Abstract
Evidence suggests that environmental substances regulating estrogenic pathways during puberty may be detrimental to the developing mammary gland (MG). Manganese (Mn) is a trace mineral required for normal physiological processes. Prepubertal exposure to Mn induces precocious puberty in rats, an event associated with early elevations in puberty-related hormones, including estradiol (E2). However, until now the effect of Mn-induced precocious MG development has not been determined. Therefore, we assessed the ability of prepubertal Mn exposure to advance normal MG development and alter E2 driven pathways involved in tumorigenesis. Sprague Dawley female rats were gavaged daily with either 10 mg/kg manganese chloride (MnCl2) or saline (control) from postnatal day (PND) 12 through PND 30. Blood and MGs were collected on PNDs 30 and 120. Compared to controls, serum E2 levels on PND 30 were elevated (p<0.05) in the Mn-treated group. Mn exposure significantly increased differentiated MG terminal ductal structures and the percentage of MG epithelial cells that stained positive for the proliferative marker, Ki67, at PND 30 (p<0.001) and PND 120 (p < 0.001). Levels of Mn (ppm) were not elevated in these MGs. Mn-treated animals (40%) exhibited reactive stroma and intra-luminal focal hyperplasia in hemotoxylin and eosin stained MGs at PND 120. Furthermore, Mn exposure resulted in elevated protein expression levels of estrogen receptor α, activator protein 2α, phosphorylated (p)-Akt, and p53 in MGs on PND 120, but not on PND 30. Collectively, these data show that exposure to a supplemental dose of Mn causes accelerated pubertal MG growth which can progress to adult hyperplasia; thus, providing evidence that early life Mn exposure may increase susceptibility to breast cancer.
Keywords: Manganese, 17β-estradiol, mammary gland, breast cancer, precocious puberty
Introduction
There is strong evidence that breast cancer incidence is influenced by interactions between the environment and unknown predisposing genes. One of the keys to preventing breast cancer is to identify environmental factors and developmental time points that increase the overall risk for developing this disease in order to eliminate overexposure. With regard to this, adolescence is a critical window of increased susceptibility to environmental influences.1–3 Altering the timing of normal developmental and reproductive events during this time has been shown to have a significant impact on a women's susceptibility to breast cancer. In that, epidemiological studies have demonstrated that early age at menarche is associated with a 10–20% increase in breast cancer risk.4–7 One explanation for the elevated risk due to precocious puberty is prolonged exposure of the breast to estrogens as a result of higher levels of estradiol (E2) during adolescence;8 thus, allowing more E2 to enter the breast tissue resulting in increased breast epithelial growth.9 Therefore, prepubertal exposure to environmental factors that regulate E2 during puberty could have a substantial impact on mammary gland (MG) development and subsequent breast cancer risk.
Manganese (Mn) is a naturally abundant environmental trace mineral that is found in all tissues and is required for normal physiological processes in all animals. In general, it is required for normal amino acid, lipid, protein, and carbohydrate metabolism.10 Furthermore, there are several Mn-dependent enzyme families including oxidoreductases, transferases, hydrolases, lyases, isomerases, and ligases.11 Mn is known to be both beneficial and harmful, but both excesses and deficiencies can cause serious health problems, including reproductive dysfunction12–15 in men and women. Numerous studies support the potential for excess prepubertal Mn exposure in humans making it plausible that the element could influence developmental processes that regulate MG development. Mn is found in a variety of foods and many geographical regions have higher levels of Mn in the drinking water.16 High levels of this element have been found in soy baby formulas leading to elevated serum levels;17–19 thus identifying infants and children as being more sensitive to excess Mn exposure.16,19
