mechanical fatiguing of the tissue [6, 9, 16, 18, 35, 36]. Slow depletion of these ECM components compounded with constant cyclic loading may lead to microtears after valve implantation. This early degeneration may then lead to calcification nodule development and/or visible tears within a few years. Our current data clearly showed that GLUT crosslinking did not stabilize GAGs and elastin in the tissue and caused more inflammatory response after implantation and heavy tissue calcification after implantation. Perhaps retaining these other ECM components by using them as active substrates for crosslinking chemistry will enable more prolonged stabilization and promote a better functional, more durable biomaterial in the long term to enable extended implant life. We show here that by using the novel crosslinking technique (TRI), a more robust and durable BHV could be obtained.
TRI, a new crosslinking method for ECM stability
TRI uses a combination of carbodiimide crosslinking chemistry, neomyocin, and PGG. Carbodiimide chemistry has been used previously to attach functional groups to surfaces of polymers [37–39]. Carbodiimide is a zero length crosslinker meaning it allows linking neighboring carboxyl and amine groups in proteins to create amide bonds [37]. Carbodiimide has previously been used alone to stabilize heart valve prosthetics with the intent of decreasing calcification [40]; however, carbodiimide crosslinking treatments pertaining to heart valve biomaterial fabrication was not clinically pursued. This was probably due to degenerative failures of these valves during early development due to insufficient crosslinks.
Neomycin historically has been used as an antibacterial agent; however, neomycin is also an effective hyalaronidase inhibitor [16]. Hyaluronic acid serves as the backbone of the aggrecan molecules of GAGs that make up much of the spongiosa in PAVs. GAGs are highly hydrated and are important to valve function as they mediate low force tension and fiber-fiber orientation [7]. If this backbone is stabilized, GAGases are unable to hydrolyze proteoglycans thus increasing the retention of GAGs in the ECM. By using neomycin in conjunction with a crosslinker that utilizes amine groups for crosslinking such as glutaraldehyde or carbodiimide, neomycin can be incorporated into the crosslinking scheme sustained protective effect to prevent GAG loss [6, 11, 16, 35, 36]. This protective ability for neomycin towards GAGs has been previously demonstrated in our laboratory in which tissues treated with neomycin combined with glutaraldehyde reduced GAG loss during enzymatic treatment and this retention in GAGs reduced the tissue’s propensity to buckle compared to glutaraldehyde only treated tissue [11]. Furthermore, tissues treated with neomycin and carbodiimide chemistry has previously been demonstrated to prevent GAG loss during enzymatic treatment and this retention has shown similar biomechanics to GLUT valves [36]. However, neomycin treatment was unable to stabilize elastin, another important component of the ECM [6, 11].
Veseley et al demonstrated the key biomechanical roles that elastin plays in the heart valve leaflet [8, 9, 41]. A large deviation and a loss of elasticity particularly in compliance of the valves were noted in tissues that were depleted of elastin [9]. PGG is a tannic acid derivative that has been shown previously to stabilize elastin in aortic root [6, 17, 42, 43]. PGG is believed to physically interact with the hydrophobic domains of elastin thus blocking cleavage sites for elastases such as matrix metalloproteinases (MMPs) and cathapsins [17, 42–44]. This interaction is also believed to block deposition sites for calcification [42, 45]. Tripi et al combined PGG with neomycine treatment and noted a retention of GAGs and elastin when challenged enzymes; however, due to gluratarldehyde crosslinking calcification of the valve was not prevented [6].
In present work, we combined neomycine, PGG, and carbodimide chemistries to create TRI crosslinking. Carbodiimide was used to replace GLUT, neomycin was used to protect GAGs, and PGG was used to protect elastin. Collectively, this novel treatment technique created a more robust stabilization of the entire composite through an irreversible meshwork of crosslinks. We show the improved efficacy of tissue composite stabilization at the biochemical level of this combination of stabilizing agents using enzymatic challenges. Collagen, GAGs, and elastin were all stabilized with this crosslinking chemistry when challenged with enzymes specifically targeting these ECM components (Figure 1). We also demonstrated this ECM stability over an extended period of storage time of up to six months (Figure 2). This is important as tissue heart valve implants need to have prolonged storage life. GLUT treated BHVs showed no protection of elastin or GAGs during storage and this may be one of the reasons for BHV failure within few years found for currently clincally used GLUT treated valves.
