Abstract
Objective
To examine the expression pattern of biomarker proteins in extravillous trophoblast (EVT) cells obtained noninvasively by transcervical retrieval and isolation from the cervix (TRIC) in patients with early pregnancy loss, compared to control patients with uncomplicated term delivery.
Design
Case-control study.
Setting
Academic medical center.
Patients
Endocervical specimens were obtained from ongoing pregnancies at gestational ages of 5 to 10 weeks to generate an archive of EVT cells isolated by TRIC.
Interventions
Medical records were examined to select specimens from patients with either early pregnancy loss (EPL; N=10) or an uncomplicated term delivery (N=10), matched for gestational age at the time of endocervical sampling.
Main Outcome Measures
Known serum biomarkers for adverse pregnancy outcome that are expressed by EVT cells were evaluated by semi-quantitative immunocytochemistry, using antibodies against endoglin (ENG), FMS-like tyrosine kinase-1 (FLT-1), alpha-fetoprotein (AFP), pregnancy-associated plasma protein-A (PAPPA), galectin-13 (LGALS13), galectin-14 (LGALS14), and placental growth factor (PGF).
Results
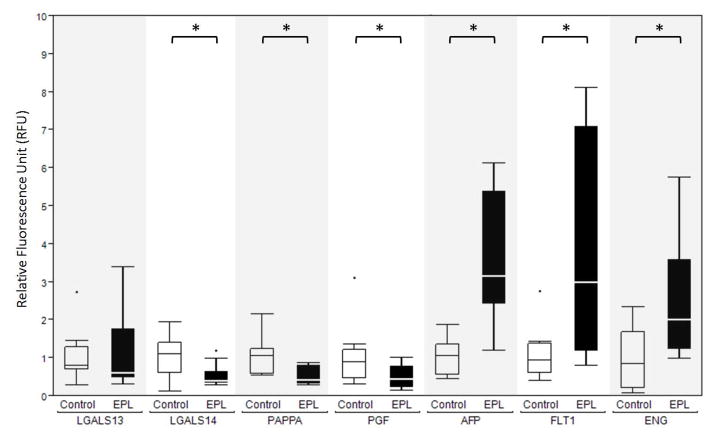
EVT purity was over 95% in all specimens, based on chorionic gonadotropin expression; however, the number of EVT cells obtained was significantly lower in women with EPL than the control group. There was significant elevation of AFP, ENG, and FLT-1, and significant reduction of PAPP-A, LGALS14, and PGF in the EPL group, compared to controls.
Conclusions
In this pilot study, EVT cells isolated by TRIC early in gestation exhibit altered protein expression patterns prior to EPL, compared to uncomplicated term pregnancies.
Keywords: early pregnancy loss, prenatal testing, trophoblast, uteroplacental insufficiency
INTRODUCTION
Early pregnancy loss (EPL) is a common pregnancy complication with the incidence of clinical pregnancy loss approaching 15% (1). Including preclinical losses, the rate of overall pregnancy loss is over 50% (2). Aneuploidy, one of the leading causes of EPL, accounts for approximately 50% of occurrences; however, a large proportion still remain idiopathic (3). Recurrent pregnancy loss, defined as two or more pregnancy losses, occurs in approximately 2–5% of patients, and despite an extensive workup, the cause remains unknown in up to 75% of cases (4).
Proper implantation and placentation are necessary for adequate fetal development. A critical step in this process throughout the first and second trimesters is the penetration of extravillous trophoblast (EVT) cells through the decidua and inner one third of the myometrium, followed by remodeling of the spiral arteries (5). Remodeling of the uterine spiral arteries transforms narrow, high-resistance blood vessels into dilated, low-resistance conduits of maternal blood into the intervillous space (6). Shallow EVT invasion and failure to convert the maternal spiral arteries contribute to uteroplacental insufficiency that can result in pregnancy complications, including intrauterine growth restriction (IUGR) and preeclampsia (7, 8). Prior to 10 weeks of gestation, maternal blood flow to the intervillous space is limited due to formation of an endovascular plug by EVT cells within the spiral arteries, reducing turbulence at the anchoring villi and lowering oxygen tension (9). It has been suggested that this environment ensures proper growth and protects the early developing placenta from oxidative and mechanical stress (9–11). Premature blood flow to the intervillous space due to inadequate EVT plug formation has been linked to EPL (9), and polymorphisms in genes related to antioxidant production are found in women with recurrent idiopathic miscarriages (12). Indeed, EPL specimens have revealed a strong incidence of deficient EVT proliferation and invasion, suggesting a central role for EVT cells in this pathology (13).
Information about first trimester EVT cells in the context of pregnancies with adverse outcomes is important in light of molecular studies of EVT cells at the time of delivery. Signaling systems that regulate EVT differentiation and survival (14–16), are disrupted in diseases related to uteroplacental insufficiency (17, 18). Whether these signaling systems are dysregulated during placentation or simply disrupted due to events arising late in the disease process can only be addressed by accessing EVT cells in the first trimester without disrupting pregnancy outcome. Obtaining intact trophoblast cells from ongoing pregnancies has been limited to chorionic villous sampling (CVS), which is almost exclusively preformed for prenatal genetic diagnostics. Moreover, CVS is invasive, and is associated with a pregnancy loss rate of approximately 0.5%-1.0% (19). Therefore, acquisition of trophoblast cells by CVS to investigate their dysfunction in EPL is not feasible.
