Abstract
The pericentriolar material (PCM) is composed of proteins responsible for microtubule nucleation/anchoring at the centrosome, some of which have been associated with genetic susceptibility to schizophrenia. Here, we show that mice haploinsufficient for Pericentriolar material 1 (Pcm1+/−), which encodes a component of the PCM found to bear rare loss of function mutations in patients with psychiatric illness, manifest neuroanatomical phenotypes and behavioral abnormalities. Using ex-vivo magnetic resonance imaging of the Pcm1+/− brain, we detect reduced whole brain volume. Pcm1 mutant mice show impairment in social interaction, specifically in the social novelty phase, but not in the sociability phase of the three-chamber social interaction test. In contrast, Pcm1+/− mice show normal preference for a novel object, suggesting specific impairment in response to novel social stimulus. In addition, Pcm1+/− mice display significantly reduced rearing activity in the open field. Pcm1+/− mice behave normally in the elevated plus maze, rotarod, prepulse inhibition, and progressive ratio tests. Together, our results suggest that haploinsufficiency at the Pcm1 locus can induce a range of neuroanatomical and behavioral phenotypes that support the candidacy of this locus in neuropsychiatric disorders.
1. Introduction
Schizophrenia is a common disorder of largely obscure etiology. Genetic analyses have highlighted the influence of susceptibility alleles for schizophrenia, which, in turn, raised the expectation that the cloning of such loci will illuminate the key pathways for the understanding of the disease pathology.
One candidate, PCM1 (Pericentriolar material 1), is attractive in molecular psychiatry for two reasons. First, we and others have linked the chromosomal location 8p22 and specifically PCM1 to schizophrenia in European populations. Family and trio samples showed significant transmission disequilibrium between a marker in the PCM1 locus and schizophrenia. A case-control sample also found significant association between PCM1 markers and schizophrenia (Gurling et al., 2006). This was followed by identification of specific non-synonymous mutations in coding and regulatory regions of PCM1 in schizophrenia (Kamiya et al., 2008; Datta et al., 2010). Of note, a meta-analysis in Japanese failed to find a significant association (Hashimoto et al., 2011). Second, we have reported previously that transient depletion of Pcm1 by in utero gene transfer can affect the neuroarchitecture of the developing cortex (Kamiya et al. 2008). PCM1 is recruited to the centrosome by Disrupted in Schizophrenia-1 (DISC1) and Bardet-Biedl syndrome 4 proteins (Kamiya et al. 2008; Narayan et al., 2013). PCM1 also interacts with Huntingtin, which is critical for regulation of ciliogenesis (Keryer et al., 2011).
Given these observations, we targeted the Pcm1 locus through homologous recombination. Here we show that mice haploinsufficient for Pcm1 show a significant reduction in brain volume. Pcm1+/− mice were impaired in response to a novel social stimulus, but not to a novel object. In addition, they displayed reduced rearing in the open field test, whereas motor function appeared unimpaired.
2. Materials and methods
2.1. Mouse colony
Mice deficient in Pcm1 were generated in a previous study from our group (Oh et al.,). In brief, the Pcm1 gene was targeted through insertion of a gene-trap cassette between exon 4 and 5. The depletion of Pcm1 protein is demonstrated in the present manuscript (Fig. 1A). Mice heterozygous for Pcm1 were backcrossed to C57BL/6N nine times. All the experiments were performed with heterozygous males and their wild-type (WT) littermates. All animal experiments were carried out in accordance with the National Institute of health Guide for Care and Use of Laboratory Animal (NIH Publications No. 823) and were approved by the Johns Hopkins IACUC.
Fig. 1.
