Abstract
The use of d-cycloserine (DCS) as a cognitive enhancer to augment exposure-based cognitive-behavioral therapy (CBT) represents a promising new translational research direction with the goal to accelerate and optimize treatment response for anxiety disorders. Some studies suggest that DCS may not only augment extinction learning but could also facilitate fear memory reconsolidation. Therefore, the effect of DCS may depend on fear levels reported at the end of exposure sessions. This paper presents the rationale and design for an ongoing randomized controlled trial examining the relative efficacy of tailoring DCS administration based on exposure success (i.e. end fear levels) during a 5-session group CBT protocol for social anxiety disorder (n = 156). Specifically, tailored post-session DCS administration will be compared against untailored post-session DCS, untailored pre-session DCS, and pill placebo in terms of reduction in social anxiety symptoms and responder status. In addition, a subset of participants (n = 96) will undergo a fear extinction retention experiment prior to the clinical trial in which they will be randomly assigned to receive either DCS or placebo prior to extinguishing a conditioned fear. The results from this experimental paradigm will clarify the mechanism of the effects of DCS on exposure procedures. This study aims to serve as the first step toward developing an algorithm for the personalized use of DCS during CBT for social anxiety disorder, with the ultimate goal of optimizing treatment outcome for anxiety disorders.
ClinicalTrials.gov identifier: NCT02066792
Keywords: cognitive-behavioral therapy, exposure therapy, d-cycloserine, social anxiety disorder, social phobia
Whereas ample evidence supports the efficacy of cognitive behavioral therapy (CBT) for anxiety disorders, response rates leave considerable room for improvement.1 Attempts to improve treatment response with combined CBT and anxiolytic pharmacotherapy have led to disappointing results.2,3 One promising strategy for strengthening the effects of CBT in recent years has been the use pharmacological agents that augment core learning processes in CBT. One important element in CBT for anxiety disorders is exposure procedures, which rely of extinction learning. Preclinical research has shown that extinction learning is blocked by antagonists at the glutametergic N-methyl-D-aspartate (NMDA) receptor, whereas d-cycloserine (DCS), a partial NMDA agonist, augments such learning in animals (for review, see 4).
In clinical settings, a number of studies have shown that DCS also appears to augment the effects of exposure therapy (for review, see 5). In one of the first human trials, patients with social anxiety disorder (SAD) who received 50 mg of DCS one hour prior to each of five exposure sessions had greater reductions in social anxiety symptoms at post-treatment and one-month follow-up than those who received exposure therapy plus pill placebo.6 These findings were replicated by Guastella and colleagues.7 Similarly promising results were also reported in the treatment of height phobia8 and panic disorder.9 In contrast, a number of studies have found minimal to no benefits for DCS augmentation of exposure procedures for various clinical and subclinical anxiety problems.10–14 In the largest DCS trial to date (n = 169),12 patients with SAD showed a significantly faster rate of symptom improvement than those receiving placebo, but no differences were observed in the response or remission rates at post-treatment and 6-month follow-up. In addition to within-session changes, between session fear reduction also appears to be of importance, as shown in a recent trial with 156 Iraq and Afghanistan veterans.15 After six sessions of virtual reality exposure therapy, participants showed a greater reduction in PTSD symptoms with d-cycloserine augmentation when extinction learning was well achieved between sessions, which was quantified as the average decrease in peak subjective distress ratings across successive exposure sessions. This effect was not observed when the exposure sessions were combined with alprazolam or placebo.
One possible explanation for these inconsistent findings is related to the extent to which extinction learning has occurred. Consistent with this hypothesis are the results of a re-analysis of the study by Hofmann and colleagues.12 The results demonstrated that relative to placebo, patients receiving DCS showed greater symptom improvement at each session when self-reported fear levels were low at the end of the prior exposure session.16 Conversely, patients who received DCS and whose end fear levels were high at the prior session showed less symptom improvement than those taking placebo. At post-treatment, patients receiving DCS whose average fear level at the end of each exposure was low to moderate showed superior outcome to those receiving placebo. Similar results were found in another trial with height phobic patients in which DCS was administered post-session.17
It may then be best to selectively administer DCS to patients only after exposures in which end fear levels are low, as DCS can make “good” exposures better and “bad” exposure worse.18 This is based on findings from animal studies suggesting that NMDA antagonists impair reconsolidation of fear memories, whereas DCS appears to enhance reconsolidation of fear memory. 19 A critical condition determining whether DCS augments extinction learning or reconsolidation appears to be the length of memory reactivation and extinction training sessions. If the extinction session (and the period of stimulus re-exposure) is brief, reconsolidation processes are dominant, whereas extinction processes dominate in longer sessions. 19 Therefore, if fear does not sufficiently decrease during exposure therapy, fear memory reconsolidation may occur and DCS can facilitate this counter-therapeutic process. To directly test the hypothesis that the success of DCS depends on the level of fear experienced at the end an exposure, the present study will examine the effect of selectively administering DCS to patients after exposures in which end fear levels are low.
Methods
Study Design and Objectives
This study is funded as a multi-site linked R34 grant (R34MH099318; ClinicalTrials.gov identifier: NCT02066792) by the National Institute of Mental Health (Principal Investigators: Stefan G. Hofmann, Mark H. Pollack, and Jasper A. J. Smits). The primary objective of this study is to optimize the application of DCS as an augmentation strategy for exposure therapy. To this end, we will examine the relative efficacy of tailoring post-session administration of DCS based on end fear levels during a five-session exposure therapy protocol for SAD. Tailored post-session DCS administration will be compared to the most common application of DCS in prior research, which is unselective DCS administration before exposure sessions, as well as unselective post-session DCS administration in order to determine whether post-session DCS administration needs to be tailored. Because results of previous trials comparing DCS to placebo have been variable, we will also include a placebo condition. We hypothesize that selectively administering DCS when fear levels are low at the end of exposures will lead to greater reductions in social anxiety symptoms at post-treatment and follow-up compared to placebo, unselective pre-session DCS administration, and unselective post-session DCS administration.
A secondary aim of this study is to examine whether DCS enhances fear extinction retention in a laboratory setting. Doing so will allow us to make inferences about the mechanism of action through which DCS enhances outcomes (i.e. whether its effects are due to increased fear extinction retention), and to better interpret any possible null results of the clinical trial (e.g. DCS may work in an experimental paradigm but not in a clinical setting). To examine the effect of DCS on fear extinction retention, a subset of participants will complete a computerized protocol involving a fear conditioning procedure, fear extinction training, and fear extinction retention testing on three separate days, while receiving DCS or pill placebo prior to extinction training. We hypothesize that participants receiving DCS will show greater fear extinction retention in the laboratory experiment than those receiving placebo.