The potential for an essential environmental dietary element, such as Mn, to regulate pubertal MG development subsequently leading to increased breast cancer risk is intriguing. The MG is unique because it forms postembryonically, mainly during adolescence, undergoing dynamic pre- and postpubertal developmental changes. This provides a critical window of susceptibility to environmental influences. Recently, we showed that prepubertal exposure to Mn induces precocious puberty in rats via centrally mediated events.20 Specifically, Mn has been shown to precociously activate the Rheb-mTOR pathway leading to increased gonadotropin-releasing hormone (GnRH) gene expression in the preoptic area and rostral hypothalamic area.21 Furthermore, Mn activates the cGMP-PKG pathway stimulating GnRH peptide release from the basal hypothalamus;22 hence, resulting in elevated puberty-related hormones including E2.20,22 During normal pubertal MG development, E2 is the main steroidogenic signal that drives growth and differentiation.23–25 In the normal adult gland, E2 has a limited influence on proliferation, at least in part, due to estrogen receptor α (ERα) degradation in cells that have entered the cell cycle.26 However, in breast cancer, ERα is highly expressed and, as a consequence of E2 stimulation, has been shown to promote growth and invasion of MG epithelial cells expressing ERα.27–30 In this regard, two-thirds of all human breast cancers are ERα positive.31 It has been suggested that centrally mediated precocious puberty resulting in earlier exposure to E2 alters MG cell fate leading to increased responsiveness to the proliferative actions of the hormone in the adult gland. Currently, there is a lack of studies that show atypical growth and altered cell fate in the adult MG as a consequence of environmental influences on centrally mediated endogenous estrogen signaling during puberty. Furthermore, no study to date has investigated whether prepubertal exposure to low levels of Mn can significantly contribute to this process. In the current study, we determined if Mn exposure only during juvenile/peripubertal development could accelerate pubertal MG development (in an estrogen-dependent manor) resulting in noted morphologic and protein changes in the adult MG that are commonly seen in breast cancer.
Material and methods
Animals
Female rats of the Sprague Dawley line raised in our colony were used for these experiments. Females were housed under controlled conditions of temperature (23°C), lighting (lights on: 6:00 h; light off: 18:00 h) and ad libitum access to food and deionized water. The diet (Harlan Teklad 2016 rodent chow; Harlan Laboratories, Indianapolis, IN) contained 91 mg/kg Mn and 197 mg/kg of iron (Fe) as determined by the Heavy Metal Analysis Laboratory, Department of Veterinary Integrative Biosciences, College of Veterinary Medicine, Texas A&M University. All procedures were approved by University Animal Care and Use Committee and in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Experimental protocol
Following normal delivery, litters were culled to 10–12 pups with at least 5–6 females per litter. Half of the female pups from each dam were designated as saline-treated (controls) and the other half Mn-treated. On postnatal day (PND) 12, the female pups began to be dosed daily via gastric gavage with 10 mg/kg MnCl2 or an equal volume of saline. The dosing regimen continued through PND 29. We have shown previously that this is the minimum effective dose resulting in female precocious puberty.20 On PND 30 half of the females in each group were killed by decapitation and the blood and MGs were collected and prepared for analysis as described below. Between PND 119 and 122 (referred to throughout as PND 120), the remaining animals in both groups were killed and their tissues collected. Using criteria we have described previously,32,33 the PND 30 animals were confirmed to be immature (anestrous) and in the late juvenile stage of rodent development, and the cycling PND 120 animals were confirmed to be in the diestrus stage of the estrous cycle.
Mammary whole gland morphological analysis
MG whole mounts were processed similarly as previously described34 with the following modifications. On PND 30, the #4 right abdominal MGs from control and Mn-treated animals were removed and placed in Carnoy's fixative at room temperature (RT) for 3 days. Next, slides were washed with 70% ethanol (EtOH) for 1 h, distilled water for 30 min, and placed in Carmine Alum stain for 4 days at RT. MGs were dehydrated with EtOH 70, 95, 100% for 1 h each and cleared in xylene for 2 days at RT. Each gland was then placed on a slide mounted in permount under a coverslip and analyzed under an Olympus CKX41 microscope.
The number of MG terminal end buds (TEB), alveolar buds (ABs), lobular type 1 (Lob1), and terminal ducts (TD) was counted in a localized exterior area (opposite of the nipple), previously described as Zone C.35 The number of each of the above ductal structures was counted in all of the MGs analyzed (N=7, saline; N=8, Mn). The mean number of each of these structures per group was calculated and statistical analysis between groups was assessed.
Mn measurements in MG tissue analysis
Mn was measured in MG tissue by the Texas A&M University Heavy Metal Analysis Laboratory. Tissues were digested following the standard protocol of da Silva et al.36 with slight modifications that we have described previously.20 Tissues were analyzed using a PerkinElmer/Sciex DCR II inductively coupled plasma-mass spectrometer.