Trophoblast cells are shed into the lower uterine segment during pregnancy and can be retrieved by minimally invasive methods from the cervix of ongoing pregnancies as early as 5 weeks of gestation (20). Historically, the limiting step in employing these cells for placental evaluation was the inability to isolate them free of maternal cervical cells (20). Recently, trophoblast retrieval and isolation from the cervix (TRIC) has enabled isolation of EVT cells after a Papanicolaou procedure with 90–100% purity in a minimally invasive approach (21). EVT cells are separated from maternal cells, using immunomagnetic isolation with antibody against human leukocyte antigen-G (HLA-G), an EVT-specific protein. The isolated cells have the expected EVT protein expression pattern (21).
We hypothesized that EVT cells obtained by TRIC from EPL patients have specific protein expression signatures compared to healthy control pregnancies. To test this hypothesis, we obtained EVT cells by TRIC and evaluated seven proteins altered in pregnancy disorders related to placental dysfunction. Galectin 13 (LGALS13), galectin 14 (LGALS14), pregnancy-associated plasma protein-A (PAPPA), and placental growth factor (PGF) are proteins secreted by the placenta that decrease in both placentas and serum of women with adverse pregnancy outcomes associated with abnormal placentation, such as preeclampsia (22–26). Elevated levels of endoglin (ENG), fms-like tyrosine kinase-1 (FLT1), and alpha-fetoprotein (AFP), have been found in placentas or serum of patients with adverse pregnancy outcomes related to abnormal placentation (27–31). EPL is theorized to share a similar pathophysiology with preeclampsia, stemming from abnormal placentation (11). Therefore, expression of these seven proteins was evaluated in EVT cells obtained by TRIC in specimens from EPL and healthy controls delivering at term.
MATERIALS AND METHODS
Patient Selection
The Institutional Review Board of Wayne State University approved this study and each participating patient was provided with informed consent. Patients were counseled for collection of endocervical samples at Wayne State University or affiliated clinics. Exclusion criteria included viable pregnancies with a history of vaginal bleeding. All the patients included in this study had singleton gestations. Medical records were evaluated to identify pregnancies with a diagnosis of an EPL (N=10), and control pregnancies, matched for gestational age (GA), were selected that resulted in healthy term deliveries (N=10). GA was determined by the date of the last menstrual period and the first ultrasound. Eight of 10 case specimens were collected at or after diagnosis of EPL, while 2 specimens were collected prior to awareness of an EPL (Supplementary Table 1).
Endocervical Sampling
Endocervical sampling was preformed as described previously (32). Briefly, a vaginal speculum was used, and endocervical specimens were collected using a cytobrush and a ThinPrep kit (Hologic, Inc., Marlborough, MA) containing 20 ml of PreservCyt fixative solution. The specimens were transferred to the laboratory, where they were acidified with 500 μl of glacial acetic acid for 5 minutes to dissolve the mucous. Samples were centrifuged at 400 × g for 5 minutes at 4°C. The supernatant was removed and the cell pellet was re-suspended in 20 ml of ice-cold sterile phosphate buffered saline (PBS). Specimens were then washed by centrifugation and re-suspension three times with 20mL of PBS, and on the final wash, the specimen was brought to 10mL with PBS at 4°C.
Isolation of EVT Cells
The endocervical specimens were centrifuged and re-suspended in 1.5 ml of PBS, combined with mouse anti-HLA-G antibody conjugated to 250 nm magnetic nanoparticles (Clemente Associates, Madison, CT), and incubated overnight at 4°C with mixing. The EVT cells bound to magnetic nanoparticles were then immobilized on a DynaMag-Spin magnet (Life Technologies) for 10 minutes. The non-bound cells were collected, followed with three washings in 1 ml of PBS. The bound cells were divided into aliquots of approximately 20–50 cells in 200 μl of PBS, and spun onto microscope slides using a Shandon Cytospin 3 centrifuge (Thermo-Fisher, Waltham, MA) at 1500 RPM for 5 minutes. The isolated cells were checked for purity by immunocytochemical labeling of the trophoblast marker, human chorionic gonadotropin β-subunit (β-hCG), and determining the percentage of cells labeled with β-hCG/DAPI, as described previously (21).
Immunocytochemistry
Slides containing isolated EVT cells were incubated for 17 hours at 4°C in Tris-buffered saline containing 0.05% Tween-20 and 5 mg/ml BSA (TTBS/BSA) with 10 μg/ml of mouse antibody against β-hCG, or 5 μg/ml of primary antibody recognizing ENG, FLT1, AFP, PAPPA, LGALS13, LGALS14, or PGF. Antibodies are described in Supplementary Table 2. Each antibody was initially titered to ensure a linear fluorescence signal with labeling (Supplementary Fig. 1) and similar antibody lots were used throughout the study. To evaluate background fluorescence, control slides were incubated with 5 μg/ml of non-immune rabbit, goat, or mouse IgG (Jackson Immuno Research), as appropriate. Slides were washed three times with TTBS/BSA and incubated for 1 hour in the dark at room temperature with similar lots of FITC- or Texas Red-conjugated, species-specific, secondary antibodies (Jackson ImmunoResearch) diluted 1:250 in TTBS/BSA. Slides were washed three times with TTBS/BSA and nuclei were counterstained with 1 ng/ml DAPI for 10 min, followed by three washes with TTBS/BSA. Slides were cover slipped with Vectashield mounting media (Vector Laboratories, Burlingame, CA) and sealed with nail polish.