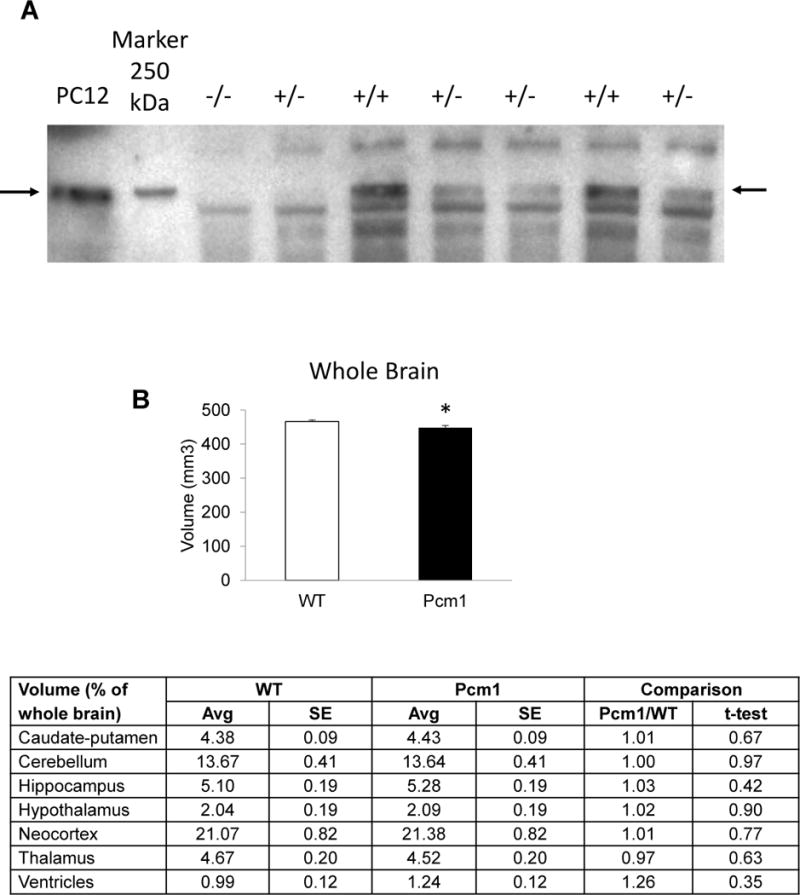
Immunoblot to demonstrate haploinsufficiency in Pcm1+/− mice (A) and gross brain anatomy by structural magnetic resonance imaging at 4 months of age (B). Whole brain volume of Pcm1+/− mice was reduced significantly compared to WT mice, *p<0.05. No significant differences in the relative volumes (out of whole brain volume) of the other regions of interest were observed in Pcm1+/− brains.
2.2. Western blot
Lysates from PC12 cells as a reference and from the cortex of 10 weeks old Pcm1+/− mice were subjected to immunoblot with Rabbit anti-Pcm1 (1:500) (Kamiya et al. 2008).
2.3. Ex vivo MRI
High resolution T2-weighted MRI of postmortem specimens was performed on an 11.7 Tesla vertical bore NMR spectrometer (Bruker Biospec, Inc. Billerica, MA, USA) using a 15 mm diameter volume coil as the radiofrequency transmitter and receiver. A three-dimensional (3D) rapid acquisition with refocused echoes (RARE) sequence was used with the following parameters: TE of 45 ms, TR of 1500 ms, RARE factor of 8, and 4 signal averages. The imaging field of view and matrix size were 16.0 mm × 12.0 mm × 10.0 mm and 200 × 152 × 128 respectively, and the native resolution was approximately 80 × 80 × 80 μm3. The total imaging time was 4 hours for each specimen. We imaged 4 WT mice and 5 Pcm1+/− mice at the age of 4 months.
2.4. Behavioral testing
Behavioral testing was performed starting at the age of three months. In order to minimize inter-trial interference the tests were performed with about a week between the tests and from less stressful to more stressful trials in the following order: open field test, elevated plus maze, three chamber social interaction, rotarod, and prepulse inhibition of acoustic startle. The above series of tests was performed on 11 WT and 16 Pcm1+/− mice. Testing was done as published (Hikida et al., 2007; Jaaro-Peled et al., 2013), except that the open field test was analyzed in bins of 10 min. Novel object recognition test was performed with a second cohort of 14 WT and 8 Pcm1+/− mice. First the mice were habituated to the testing box over 3 days. On the fourth day the mice were exposed to two identical objects for 10 min. After 1 h one of the familiar objects was replaced by a novel object and the mice were allowed to explore them for 5 min. Progressive ratio was performed on a third cohort of 11 WT and 8 Pcm1+/− mice as published (Johnson et al., 2013).
2.5. Statistics
Statistical analysis was done with student t-test, except for social interaction and prepulse inhibition tests for which we used two-way repeated measures ANOVA (RM ANOVA). p<0.05 was considered significant.
