Abstract
Objectives
Epithelial ovarian cancer (EOC) typically presents with advanced disease. Even with optimal debulking and response to adjuvant chemotherapy, the majority of patients will have disease relapse. We evaluated cytokine and chemokine profiles in ascites at primary surgery as biomarkers for progression-free survival (PFS) and overall survival (OS) in patients with advanced EOC.
Methods
Retrospective analysis of patients (n =70) who underwent surgery at Roswell Park Cancer Institute between 2002-12, followed by platinum-based chemotherapy.
Results
The mean age at diagnosis was 61.8 years, 85.3% had serous EOC, and 95.7% had stage IIIB, IIIC, or IV disease. Univariate analysis showed that ascites levels of tumor necrosis factor (TNF)-α were associated with reduced PFS after primary surgery. Although the ascites concentration of interleukin (IL)-6 was not by itself predictive of PFS, we found that stratifying patients by high TNF-α and high IL-6 levels identified a sub-group of patients at high risk for rapid disease relapse. This effect was largely independent of clinical prognostic variables.
Conclusions
The combination of high TNF-α and high IL-6 ascites levels at primary surgery predicts worse PFS in patients with advanced EOC. These results suggest an interaction between ascites TNF-α and IL-6 in driving tumor progression and resistance to chemotherapy in advanced EOC, and raise the potential for pre-treatment ascites levels of these cytokines as prognostic biomarkers. This study involved a small sample of patients and was an exploratory analysis; therefore, findings require validation in a larger independent cohort.
Keywords: Ascites, TNF-α, IL-6, ovarian cancer, prognosis
Introduction
Ovarian cancer is the leading cause of death from gynecological malignancies. Based on American Cancer Society estimates, 21,980 new cases of ovarian cancer and 14,270 deaths are expected in the U.S. in 2014 [1]. Epithelial ovarian cancer (EOC) is by far the most common type of ovarian cancer, and among these tumors, serous EOC is the most common subtype. The majority of patients present with advanced disease, and even with optimal debulking and response to adjuvant therapy, relapse of disease is expected. In an analysis of 1,895 patients with stage III EOC treated with primary surgery followed by standard adjuvant chemotherapy, the median progression-free survival (PFS) was 17 months and the median overall survival (OS) was 45 months [2]. However, there is substantial variability in PFS among women with the same disease stage. Validated prognostic factors for advanced EOC principally rely on age, performance status, residual tumor volume following primary surgery, and tumor histology [2, 3]. Serum CA-125 at diagnosis and changes in levels following treatment have also been used as outcome markers [4]. It is a priority to develop novel pre-treatment prognostic markers that will identify patients who are likely to have a poor response to standard platinum-based chemotherapy who might be considered for investigational protocols.
There is growing recognition that immune responses in the tumor microenvironment are both prognostic biomarkers and potential targets for therapeutic modulation. The critical role of immune surveillance in EOC was demonstrated by correlation of survival with tumor-infiltrating lymphocytes (TILs) [5]. However, the tumor microenvironment in EOC is inflammatory, characterized by myeloid cell accumulation and expression of pro-inflammatory cytokines and chemokines, while also being immunosuppressive [6-10]. Ascites from patients with EOC inhibited T cell receptor-induced NF-kB and NFAT signaling in tumor-associated T cells [6]. Infiltrating regulatory T cells (Tregs) abrogate T cell-driven anti-tumor immunity. Intraepithelial CD8+ TILs and a high CD8+/Treg ratio were associated with favorable prognosis in patients with EOC [11]. Tumor cells and macrophages in the tumor microenvironment produce CCL22, which mediates trafficking of Tregs to the tumor and poorer prognosis in EOC [12]. Accumulation of H4-expressing macrophages in the tumor environment can impede T cell responses and correlate with more rapid tumor recurrence in EOC [13, 14]. Myeloid-derived suppressor cells (MDSCs) also accumulate in the ascites of patients with advanced EOC [7, 10]. In addition to being obstacles to antitumor immunity, tumor-infiltrating myeloid cells can release matrix metalloproteinases and pro-angiogenic factors (e.g., vascular endothelial growth factor) as part of a wound repair response that can promote tumor invasion and spread [15].
As much as one third of patients with EOC present with ascites at initial diagnosis and the majority have ascites with relapse [16]. The etiology of ascites production is multi-factorial including lymphatic obstruction, increased vascular permeability and secretion of soluble factors by tumor and activated mesothelial cells [16]. Understanding the interaction of ascites constituents, including the cellular populations and soluble factors (e.g., cytokines, chemokines, pro-angiogenic factors, proteases, products of necrosis) may yield important knowledge about mechanisms that drive tumor progression that can be potential prognostic biomarkers and therapeutic targets.