Protein Marker Quantification by Image Analysis
Fluorescent antibody labeling was imaged using a Hamamatsu Orca cooled-chip digital camera and a Leica DM IRB microscope with filter sets for DAPI, FITC and Texas Red. Cells in each field were imaged at an objective magnification of 20 × and an exposure time of 2.0 seconds. The FITC or Texas Red stain intensities were quantified using Simple PCI (Hamamatsu) imaging software. Fluorescence intensities (grey levels) were determined for each antibody and non-immune IgG (background) by circumscribing at least 10 cells. The background values were averaged and subtracted from each fluorescent value. The background-subtracted values for each specimen were averaged to calculate the average fluorescent units (AFU). Each AFU value was divided by the average of the AFUs for the control cohort to generate the relative fluorescent unit (RFU) values.
Statistical Analysis and Data Modeling
All statistical analyses were performed using JMP (version 10.0; SAS Institute, Cary, NC). Data were initially examined for normality using the Shapiro-Wilk test and between-group differences in RFU for each protein biomarker were analyzed with the non-parametric Wilcoxon rank-sum test, as the data were not normally distributed. Spearman’s rho was used to assess for correlation between GA and trophoblast yield. A value of p < 0.05 was considered statistically significant.
Principal component analysis (PCA) was implemented to assess the ability of the biomarker panel to separate individual data points (score plot) by projection of the original data on the first two principal components (PC1 and PC2), as well as correlations among biomarkers (loading plot). This useful statistical tool reduces the complexity of multidimensional data models by linearly combining multiple factors and downsizing them into new variables or principal components, while retaining the original information (33).
RESULTS
Isolation of EVT Cells
The GA of pregnancies at the time that TRIC specimens were obtained ranged between 5 and 8 weeks in the EPL cohort, and between 6 and 10 weeks in the control cohort, with similar median GAs of 7.5 and 7.8 weeks, respectively (Table 1). The median maternal ages in the EPL and control cohort were not significantly different, at 29.5 and 25.0, respectively. However, the median number of EVT cells recovered by TRIC in the EPL group was approximately 25% (p<0.05) of that recovered in the control group (Table 1). Immunofluorescence labeling of β-hCG revealed a comparable median EVT purity of 96.9% in EPL specimens and 99.1% in the control group (Table 1, Supplementary Fig. 2). There was no significant correlation between GA and trophoblast yield in the group of 20 patients that provided endocervical specimens between 5–10 weeks GA.
Table 1. Patient and specimen characteristics.
The differences in parameters related to recovered fetal cells (Count, Purity) were compared using the non-parametric Wilcoxon test.
| Control | EPL | |
|---|---|---|
| Number, n (% of total) | 10 (50) | 10 (50) |
|
| ||
| Maternal age in years, median (IQR) | 25 (21.75 – 29) | 29.5 (26.75 – 34) |
|
| ||
| Smoking/Drug habit | ||
| Marijuana, Tobacco, n (%) | 0 (0) | 1 (10) |
| Marijuana, n (%) | 1 (10) | 1 (10) |
| None, n (%) | 9 (90) | 8 (80) |
|
| ||
| Pre-existing conditions | ||
| Epilepsy, n (%) | 1 (10) | 0 (0) |
| Asthma, n (%) | 1 (10) | 0 (0) |
| Diabetes Mellitus Type II, n (%) | 1 (10) | 0 (0) |
| None, n (%) | 7 (70) | 10 (100} |
|
| ||
| Ethnicity | ||
| Caucasian, n (%) | 1 (10) | 3 (30) |
| African, n (%) | 8 (80) | 7 (70) |
| Asian, n (%) | 1 (10) | 0 (0) |
|
| ||
| Gestational age at sampling in weeks, median (IQR) | 7.78 (7.42 – 9.28) | 7.495 (6.85 – 7.71) |
|
| ||
| Gestational age at delivery in weeks, median (IQR) | 38.07 (37.86 – 38.14) | N/R |
|
| ||
| Gravity, median (IQR) | 2 (2 – 2.75) | 2.5 (1 – 3.75) |
|
| ||
| Parity, median (IQR) | 1 (0 – 1.75) | 1.5 (0 – 2) |
|
| ||
| Delivery mode | ||
| Normal Spontaneous Vaginal Delivery (NSVD), n (%) | 8 (80) | N/R |
| Cesarean section (CS), n (%) | 1 (10) | N/R |
| Unknown, n (%) | 1 (10) | N/R |
|
| ||
| Recovered fetal cells | ||
| Count, median (IQR) | 942.5 (543.75 – 1047.5) | 250 (227.5 – 500) * |
| Purity (percentage of βhCG positive cells), median (IQR) | 98 (95.77 – 100) | 96.875 (92.025 – 100} |
The significant differences (p<0.05) are bolded and labeled with an asterisk. N/R, not reported; IQR, interquartile range.