TRI vs. GLUT biomechanics
Our biomechanical results for GLUT treated leaflets match closely to prior results [35, 46] with the maximum areal stretch being ~1.7 for both directions. Subtle differences may arise due to slight variations in tissue preparation. We do however note that there is a significant difference between TRI and GLUT. This was expected considering that TRI leaflets have a higher level of crosslinking. We also note that the main differences in strain occur in the low stress region of the leaflet (T < 10 N/m) similar to Friebe et al.’s study on neomycin-glutaraldehyde crosslinked valves [35]. Based on conventional theories involving collagenous tissue [19, 20, 22], collagen fibers are crimped structures that bear nearly no load until straightened. Thus, in this region collagen is mostly undulated and the load bearing contribution comes from remaining ECM structures such as elastin, proteoglycans and GAGs. Thus increase in strain at lower stress region in TRI leaflets may be a result of a more extensive ECM stabilization. The exact contribution from each ECM component is not known and requires rigorious analysis via constitutive modeling. However, we do note that the nonlinear/exponential like behavior and anisotropy of the leaflet remain similar to GLUT treated tissues. Furthermore, there is no statistical difference in thickness measured after the treatments (Figure 3C) which suggests that TRI does not produce a drastic change in leaflet geometry. This suggests that the leaflets will experience similar stress during physiological loading. We also note from observation that there is no visible difference between the leaflets at a tissue level, except slight discoloration in GLUT leaflets as compared to white TRI leaflets. As such, the mechanical differences are a result of the mechanism at the fiber (elastin and collgen fiber composition) and molecular (crosslinking bounds) scale. Long-term cyclic fatigue results are underway to see if TRI treatment makes tissue more fatigue resistant.
TRI vs GLUT tear stress test
It is important to note that treatment crosslinkers although make tissues an order of magnitude stiffer than fresh tissues, crosslinking does not have a statistically significant effect on the suture pull out stress, clearly suggesting tearing between collagen bundles. The suture pull out study suggests that retaining key ECM components such as GAGs or utilizing a more extensive crosslinking chemistry in lieu of glutaraldehyde may enable a biomaterial to resist tear formations by preventing shunting between collagen fibers and thus resist the process of structural degradation, one of the main failure modes of BHVs to date. The data suggested that losing GAGs within the tissue composite decreases the biomaterial’s resistance to tearing demonstrated by the reduction of peak stress to cause tearing in both Fresh and GLUT leaflets after treatment with GAGase enzyme. However when GAGs are actively retained in the material using the TRI treatment, treatment with GAGase produced no significantly reduction in peak stress to cause suture pull out (Figure 4). This may be due to GAGs playing a roll in ECM-ECM component coordination and acting as articulation points between ECM components such as collagen-collagen interactions [7]. Retaining this structure may provide for a more robust biomaterial that resists tearing.
In the GLUT samples, treatment with elastase significantly reduced the peak stress required to cause suture pull out. Therefore, elastin loss may shunt stress more quickly along collagen bundles in GLUT crosslinked tissues as comapred to fresh uncrosslinked leaflets. Fresh tissues did show reduction in peak stress for suture pull out after elastase but it did not reach statistical significance. TRI on the other hand demonstrated no statistical significat reduction in peak stress required to cause suture pull out due to elastin stabilization again showing elastin stabilization can also aid in preventing tissue tearing. To our knowledge, this is the first time anyone has tested for tear resistance of bioprosthetic heart valve biomaterials and its dependence on ECM crosslinking.