A number of studies have shown correlations between ascitic cytokine profiles and outcome in patients with EOC [17-20]. In general, these studies involved small numbers of patients, and did not examine the interaction between cytokines in predicting PFS. We therefore began our analysis with a straightforward univariate analysis of levels of a panel of cytokines and chemokines in ascites collected at primary surgery for advanced EOC. The inflammatory mediators we examined were specifically selected based on their known biological roles in myeloid cell recruitment and activation. Based on the univariate results, we performed additional modeling of interactions between cytokine levels using pairwise comparisons. We found an interaction between ascites TNF-α and IL-6 levels such that high levels (> 50th percentile) of both cytokines were highly predictive of rapid disease recurrence following primary surgery.
Methods
Patients and ascites collection
This study was approved by the Institutional Review Board of Roswell Park Cancer Institute, Buffalo, NY, and was in compliance with federal and state requirements. All participants gave informed consent to use samples for research. Ascites was collected from 70 patients newly diagnosed with ovarian, primary peritoneal, and fallopian tube carcinoma at the time of primary surgery (2002-2012). All cases were reviewed by a pathologist. Staging was based on the 2014 International Federation of Gynecology and Obstetrics (FIGO) criteria, and grade was based on the Shimizu/Silverberg system, with high grade defined as grades 2 or 3 [21]. Ascitic fluid was obtained at the time of initial cytoreductive surgery and centrifuged at 500g for 15 minutes. Supernatants were stored at −80°C until assayed. All samples were deidentified.
Electronic medical records were retrospectively reviewed and baseline demographic and clinico-pathologic data were collected for all patients. Postoperatively, patients underwent adjuvant platinum-based chemotherapy, and were observed for an average of 35 months. The primary endpoint was PFS, which was defined as the time from primary surgery until disease recurrence or death. Overall survival was defined from the day of diagnosis to the date of the last follow-up or death.
Cytokine measurements
Cytokine concentrations in undiluted ascites specimens were determined using human cytokine/chemokine magnetic bead kit (EMD Millipore, St Charles, MO) by Luminex multiplex assay at the Roswell Park Cancer Institute Flow Cytometry and Imaging facility. We selected a panel of cytokines and chemokines involved in the expansion, recruitment, and activation of macrophages and neutrophils: G-CSF, GM-CSF, IL-1β, IL-6, IL-17Α, TNF-α, IL-8/CXCL8, MCP-1/CCL2, MCP-3/CCL7, MDC/CCL22, MIP-1α/CCL3, MIP-1β/CCL4, and VEGF. The detection thresholds were 1.2 – 7.5 pg/ml for pro-inflammatory cytokines and colony-stimulating factors (G-CSF, GM-CSF, IL-1β, IL-6, IL-17Α, TNF-α); 3.5 – 17.5 pg/ml for chemokines (IL-8/CXCL8, MCP-1/CCL2, MCP-3/CCL7, MDC/CCL22, MIP-1α/CCL3, MIP-1β/CCL4); and 40 pg/ml for the pro-angiogenic factor, VEGF. Average values from duplicate wells per samples are presented. Levels of IL-6 and MCP-1 in all samples were also evaluated by standard ELISA since levels of IL-6 and MCP-1 in several samples exceeded the upper assay limit of the Luminex platform. Anti-human IL-6 mAb (mouse capture Ab) with biotinylated anti-human IL-6 pAb (goat detection Ab) and anti-human MCP-1 mAb (mouse capture Ab) with biotinylated anti-human MCP-1 pAb (goat detection Ab) (R & D System, Minneapolis, MN) were used in ELISA.
Statistical analysis and clinical correlation
Because concentration levels are strongly right-tailed, our univariate analysis of cytokine/chemokine association with overall survival and PFS regression models used the rank of each cytokine to provide a non-parametric analysis via Cox’s regression model. Subsequently, we stratified the level of each cytokine at the observed median value to form low and high level sets. Where appropriate, we have presented median survival times and tested the difference between survival curves via the log-rank test. Association between cytokine/chemokine risk categories and continuous clinical variables were tested by ANOVA, and categorical variables were analyzed by chi-square test. We considered a p<0.05 level of significance and interval estimates are 95% CI. We initially performed a univariate analysis to identify those cytokines and chemokines that correlated with PFS. Based on the univariate results, we analyzed pairwise combinations to evaluate interactions between cytokines and chemokines in predicting PFS. All analyses were performed in the R3.1.2 statistical programming environment.