Justification of Mn dose
We previously established that the 10 mg/kg dose of Mn used in this study was the lowest effective dose capable of centrally mediating elevated levels of E2, resulting in precocious puberty.20 In addition, this dose has no known toxic effects in rats when administered as described. Importantly the dose used and route of exposure are relevant to human consumption habits. The U.S National Research Council sets “Estimated Safe and Adequate Daily Dietary Intake” (ESADDI) age-related levels of Mn for infants and children as follows: 0.6–1.5mg of Mn (infants to children 3 years of age), 1–2 mg of Mn (children 4–10 years of age), and 2–5 mg of Mn (children 10 years and older).37 Based on our previous studies, Mn taken in by pups consuming commercial rodent chow on an ad lib basis increases from about 0.28 mg/day to about 1.4 mg/day. During this same time (PND 12-30), treated female rats in our study were supplemented by gavage with 10 mg of Mn/kg animal/day. Thus, at 15 days, they are exposed to a total of 0.53 mg of Mn/day (food + Mn supplement) and this amount gradually rises to a total of 2.4 mg of Mn/day (food + Mn supplement) just before puberty. Collectively, the total daily amount of Mn consumed by rats in our study did not exceed the ESADDI intake amounts of this element for infants and children,37 which highlights the relevance of this study to human health concerns.
Histological analysis and immunohistochemistry (IHC)
The #4 abdominal MG was harvested, placed in 4% paraformaldehyde in phosphate buffered saline (PBS) and were sent to the Histology Core, Texas A&M University, for paraffin embedding. Ten serial sections (5 μm thick) were cut, placed on slides, deparaffinized using xylene, and gradually hydrated using a series of ethanol washes. Five of the sections per animal in each group were stained with hemotoxylin and eosin (H&E) and the other five section used for IHC.
IHC
Following rehydration, sections were place in 0.01 M citrate buffer (pH 6.0) in stainless steel pressure cooker for 5 min for antigen retrieval. Sections were washed in tap water and tris buffer (pH 7.6) for 5 min each and then placed in 1% H2O2 in tris-buffered saline (TBS) for 30 min to block endogenous peroxidase activity. After a 1 h incubation in 2.5% normal goat serum, sections were incubated with Ki67 antibody (1:1000; Novacastra Laboratories Ltd, New Castle, United Kingdom) overnight at 4°C. Sections were washed in TBS, incubated in biotinylated goat anti-rabbit (Vector Laboratories, Burlingame, CA) for 1 h at RT, followed by avidin peroxidase using the Vector ABC Elite Kit (Vector Laboratories) for 30 min and then washed in TBS and water. Chromogenic reaction was carried out by 3′-3′ diaminobenzidine solution (Vector Laboratories), washed with water and counterstained with methyl green (Vector Laboratories) then dehydrated with graded ethanols, xylene, and coverslipped. Microscope images were taken using a Leica DM500.
To determine the percentage of MG epithelial cells that stained positive for Ki67 at PND 30, three serial sections per animal (N = 5) per treatment group were analyzed. At PND 120, five serial sections per animal (N = 6) per treatment group were analyzed. The number of Ki67 positively stained ductal epithelial cells relative to the total number of ductal epithelial cells per section was averaged per animal and compared between groups.