3. Results
To achieve a homogeneous genetic background, Pcm1+/− mice were backcrossed nine generations to a C57BL/6N (Charles River) background. Pcm1−/− animals were produced at a low frequency from Pcm1+/− mating, suggesting early lethality. Gross histological analyses of Pcm1+/− and wild-type littermates showed no overt pathology; therefore this genotype was used for subsequent experiments to address key significant questions in molecular psychiatry.
First we confirmed by immunoblot that the mice were indeed haploinsufficient for Pcm1 (Fig. 1A). To detect potential neuroanatomical abnormalities in the Pcm1−/+ mice, we performed ex-vivo MRI and measured the volume of the following brain regions: caudate-putamen; cerebellum; hippocampus; hypothalamus; neocortex; thalamus; and lateral ventricles. A significant reduction in total brain volume of Pcm1+/− samples was observed and a similar trend in discrete brain regions was annotated (Fig. 1B). To control for overall lower whole brain volume, we also compared the relative volume of the brain regions out of the whole brain volume, but did not detect any differences.
Next we tested the Pcm1 mice in a series of behavioral tests. Pcm1+/− mice did not differ from WT controls in total horizontal locomotion in the open field test (Fig. 2A, left). However, when we analyzed activity over time, it became evident that although WT mice continued habituating till the end of the 2 h testing period and reached a low activity level of 50 counts/10 min, Pcm1+/− mice stopped habituating at 80 min and kept that level of activity (~200 counts/10 min) until the end of the 2 h testing (Fig. 2A, right). Analysis of the vertical activity showed that the Pcm1+/− mice reared significantly less than WT mice (Fig. 2B). Pcm1+/− mice did not show signs of anxiety based on the time spent in the center of the open field (Fig 2C, left) and performance in the elevated plus maze (Fig 2C, right).
Fig. 2.
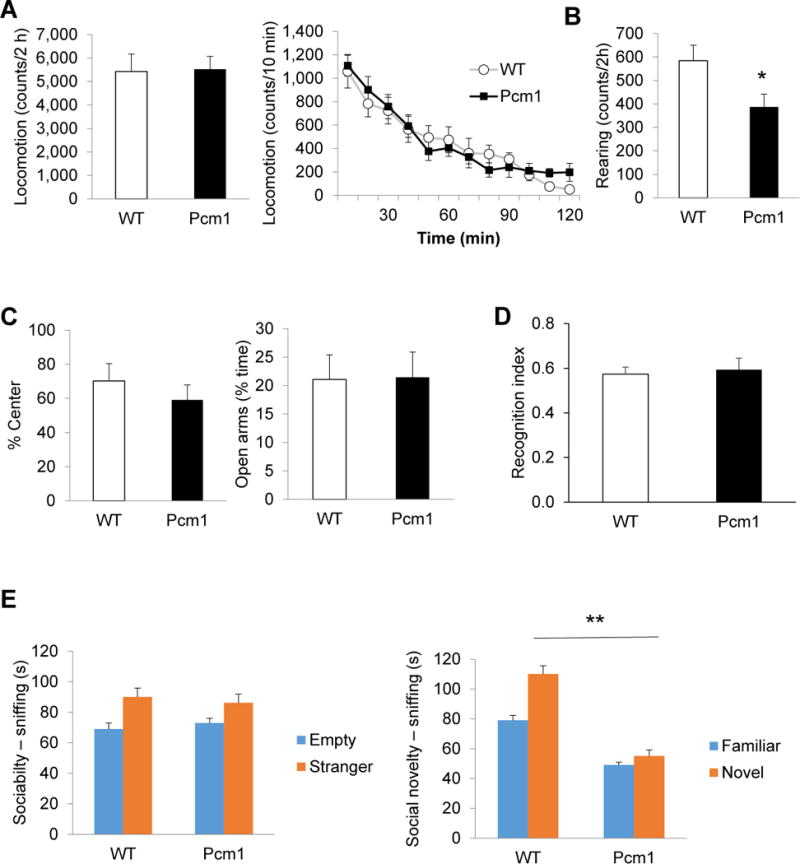
Pcm1+/− mice were impaired in recognition of a novel social stimulus, but not a novel object. (A) No difference in locomotion in the open field (left). Pcm1+/− mice stopped habituating at 80 min, ttest comparing 80 min vs. 120 min: p<0.01 for WT, p=0.85 for Pcm1+/− mice (right). (B) Pcm1+/− mice reared less in the open field, *p<0.05. (C) No change in measurements of anxiety based on % center activity in the open field (left) and % time in the open arms of the elevated plus (right). (D) In the novel object recognition test, Pcm1+/− mice showed normal preference for the novel object after a 1 h inter-trial interval. Presented as interaction with novel object/total interaction time. (E) The three-chamber social interaction test. In the sociability phase only WT mice interacted more with the mouse than with an empty enclosure (p<0.05), but ANOVA did not detect a significant effect of Pcm1+/− on sociability (left). In the social novelty phase the Pcm1+/− mice were impaired, F(1,25)=12.38, **p<0.01 (right). WT mice interacted both with the familiar and with the novel mouse significantly longer than the Pcm1+/− mice interacted with each (Bonferroni post-test analysis, p<0.001).