Results
Univariate association between cytokines and prognosis
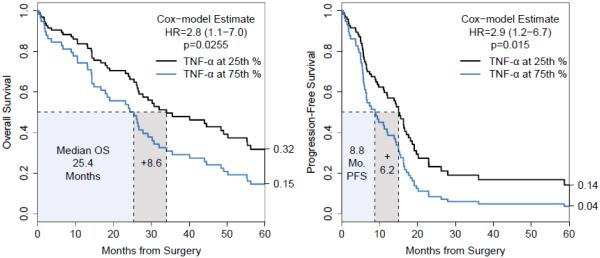
Baseline clinical characteristics and response to therapy for all of the subjects are summarized in Table 1. While most of the cytokines were detected in all samples, IL-17A (41/70, 59%) and IL-β (52/70, 74%) had greater than 50% non-detection rates and were excluded from further analysis. The frequency of non-detection of GM-CSF, MCP-3, and MIP-1β in ascites samples was 20% (14/70), 3% (2/70), and 4% (3/70), respectively. Cytokine levels were defined as high or low based on the observed median. We tested the univariate association of each cytokine with PFS and OS. Only TNF-α had a significant association with worse OS (HR= 2.8, 95% CI: 1.1-7.0, p= 0.0255) and PFS (HR=2.9, 95% CI: 1.2-6.7, p=0.0154); the Cox model-based curves in Figure 1 are the Breslow estimated survival functions at the 25th and 75th quantiles (29.5 and 53.8 pg/ml, respectively). These results imply that the clinical magnitude is mild when TNF-α levels are considered continuously. These effects were not significant after multiple testing correction. Notably, there was no univariate relationship with survival for IL-6 (OS, p=0.779; PFS, p=0.510) or IL-8 (OS, p=0.691; PFS, p=0.933).
Table 1.
Clinical characteristics and outcomes of patients with advanced ovarian cancer(n=70)
| Median Age | 62 (range 21-85) | ||
|---|---|---|---|
|
| |||
| n | % | ||
| Advanced Stage IIIB-IV | 68 | 97.14 | |
|
| |||
| Grade 1 | 6 | 8.57 | |
| Grade 2 | 11 | 15.71 | |
| Grade 3 | 53 | 75.71 | |
|
| |||
| Serous | All grades | 55 | 78.57 |
| Grade 1 | 2 | 3.64 | |
| Grade 2 | 8 | 14.54 | |
| Grade 3 | 45 | 81.82 | |
|
| |||
| Non-serous | 6 | 8.57 | |
| Clear Cell | 2 | 2.86 | |
| Endometrioid | 1 | 1.43 | |
| Mucinous | 3 | 4.29 | |
|
| |||
| Mixed | 9 | 12.86 | |
|
| |||
| Optimal Debulking, <1cm | 53 | 75.71 | |
| Platinum sensitive response | n=36 (51.4%) | ||
|
| |||
|
Median OS from time of
primary surgery |
26.7 months (range 24.0-44.0) |
||
|
| |||
|
Median PFS from time of
primary surgery |
11.6 months (range 7.3-16.3) |
||
Figure 1.
Model based estimates for pre-treatment ascites TNF-α levels at the 25th percentile (29.5 pg/ml) and 75th percentile (53.75 pg/ml). TNF-α concentration had a significant association with worse OS (HR= 2.8, 95% CI:1.1-7.0, p= 0.0255) and PFS (HR=2.9 95%CI:1.2-6.7 p=0.0154).
Pairwise associations between TNF-α, IL-6, and IL-8
Stratifying patients into high and low groups based on the median of each of IL-6, IL-8 and TNF-α, we considered the combined levels of IL-6 and IL-8; of IL-8 and TNF-α; and of IL-6 and TNF-α. The median PFS and OS are shown in Table 2 where each subsection reflects a different way to classify all 70 patients. Notably, we observed strong deleterious PFS trends for patients with high ascites levels of any combination of IL-8 or IL-6 with TNF-α. These stratifications are likely detecting the same set of 13 patients who express high levels of all three cytokines: these 13 patients had a shortened PFS interval (median 6.0 versus 15.0 months) and a shorter OS time (median 16.7 versus 30.2 months) (Table 2). We did not perform additional multivariate analysis of combinations of ≥ 3 cytokines and chemokines because of the small group sizes. Because of the significant OS and PFS associations, we consider the stratification defined by low IL-6, and high IL-6 split by TNF-α level for further study.
Table 2.