TRI vs. GLUT stabilization: in vivo study
Research indicates that glutaraldehyde may not be as tolerable long-term to the body [13, 23, 47, 48]. As Schiff base bonds are degraded, free aldehyde molecules created could be released that could activate additional host cellular response [48–51]. We evaluated the retention of key ECM components (collagen, elastin, and GAGs) and biocompatibility after subcutaneous implantation into a rat model (Figure 5). GLUT treated leaflets experienced complete fragmentation of the elastin fibers and complete depletion of GAGs. This depletion of ECM could be a large contributing factor to structural degradation of GLUT treated valves. On the other hand, TRI treated leaflets showed retention of collagen, elastin, and GAGs. In addition, TRI treatment effectively curbed calcification of BHV tissue compared to glutaraldehyde treated tissues after subcutaneous implantation for ninty days. No signs of calcification were found in the alizarin red staining in TRI treated valves (Figure 6). GLUT samples presented with intense red staining with Alizarin stain indicative of precipitous calcification in all layers of the leaflet. This accumulation of calcification deposition in the subcutaneous rat model corresponds to over 10 years of clinical implantation [15].
When implanted subdermally, GLUT tissues showed thick fibrous capsule formation around the implant with many inflammatory cells. TRI presented with minimal fibrous capsule formation with minimal cellular activation present. This data clearly suggests that due to stable crosslinks in TRI, ECM compoments and unstable crosslinkers do not leach out and cause inflammatory response as seen in GLUT tissues.
Limitations of Study
Although we show improved crosslinking and stability of TRI treated leaflets to enzymatic degradation in vitro and complete resistance towards calcification after subdermal implantation, more studies are needed establish the viability and functionality in vivo under long term cyclic strain. This includes demonstrating the efficacy of the this novel biomaterial via accelerated in vitro fatigue testing. Furthermore, these valves need to be examined in large animal studies by implanting as heart valve replacements to assertain their function and resistance to degeneration and calcification under blood contact. By gaining more insight into this novel technique and optimizing the treatment to produce superior durability and biomechanical behavior, a more robust BHV can become commercially viable.
CONCLUSIONS
A novel treatment technique for BHV fabrication, TRI, has been shown to produce a biomaterial that is less susceptible to enzymatic structural degradation, tearing, and calcification. Furthermore, the data suggest that TRI produces a more biocompatible material than glutaraldehyde treated tissues. Such newly developed biomaterial may provide more durable heart valve implants in future.
Acknowledgement
Research reported in this publication was supported by [National Heart, Lung, and Blood Institute and National Institutes of General Medical Sciences] of the National Institutes of Health under award numbers R01HL108330, P20RR021949.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Schoen FJ. Evolving concepts of cardiac valve dynamics: the continuum of development, functional structure, pathobiology, and tissue engineering. Circulation. 2008;118:1864–1880. doi: 10.1161/CIRCULATIONAHA.108.805911. [DOI] [PubMed] [Google Scholar]
- 2.Manji RA, Menkis AH, Ekser B, Cooper DK. Porcine bioprosthetic heart valves: The next generation. American heart journal. 2012;164:177–185. doi: 10.1016/j.ahj.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 3.Siddiqui RF, Abraham JR, Butany J. Bioprosthetic heart valves: modes of failure. Histopathology. 2009;55:135–144. doi: 10.1111/j.1365-2559.2008.03190.x. [DOI] [PubMed] [Google Scholar]