Given the candidacy of Pcm1 for contributing mutations to psychiatric illness, we tested the mice for cognitive function using the novel object recognition test. The Pcm1+/− mice behaved normally in this test and preferred the novel object over the familiar object (Fig. 2D). Next we assessed social function using the three-chamber social interaction test. Although only WT mice interacted more with the mouse than with an empty enclosure, ANOVA did not detect a significant effect of the genotype on sociability (Fig. 2E, left). In the social novelty phase of the test, however, the social impairment was clear; Pcm1+/− mice did not interact more with a novel stranger than with the by-then familiar mouse and RM ANOVA detected a significant genotype x chamber interaction (Fig. 2E, right). Bonferroni post-test analysis indicated that WT mice interacted both with the familiar and the novel mouse significantly longer than the Pcm1+/− mice interacted with each.
Pcm1+/− mice performed normally in the rotarod test for motor function and learning (Fig. 3A). They displayed a lower startle response to an auditory stimulus of 120 dB, but normal prepulse inhibition, suggesting normal sensorimotor gating (Fig. 3B). Pcm1+/− mice also behaved normally in the progressive ratio test in which motivation is assessed by gradually increasing the number of licks required to get a reward (Fig. 3C).
Fig. 3.
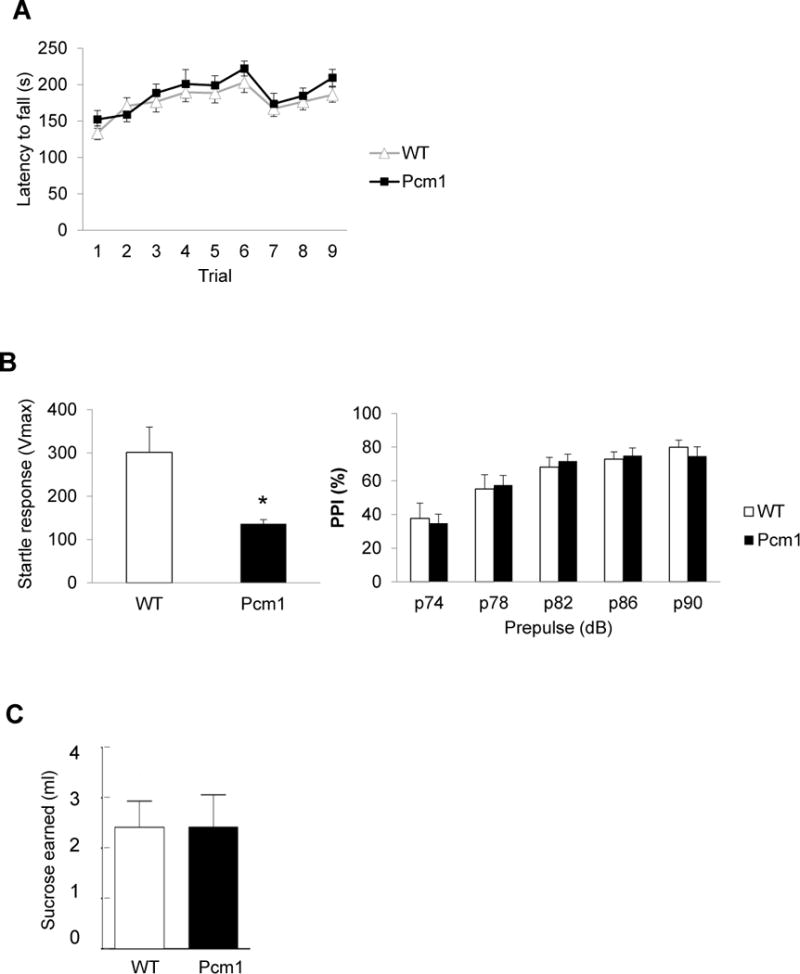
Normal motor and reinforcement behavioral responses in Pcm1+/− mice. (A) Similar rotarod performance was observed between Pcm1+/− and WT littermates. (B) Sensorimotor gating of acoustic startle: Pcm1+/− mice had reduced startle response (left, p*<0.05), but normal prepulse inhibition, right). (C) Progressive ratio test: Pcm1+/− mice earned the same amount of sucrose indicating that they were as willing as WT mice to work for a reward.