Association or pre-treatment pairwise combinations of ascites cytokine levels on PFS and OS in months (n=70)
| IL-8 and IL-6 | n | PFS (p=0.732) | OS (p=0.779) |
|
| |||
| IL-8low/IL-6low | 22 | 15.2 (6.7-19.1) | 26.1 (17.9-49.1) |
| IL-8low/IL-6high | 13 | 14.4 (4.5-NA) | 34.0 (23.2-NA) |
| IL-8high/IL-6low | 12 | 11.1 (7.8-NA) | 24.6 (13.1-NA) |
| IL-8high/IL-6high | 23 | 6.5 (5.7-18.6) | 27.8 (14.3-67.6) |
|
| |||
| TNF-α and IL-8 | n | PFS (p=0.090) | OS (p=0.223) |
|
| |||
| TNF-αlow/IL-8low | 19 | 15 (5.1-26.2) | 26.3 (17.9-NA) |
| TNF-αlow/IL-8high | 16 | 15 (7.8-NA) | 40.1 (19-NA) |
| TNF-αhigh/IL-8low | 16 | 15 (6-19.7) | 26.7 (24.3-NA) |
| TNF-αhigh/IL-8high | 19 | 6.3 (5.6-12.1) | 16.7 (13.2-56.2) |
|
| |||
| TNF-α and IL-6 | n | PFS (p<0.001) | OS (p=0.044) |
|
| |||
| TNF-αlow/IL-6low | 17 | 11.2 (5.7-16.6) | 25.4 (14.3-55.2) |
| TNF-αlow/IL-6high | 18 | 18.1 (12.0-NA) | 48.4 (26.5-NA) |
| TNF-αhigh/IL-6low | 17 | 16.3 (9.4-NA) | 26.1 (18.4-NA) |
| TNF-αhigh/IL-6high | 18 | 6.2 (5.3-12.1) | 25 (13.2-34) |
|
| |||
| n | PFS (p=0.001) | OS (p=0.039) | |
|
| |||
| All 3 high | 13 | 6.0 (5.32-NA) | 16.7 (6.31-NA) |
| All other combinations | 57 | 15.0 (9.4-17.1) | 30.2 (25.4-48.4) |
NA: estimate does not drop below; p-values are 4-group log-rank tests; Italic = individually significant log-rank p<0.05
PFS and survival analysis in relation to subsets of ascites levels of IL-6 and TNF-α
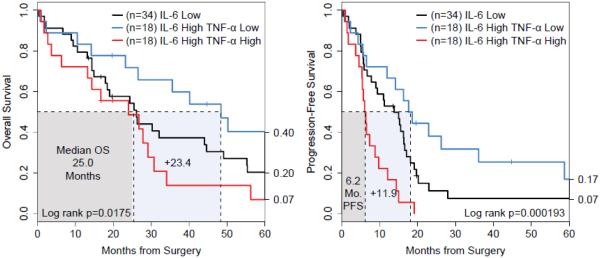
Patients who had high ascites levels of both IL-6 and TNF-α had shorter PFS intervals versus all other patients (median 6.2 versus 15.2 months, log-rank p=0.00015) but the threat to median overall survival was transient (25.0 versus 30.2 months, log-rank p=0.0278) as there was a comparatively small reduction in overall survival. Conversely, high IL-6 patients with low TNF-α had similarly improved median PFS (18.1 versus 9.2 months, log-rank p=0.0101) and a durable increase in overall survival (48.4 months, log-rank p=0.016) (Table 2).
Given a high ascites level of IL-6, patients with high or low TNF-α had the strongest differences in OS (48.4 vs. 25 months, log-rank p=0.0175) and PFS (18.1 vs 6.2 months, p=0.000193) (Figure 2). The PFS curves were well-ordered and the differences appear after 8 months where the high IL-6 and high TNF-α subset decreased rapidly to 22% progression-free at 10 months versus 72% for the high IL-6 and low TNF-α subset. For overall survival, prognosis for the IL-6 high subsets was not distinct at 10 months (89% TNF-α low versus 72% TNF-α high, Z-test p=0.098), but a significant difference was observed by 30 months (TNF-α high 28% versus 66%, Z-test p=0.0092).
Figure 2.
High TNF-α and high IL-6 ascites levels correlated with worse OS and PFS. The median concentrations of TNF-α and IL-6 were 38.5 pg/ml and 4741.6 pg/ml, respectively. In contrast, all other groups (TNF-αhigh/IL-6low, TNF-αlow/IL-6high, and TNF-αlow/IL-6low) had similar outcomes.
Association between IL-6- and TNF-α-defined risk sets and clinical factors
Stratifying patients by high IL-6 levels identified a strong prognostic effect for TNF-α. Table 3 describes the stratification of patients into IL-6low, IL-6high/TNF-αlow, and IL-6-high/TNF-αhigh level groups and their association with clinical factors. Of the clinical variables, stage varied little in this data (all but 3 patients were stage IIIB/C or IV). Approximately 90% of tumors were high grade (2 or 3) in all 3 groups, but the IL-6high/TNF-αlow group had a lower proportion of grade 3 histology (Chi-square p=0.03). No other cytokines were associated with the IL-6/TNF-α subgroups.