Western blot analysis
Mammary tissues were homogenized in 1% Igepal CA-630, 20 mM Tris–Cl, pH (8.0), 137 mM NaCl, 2 mM ethylenediaminetetraactic acid (EDTA), 10% glycerol, 10 mM sodium pyruvate, 10 mM sodium fluoride, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride and 0.25% protease inhibitor cocktail (Sigma-Aldrich, St Louis, MO) at 4°C. The homogenates were incubated on ice for 30 min and centrifuged at 12,000g for 15 min. The concentration of total protein in the resulting supernatant was determined by the RC DC protein assay (Bio-Rad Laboratories) using bovine serum albumin as standard. Immunoblot analysis was performed by solubilizing the proteins (100 μg) in a sample buffer containing 25 mM Tris–Cl, pH 6.8, 1% sodium dodecyl sulfate (SDS), 5% β-mercaptoethanol, 1 mM EDTA, 4% glycerol, and 0.01% bromophenol blue and electrophoresed through 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions for phospho-ERK1/2 (mouse monoclonal 1:5000; Abcam Inc., Cambridge, MA), extracellular signal-regulated kinase 1/2 (ERK1/2) (mouse monoclonal 1:5000; Abcam Inc. Cambridge, MA), phospho-Akt (rabbit polyclonal 0.4 μg/mL; Abcam Inc., Cambridge, MA, Akt (rabbit polyclonal 1:1000; Abcam Inc., Cambridge, MA), ERα (mouse monoclonal 1 μg/mL; Abcam Inc., Cambridge, MA), p53 (goat polyclonal 1:700; R&D Systems, Minneapolis, MN), and activator protein 2α (AP2α; rabbit monoclonal 1:250; Cell Signaling Tech, MA). The separated proteins were electrophoretically transblotted onto polyvinylidene difluoride membranes. Following transfer, membranes were blocked with 5% nonfat dried milk/0.1% Tween 20 in PBS (pH 7.4) for 3 h and subsequently incubated at 4°C overnight with primary antibodies. After the incubation, membranes were washed in PBS/0.1%Tween-20 and then incubated for 2 h at RT with secondary antibodies: ERK1/2, goat anti-mouse 1:30,000; Akt, goat anti-rabbit 1:40,000; ERα, goat anti-mouse 1:10,000; p53, donkey anti-goat 1:20,000; AP2α, goat anti-rabbit 1:10,000 (all purchased from Santa Cruz Biotech, CA). Following washing, the specific signals were detected with the enhanced chemiluminescence (Western Lightning Plus-ECL, PerkinElmer, Shelton, CT) and quantified with NIH Image J software version 1.43 (National Institutes of Health, MD). Subsequently, membranes were stripped using Re-Blot Plus kit (EMD Millipore, Temecula, CA) and reprobed with a mouse monoclonal antibody to the β-actin (1:50,000; Sigma-Aldrich, MO) and goat anti-mouse secondary antibody (1:50,000; Santa Cruz Biotech, CA) to normalize for the amount of sample loading. Following washing, the detection and quantitation of β-actin was done as described earlier.
Hormone and statistical analysis
Blood was collected from saline controls and Mn-treated animals, centrifuged at 3,000g for 30 min, and serum was removed for measurement of E2 using a mouse/rat estradiol enzyme-linked immunosorbent assay (ELISA) assay from Calbiotech (Spring Valley, CA). The assay sensitivity was 3 pg/mL.
Differences between saline control and Mn-treated groups were analyzed by the unpaired Student's t-test assuming random sampling. Probability values<0.05 were considered to be statistically significant. The IBM PC programs INSTAT and PRISM software (GraphPad, San Diego, CA, USA) were used to calculate and graph the results.
Results
Effects of Mn on MG ductal differentiation at PND 30
Previously we showed that prepubertal Mn exposure activates GnRH and gonadotropin release, resulting in elevated serum E2 levels and subsequently, precocious puberty in female rats.20 Because of this action, we assessed whether this early Mn exposure model would cause advanced mammary development. As expected, 30-day-old Mn-treated rats showed a twofold increase (p < 0.05) in serum E2 levels compared to saline-treated controls (Figure 1(a)). This coincided with morphological changes observed in the MG at PND 30. In Figure 2, whole mount analysis revealed that while Mn treatments did not alter MG ductal elongation (not shown), it did increase differentiation of the gland, as indicated by the number of terminal ductal epithelial structures present on PND 30. Representative whole mount images of the right #4 abdominal MG illustrate the noted morphological changes in Mn-exposed animals (Figure 2(b) and (d)) compared to saline-treated controls (Figure 2(a) and (c)). Specifically, Figure 2(e) shows that the mean number of TEB, undifferentiated progenitor cells, was lower (p < 0.001) in prepubertal animals receing Mn supplementation compared to the saline controls on PND 30. As such, Figure 2(f) shows that Mn exposure resulted in a marked increase (p < 0.0001) in the mean number of ABs compared to controls. Furthermore, Figure 2(g) and (h), respectively, shows that Mn caused an increase (p<0.01) in the mean number of Lob1 structures, as well as an increase (p < 0.05) in the mean number of TD compared to their respective saline controls on PND 30. It is important to note that the concentration of Mn (mean ± standard error of the mean (SEM)) within the MGs was not different on PND 30 between saline (0.12 ± 0.02 ppm) and Mn-treated (0.16 ± 0.06 ppm) rats, indicating that Mn did not accumulate in the gland.