Protein Expression in Isolated EVT Cells
In the EPL cohort, compared to the controls, expression levels for AFP, FLT1 and ENG were elevated (P< 0.05), while PGF, LGALS14, and PAPPA were significantly (P< 0.05) reduced (Figs. 1 & 2). LGALS13 labeling revealed equivalent expression in the EPL and control cohorts.
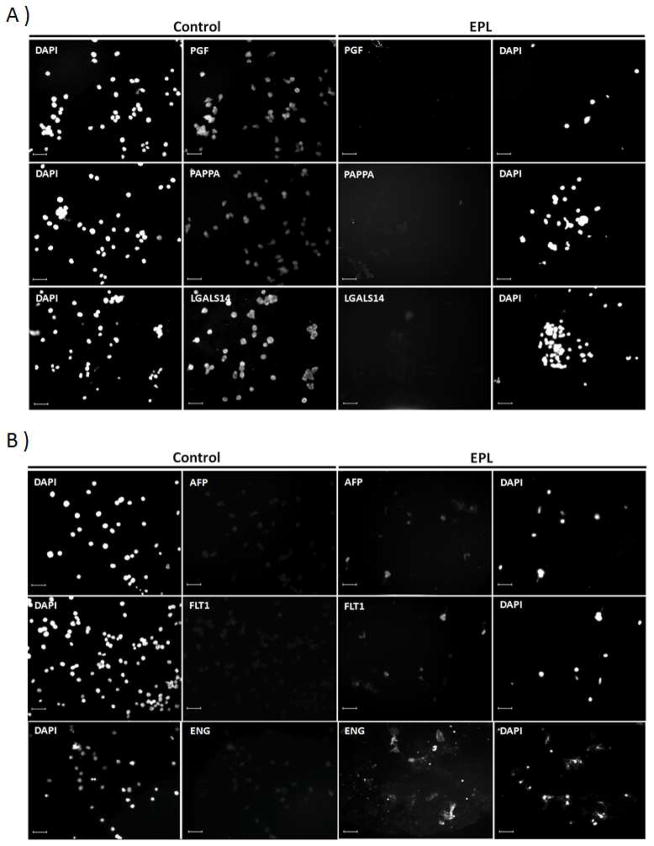
Figure 1.
TRIC-isolated EVT cells imaged for biomarker expression by immunocytochemistry. Shown are examples of EVT cells labeled with antibodies against PGF, PAPPA and LGALS14 in A, and ALF, FLT1 and ENG in B. The first column of images depicts DAPI-labeled nuclei for the control group. The second column depicts the corresponding protein expression for the control group. The third column depicts protein expression for the EPL group, and the fourth column depicts the corresponding DAPI staining for the EPL group. Size bars represent 100 μm.
Figure 2.
Quantification of biomarker expression in TRIC-isolated EVT cells. EVT cells labeled with antibodies against the indicated proteins were imaged to obtain the relative fluorescence unit (RFU) values, as described in the Materials and Methods. The non-parametric Wilcoxon test was employed to compare the expression of each protein marker between control (white) and Early Pregnancy Loss (EPL) (black) groups. The significant differences (p<0.05) are indicated by a bar and asterisk above the control/EPL pairs.. Box = 25th to 75th percentiles, horizontal line within the box = median, whisker = 1.5 × Inter Quartile Range (3rd quartile – 1st quartile). The dots represent individual outliers.
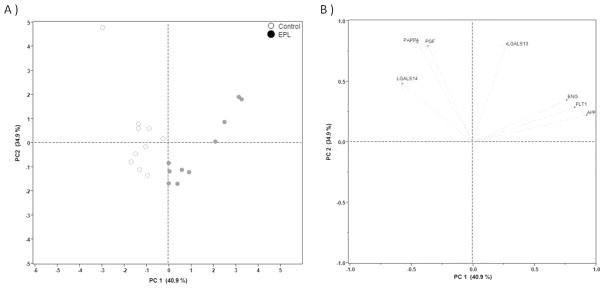
Principal component analysis (PCA)
PCA was employed to examine whether the two cohorts could be distinguished with the protein expression panel. The PCA score plot was generated over the first two PCs, accounting for 75.8% of the total variance, which separated the EPL and control specimens (Fig. 3A). The loading plot revealed two distinct groups of biomarkers (AFP, FLT1, and ENG; and LGALS14, PAPPA, and PGF; Fig. 3B).
Figure 3.
Principal component analysis (PCA) plots. (A) PCA scores were calculated and plotted for individual patients over the first two principal components (PC1 and PC2). The PCA score plot demonstrates a separation of EPL (closed circles) and control cases (open circles). (B) The loading plot was generated for individual biomarkers to determine their relationships over PC1 and PC2. Two groups of factors composed of associated members (AFP, FLT1, and ENG; and LGALS14, PAPPA, and PGF) were identified by their positions along the x-axis. Note that LGALS13 did not cluster with the other proteins.