Table 3.
Baseline characteristics and outcomes in patients with advanced ovarian cancer in relation to pre-treatment TNF-α and IL-6 ascites levels
| IL-6 low | IL-6 high/TNF-α low | IL-6 high/TNF-α high | p-value | ||
|---|---|---|---|---|---|
| Number of patients | 34 | 18 | 18 | ||
| Mean age in years | 64.5 | 57.8 | 60.7 | p=0.135 | |
|
| |||||
| Grade | 1 | 3 | 1 | 1 | p=0.030 |
| 2 | 3 | 7 | 1 | ||
| 3 | 27 | 9 | 15 | ||
| Serous % | 85.3 | 66.7 | 77.8 | p=0.266 | |
| <1cm residual disease % | 75.8 | 77.8 | 77.8 | p=0.981 | |
|
| |||||
| OS, Months | 26.1 (17.9-49.1) | 48.4 (26.5-NA) | 24.0 (13.2-34.0) | p=0.018 | |
| PFS, Months | 13.8 (9.0-17.1) | 18.1 (11.9-NA) | 6.2 (5.3-12.1) | p<0.001 | |
NA: survival estimate does not reach 50%; Italic added for emphasis
Discussion
We evaluated the prognostic value of cytokine and chemokine profiles in ascites of patients undergoing primary surgery for advanced EOC. The panel of cytokines and chemokines was selected based on their importance to myeloid cell accumulation and phenotypes. Univariate analysis showed that ascites levels of TNF-α were associated with reduced PFS after primary surgery. Although the ascites concentration of IL-6 was not by itself predictive of PFS, we found that high TNF-α and high IL-6 ascites levels correlated with rapid disease relapse. In contrast, all other groups (TNF-αhigh/IL-6low, TNF-αlow/IL-6high, and TNF-αlow/IL-6low) had similar durations of PFS. These results suggest an interaction between ascites TNF-α and IL-6 in driving tumor progression in advanced EOC. We did not observe a consistent interaction of these cytokine strata with clinical variables (e.g., age, stage, histology, and optimal tumor debulking). Additional exploratory analysis, including multivariate models with clinical variables, showed trends to interactions between levels of TNF-α, IL-6, and IL-8. In each of these interactions, high ascites levels of two or all three of these cytokines correlated with reduced PFS. In each of these scenarios, median PFS after surgery ranged between 6.0 – 6.5 months, versus a median of 11.1 – 18.1 months in all other strata (Table 2). If confirmed in a larger independent cohort, pre-treatment ascites levels of these cytokines will be useful prognostic biomarkers for rapid tumor recurrence and resistance to standard chemotherapy.
Tumor-derived factors are capable of activating macrophages, leading to production of pro-inflammatory cytokines, including TNF-α and IL-6, to generate an inflammatory microenvironment that promotes metastatic growth [22]. Conversely, these same cytokines can activate other pathways that augment anti-tumor immunity, an illustration of the “two faces” of inflammatory mediators in tumor progression. It is therefore not surprising that conflicting reports exist regarding the potential protective versus deleterious role of inflammatory mediators during cancer. TNF-α production in the ovarian cancer microenvironment has been recognized for decades, with tumor-infiltrating macrophages likely to be the major source [23]. In ex vivo studies, exogenous TNF-α stimulated ovarian tumor cells to produce TNF-α and to proliferate [24]. Charles et al. [25] showed that TNF-α production in the ovarian cancer microenvironment increased myeloid cell recruitment in an IL-17-dependent manner, and promoted tumor progression in mice. In patients with advanced ovarian cancer, treatment with infliximab (anti-TNF-α antibody) reduced IL-1R and IL-23R expression in CD4+CD25− cells isolated from ascites, and reduced plasma IL-17 levels [25]. Our findings differ from those of Bamias et al. [26] who reported that in patients receiving first-line chemotherapy, ascites TNFα levels >35 pg/ml were associated with improved PFS. They observed an inverse relationship between ascites TNF-α levels and accumulation of CD4+CD25+(hi) cells, a population that includes Tregs [26].