Participants
Our sample will consist of 156 participants for the clinical trial portion of the study, with a subset of 96 participants completing the optional laboratory-based fear extinction paradigm. Recruitment, which began January 2015 and is expected to continue through the end of 2017, will be split equally amongst the three study sites: Boston University, University of Texas at Austin, and Rush University Medical Center in Chicago. Inclusion criteria include (1) a primary diagnosis of SAD as defined by DSM-5 criteria.20 (2) A total score > 60 on the Liebowitz Social Anxiety Scale (LSAS).21 (3) At least 18 years of age. (4) A physical examination and laboratory findings without clinically significant abnormalities. (5) Willingness and ability to provide informed consent and comply with the requirements of the study protocol.
Exclusion criteria include: (1) A lifetime history of bipolar or psychotic disorders or obsessive-compulsive disorder; an eating disorder, PTSD, or substance abuse or dependence (other than nicotine) in the past 6 months; organic brain syndrome, mental retardation or other potentially interfering cognitive dysfunction. (2) Significant suicidal ideation or suicidal behaviors within 6 months prior to intake. (3) Concurrent psychotropic medication (e.g., antidepressants, anxiolytics, beta blockers) within the 2 weeks prior to initiation of treatment. (4) Significant personality dysfunction likely to interfere with study participation (e.g., being overly aggressive or abusive towards the therapists or group members). (5) Serious medical illness or instability for which hospitalization may be likely within the next year. (6) A history of seizures. (7) Pregnant women, lactating women, and women of childbearing potential who are not using medically accepted forms of contraception. (8) Concurrent psychotherapy initiated within 3 months of baseline, or ongoing psychotherapy of any duration directed specifically toward treatment of the SAD. (9) Prior non-response to adequately-delivered exposure therapy. (10) A history of head trauma causing loss of consciousness, seizure or ongoing cognitive impairment.
Screening and Randomization
Individuals interested in the study will undergo a psychiatric evaluation consisting of a diagnostic clinical interview and an administration of the LSAS to evaluate psychiatric inclusion and exclusion criteria. Participants will also be medically cleared by a study physician.
Participants in the optional fear extinction retention experiment will be randomized to receive either DCS or placebo prior to fear extinction procedures. For the clinical trial portion of the study, participants will be randomized to one of four conditions: 1) tailored post-session DCS, 2) unselective pre-session DCS, 3) unselective post-session DCS, or 4) pill placebo. The conditions of the fear extinction experiment will be equally distributed across the four clinical trial conditions such that participants will be equally likely to receive any combination of fear extinction condition and trial condition. Randomization will occur by site using variable-sized permuted block randomization (block sizes varying from 4 to 12). Randomization tables will be created before the first subject is run and sent to the study pharmacist to make the appropriate medication kits. Prior to data analysis, the project statistician will check the balance of randomization to control for any unbalanced factors.
Fear Extinction Retention Experiment
The fear extinction retention experiment will occur on three separate days prior to the initiation of therapy. Participants who partake in this portion of the study will undergo conditioning procedures on Day 1, extinction learning on Day 2, and extinction recall and fear renewal on Day 3 (see figure 1). Day 1 and Day 3 sessions will take place no more than seven days apart, and ideally sessions will occur on consecutive days. DCS or placebo will be administered immediately prior to the Day 2 procedures to examine whether DCS enhances extinction recall and reduces fear renewal on Day 3.
Figure 1.
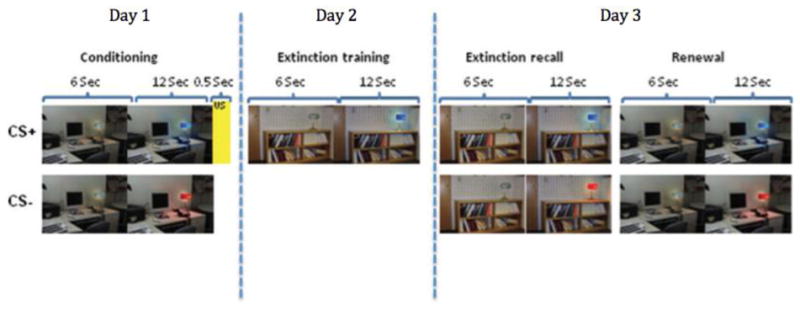
Schematic depiction of the fear extinction retention paradigm. In each phase, participants view a computer monitor with an image of a room containing an unlit lamp for 6 seconds. The lamp then lights up to one of two colors for 12 seconds. During conditioning, one color of the lamp (CS+) is paired with a 500 ms electric shock in context 1. During extinction training, the CS+ is presented in context 2 without the US, as is the CS- (not shown). This procedure is repeated in context 2 during extinction recall, and in context 1 during renewal.
The procedures for this experiment are based off a previously validated fear conditioning and extinction paradigm.22 During each portion of the experiment, recording electrodes will be attached to the palm of the participant’s left hand to measure skin conductance response (SCR), and stimulating electrodes will be connected to two fingers of the participant’s right hand to deliver an electric shock. SCR will be measured through a 9-mm (sensor diameter) Sensor Medics Ag/AgCl electrodes. For each trial, participants will view a computer monitor with an image of one of two different rooms containing an unlit lamp for 6 seconds. The lamp will then be “switched on” to one of two colors for 12 seconds, which will serve as the conditioned stimulus (CS). One of the two colors will be paired with an electric shock (the unconditioned stimulus, or US), while the other color will not. The shock will have been previously selected by the participant to be “highly annoying but not painful” and delivered for 500 millisecond through electrodes attached to the second and third finger of the right hand. The inter-trial interval will last between 12 and 18 seconds, with an average of 15 seconds. The stimulating electrodes will remain attached to the fingertips throughout all three days of the experiment, but the US will be administered only during the Conditioning session on Day 1.
On Day 1, the to-be CS+ and the to-be CS− (four trials of each) will be presented within each room in a counterbalanced manner without presentation of the US (Habituation phase). The Conditioning phase will follow with five CS+ trials that will be immediately followed by the US (100% reinforcement), and five CS− trials (i.e., not followed by shock). All conditioning trials will use the same context (one of the two rooms). On Day 2 (Extinction phase), five CS+ trials and five CS− trials will be presented within the extinction context (the room not used during conditioning) with no US presentation. Day 3 consists of the Extinction Recall phase and the Renewal phase. Extinction Recall is identical to the Extinction phase on day 2, while Renewal is identical to the Conditioning phase except without presentation of the US. Upon completion of each day of the fear extinction retention experiment participants will be compensated $40 (i.e., $120 total).