- 4.Takkenberg JJ, van Herwerden LA, Eijkemans MJ, Bekkers JA, Bogers AJ. Evolution of allograft aortic valve replacement over 13 years: results of 275 procedures. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2002;21:683–691. doi: 10.1016/s1010-7940(02)00025-8. discussion 91. [DOI] [PubMed] [Google Scholar]
- 5.Sacks MS, Schoen FJ. Collagen fiber disruption occurs independent of calcification in clinically explanted bioprosthetic heart valves. Journal of biomedical materials research. 2002;62:359–371. doi: 10.1002/jbm.10293. [DOI] [PubMed] [Google Scholar]
- 6.Tripi DR, Vyavahare NR. Neomycin and pentagalloyl glucose enhanced cross-linking for elastin and glycosaminoglycans preservation in bioprosthetic heart valves. Journal of biomaterials applications. 2014;28:757–766. doi: 10.1177/0885328213479047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eckert CE, Fan R, Mikulis B, Barron M, Carruthers CA, Friebe VM, et al. On the biomechanical role of glycosaminoglycans in the aortic heart valve leaflet. Acta biomaterialia. 2013;9:4653–460. doi: 10.1016/j.actbio.2012.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vesely I. The role of elastin in aortic valve mechanics. Journal of biomechanics. 1998;31:115–123. doi: 10.1016/s0021-9290(97)00122-x. [DOI] [PubMed] [Google Scholar]
- 9.Lee TC, Midura RJ, Hascall VC, Vesely I. The effect of elastin damage on the mechanics of the aortic valve. Journal of biomechanics. 2001;34:203–210. doi: 10.1016/s0021-9290(00)00187-1. [DOI] [PubMed] [Google Scholar]
- 10.Lovekamp JJ, Simionescu DT, Mercuri JJ, Zubiate B, Sacks MS, Vyavahare NR. Stability and function of glycosaminoglycans in porcine bioprosthetic heart valves. Biomaterials. 2006;27:1507–1518. doi: 10.1016/j.biomaterials.2005.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah SR, Vyavahare NR. The effect of glycosaminoglycan stabilization on tissue buckling in bioprosthetic heart valves. Biomaterials. 2008;29:1645–1653. doi: 10.1016/j.biomaterials.2007.12.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zilla P, Brink J, Human P, Bezuidenhout D. Prosthetic heart valves: catering for the few. Biomaterials. 2008;29:385–406. doi: 10.1016/j.biomaterials.2007.09.033. [DOI] [PubMed] [Google Scholar]
- 13.Human P, Zilla P. The possible role of immune responses in bioprosthetic heart valve failure. The Journal of heart valve disease. 2001;10:460–466. [PubMed] [Google Scholar]
- 14.Pomerance A. Pathology and valvular heart disease. British heart journal. 1972;34:437–443. doi: 10.1136/hrt.34.5.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schoen FJ, Levy RJ, Nelson AC, Bernhard WF, Nashef A, Hawley M. Onset and progression of experimental bioprosthetic heart valve calcification. Laboratory investigation; a journal of technical methods and pathology. 1985;52:523–532. [PubMed] [Google Scholar]
- 16.Raghavan DSD, Vyavahare NR. Neomycin prevents enzyme-mediated glycosaminoglycan degradation in bioprosthetic heart valves. Biomaterials. 2007;28:2861–2868. doi: 10.1016/j.biomaterials.2007.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isenburg JC, Simionescu DT, Vyavahare NR. Elastin stabilization in cardiovascular implants: improved resistance to enzymatic degradation by treatment with tannic acid. Biomaterials. 2004;25:3293–3302. doi: 10.1016/j.biomaterials.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Shah SRVN. The effect of glycosaminoglycan stabilization on tissue buckling in bioprosthetic heart valves. Biomaterials. 2008;29:1645–1653. doi: 10.1016/j.biomaterials.2007.12.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grashow JS, Sacks MS, Liao J, Yoganathan AP. Planar biaxial creep and stress relaxation of the mitral valve anterior leaflet. Annals of biomedical engineering. 2006;34:1509–1518. doi: 10.1007/s10439-006-9183-8. [DOI] [PubMed] [Google Scholar]
- 20.Grashow JS, Yoganathan AP, Sacks MS. Biaixal stress-stretch behavior of the mitral valve anterior leaflet at physiologic strain rates. Annals of biomedical engineering. 2006;34:315–325. doi: 10.1007/s10439-005-9027-y. [DOI] [PubMed] [Google Scholar]