DISCUSSION
We evaluated EVT cell protein expression patterns from ongoing pregnancies in “real time” by a minimally invasive method, using TRIC. This pilot study reveals that EVT protein expression patterns are dysregulated in EPL pregnancies, compared to normal healthy controls. These findings support the hypothesis that dysregulated EVT function is associated with EPL, and more generally to uteroplacental insufficiency.
In agreement with our previous study (21), we are able isolate EVT cells from the cervix of ongoing pregnancies with a high degree of purity, based on β-hCG immunostaining. Interestingly, significantly fewer trophoblast cells were isolated from pregnancies with EPL than from the control group. This is consistent with our prior report that reduced numbers of EVT cells can be detected in cervical smears from pregnant patients with ectopic pregnancies or a blighted ovum EPL (32). Although patients in this study were within a narrow GA range of 5–10 weeks at the time of sampling, there was no correlation with trophoblast yield. Previously, in a larger cohort of 56 patients between 5 and 20 weeks GA, we found no correlation between GA and trophoblast yield (21).
By evaluating expression patterns of proteins known to play a role in EVT function and pathology, we provide evidence of EVT dysfunction in EPL. Our findings revealed decreased expression of PGF, PAPPA and LGALS14. PGF has a similar amino acid sequence to VEGF (34), with an important role in placental vasculogenesis and angiogenesis. It is decreased in maternal serum from pregnancies with complications related to abnormal EVT invasion, including preeclampsia and IUGR (25, 26). PAPPA is produced by the placenta and can be measured in maternal serum as early as five weeks of gestation (35, 36). It is decreased in maternal circulation in pregnancies with adverse outcomes, including preeclampsia, IUGR, preterm labor, and stillbirth (24). Low levels of PAPPA, a protease targeting insulin-like growth factor binding protein-4 (37), permit accumulation of its circulating substrate, thereby decreasing the bioavailability of IGF, which negatively impacts trophoblast growth and extravillous differentiation (38). Altered maternal immunity is critical for EVT invasion, and the galectins LGALS13 and LGALS14 are known to have an immunoregulatory role (39–42). Furthermore, both are decreased in placentas with severe early onset preeclampsia (23), a syndrome that, like EPL, is associated with early EVT dysfunction (11). While LGALS14 is significantly reduced in EVT cells from pregnancies that suffer EPL, no such change was observed for LGALS13, suggesting there could be subtle differences in the molecular mechanism of EPL and preeclampsia.
Levels of FLT1, AFP and ENG were significantly elevated in the EPL cohort. Soluble forms of FLT1 antagonize VEGF, and are significantly elevated in serum of preeclamptic patients (28). FLT1 mRNA and protein expression increase in placentas from preeclamptic patients, compared to controls (29). The precise function of AFP is not known; however, it is produced by the placenta, and recent evidence has linked AFP to anti-oxidant activity (43). The increased expression of AFP could represent a compensatory response to precocious oxygen radical formation. ENG is a co-receptor of transforming growth factor-β that functions in angiogenesis (44), and is expressed by trophoblast cells as early as 6 weeks of gestation (45). ENG increases in placentas with IUGR (46), and first trimester chorionic villous sampling revealed increased levels of ENG mRNA in patients destined to develop preeclampsia (27). Furthermore, ENG expression negatively regulates EVT differentiation and invasiveness in vitro, based on a human chorionic villous explant model (47).
For classification and visualization of protein relationships in the control and EPL cohorts, a two dimensional PCA score plot was generated using two principal components (PC1 and PC2). This model demonstrated that the individual components of the two cohorts have a strong tendency to dissociate. The loading plot showed that there were appreciable correlations among the proteins measured. A positive correlation was detected among AFP, FLT1 and ENG, and another among LGALS14, PAPPA and PGF in the opposite direction. The ability of the current model to distinguish the two groups of proteins is consistent with their levels in the circulation. The panel of six responsive biomarkers could prove useful for risk assessment and early diagnosis of EPL.
A limitation of this pilot study is the lack of cytogenetic information to distinguish EPL pregnancies associated with chromosomal anomalies. Cohort sizes were not adequate to identify subgroups within the EPL or control groups. Interestingly, the early onset of intervillous blood flow is similar in EPL with and without aneuploidy (48), suggesting a convergent etiology. It would also have been useful to obtain the time of last vaginal intercourse before endocervical specimen collection, as growing evidence has demonstrated a significant effect of seminal fluid on the reproductive tract, including the cervix (49, 50).
As depicted in Supplementary Table 1, eight out of the ten EPL samples in this study were obtained after diagnosis, while two samples were obtained prior to diagnosis. It is difficult to evaluate whether those two pregnancies were viable at the time of sampling. In the future, it will not only be important to correlate the findings in relation to karyotype, but also to the timing of EPL diagnosis. With a larger cohort, it might be possible to distinguish changes in protein expression that precede pregnancy failure from those that follow it. Obtaining an abnormal EVT protein expression pattern prior to an adverse outcome could enable clinicians to screen for adverse outcomes related to abnormal placental function and better evaluate therapeutic options.