For each trial, SCR will be calculated by taking the difference between the highest skin conductance level (SCL) during each 12-second CS+/CS− presentation and the mean SCL during the 2 seconds immediately preceding that trial. The SCR for the CS− will be subtracted from the SCR for the CS+ to create a differential SCR. Performance during Extinction Training and Recall will be assessed by comparing SCR during CS+ trials on Day 2 and 3 with the maximum SCR during the Conditioning phase. Our main outcome of interest will be Extinction Retention, for which we will calculate an extinction retention index based on the ratio of the mean SCR during the first two trials of the Extinction Recall phase to the largest SCR during the Day 1 Conditioning phase. This ratio will be multiplied by 100 to yield a percentage of the maximum conditioned response, and this value will be subtracted from 100% to yield the extinction retention index.
Therapy Procedures
Consistent with prior clinical trials examining DCS for SAD,6,7 we will use a 5-session group CBT protocol emphasizing repeated exposure practice. Each group will consist of 4–6 patients and 2 therapists, who will be PhDs or advanced, trained doctoral students supervised by the first and fifth authors. In the first session (60 minutes), patients will be introduced to the cognitive-behavioral model of SAD and provided a rationale for treatment with exposure therapy. Sessions 2–5 (90 minutes each) will consist of increasingly difficult public speaking exposure tasks in which each participant in the group will give an impromptu speech on a topic chosen by the therapist in front of the other group members, confederates and a video camera. Video recordings of each exposure will then be reviewed, and participants will compare pre-exposure predictions about their behavior and appearance during the speech task with their actual performance. At the conclusion of each exposure session, patients will be encouraged to continue to apply home-practice strategies (such as giving speeches in front of a mirror). Continued practice of the interventions will be considered part of treatment, and patients will be asked to refrain from alternative treatment for four weeks following completion of the last treatment session.
Pill Administration
Following prior research demonstrating DCS to augment the efficacy of exposure therapy,6–9 DCS will be administered in 50 mg doses. Administration of DCS at such a dose has been shown to have a benign side effect profile,23,24 and increasing the dose to as high as 500 mg has not been shown to produce an additive effect in enhancing exposure-based treatment.8 Study capsules containing DCS and placebo will be identical in appearance, and will be administered by study staff blind to study condition and not involved in the assessment or treatment of study participants. Participants who elect to undergo the fear extinction retention experiment will take one study pill of either 50 mg DCS or placebo immediately before Day 2 of the experiment.
For the clinical trial, the pharmacist will fill three bottles (with one pill type in each) for each patient for each exposure session according to the schedule in Table 1. Patient, therapist, and a staff member will be blind to the drug condition (DSC vs. placebo) at pre-exposure; and therapists and patients will be blind to the drug condition at post-exposure. Regardless of study condition, research staff will administer a pill (Pill 1) to each participant one-hour before the exposure session and administer a second pill (Pill 2) immediately after the exposure session. In the tailored administration condition, the selection of Pill 2 will be guided by the end fear level. Specifically, DCS will be administered by the research staff if the end fear is ≤40 (of 100) on the Subjective Units of Distress Scale (SUDS), whereas placebo will be selected if the end fear is >40. A cutoff of 40 and below was selected based on results of our prior clinical trial, which demonstrated that when end SUDS ratings were below 47, DCS showed an advantage over placebo.16 Two prior studies using the same 5-session CBT protocol found that mean end SUDS ratings were 49.5 (SD = 19.2)12 and 39.1 (SD = 17.7).25 In the latter study, 85% of patients had at least one end SUDS rating of 40 or below, and 67.5% had at least 2 end SUDS ratings of 40 or below, demonstrating that there is sufficient variability in end fear ratings to justify the examination of tailored and non-tailored post-session DCS administration.
Table 1.
Pill administration schedule
| Condition | Pre-exposure (1st Pill ) | Post-exposure (2nd Pill) | |
|---|---|---|---|
|
| |||
| If SUDS ≤ 40 | If SUDS > 40 | ||
| 1. Pre-session DCS | DCS | PBO | PBO |
| 2. Placebo | PBO | PBO | PBO |
| 3. Non-tailored post-session DCS | PBO | DCS | DCS |
| 4. Tailored administration | PBO | DCS | PBO |
Note: The Table shows the pill administration schedule. All patients receive 2 pills, one before and one after the exposure session. Depending on experimental condition and SUDS at end of exposure, patients receive one of 4 pill combinations. SUDS: Subjective Units of Distress Scale (rated 1–100); PBO: placebo; DCS: D-cylcoserine (50 mg).
Primary Outcome Measures
The timing of the administration of assessments is detailed in Table 2. Clinician-administered instruments (LSAS, SPD-SC Form and MADRS) will be delivered by trained independent evaluators who are blind to treatment condition.
Table 2.
Schedule of Assessments
| Measures | Screening | Baseline | Each Session | Post-treatment | 1 mo. Follow-up | 3 mo. Follow-up |
|---|---|---|---|---|---|---|
| SCID or ADIS | X | |||||
| Medical Screening | X | |||||
| Primary Outcomes | ||||||
| LSAS | X | X | X | X | X | X |
| SPD-SC Form | X | X | X | X | X | X |
| Fear Extinction Paradigm (optional) | Xa | |||||
| Secondary Outcomes | ||||||
| MADRS | X | X | X | X | X | X |
| Q-LES-Q | X | X | X | X | ||
| ASC | X | X | X | X | X | |
| SAFE | X | X | X | X | X | |
| SAQ | X | X | X | X | ||
| Exposure Success | ||||||
| SUDS | Xb | |||||
| Heart Rate | Xb | |||||
| Salivary Cortisol and Alpha Amylase | Xb | |||||
| Moderators and Predictors | ||||||
| Demographics | X | |||||
| NEO-FFI | X | |||||
| PSQI | X | |||||
| CSD | Xc | |||||
| Audio-recorded speech | Xd | |||||
| CEQ | Xe | |||||
| Adherence | X | |||||
Note: ADIS = Anxiety Disorder Interview Schedule; SCID = Structured Clinical Interview for DSM Disorders; LSAS = Liebowitz Social Anxiety Scale; SPD-SC Form = Social Phobic Disorders Severity and Change Form; ASC = Appraisal of Social Concerns; SAFE = Subtle Avoidance Frequency Examination; SAQ = Social Anxiety Questionnaire for Adults; MADRS = Montgomery Asberg Depression Rating Scale; Q-LES-Q = Quality of Life Enjoyment and Satisfaction Questionnaire; PSQI = Pittsburgh Sleep Quality Index; CSD = Consensus Sleep Diary. SUDS = Subjective Units of Distress Scale; NEO-FFI = NEO Five Factor Inventory of Personality; CEQ = Credibility and Expectancy Questionnaire.
The fear extinction experiment will be scheduled after participants are deemed eligible from the ADIS/SCID and medical examination.