- 21.Stella JA, Liao J, Sacks MS. Time-dependent biaxial mechanical behavior of the aortic heart valve leaflet. Journal of biomechanics. 2007;40:3169–3177. doi: 10.1016/j.jbiomech.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Billiar KL, Sacks MS. Biaxial mechanical properties of the native and glutaraldehyde-treated aortic valve cusp: Part II--A structural constitutive model. Journal of biomechanical engineering. 2000;122:327–335. doi: 10.1115/1.1287158. [DOI] [PubMed] [Google Scholar]
- 23.Nimni ME, Cheung D, Strates B, Kodama M, Sheikh K. Chemically modified collagen: a natural biomaterial for tissue replacement. Journal of biomedical materials research. 1987;21:741–771. doi: 10.1002/jbm.820210606. [DOI] [PubMed] [Google Scholar]
- 24.Mendoza-novelo B, Cauich-rodríguez JV. Decellularization stabilization and functionalization of collagenous tissues used as cardiovascular biomaterials. Biomaterials -Physics and Chemistry. 2011:159–182. [Google Scholar]
- 25.Mirnajafi A, Raymer JM, McClure LR, Sacks MS. The flexural rigidity of the aortic valve leaflet in the commissural region. Journal of biomechanics. 2006;39:2966–2973. doi: 10.1016/j.jbiomech.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 26.Gott JP, Girardot MN, Girardot JM, Hall JD, Whitlark JD, Horsley WS, et al. Refinement of the alpha aminooleic acid bioprosthetic valve anticalcification technique. The Annals of thoracic surgery. 1997;64:50–58. doi: 10.1016/s0003-4975(97)00118-5. [DOI] [PubMed] [Google Scholar]
- 27.Ogle MF, Kelly SJ, Bianco RW, Levy RJ. Calcification resistance with aluminum-ethanol treated porcine aortic valve bioprostheses in juvenile sheep. The Annals of thoracic surgery. 2003;75:1267–1273. doi: 10.1016/s0003-4975(02)04489-2. [DOI] [PubMed] [Google Scholar]
- 28.Vyavahare N, Hirsch D, Lerner E, Baskin JZ, Schoen FJ, Bianco R, et al. Prevention of bioprosthetic heart valve calcification by ethanol preincubation Efficacy and mechanisms. Circulation. 1997;95:479–488. doi: 10.1161/01.cir.95.2.479. [DOI] [PubMed] [Google Scholar]
- 29.Levy RJ, Schoen FJ, Sherman FS, Nichols J, Hawley MA, Lund SA. Calcification of subcutaneously implanted type I collagen sponges. Effects of formaldehyde and glutaraldehyde pretreatments. The American journal of pathology. 1986;122:71–82. [PMC free article] [PubMed] [Google Scholar]
- 30.Golomb G, Schoen F, Smith M, Linden J, Dixon M, Levy R. The role of glutaraldehyde-induced cross-links in calcification of bovine pericardium used in cardiac valve bioprostheses. Am J Pathol. 1987;127:122–130. [PMC free article] [PubMed] [Google Scholar]
- 31.Bezuidenhout D, Oosthuysen A, Human P, Weissenstein C, Zilla P. The effects of crosslink density and chemistry on the calcification potential of diamine-extended glutaraldehyde-fixed bioprosthetic heart-valve materials. Biotechnology and applied biochemistry. 2009;54:133–140. doi: 10.1042/ba20090101. [DOI] [PubMed] [Google Scholar]
- 32.Deshmukh A, Deshmukh K, Nimni ME. Synthesis of aldehydes and their interactions during the in vitro aging of collagen. Biochemistry. 1971;10:2337–2342. doi: 10.1021/bi00788a025. [DOI] [PubMed] [Google Scholar]
- 33.Connolly J, Alferiev I, Kronsteiner A, Lu Z, Levy R. Ethanol inhibition of porcine bioprosthetic heart valve cusp calcification is enhanced by reduction with sodium borohydride. The Journal of heart valve disease. 2004;13:487–493. [PubMed] [Google Scholar]
- 34.Vyavahare N, Ogle M, Schoen FJ, Zand R, Gloeckner DC, Sacks M, et al. Mechanisms of bioprosthetic heart valve failure: fatigue causes collagen denaturation and glycosaminoglycan loss. Journal of biomedical materials research. 1999;46:44–50. doi: 10.1002/(sici)1097-4636(199907)46:1<44::aid-jbm5>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 35.Friebe VMMB, Kole S, Ruffing CS, Sacks MS, Vyavahare NR. Neomycin enhances extracellular matrix stability of glutaraldehyde crosslinked bioprosthetic heart valves. J Biomed Mater Res B Appl Biomater. 2011;99:217–229. doi: 10.1002/jbm.b.31889. [DOI] [PubMed] [Google Scholar]