4. Discussion
The significance of the present study is to report brain and psychiatry-associated phenotypes (gross anatomical changes and unique set of behavioral alteration) in Pcm1+/− mice that our group originally developed (Oh et al.).
The two most prominent behavioral phenotypes of Pcm1+/− mice were reduced rearing in the open field and reduced social interaction in the three-chamber social interaction test. Rearing is a motor function aimed at the visual scanning of the (novel) environment. Reduced rearing was not due to a motor dysfunction, since horizontal activity (locomotion) and rotarod performance were normal. Pcm1+/− mice did not show any preference for a novel social stimulus vs. a familiar social stimulus. This was not due to anxiety based on both open field and elevated plus maze measures. Interestingly, this deficit was specific to the social context, as the mice behaved normally in a test of novel object recognition. The phenotype of Pcm1+/− mice was relatively mild as expected for heterozygotes. The mild phenotype provides a good background for combining this model of a genetic risk factor with environmental risk factors to study geneenvironment interactions in schizophrenia.
Since PCM1 is an interactor of DISC1, it is of interest to compare the behavioral profile of the Pcm1+/− mice with the many published DISC1 mouse models (Jaaro-Peled 2009; Brandon and Sawa 2011). Impaired social interaction is shared by many of the Disc1 mutant mouse lines and the Pcm1+/− mice. Whether the DISC1-PCM1 pathway mediates social function is an interesting question for future studies.
The gray matter volume of the orbitofrontal cortex is reduced in patients with schizophrenia who carry a disease-associated risk allele of the PCM1 gene (Gurling et al. 2006). This may potentially be relevant to the social novelty deficit we found in Pcm1+/− mice, as the orbitofrontal cortex can underlie, at least in part, flexible decision making in face of changing environment, especially in social context (Rushworth et al., 2007; Nelson and Guyer 2011).
Acknowledgments
We thank Drs. Akiharu Kubo and Sachiko Tsukita for the Pcm1 antibody and Ms. Yukiko Lema for organizing the figures.
Funding statement
This research was funded by MH-084018, MH-094268 Silvo O. Conte center, MH-069853, MH-085226, MH-088753, MH-092443, as well as grants from Stanley, S-R, RUSK, NARSAD, MSCRF.
Footnotes
Conflict of interest statement
There are no known conflicts of interest for any author.