The ability to obtain EVT cells from early, ongoing pregnancies and determine the eventual outcomes provides opportunities to discover novel biomarkers through global analytic approaches. In addition to providing insights into the etiology of placental insufficiency, identification of differentially expressed genes could be used for development of new biomarkers panels, similar to the one used in the present study, to better distinguish patients at low risk of pregnancy disorder from those at risk for specific pathologies. Early identification of at-risk patients would greatly facilitate the evaluation of potential therapeutic interventions for obstetrical disorders by selecting study cohorts more likely to develop disease than the general population.
Supplementary Material
Acknowledgments
Study funding/competing interest(s): This research was supported in part by the Intramural Research Program of the NICHD, NIH Grant HD071408 and the W.K. Kellogg Foundation. Drs. Armant and Diamond have a pending patent. They and Dr. Drewlo receive payment from intellectual property that has been licensed on their behalf by Wayne State University to Perkin Elmer, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Larsen EC, Christiansen OB, Kolte AM, Macklon N. New insights into mechanisms behind miscarriage. BMC Med. 2013;11:154. doi: 10.1186/1741-7015-11-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Norwitz ER. Defective implantation and placentation: laying the blueprint for pregnancy complications. Reprod Biomed Online. 2006;13:591–9. doi: 10.1016/s1472-6483(10)60649-9. [DOI] [PubMed] [Google Scholar]
- 3.Brown S. Miscarriage and its associations. Semin Reprod Med. 2008;26:391–400. doi: 10.1055/s-0028-1087105. [DOI] [PubMed] [Google Scholar]
- 4.Practice Committee of the American Society for Reproductive M. Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil Steril. 2012;98:1103–11. doi: 10.1016/j.fertnstert.2012.06.048. [DOI] [PubMed] [Google Scholar]
- 5.Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta. 2006;27:939–58. doi: 10.1016/j.placenta.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Lala PK, Chakraborty C. Factors regulating trophoblast migration and invasiveness: possible derangements contributing to pre-eclampsia and fetal injury. Placenta. 2003;24:575–87. doi: 10.1016/s0143-4004(03)00063-8. [DOI] [PubMed] [Google Scholar]
- 7.Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376:631–44. doi: 10.1016/S0140-6736(10)60279-6. [DOI] [PubMed] [Google Scholar]
- 8.Ishihara N, Matsuo H, Murakoshi H, Laoag-Fernandez JB, Samoto T, Maruo T. Increased apoptosis in the syncytiotrophoblast in human term placentas complicated by either preeclampsia or intrauterine growth retardation. Am J Obstet Gynecol. 2002;186:158–66. doi: 10.1067/mob.2002.119176. [DOI] [PubMed] [Google Scholar]
- 9.Hempstock J, Jauniaux E, Greenwold N, Burton GJ. The contribution of placental oxidative stress to early pregnancy failure. Hum Pathol. 2003;34:1265–75. doi: 10.1016/j.humpath.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Jauniaux E, Watson AL, Hempstock J, Bao YP, Skepper JN, Burton GJ. Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am J Pathol. 2000;157:2111–22. doi: 10.1016/S0002-9440(10)64849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burton GJ, Jauniaux E. Placental oxidative stress: from miscarriage to preeclampsia. J Soc Gynecol Investig. 2004;11:342–52. doi: 10.1016/j.jsgi.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Tempfer C, Unfried G, Zeillinger R, Hefler L, Nagele F, Huber JC. Endothelial nitric oxide synthase gene polymorphism in women with idiopathic recurrent miscarriage. Hum Reprod. 2001;16:1644–7. doi: 10.1093/humrep/16.8.1644. [DOI] [PubMed] [Google Scholar]
- 13.Hustin J, Jauniaux E, Schaaps JP. Histological study of the materno-embryonic interface in spontaneous abortion. Placenta. 1990;11:477–86. doi: 10.1016/s0143-4004(05)80193-6. [DOI] [PubMed] [Google Scholar]
- 14.Jessmon P, Leach RE, Armant DR. Diverse functions of HBEGF during pregnancy. Mol Reprod Dev. 2009;76:1116–27. doi: 10.1002/mrd.21066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith S, Francis R, Guilbert L, Baker PN. Growth factor rescue of cytokine mediated trophoblast apoptosis. Placenta. 2002;23:322–30. doi: 10.1053/plac.2001.0783. [DOI] [PubMed] [Google Scholar]
- 16.Bass KE, Morrish D, Roth I, Bhardwaj D, Taylor R, Zhou Y, et al. Human cytotrophoblast invasion is up-regulated by epidermal growth factor: evidence that paracrine factors modify this process. Dev Biol. 1994;164:550–61. doi: 10.1006/dbio.1994.1223. [DOI] [PubMed] [Google Scholar]