Indicators of exposure success are measured during sessions 2–5 only, and salivary cortisol and alpha amylase samples will be collected at sessions 2 and 5 only.
Subjects will complete the CSD the night before and the night after each session.
Recordings of speeches from session 2 only will be saved for further analysis.
The CEQ will be administered after the first session only.
Liebowitz Social Anxiety Scale (LSAS)
The Liebowitz Social Anxiety Scale21 is a 24-item clinician-administered scale that assesses fear and avoidance in social and performance situations within the last week. Fear and avoidance are rated separately on a scale ranging from 0 (no fear/never avoids) to 3 (severe fear/usually avoids), and ratings are combined to form a total score (range 0 – 144). The instrument is widely used in social anxiety treatment studies and shows very good psychometric properties.26,27
Social Phobic Disorders Severity and Change Form (SPD-SC Form)
The Social Phobic Disorders Severity and Change Form28 is an expansion and adaptation of the Clinical Global Impression Scale (CGI)29 for social anxiety disorder. Similar to the original CGI scale, the SPD-SC Form is rated on a 7-point scale to indicate severity and improvement. We chose this scale over the original CGI scale because it provides a more detailed analysis of psychological functioning for individuals with SAD. Furthermore, past social anxiety treatment studies12,30 have used the SPD-SC Form rather than the CGI.
Secondary Outcome Measures
Appraisal of Social Concerns (ASC)
The Appraisal of Social Concerns measure31 is a 20-item self-report questionnaire that assesses concern regarding potential outcomes of social situations that are commonly feared among socially anxious individuals. The types of concerns measured by the ASC load on to three factors: negative evaluation (e.g. “appearing stupid”), observable symptoms (e.g. “trembling”) and social helplessness (e.g. “people ignoring you”). Each potential outcome is rated in terms of amount of concern on a 0–100 scale (in increments of 10) with the following anchors: 0=Not at all concerned, 20=Mildly concerned, 50=Moderately concerned, 80=Very concerned, and 100=Extremely concerned. Data from both clinical and nonclinical samples have indicated that the ASC has very good psychometric properties.31
Subtle Avoidance Frequency Examination (SAFE)
The Subtle Avoidance Frequency Examination32 measures safety behaviors that people with social anxiety typically engage in as a way of diminishing anxiety. The SAFE is a 32-item self-report scored on a Likert scale from 1 to 5, with a score of 1 indicating that the individual “Never” engages in the behavior and a score of 5 indicating that the individual “Always” engages in the behavior. This measure has three factors: active safety behaviors (e.g. “rehearsing sentences in your mind”), subtle restriction of behavior (e.g. “remain silent”), and behaviors aimed at avoiding or concealing physical symptoms (e.g. “say ‘it’s hot’ to explain sweating or blushing”). The SAFE has been shown to have excellent psychometric properties, the ability to distinguish between clinical and nonclinical populations, and is able to successfully assess treatment outcome.32
Social Anxiety Questionnaire for Adults (SAQ)
The Social Anxiety Questionnaire for Adults33 is a 30-item self-report measure that assesses the level of unease, stress, or nervousness experienced during different social situations. The SAQ assesses five dimensions of social anxiety that are considered not well addressed in extant measures of social anxiety. These dimensions are 1) interactions with strangers, 2) speaking in public/talking with people in authority, 3) interactions with the opposite sex, 4) criticism and embarrassment, and 5) assertive expression of annoyance, disgust, or displeasure. Items are rated on a 5-point Likert scale ranging from 1 (no unease, stress, or nervousness) to 5 (very high or extreme unease, stress, or nervousness). The measure demonstrates excellent psychometric properties.33
Montgomery Asberg Depression Rating Scale (MADRS)
The Montgomery Asberg Depression Rating Scale34 is a clinician-administered measure designed to assess overall severity of depressive symptoms. The MADRS consists of 10 symptoms of depression rated on a scale from 0 to 6, with anchors specific to each item. It has demonstrated good reliability, specificity for symptoms of depressive compared to anxiety, and sensitivity to change during treatment.34 The MADRS will be used to assess depression both as a secondary outcome and as a potential treatment moderator.
Quality of Life Enjoyment and Satisfaction Questionnaire (Q-LES-Q)
The Quality of Life Enjoyment and Satisfaction Questionnaire35 is a questionnaire rating 16 aspects of quality of life, including physical health, mood, activities of daily living, and overall life satisfaction. The Q-LES-Q has been shown to be a valid and reliable instrument that measures a construct related to but distinct from severity of illness.35
Measures of Exposures Success
Subjective Units of Distress Scale (SUDS)
Participants will provide fear ratings at the beginning of an exposure exercise (i.e., Beginning Fear) and just prior to the conclusion of an exposure exercise (i.e., End Fear). In addition, after the exercise they will indicate their highest level of fear experienced during exposure (i.e., Peak Fear). Fear ratings will be assessed using the subjective units of distress scale (SUDS),36 which ranges from 0 to 100 (0=no fear, relaxed; 25=mild fear, able to cope; 50=moderate fear, trouble concentrating; 75=severe fear, thoughts of leaving; 100=very severe fear, worst ever experienced). The procedures for collecting fear ratings will be similar to that in previous social anxiety disorder treatment studies from our group and other groups.16,37–40 Specifically, during the first session, therapists will introduce patients to the SUDS as they work together to develop a fear and avoidance hierarchy. Attention will be given to the anchors such that patients can distinguish the different levels along the scale. Accordingly, by the time patients initiate exposure practice (i.e., session 2), they will have had had ample practice using the scale. We will use a fear rating at the end of exposure of ≤40 as an index of exposure success (see Table 1).
Heart Rate
In addition to providing SUDS ratings, participants will also wear a Polar heart rate monitoring watch and chest band during the exposure exercise during sessions 2 through 5 as a secondary, physiological measure of anxiety within the session. A sweatband will be placed over the watch so that participants will not see their heart rate during the exposure (as this could be a distraction). Maximum and average heart rate will be recorded over the course of the exposure, and compared to baseline heart rate at the start of the session.
Salivary Samples
Saliva will be collected and assayed for salivary cortisol (ng/mL) and salivary alpha amylase (U/ml) using a highly sensitive enzyme immunoassay. Patients will be asked not to brush their teeth, eat, drink or smoke 30 minutes prior to collection of saliva samples. Saliva samples of 0.5ml will be collected prior to and directly after the first and last exposure tasks, sessions two and five respectively. The post-exposure amylase salivary samples will be collected within five minutes of the end of the exposure, while the post-exposure cortisol level will be collected twenty minutes post exposure. Saliva samples will be frozen at −80°C and be sent frozen to a central processing laboratory at the conclusion of enrollment. We will use cortisol and alpha amylase levels as additional indicators of the stress response to exposure. Specifically we will investigate the relationship between these indicators and heart rate and SUDS levels, and as a moderator of the relationship between DCS and treatment outcome.