Members of the IL-6 family of cytokines are highly up-regulated in several cancers and are considered to be key links between inflammation, tumorigenesis and metastasis [27]. Conversely, IL-6 can enhance anti-tumor immunity by augmenting activation, proliferation and survival of lymphocytes and their trafficking to the tumor and draining lymph nodes [28]. Yigit et al. [20] noted a positive correlation between IL-6 concentration in ascites and residual disease after debulking. In addition, IL-6 levels were higher at recurrence compared to primary advanced disease. In another study, IL-6 levels in ascites correlated significantly with ascites volume and initial tumor size, but not with survival [29]. In contrast, Lane et al. [17] reported that ascites IL-6 levels predicted shorter PFS in patients with EOC. Soluble IL-6-receptor-α stimulated IL-6 trans-signaling in endothelial cells, and increased ascites levels of soluble IL-6-receptor-α correlated with poor prognosis in patients with ovarian cancer [30]. In addition to its local effects in the tumor microenvironment, tumor-derived IL-6 can stimulate paraneoplastic thrombocytosis in patients with advanced EOC that, in turn, is associated with poor prognosis [31]. The therapeutic potential of targeting IL-6 and downstream signaling has been shown in mouse models of ovarian cancer [30, 31].
Although we did not observe a prognostic influence of ascites IL-6 by itself, we identified a putative interaction between IL-6 and TNF-α. Patients with both high TNF-α and high IL-6 ascites levels had significantly shorter PFS than other groups. All members of the TNF superfamily exhibit pro-inflammatory activity, in part through activation of NF-κB. They regulate numerous signaling pathways that modulate inflammation, proliferation, apoptosis, and angiogenesis that can affect tumor progression and metastasis [32]. IL-6 signals through the IL-6 receptor and gp130 to initiate JAK2/STAT3 signaling [33, 34]. STAT3 activation has been implicated in stimulating stem cell-like characteristics in ovarian tumor cells associated with chemotherapy resistance [35]. In addition, tumor-derived products can activate STAT3 signaling in myeloid cells that can lead to inhibition of DC maturation and stimulation of MDSC development [36-39]. Potentially, the dual effects of TNF-α and IL-6 on activation of NF-κB and STAT3, respectively, promote tumor growth [40].
Our study has a number of limitations. This study involved a small sample of patients and was an exploratory analysis, i.e., we did not pre-specify a specific effect (positive or negative) of individual cytokines or paired cytokines on PFS. In addition, we divided patients into high and low cytokine groups based on values above or below the median, respectively; this division is arbitrary and is not designed to model all of the cytokine interactions that can influence PFS. Our results were limited to patients treated at a single center. Thus, our results should be considered hypothesis-generating, and findings require validation in a larger independent cohort. In addition, the correlative design of our study does not permit conclusions about immunologic mechanisms affecting tumor progression. If our results are confirmed in a larger independent cohort, they would establish ascites TNF-α and IL-6 as novel prognostic biomarkers that may be combined with standard clinical variables as a composite prognostic signature, and would provide additional support for evaluating these cytokines as therapeutic targets in patients with advanced EOC.
Highlights.
High levels of TNF-α in ascites at primary surgery correlate with poor prognosis in patients with epithelial ovarian cancer.
The combination of high TNF-α and high IL-6 levels identified a sub-group at high risk for rapid disease relapse.
These results suggest an interaction between ascites TNF-α and IL-6 in driving tumor progression.
Acknowledgments
This work was funded by RPCI-UPCI Ovarian Cancer SPORE NIH P50CA159981-01A1, NIH T32CA108456 (NK and KG), Roswell Park Alliance Foundation (KM), 2014-2015 Developmental Funds award from the Roswell Park Alliance Foundation (BHS and KHE), University at Buffalo School of Medicine Pilot Research Award (BHS), K01LM012100 (KHE), and NCI Cancer Center Support Grant CA016056 to Roswell Park Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no financial conflict of interest.
References
- [1].American Cancer Society Cancer Facts & Figures 2014.