- 17.Leach RE, Romero R, Kim YM, Chaiworapongsa T, Kilburn BA, Das SK, et al. Pre- eclampsia and expression of heparin-binding EGF-like growth factor. Lancet. 2002;360:1215–9. doi: 10.1016/S0140-6736(02)11283-9. [DOI] [PubMed] [Google Scholar]
- 18.Armant DR, Fritz R, Kilburn BA, Kim YM, Nien JK, Maihle NJ, et al. Reduced expression of the epidermal growth factor signaling system in preeclampsia. Placenta. 2014 doi: 10.1016/j.placenta.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American College of O Gynecologists. ACOG Practice Bulletin No. 88, December 2007. Invasive prenatal testing for aneuploidy. Obstet Gynecol. 2007;110:1459–67. doi: 10.1097/01.AOG.0000291570.63450.44. [DOI] [PubMed] [Google Scholar]
- 20.Imudia AN, Kumar S, Diamond MP, Decherney AH, Armant DR. Transcervical retrieval of fetal cells in the practice of modern medicine: a review of the current literature and future direction. Fertil Steril. 2010;93:1725–30. doi: 10.1016/j.fertnstert.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bolnick JM, Kilburn BA, Bajpayee S, Reddy N, Jeelani R, Crone B, et al. Trophoblast retrieval and isolation from the cervix (TRIC) for noninvasive prenatal screening at 5 to 20 weeks of gestation. Fertil Steril. 2014 doi: 10.1016/j.fertnstert.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huppertz B, Meiri H, Gizurarson S, Osol G, Sammar M. Placental protein 13 (PP13): a new biological target shifting individualized risk assessment to personalized drug design combating pre-eclampsia. Hum Reprod Update. 2013;19:391–405. doi: 10.1093/humupd/dmt003. [DOI] [PubMed] [Google Scholar]
- 23.Than NG, Romero R, Xu Y, Erez O, Xu Z, Bhatti G, et al. Evolutionary origins of the placental expression of chromosome 19 cluster galectins and their complex dysregulation in preeclampsia. Placenta. 2014;35:855–65. doi: 10.1016/j.placenta.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith GC, Stenhouse EJ, Crossley JA, Aitken DA, Cameron AD, Connor JM. Early pregnancy levels of pregnancy-associated plasma protein a and the risk of intrauterine growth restriction, premature birth, preeclampsia, and stillbirth. J Clin Endocrinol Metab. 2002;87:1762–7. doi: 10.1210/jcem.87.4.8430. [DOI] [PubMed] [Google Scholar]
- 25.Nucci M, Poon LC, Demirdjian G, Darbouret B, Nicolaides KH. Maternal Serum Placental Growth Factor (PlGF) Isoforms 1 and 2 at 11–13 Weeks' Gestation in Normal and Pathological Pregnancies. Fetal Diagn Ther. 2014 doi: 10.1159/000357842. [DOI] [PubMed] [Google Scholar]
- 26.Ghosh SK, Raheja S, Tuli A, Raghunandan C, Agarwal S. Can maternal serum placental growth factor estimation in early second trimester predict the occurrence of early onset preeclampsia and/or early onset intrauterine growth restriction? A prospective cohort study. J Obstet Gynaecol Res. 2013;39:881–90. doi: 10.1111/jog.12006. [DOI] [PubMed] [Google Scholar]
- 27.Farina A, Sekizawa A, De Sanctis P, Purwosunu Y, Okai T, Cha DH, et al. Gene expression in chorionic villous samples at 11 weeks' gestation from women destined to develop preeclampsia. Prenat Diagn. 2008;28:956–61. doi: 10.1002/pd.2109. [DOI] [PubMed] [Google Scholar]
- 28.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–83. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 29.Chung JY, Song Y, Wang Y, Magness RR, Zheng J. Differential expression of vascular endothelial growth factor (VEGF), endocrine gland derived-VEGF, and VEGF receptors in human placentas from normal and preeclamptic pregnancies. J Clin Endocrinol Metab. 2004;89:2484–90. doi: 10.1210/jc.2003-031580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams MA, Hickok DE, Zingheim RW, Luthy DA, Kimelman J, Nyberg DA, et al. Elevated maternal serum alpha-fetoprotein levels and midtrimester placental abnormalities in relation to subsequent adverse pregnancy outcomes. Am J Obstet Gynecol. 1992;167:1032–7. doi: 10.1016/s0002-9378(12)80033-0. [DOI] [PubMed] [Google Scholar]
- 31.Waller DK, Lustig LS, Cunningham GC, Feuchtbaum LB, Hook EB. The association between maternal serum alpha-fetoprotein and preterm birth, small for gestational age infants, preeclampsia, and placental complications. Obstet Gynecol. 1996;88:816–22. doi: 10.1016/0029-7844(96)00310-9. [DOI] [PubMed] [Google Scholar]
- 32.Imudia AN, Suzuki Y, Kilburn BA, Yelian FD, Diamond MP, Romero R, et al. Retrieval of trophoblast cells from the cervical canal for prediction of abnormal pregnancy: a pilot study. Hum Reprod. 2009;24:2086–92. doi: 10.1093/humrep/dep206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olliffe IT. Principal Component Analysis. 2. New York, NY: Springer; 2002. [Google Scholar]