- 36.Leong J, Munnelly A, Liberio B, Cochrane L, Vyavahare N. Neomycin and carbodiimide crosslinking as an alternative to glutaraldehyde for enhanced durability of bioprosthetic heart valves. Journal of biomaterials applications. 2013;27:948–960. doi: 10.1177/0885328211430542. [DOI] [PubMed] [Google Scholar]
- 37.Grabarek Z, Gergely J. Zero-Length Crosslinking Procedure with the Use of Active Esters. Anal Biochem. 1990;185:131–135. doi: 10.1016/0003-2697(90)90267-d. [DOI] [PubMed] [Google Scholar]
- 38.Griehl C, Kolbe A, Merkel S. Quantitative description of epimerization pathways using the carbodiimide method in the synthesis of peptides. J Chem Soc Perk T 2. 1996:2525–2529. [Google Scholar]
- 39.Damink LHHO, Dijkstra PJ, vanLuyn MJA, vanWachem PB, Nieuwenhuis P, Feijen J. Cross-linking of dermal sheep collagen using a water-soluble carbodiimide. Biomaterials. 1996;17:765–773. doi: 10.1016/0142-9612(96)81413-x. [DOI] [PubMed] [Google Scholar]
- 40.Girardot JM, Girardot MN. Amide cross-linking: an alternative to glutaraldehyde fixation. The Journal of heart valve disease. 1996;5:518–525. [PubMed] [Google Scholar]
- 41.Vesely I. The evolution of bioprosthetic heart valve design and its impact on durability. Cardiovascular pathology : the official journal of the Society for Cardiovascular Pathology. 2003;12:277–286. doi: 10.1016/s1054-8807(03)00075-9. [DOI] [PubMed] [Google Scholar]
- 42.Isenburg JC, Simionescu DT, Vyavahare NR. Tannic acid treatment enhances biostability and reduces calcification of glutaraldehyde fixed aortic wall. Biomaterials. 2005;26:1237–1245. doi: 10.1016/j.biomaterials.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 43.Isenburg JC, Karamchandani NV, Simionescu DT, Vyavahare NR. Structural requirements for stabilization of vascular elastin by polyphenolic tannins. Biomaterials. 2006;27:3645–3651. doi: 10.1016/j.biomaterials.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 44.Dobreva MA, Frazier RA, Mueller-Harvey I, Clifton LA, Gea A, Green RJ. Binding of pentagalloyl glucose to two globular proteins occurs via multiple surface sites. Biomacromolecules. 2011;12:710–715. doi: 10.1021/bm101341s. [DOI] [PubMed] [Google Scholar]
- 45.Tripi DRVN. Neomycin and Pentagalloyl Glucose Enhanced Cross-linking for Elastin and Glycosaminoglycans Preservation in Bioprosthetic Heart Valves. Journal of Biomaterials Applications. 2013;28:757–766. doi: 10.1177/0885328213479047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stella JA, Sacks MS. On the biaxial mechanical properties of the layers of the aortic valve leaflet. Journal of biomechanical engineering. 2007;129:757–766. doi: 10.1115/1.2768111. [DOI] [PubMed] [Google Scholar]
- 47.Carpentier A, Lemaigre G, Robert L, Carpentier S, Dubost C. Biological factors affecting long-term results of valvular heterografts. The Journal of thoracic and cardiovascular surgery. 1969;58:467–483. [PubMed] [Google Scholar]
- 48.Dahm M, Lyman WD, Schwell AB, Factor SM, Frater RW. Immunogenicity of glutaraldehyde-tanned bovine pericardium. The Journal of thoracic and cardiovascular surgery. 1990;99:1082–1090. [PubMed] [Google Scholar]
- 49.Dahm M, Husmann M, Eckhard M, Prufer D, Groh E, Oelert H. Relevance of immunologic reactions for tissue failure of bioprosthetic heart valves. The Annals of thoracic surgery. 1995;60:S348–S352. doi: 10.1016/0003-4975(95)00291-r. [DOI] [PubMed] [Google Scholar]
- 50.Trantina-Yates A, Weissenstein C, Human P, Zilla P. Stentless bioprosthetic heart valve research: sheep versus primate model. The Annals of thoracic surgery. 2001;71:S422–S427. doi: 10.1016/s0003-4975(01)02502-4. [DOI] [PubMed] [Google Scholar]
- 51.Everts V, van der Zee E, Creemers L, Beertsen W. Phagocytosis and intracellular digestion of collagen, its role in turnover and remodelling. The Histochemical journal. 1996;28:229–245. doi: 10.1007/BF02409011. [DOI] [PubMed] [Google Scholar]