References
- Brandon NJ, Sawa A. Linking neurodevelopmental and synaptic theories of mental illness through DISC1. Nat Rev Neurosci. 2011;12:707–722. doi: 10.1038/nrn3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta SR, McQuillin A, Rizig M, Blaveri E, Thirumalai S, Kalsi G, Lawrence J, Bass NJ, Puri V, Choudhury K, Pimm J, Crombie C, Fraser G, Walker N, Curtis D, Zvelebil M, Pereira A, Kandaswamy R, St Clair D, Gurling HM. A threonine to isoleucine missense mutation in the pericentriolar material 1 gene is strongly associated with schizophrenia. Mol Psychiatry. 2010;15:615–628. doi: 10.1038/mp.2008.128. [DOI] [PubMed] [Google Scholar]
- Gurling HM, Critchley H, Datta SR, McQuillin A, Blaveri E, Thirumalai S, Pimm J, Krasucki R, Kalsi G, Quested D, Lawrence J, Bass N, Choudhury K, Puri V, O’Daly O, Curtis D, Blackwood D, Muir W, Malhotra AK, Buchanan RW, Good CD, Frackowiak RS, Dolan RJ. Genetic association and brain morphology studies and the chromosome 8p22 pericentriolar material 1 (PCM1) gene in susceptibility to schizophrenia. Arch Gen Psychiatry. 2006;63:844–854. doi: 10.1001/archpsyc.63.8.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto R, Ohi K, Yasuda Y, Fukumoto M, Yamamori H, Kamino K, Morihara T, Iwase M, Kazui H, Numata S, Ikeda M, Ueno S, Ohmori T, Iwata N, Ozaki N, Takeda M. No association between the PCM1 gene and schizophrenia: a multi-center case-control study and a meta-analysis. Schizophr Res. 2011;129:80–84. doi: 10.1016/j.schres.2011.03.024. [DOI] [PubMed] [Google Scholar]
- Hikida T, Jaaro-Peled H, Seshadri S, Oishi K, Hookway C, Kong S, Wu D, Xue R, Andrade M, Tankou S, Mori S, Gallagher M, Ishizuka K, Pletnikov M, Kida S, Sawa A. Dominant-negative DISC1 transgenic mice display schizophrenia-associated phenotypes detected by measures translatable to humans. Proc Natl Acad Sci U S A. 2007;104:14501–14506. doi: 10.1073/pnas.0704774104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaaro-Peled H. Gene models of schizophrenia: DISC1 mouse models. Prog Brain Res. 2009;179:75–86. doi: 10.1016/S0079-6123(09)17909-8. [DOI] [PubMed] [Google Scholar]
- Jaaro-Peled H, Niwa M, Foss CA, Murai R, de Los Reyes S, Kamiya A, Mateo Y, O’Donnell P, Cascella NG, Nabeshima T, Guilarte TR, Pomper MG, Sawa A. Subcortical dopaminergic deficits in a DISC1 mutant model: a study in direct reference to human molecular brain imaging. Hum Mol Genet. 2013;22:1574–1580. doi: 10.1093/hmg/ddt007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AW, Jaaro-Peled H, Shahani N, Sedlak TW, Zoubovsky S, Burruss D, Emiliani F, Sawa A, Gallagher M. Cognitive and motivational deficits together with prefrontal oxidative stress in a mouse model for neuropsychiatric illness. Proc Natl Acad Sci U S A. 2013;110:12462–12467. doi: 10.1073/pnas.1307925110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya A, Tan PL, Kubo K, Engelhard C, Ishizuka K, Kubo A, Tsukita S, Pulver AE, Nakajima K, Cascella NG, Katsanis N, Sawa A. Recruitment of PCM1 to the centrosome by the cooperative action of DISC1 and BBS4: a candidate for psychiatric illnesses. Arch Gen Psychiatry. 2008;65:996–1006. doi: 10.1001/archpsyc.65.9.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keryer G, Pineda JR, Liot G, Kim J, Dietrich P, Benstaali C, Smith K, Cordelieres FP, Spassky N, Ferrante RJ, Dragatsis I, Saudou F. Ciliogenesis is regulated by a huntingtin-HAP1-PCM1 pathway and is altered in Huntington disease. J Clin Invest. 2011;121:4372–4382. doi: 10.1172/JCI57552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan S, Nakajima K, Sawa A. DISC1: a key lead in studying cortical development and associated brain disorders. Neuroscientist. 2013;19:451–464. doi: 10.1177/1073858412470168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EE, Guyer AE. The development of the ventral prefrontal cortex and social flexibility. Developmental cognitive neuroscience. 2011;1:233–245. doi: 10.1016/j.dcn.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh EC, Brodar SC, Garrett M, Rodriguiz R, Savage J, Maria Kousi M, Patrick Sullivan P, Sawa A, Ashley-Koch A, Werge T, Wetsel W, Katsanis N. Treatment-resistant schizophrenia due to loss of function mutations in PCM1 and defective trafficking of G-protein coupled receptors [Google Scholar]
- Rushworth MF, Behrens TE, Rudebeck PH, Walton ME. Contrasting roles for cingulate and orbitofrontal cortex in decisions and social behaviour. Trends in cognitive sciences. 2007;11:168–176. doi: 10.1016/j.tics.2007.01.004. [DOI] [PubMed] [Google Scholar]