- 34.Shore VH, Wang TH, Wang CL, Torry RJ, Caudle MR, Torry DS. Vascular endothelial growth factor, placenta growth factor and their receptors in isolated human trophoblast. Placenta. 1997;18:657–65. doi: 10.1016/s0143-4004(97)90007-2. [DOI] [PubMed] [Google Scholar]
- 35.Folkersen J, Grudzinskas JG, Hindersson P, Teisner B, Westergaard JG. Pregnancy-associated plasma protein A: circulating levels during normal pregnancy. Am J Obstet Gynecol. 1981;139:910–4. doi: 10.1016/0002-9378(81)90957-1. [DOI] [PubMed] [Google Scholar]
- 36.Schindler AM, Bordignon P, Bischof P. Immunohistochemical localization of pregnancy-associated plasma protein A in decidua and trophoblast: comparison with human chorionic gonadotrophin and fibrin. Placenta. 1984;5:227–35. doi: 10.1016/s0143-4004(84)80032-6. [DOI] [PubMed] [Google Scholar]
- 37.Lawrence JB, Oxvig C, Overgaard MT, Sottrup-Jensen L, Gleich GJ, Hays LG, et al. The insulin-like growth factor (IGF)-dependent IGF binding protein-4 protease secreted by human fibroblasts is pregnancy-associated plasma protein-A. Proc Natl Acad Sci U S A. 1999;96:3149–53. doi: 10.1073/pnas.96.6.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Irwin JC, Suen LF, Martina NA, Mark SP, Giudice LC. Role of the IGF system in trophoblast invasion and pre-eclampsia. Hum Reprod. 1999;14(Suppl 2):90–6. doi: 10.1093/humrep/14.suppl_2.90. [DOI] [PubMed] [Google Scholar]
- 39.Than NG, Romero R, Goodman M, Weckle A, Xing J, Dong Z, et al. A primate subfamily of galectins expressed at the maternal-fetal interface that promote immune cell death. Proc Natl Acad Sci U S A. 2009;106:9731–6. doi: 10.1073/pnas.0903568106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Than NG, Balogh A, Romero R, Karpati E, Erez O, Szilagyi A, et al. Placental Protein 13 (PP13) - A Placental Immunoregulatory Galectin Protecting Pregnancy. Front Immunol. 2014;5:348. doi: 10.3389/fimmu.2014.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skornicka EL, Kiyatkina N, Weber MC, Tykocinski ML, Koo PH. Pregnancy zone protein is a carrier and modulator of placental protein-14 in T-cell growth and cytokine production. Cell Immunol. 2004;232:144–56. doi: 10.1016/j.cellimm.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 42.Mishan-Eisenberg G, Borovsky Z, Weber MC, Gazit R, Tykocinski ML, Rachmilewitz J. Differential regulation of Th1/Th2 cytokine responses by placental protein 14. J Immunol. 2004;173:5524–30. doi: 10.4049/jimmunol.173.9.5524. [DOI] [PubMed] [Google Scholar]
- 43.Choi HY, Kim SW, Kim B, Lee HN, Kim SJ, Song M, et al. Alpha-fetoprotein, identified as a novel marker for the antioxidant effect of placental extract, exhibits synergistic antioxidant activity in the presence of estradiol. PLoS One. 2014;9:e99421. doi: 10.1371/journal.pone.0099421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Z, Lebrin F, Maring JA, van den Driesche S, van der Brink S, van Dinther M, et al. ENDOGLIN is dispensable for vasculogenesis, but required for vascular endothelial growth factor-induced angiogenesis. PLoS One. 2014;9:e86273. doi: 10.1371/journal.pone.0086273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.St-Jacques S, Forte M, Lye SJ, Letarte M. Localization of endoglin, a transforming growth factor-beta binding protein, and of CD44 and integrins in placenta during the first trimester of pregnancy. Biol Reprod. 1994;51:405–13. doi: 10.1095/biolreprod51.3.405. [DOI] [PubMed] [Google Scholar]
- 46.Szentpeteri I, Rab A, Kornya L, Kovacs P, Brubel R, Joo JG. Placental gene expression patterns of endoglin (CD105) in intrauterine growth restriction. J Matern Fetal Neonatal Med. 2014;27:350–4. doi: 10.3109/14767058.2013.818125. [DOI] [PubMed] [Google Scholar]
- 47.Caniggia I, Taylor CV, Ritchie JW, Lye SJ, Letarte M. Endoglin regulates trophoblast differentiation along the invasive pathway in human placental villous explants. Endocrinology. 1997;138:4977–88. doi: 10.1210/endo.138.11.5475. [DOI] [PubMed] [Google Scholar]
- 48.Greenwold N, Jauniaux E, Gulbis B, Hempstock J, Gervy C, Burton GJ. Relationship among maternal serum endocrinology, placental karyotype, and intervillous circulation in early pregnancy failure. Fertil Steril. 2003;79:1373–9. doi: 10.1016/s0015-0282(03)00364-9. [DOI] [PubMed] [Google Scholar]
- 49.Sharkey DJ, Tremellen KP, Jasper MJ, Gemzell-Danielsson K, Robertson SA. Seminal fluid induces leukocyte recruitment and cytokine and chemokine mRNA expression in the human cervix after coitus. J Immunol. 2012;188:2445–54. doi: 10.4049/jimmunol.1102736. [DOI] [PubMed] [Google Scholar]
- 50.Robertson SA. Seminal fluid signaling in the female reproductive tract: lessons from rodents and pigs. J Anim Sci. 2007;85:E36–44. doi: 10.2527/jas.2006-578. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.