Potential Moderators and Predictors
Pittsburgh Sleep Quality Index (PSQI)
The PSQI is a well-validated self-report instrument that assesses sleep quality over the past month.41 The measure consists of seven items rated on a 4-point scale: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction. These items are summed to create a global PSQI score with higher scores reflecting poorer sleep quality (range 0–21). The use of the PSQI at baseline is based on prior research that found poorer baseline sleep to predict worse treatment outcome during CBT for SAD.42
Consensus Sleep Diary (CSD)
The Consensus Sleep Diary was developed amongst a collaboration of insomnia experts in order to create a standardized assessment of nightly sleep quality.43 The core CSD contains 9 consensus-determined critical parameters of sleep related to the time of day one goes to bed and gets up, and the duration and quality of sleep. The CSD will be administered the night before and after each session based on researching showing that that higher levels of restorative sleep the night following an exposure session predicted lower social anxiety symptoms at the next session.42 Post-session sleep may then affect the consolidation of in-session learning, and will be explored as a moderator of the relationship between in-session learning (i.e. fear reduction) and treatment outcome.
NEO-Five-Factor Inventory
At the baseline session, participants will complete the 60-item NEO-Five-Factor Inventory (NEO-FFI),44 which is a psychometrically-sound measure of the five traits from the Five-Factor model of personality: agreeableness, conscientiousness, extraversion, neuroticism, and openness. Using the NEO-FFI will enable this study to potentially replicate prior research that found DCS augmentation to be more effective in SAD for individuals who score low in conscientiousness and high in agreeableness.38
Analysis of audio-recorded speech
The speech delivered in session 2 (the first exposure session) will be audio recorded and saved for later analysis (including analysis of vocal tone and content). For vocal analyses, these recordings will be analyzed using methodology similar to that used in recent studies,45 which involves taking selections of speech and using an audio analyzer to examine audio properties such as pitch, frequency, and silences. The speeches will also be transcribed for content analysis using a software program called the Linguistic Inquiry and Word Count (LIWC), which computes percentages of words that fall into various categories.46
Clinical Characteristics
Baseline psychiatric functioning will be assessed by clinician-rated measures. This domain comprises clinical severity as assessed by the Social Phobic Disorders Severity Form; (SPD-SC),28 depressive symptom severity as assessed by the MADRS,34 Axis I comorbidity (i.e., number of comorbid Axis I disorders) as well as history of antidepressant and other psychotropic medication use. There is some evidence to suggest that antidepressants interact with DSC to block its augmentation effect on fear extinction.47 Therefore, consistent with our earlier trial12 we decided to exclude participants with concurrent psychotropic medication use. Our experience with this earlier trial indicates that this exclusion criterion is unlikely to negatively affect recruitment or generalizability of the findings. Comorbidity and medication use will be assessed with the Structured Clinical Interview (SCID)48 or the Anxiety and Related Disorders Interview Schedule for DSM-5 (ADIS-5).49
Credibility and Expectancy
The Credibility/Expectancy Questionnaire (CEQ) is a widely used 6-item measure assesses treatment credibility and expectancy.50 Credibility is thought to be a cognitive assessment of the rationale for treatment, while expectancy is an affectively-based belief about the likelihood of success in treatment. The CEQ has demonstrated high internal consistency within each of the credibility and expectancy and good test-retest reliability.50
Patient Adherence
Patient adherence to each intervention will be assessed as the number of total sessions attended.
Safety Monitoring
A review of medical history, a physical examination, and laboratory tests will be performed at admission, and vital signs measured at the baseline visit. Patients with clinically significant abnormalities in vital signs (e.g., systolic blood pressure >150 mm Hg or diastolic blood pressure<50 mm Hg) at baseline will be excluded from further study participation and referred for appropriate clinical management. Pregnancy tests for all female participants of childbearing potential will be administered at intake and each month following randomization. Patients will be queried at each visit regarding the presence of adverse effects associated with the study medication. On Day 2 of the Fear Extinction Experiment and at each of the four study visits during which participants are asked to take study medication, participants will be asked to breathe into an AlcoBreath tube in order to assess for the presence of alcohol before they are administered the study medication.
Data Analysis
We will assess the equivalence of the treatment groups on key baseline variables (demographics and psychological variables), and any variables that differ among groups will be used as covariates in the final analyses. We will then examine missing data patterns, dropout rates, and distributional properties of measures and use transformations to improve distributions if necessary. We will use pattern mixture modeling to assess the effect of missing data.51 We will rerun our analyses coding for various missing data patterns (no missing data, sporadic missing, dropouts, etc.) to determine 1) if missingness impacts our findings and 2) how the differences between treatment conditions depends on the missing data pattern.
We will use multilevel modeling (MLM) to evaluate the effect of condition on our continuous outcome measures (LSAS, SPD-SC severity, MADRS, QLES-Q) and generalized linear mixed modeling (GLMM, which is MLM with a logistic linking function) to examine DCS’s effect on the dichotomous outcomes (“response” and “remission,” explained below). MLM is the recommended method for analyzing longitudinal psychiatric data,52 easily accommodates missing data, and allows inclusion of all subjects in the analysis even if they drop out. In our MLM analyses, the repeated assessments over time will be nested within individuals, which will be nested within treatment cohort, thereby appropriately accounting for correlated scores within cohorts. We will use a piecewise growth curve model, separately modeling change over time during treatment and follow-up. Although our prior large DCS study12 found each “piece” of the growth curve to be linear, we will test for quadratic trends and include them if significant. Other non-linear models (e.g., exponential models) will be also be tested, and the model that best fits the data (based on AIC and BIC) will be used. Our “time” variable in these models will be coded as “assessment week” and will reflect the number of weeks since baseline. Using this model, we can test differences between tailored DCS administration and the 3 comparison groups (pre-session DCS, post-session DCS, and placebo) by including 3 dummy coded variables as predictors of the growth curve parameters. Each dummy variable will contrast tailored DCS to each of the other 3 conditions.
Definition of Treatment Response and Remission
The definition of treatment “response” is based on the SPD-SC and is defined by an overall change score of 2 (much improved) or 1 (very much improved) as compared to the pre-treatment assessment. “Remission” is defined as an SPD-SC of 2 or 1 and an LSAS total score of < 30. This LSAS cutoff score is supported by a study of 364 patients that used receiver-operating characteristics in diagnosing SAD,53 and has generally been adopted as the boundary between remitted and symptomatic patients.54 It should be noted that the goal of this study is to determine effect size estimates for these gold-standard response and remission outcomes. Detecting differences on these dichotomous outcomes would require a very large sample size, and thus these estimates will be used to evaluate whether a larger-scale trial is warranted.