- [2].Winter WE, 3rd, Maxwell GL, Tian C, Carlson JW, Ozols RF, Rose PG, et al. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:3621–7. doi: 10.1200/JCO.2006.10.2517. [DOI] [PubMed] [Google Scholar]
- [3].Winter WE, 3rd, Maxwell GL, Tian C, Sundborg MJ, Rose GS, Rose PG, et al. Tumor residual after surgical cytoreduction in prediction of clinical outcome in stage IV epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2008;26:83–9. doi: 10.1200/JCO.2007.13.1953. [DOI] [PubMed] [Google Scholar]
- [4].Zorn KK, Tian C, McGuire WP, Hoskins WJ, Markman M, Muggia FM, et al. The prognostic value of pretreatment CA 125 in patients with advanced ovarian carcinoma: a Gynecologic Oncology Group study. Cancer. 2009;115:1028–35. doi: 10.1002/cncr.24084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–13. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- [6].Simpson-Abelson MR, Loyall JL, Lehman HK, Barnas JL, Minderman H, O'Loughlin KL, et al. 2013;13:14. [PMC free article] [PubMed] [Google Scholar]
- [7].Obermajer N, Muthuswamy R, Odunsi K, Edwards RP, Kalinski P. PGE(2)-induced CXCL12 production and CXCR4 expression controls the accumulation of human MDSCs in ovarian cancer environment. Cancer Res. 2011;71:7463–70. doi: 10.1158/0008-5472.CAN-11-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mhawech-Fauceglia P, Wang D, Ali L, Lele S, Huba MA, Liu S, et al. Intraepithelial T cells and tumor-associated macrophages in ovarian cancer patients. Cancer Immun. 2013;13:1. [PMC free article] [PubMed] [Google Scholar]
- [9].Giuntoli RL, 2nd, Webb TJ, Zoso A, Rogers O, Diaz-Montes TP, Bristow RE, et al. Ovarian cancer-associated ascites demonstrates altered immune environment: implications for antitumor immunity. Anticancer Res. 2009;29:2875–84. [PubMed] [Google Scholar]
- [10].Khan AN, Kolomeyevskaya N, Singel KL, Grimm MJ, Moysich KB, Daudi S, et al. Targeting myeloid cells in the tumor microenvironment enhances vaccine efficacy in murine epithelial ovarian cancer. Oncotarget. 2015 doi: 10.18632/oncotarget.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:18538–43. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- [13].Kryczek I, Wei S, Zhu G, Myers L, Mottram P, Cheng P, et al. Relationship between B7-H4, regulatory T cells, and patient outcome in human ovarian carcinoma. Cancer Res. 2007;67:8900–5. doi: 10.1158/0008-5472.CAN-07-1866. [DOI] [PubMed] [Google Scholar]
- [14].Kryczek I, Zou L, Rodriguez P, Zhu G, Wei S, Mottram P, et al. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med. 2006;203:871–81. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Priceman SJ, Sung JL, Shaposhnik Z, Burton JB, Torres-Collado AX, Moughon DL, et al. Targeting distinct tumor-infiltrating myeloid cells by inhibiting CSF-1 receptor: combating tumor evasion of antiangiogenic therapy. Blood. 2010;115:1461–71. doi: 10.1182/blood-2009-08-237412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ahmed N, Stenvers KL. Getting to know ovarian cancer ascites: opportunities for targeted therapy-based translational research. Frontiers in oncology. 2013;3:256. doi: 10.3389/fonc.2013.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lane D, Matte I, Rancourt C, Piche A. Prognostic significance of IL-6 and IL-8 ascites levels in ovarian cancer patients. BMC Cancer. 2011;11:210. doi: 10.1186/1471-2407-11-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chen YL, Cheng WF, Chang MC, Lin HW, Huang CT, Chien CL, et al. Interferon-gamma in ascites could be a predictive biomarker of outcome in ovarian carcinoma. Gynecol Oncol. 2013;131:63–8. doi: 10.1016/j.ygyno.2013.07.105. [DOI] [PubMed] [Google Scholar]
- [19].Matte I, Lane D, Laplante C, Rancourt C, Piche A. Profiling of cytokines in human epithelial ovarian cancer ascites. American journal of cancer research. 2012;2:566–80. [PMC free article] [PubMed] [Google Scholar]
- [20].Yigit R, Figdor CG, Zusterzeel PL, Pots JM, Torensma R, Massuger LF. Cytokine analysis as a tool to understand tumour-host interaction in ovarian cancer. Eur J Cancer. 2011;47:1883–9. doi: 10.1016/j.ejca.2011.03.026. [DOI] [PubMed] [Google Scholar]
- [21].Malpica A, Deavers MT, Lu K, Bodurka DC, Atkinson EN, Gershenson DM, et al. Grading ovarian serous carcinoma using a two-tier system. Am J Surg Pathol. 2004;28:496–504. doi: 10.1097/00000478-200404000-00009. [DOI] [PubMed] [Google Scholar]