Outcome Analysis
In order to evaluate whether tailored DCS administration produces superior outcomes to pre-session DCS, post-session DCS, and placebo, we will examine if the dummy variable contrasting tailored DCS to the relevant comparison condition is a significant predictor of the intercept. We will alternately “center” the “assessment week” variable at either posttreatment, 1-mo FU, or 3-mo FU to test for significant differences between conditions at each of these time points.55
We will also examine a number of possible moderators (demographic, clinical, and personality variables) of the potential superiority of tailored DCS over the comparison conditions (and, secondarily, of the superiority of pre-and post-DCS administration over PBO). We will use the Fournier approach56 to identify important moderators. This approach uses an algorithmic method to select significant predictors and moderators within each group of potential moderators (the groups being demographics, clinical characteristics, personality variables). These selected moderators are then combined in a final analysis, which identifies moderators that are significant over and above the other potential moderators. This approach strikes a balance between testing each moderator separately (which substantially increases Type I error due to the multiple tests) and testing all moderators simultaneously (which may substantially increase the likelihood of Type II error). We have successfully used this approach to examining the moderators of pre-session administration of DCS.38
Power analysis
As mentioned above, the primary goals of this study are to 1) determine the efficacy of tailored DCS administration and 2) determine whether a larger scale trial is warranted. As a result, this study is not powered to detect small differences between treatment conditions, nor is it powered to detect differences on our dichotomous outcomes. Accordingly, we performed power analyses only for our primary outcomes, which were continuous measures of LSAS and SPD-SC. MLM allows the inclusion of all subjects with at least one data point, regardless of missing data and regardless of whether they drop out. Thus, we based our power analysis on 156 participants and conservatively assumed that, on average, we will obtain 5 out of the 7 total assessments from each subject. The power analyses were calculated using the program PinT 2.12 (Power in Two Level Models).57
For our main outcome analysis, we used our similarly sized DCS trial12 to estimate the variances and covariances required by PinT, and calculated detectable effect sizes for both LSAS and SPD-SC. PinT indicated we would have a power of .80 to detect an effect size as low as d=.296, between a small (d=.20) and a medium (d=.50) effect size. This translates into a difference of 6.59 points on the LSAS and .42 points on the SPD-SC (at post-treatment, 1-month follow-up, or 3-month follow-up). For the Fournier moderator analysis, we assumed five simultaneous moderators in each group, and included their 15 interactions with the 3 dummy variable contrasts. Moderators were modeled as predictors of both the intercept and slope. Again using our prior DCS study to calculate the variances and covariances required by PiNT, we found that, with .80 power, we could detect an effect size of d=.466, slightly smaller than a medium effect size.
Discussion
This study aims to serve as the first step toward developing an algorithm for personalizing the use of DCS in CBT for anxiety, with the ultimate goal of maximizing the efficacy and cost-effectiveness of the treatment of anxiety disorders. Although prior clinical trials examining DCS as an augmentation strategy for exposure therapy have shown mixed results,6,12 recent findings suggest that the effect of DCS depends on the success of exposure sessions.15,16 Following such research, as well as evidence suggesting that DCS might facilitate fear memory reconsolidation if fear does not sufficiently decrease during exposure therapy,19 the present study will test the relative efficacy of tailoring DCS administration based on end fear levels as a strategy for optimizing treatment outcome. Specifically, we will compare tailored post-session DCS administration with untailored pre-session DCS administration, untailored post-session DCS administration, and pill placebo in the context of a 5-session CBT protocol for SAD. The results from this trial will add to the body of research examining the extent to which DCS can enhance exposure-based CBT for anxiety, and also illuminate the optimal use of DCS in the context of CBT.
Beyond examining the efficacy of different DCS administration strategies, this study will contribute to our understanding of the use of the DCS and CBT for anxiety disorders in a number of additional ways. For one, we will use an experimental fear extinction retention paradigm to help clarify the mechanism through which DCS might enhance treatment outcome in this study. By testing the effect of DCS on fear extinction retention in a laboratory setting with a subset of participants in the clinical trial, this study has the potential to make a strong claim that DCS is efficacious in a clinical setting because it enhances fear extinction retention (the purported mechanism). On the other hand, this experiment could also help clarify results if the findings from trial are null, as we will know whether DCS had any impact on extinction retention in the context of laboratory conditioning paradigm. A relative limitation, however, is that clinical fear and de novo conditioned fears in humans may not similarly engage fear networks,58 hence dissociation in results between these paradigms may further clarify the role of de novo fear conditioning as a model for clinical fears.
This study embodies the goals of personalized medicine by examining tailored use of DCS and by investigating a number of potential predictors and moderators of the effect of CBT and DCS on social anxiety. Specifically, the impact of sleep quality on the relationship between in-session learning and symptom reduction will be tested as a way of understanding a potential factor that could interfere with treatment success. Physiological measures including heart rate, salivary cortisol and alpha amylase will also be investigated as potential moderators of the impact of DCS on treatment outcome, and as correlates of subjective distress ratings.
The use of DCS in conjunction with CBT represents an innovative approach toward maximizing psychotherapy treatment outcomes. Rather than directly targeting anxiety symptoms themselves, DCS enhances symptom reduction by augmenting the learning processes involved in exposure therapy. This line of research results from a promising translation of research on fear extinction from preclinical neuroscience to clinical settings, but clinical research on DCS augmentation thus far demonstrates that the application of DCS to clinical settings requires further refinement. The present study aims to test one such refinement by comparing the effects of tailored DCS administration (based on exposure success) with administration strategies used in prior research (untailored pre- and post-session administration). This research is a step toward precision medicine that could lead to an algorithm for personalized DCS administration during exposure therapy, thereby optimizing treatment outcomes for anxiety disorders and significantly reducing the enormous suffering and disability caused by pathological anxiety.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Stefan G. Hofmann, Email: shofmann@bu.edu.
Joseph K. Carpenter, Email: jcarpen@bu.edu.
Michael W. Otto, Email: mwotto@bu.edu.
David Rosenfield, Email: drosenfi@smu.edu.
Jasper A. J. Smits, Email: smits@utexas.edu.
Mark H. Pollack, Email: mark_pollack@rush.edu.
References
- 1.Hofmann SG, Smits JAJ. Cognitive-behavioral therapy for adult anxiety disorders: a meta analysis of randomized placebo-controlled trials. J Clin Psychiatry. 2008;69:621–32. doi: 10.4088/jcp.v69n0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hofmann SG, Sawyer AT, Korte K, Smits JAJ. Is it beneficial to add pharmacotherapy to cognitive-behavioral therapy when treating anxiety disorders? A meta-analytic review. Int J Cogn Ther. 2009;2:160–175. doi: 10.1521/ijct.2009.2.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Otto MW, Smits JAJ, Reese HE. Combined psychotherapy and pharmacotherapy for mood and anxiety disorders in adults: review and analysis. Clin Psychol Sc Pract. 2005;12:72–86. [Google Scholar]
- 4.Myers KM, Davis M. Behavioral and neural analysis of extinction: a review. Neuron. 2002;36:567–584. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- 5.Hofmann SG, Wu QJ, Boettcher H. D-cycloserine as an augmentation strategy for cognitive behavioral therapy of anxiety disorders. Biol Mood Anxiety Disord. 2013;3:11. doi: 10.1186/2045-5380-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hofmann SG, Meuret AE, Smits JAJ, et al. Augmentation of exposure therapy with D-cycloserine for social anxiety disorder. Arch Gen Psychiatry. 2006;63:298–304. doi: 10.1001/archpsyc.63.3.298. [DOI] [PubMed] [Google Scholar]
- 7.Guastella AJ, Richardson R, Lovibond PF, et al. A randomized controlled trial of the effect of d-cycloserine on enhancement of exposure therapy for social anxiety disorder. Biol Psychiatry. 2008;63:544–549. doi: 10.1016/j.biopsych.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 8.Ressler KJ, Rothbaum BO, Tannenbaum L, et al. Cognitive enhancers as adjuncts to psychotherapy: Use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61:1136–44. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- 9.Otto MW, Tolin DF, Simon NM, et al. Efficacy of D-cycloserine for enhancing response to cognitive-behavior therapy for panic disorder. Biol Psychiatry. 2010;67:365–70. doi: 10.1016/j.biopsych.2009.07.036. [DOI] [PubMed] [Google Scholar]
- 10.Guastella AJ, Dadds MR, Lovibond PF, Mitchell P, Richardson R. A randomized controlled trial of the effect of D-cycloserine on exposure therapy for spider fear. J Psychiatr Res. 2007;41:466–71. doi: 10.1016/j.jpsychires.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Guastella AJ, Lovibond PF, Dadds MR, Mitchell P, Richardson R. A randomized controlled trial of the effect of D-cycloserine on extinction and fear conditioning in humans. Behav Res Ther. 2007;45:663–72. doi: 10.1016/j.brat.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Hofmann SG, Smits JAJ, Rosenfield D, et al. D-Cycloserine as an augmentation strategy with cognitive-behavioral therapy for social anxiety disorder. Am J Psychiatry. 2013;170:751–58. doi: 10.1176/appi.ajp.2013.12070974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Litz BT, Salters-Pedneault K, Steenkamp MM, et al. A randomized placebo-controlled trial of d-cycloserine and exposure therapy for posttraumatic stress disorder. J Psychiatr Res. 2012;46:1184–1190. doi: 10.1016/j.jpsychires.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Tart C, Handelsman P, Smits JAJ, et al. Augmentation of exposure therapy with post-session administration of D-cycloserine. J Psychiatr Res. 2013;47:168–174. doi: 10.1016/j.jpsychires.2012.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rothbaum BO, Price M, Jovanovic T, et al. A randomized, double-blind evaluation of D-cycloserine or alprazolam combined with virtual reality exposure therapy for posttraumatic stress disorder in Iraq and Afghanistan War veterans. Am J Psychiatr. 2014;171:640–648. doi: 10.1176/appi.ajp.2014.13121625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smits JAJ, Rosenfield D, Hofmann SG, et al. D-cycloserine enhancement of exposure therapy for social anxiety disorder depends on the success of exposure sessions. J Psychiatr Res. 2013;47:1455–1461. doi: 10.1016/j.jpsychires.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smits JAJ, Rosenfield D, Otto MW, et al. D-Cycloserine enhancement of fear extinction is specific to successful exposure sessions: Evidence from the treatment of height phobia. Biol Psychiatry. 2013;73:1054–58. doi: 10.1016/j.biopsych.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hofmann SG. D-cycloserine for treating anxiety disorders: Making good exposures better and bad exposures worse. Depression and Anxiety. 2014;31:175–177. doi: 10.1002/da.22257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JL, Milton AL, Everitt BJ. Reconsolidation and extinction of conditioned fear: inhibition and potentiation. J Neurosci. 2006;26:10051–10056. doi: 10.1523/JNEUROSCI.2466-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Washington, DC: 2013. [Google Scholar]
- 21.Liebowitz MR. Social phobia. Mod Probl Pharmacopsychiatry. 1987;22:141–173. doi: 10.1159/000414022. [DOI] [PubMed] [Google Scholar]
- 22.Zeidan M, Lebron-Milad K, Milad M, et al. Test–retest reliability during fear acquisition and fear extinction in humans. CNS Neurosci Ther. 2012;18(4):313–317. doi: 10.1111/j.1755-5949.2011.00238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D’Souza DC, Gil R, Cassello K, et al. IV glycine and oral D-cycloserine effects on plasma and CSF amino acids in healthy humans. Biol Psychiatry. 2000;47:450–62. doi: 10.1016/s0006-3223(99)00133-x. [DOI] [PubMed] [Google Scholar]
- 24.van Berckel BN, Lipsch C, Timp S, et al. Behavioral and neuroendocrine effects of the partial NMDA agonist D-cycloserine in healthy subjects. Neuropsychopharmacology. 1997;16:317–24. doi: 10.1016/S0893-133X(96)00196-0. [DOI] [PubMed] [Google Scholar]
- 25.Smits JAJ, Rosenfield D, Powers MB, et al. Yohimbine enhancement of exposure therapy for social anxiety disorder: A randomized controlled trial. Biol Psychiatry. 2014;75:840–846. doi: 10.1016/j.biopsych.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 26.Heimberg RG, Horner K, Liebowitz MR, et al. Psychometric properties of the Liebowitz Social Anxiety Scale. Psychol Med. 1999;29:199–212. doi: 10.1017/s0033291798007879. [DOI] [PubMed] [Google Scholar]
- 27.Safren S, Heimberg RG, Horner K, Juster H, Schneier FR, Liebowitz MR. Factor structure of social fears: The Liebowitz Social Anxiety Scale. J Anxiety Disord. 1999;13:253–270. doi: 10.1016/s0887-6185(99)00003-1. [DOI] [PubMed] [Google Scholar]