- [22].Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y, et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–6. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Naylor MS, Stamp GW, Foulkes WD, Eccles D, Balkwill FR. Tumor necrosis factor and its receptors in human ovarian cancer. Potential role in disease progression. J Clin Invest. 1993;91:2194–206. doi: 10.1172/JCI116446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wu S, Boyer CM, Whitaker RS, Berchuck A, Wiener JR, Weinberg JB, et al. Tumor necrosis factor alpha as an autocrine and paracrine growth factor for ovarian cancer: monokine induction of tumor cell proliferation and tumor necrosis factor alpha expression. Cancer Res. 1993;53:1939–44. [PubMed] [Google Scholar]
- [25].Charles KA, Kulbe H, Soper R, Escorcio-Correia M, Lawrence T, Schultheis A, et al. The tumor-promoting actions of TNF-alpha involve TNFR1 and IL-17 in ovarian cancer in mice and humans. J Clin Invest. 2009;119:3011–23. doi: 10.1172/JCI39065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bamias A, Koutsoukou V, Terpos E, Tsiatas ML, Liakos C, Tsitsilonis O, et al. Correlation of NK T-like CD3+CD56+ cells and CD4+CD25+(hi) regulatory T cells with VEGF and TNFalpha in ascites from advanced ovarian cancer: Association with platinum resistance and prognosis in patients receiving first-line, platinum-based chemotherapy. Gynecol Oncol. 2008;108:421–7. doi: 10.1016/j.ygyno.2007.10.018. [DOI] [PubMed] [Google Scholar]
- [27].Taniguchi K, Karin M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin Immunol. 2014;26:54–74. doi: 10.1016/j.smim.2014.01.001. [DOI] [PubMed] [Google Scholar]
- [28].Fisher DT, Chen Q, Skitzki JJ, Muhitch JB, Zhou L, Appenheimer MM, et al. IL-6 trans-signaling licenses mouse and human tumor microvascular gateways for trafficking of cytotoxic T cells. J Clin Invest. 2011;121:3846–59. doi: 10.1172/JCI44952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Plante M, Rubin SC, Wong GY, Federici MG, Finstad CL, Gastl GA. Interleukin-6 level in serum and ascites as a prognostic factor in patients with epithelial ovarian cancer. Cancer. 1994;73:1882–8. doi: 10.1002/1097-0142(19940401)73:7<1882::aid-cncr2820730718>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- [30].Lo CW, Chen MW, Hsiao M, Wang S, Chen CA, Hsiao SM, et al. IL-6 trans-signaling in formation and progression of malignant ascites in ovarian cancer. Cancer Res. 2011;71:424–34. doi: 10.1158/0008-5472.CAN-10-1496. [DOI] [PubMed] [Google Scholar]
- [31].Stone RL, Nick AM, McNeish IA, Balkwill F, Han HD, Bottsford-Miller J, et al. Paraneoplastic thrombocytosis in ovarian cancer. N Engl J Med. 2012;366:610–8. doi: 10.1056/NEJMoa1110352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Aggarwal BB, Gupta SC, Kim JH. Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood. 2012;119:651–65. doi: 10.1182/blood-2011-04-325225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lutticken C, Wegenka UM, Yuan J, Buschmann J, Schindler C, Ziemiecki A, et al. Association of transcription factor APRF and protein kinase Jak1 with the interleukin-6 signal transducer gp130. Science. 1994;263:89–92. doi: 10.1126/science.8272872. [DOI] [PubMed] [Google Scholar]
- [34].Zhong Z, Wen Z, Darnell JE., Jr Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264:95–8. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- [35].Abubaker K, Luwor RB, Zhu H, McNally O, Quinn MA, Burns CJ, et al. Inhibition of the JAK2/STAT3 pathway in ovarian cancer results in the loss of cancer stem cell-like characteristics and a reduced tumor burden. BMC Cancer. 2014;14:317. doi: 10.1186/1471-2407-14-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Farren MR, Carlson LM, Netherby CS, Lindner I, Li PK, Gabrilovich DI, et al. Tumor-induced STAT3 signaling in myeloid cells impairs dendritic cell generation by decreasing PKCbetaII abundance. Sci Signal. 2014;7:ra16. doi: 10.1126/scisignal.2004656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Waight JD, Netherby C, Hensen ML, Miller A, Hu Q, Liu S, et al. Myeloid-derived suppressor cell development is regulated by a STAT/IRF-8 axis. J Clin Invest. 2013;123:4464–78. doi: 10.1172/JCI68189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Vasquez-Dunddel D, Pan F, Zeng Q, Gorbounov M, Albesiano E, Fu J, et al. STAT3 regulates arginase-I in myeloid-derived suppressor cells from cancer patients. J Clin Invest. 2013;123:1580–9. doi: 10.1172/JCI60083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Mace TA, Ameen Z, Collins A, Wojcik S, Mair M, Young GS, et al. Pancreatic cancer-associated stellate cells promote differentiation of myeloid-derived suppressor cells in a STAT3-dependent manner. Cancer Res. 2013;73:3007–18. doi: 10.1158/0008-5472.CAN-12-4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].De Simone V, Franze E, Ronchetti G, Colantoni A, Fantini MC, Di Fusco D, et al. Th17-type cytokines, IL-6 and TNF-alpha synergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth. Oncogene. 2014 doi: 10.1038/onc.2014.286. [DOI] [PMC free article] [PubMed] [Google Scholar]