- 28.Liebowitz MR, Schneier FR, Campeas R, et al. Phenelzine vs atenolol in social phobia. A placebo controlled comparison. Arch Gen Psychiatry. 1992;49:290–300. doi: 10.1001/archpsyc.49.4.290. [DOI] [PubMed] [Google Scholar]
- 29.Guy W. Clinical global impression. In: Guy W, editor. Manual for the ECDEU Assessment Battery - Revised. National Institute of Mental Health; Rockville, MD: 1970. pp. 218–22. [Google Scholar]
- 30.Heimberg RG, Liebowitz MR, Klein DF, et al. Cognitive behavioral group therapy vs phenelzine therapy for social phobia: 12-week outcome. Arch Gen Psychiatry. 1998;55:1133–1141. doi: 10.1001/archpsyc.55.12.1133. [DOI] [PubMed] [Google Scholar]
- 31.Telch MJ, Lucas RA, Smits JAJ, Powers MB, Heimberg RG, Hart T. Appraisal of social concerns: A cognitive assessment instrument for social phobia. Depress Anxiety. 2004;19:217–224. doi: 10.1002/da.20004. [DOI] [PubMed] [Google Scholar]
- 32.Cuming S, Rapee RM, Kemp N, Abbott MJ, Peters L, Gaston JE. A self-report measure of subtle avoidance and safety behaviors relevant to social anxiety: Development and psychometric properties. J Anxiety Disord. 2009;23:879–883. doi: 10.1016/j.janxdis.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 33.Caballo VE, Arias B, Salazar IC, Irurtia MJ, Hofmann SG CISO-A Research Team. Psychometric properties of an innovative self-report measure: The Social Anxiety Questionnaire for Adults. Psychol Assess. doi: 10.1037/a0038828. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 35.Endicott J, Nee J, Harrison W, Blumenthal R. Quality of Life Enjoyment and Satisfaction Questionnaire: A new measure. Psychopharmacol Bull. 1993;29:321–26. [PubMed] [Google Scholar]
- 36.Wolpe J. Psychotherapy by reciprocal inhibition. Stanford University Press; 1958. [Google Scholar]
- 37.Hayes SA, Hope DA, Heimberg RG. The pattern of subjective anxiety during in-session exposures over the course of cognitive-behavioral therapy for clients with social anxiety disorder. Behav Ther. 2008;39:286–99. doi: 10.1016/j.beth.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smits JAJ, Hofmann SG, Pollack MH, et al. D-cycloserine augmentation of cognitive behavioral group therapy of social anxiety disorder: Prognostic and prescriptive variables. J Consult Clin Psychol. 2013;81:1100–1112. doi: 10.1037/a0034120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smits JAJ, Powers MB, Buxkamper R, Telch MJ. The efficacy of videotape feedback for enhancing the effects of exposure-based treatment for social anxiety disorder: a controlled investigation. Behav Res Ther. 2006;44:1773–85. doi: 10.1016/j.brat.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 40.Smits JAJ, Rosenfield D, McDonald R, Telch MJ. Cognitive mechanisms of social anxiety reduction: an examination of specificity and temporality. J Consult Clin Psychol. 2006;74:1203–12. doi: 10.1037/0022-006X.74.6.1203. [DOI] [PubMed] [Google Scholar]
- 41.Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 42.Zalta A, Dowd S, Pollack MH, et al. Sleep quality predicts treatment outcome in CBT for social anxiety disorder. Depress Anxiety. 2013;30:1114–1120. doi: 10.1002/da.22170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carney CE, Buysse DJ, Morin CM, et al. The Consensus Sleep Diary: Standardizing prospective sleep self-monitoring. Sleep. 2012;35:287–302. doi: 10.5665/sleep.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Costa PT, McCrae RR. The five-factor model of personality and its relevance to personality disorders. J Pers Disord. 1992;6:343–359. [Google Scholar]
- 45.Laukka P, Linnman C, Furmark T, et al. In a nervous voice: Acoustic analysis and perception of anxiety in social phobics’ speech. J Nonverbal Behav. 2008;32:195–214. [Google Scholar]
- 46.Pennebaker JW, Francis ME, Booth RJ. Linguistic Inquiry and Word Count (LIWC): A computerized text analysis program. Mahwah, NJ: 2001. [Google Scholar]
- 47.Andersson E, Hedman E, Enander J, et al. D-Cycloserine vs placebo as adjunct to cognitive behavioral therapy for obsessive-compulsive disorder and interaction with antidepressants: A randomized clinical trial. JAMA Psychiatry. 2015 May 13; doi: 10.1001/jamapsychiatry.2015.0546. [DOI] [PubMed] [Google Scholar]
- 48.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 49.Brown TA, Barlow DH. Client Interview Schedule. Oxford University Press; 2014. Anxiety and Related Disorders Interview Schedule for DSM-5 (ADIS-5L): Lifetime Version. [Google Scholar]
- 50.Devilly GJ, Borkovec TD. Psychometric properties of the credibility/expectancy questionnaire. J Behav Ther Exp Psychiatry. 2000;31:73–86. doi: 10.1016/s0005-7916(00)00012-4. [DOI] [PubMed] [Google Scholar]
- 51.Enders CK. Missing not at random models for latent growth curve analyses. Psychol Methods. 2011;16:1–16. doi: 10.1037/a0022640. [DOI] [PubMed] [Google Scholar]
- 52.Hamer RM, Simpson PM. Last observation carried forward versus mixed models in the analysis of psychiatric clinical trials. Am J Psychiatry. 2009;166:639–41. doi: 10.1176/appi.ajp.2009.09040458. [DOI] [PubMed] [Google Scholar]
- 53.Mennin DS, Fresco DM, Heimberg RG, Schneier FR, Davies SO, Liebowitz MR. Screening for social anxiety disorder in the clinical setting: using the Liebowitz Social Anxiety Scale. J Anxiety Disord. 2002;16:661–73. doi: 10.1016/s0887-6185(02)00134-2. [DOI] [PubMed] [Google Scholar]
- 54.Ballenger JC, Davidson JR, Westenberg HG, et al. Consensus statement on social anxiety disorder from the international consensus group on depression and anxiety. J Clin Psychiatry. 1998;59:54–60. [PubMed] [Google Scholar]
- 55.Singer JD, Willett JB. Applied longitudinal data analysis: Methods for studying change and event occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- 56.Fournier JC, DeRubeis RJ, Shelton RC, Hollon SD, Amsterdam JD, Gallop R. Prediction of response to medication and cognitive therapy in the treatment of moderate to severe depression. J Consult Clin Psychol. 2009;77:775–87. doi: 10.1037/a0015401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Snijders TAB, Bosker RJ. Standard errors and sample sizes for two-level research. J Educ Stat. 1993;18:237–59. [Google Scholar]
- 58.Grillon C. D-cycloserine facilitation of fear extinction and exposure-based therapy might rely on lower-level, automatic mechanisms. Biol Psychiatry. 2009;66:636–41. doi: 10.1016/j.biopsych.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]