Figure 1.
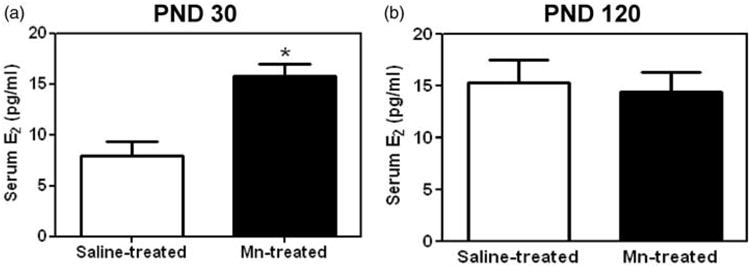
Effects of prepubertal exposure to Mn on serum levels of E2 at PND 30 and 120. (a) Mean (±SEM) serum levels of E2 were significantly increased in prepubertal females treated with Mn (10 mg/kg) compared to saline controls at PND 30. (b) There was no difference in mean (±SEM) serum E2 levels between groups at PND 120. Bars represent an N of 7 animals per group at PND 30 and an N of 17 animals per group at PND 120. * = p < 0.05
Figure 2.
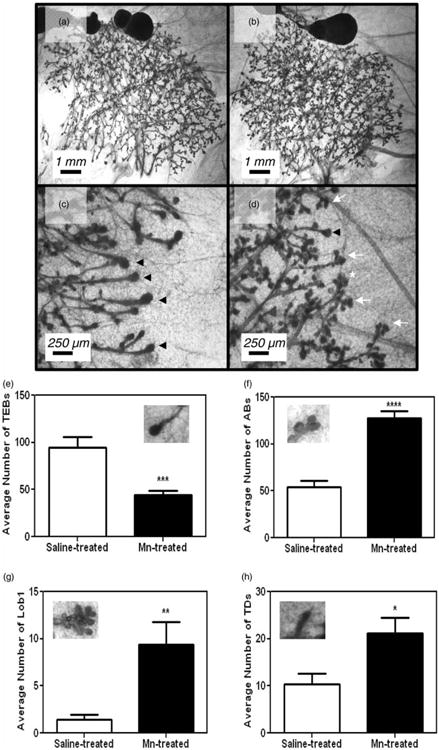
Mn exposure increases MG differentiation at PND 30. Representative images of abdominal mammary gland #4 taken from rats dosed from PND 12 until PND 29 with either 0.9% saline solution (a and c) or 10 mg/kg MnCl2 (b and d). Note the increased expression of TEBs in controls (c) compared to increases in the differentiated gland structures: ABs and Lob1s in Mn-treated glands (d). (e) Mn exposure resulted in a significant decrease in the mean (±SEM) number of TEBs compared to saline controls at PND 30. (f) to (h) The mean (±SEM) number of differentiated terminal MG structures: ABs, Lob1, and TDs, respectively, was all increased in the Mn-treated versus saline control animals at PND 30. Bars represent an N of 7 (saline) and N of 8 (Mn) animals per group. Representative image of MG structures within graphs. AB: alveolar bud; Lob1: lobular type 1; PND: postnatal day; TD: terminal duct; TEB: terminal end bud; ABs = white arrow heads; Lobs = white asterisk; TEBs = black arrow heads. * = p <0.05; ** = p <0.01; *** = p < 0.001; **** = p < 0.0001
Effects of Mn on MG ductal proliferation and protein expression at PND 30
To determine if the increased MG growth corresponded to changes in proliferation, we compared Ki67 staining from saline controls and Mn-treated rats. Figure 3(a) and (b) depicts representative IHC images and cell counts from all sections, respectively, demonstrating the increased (p < 0.001) expression of Ki67 positive staining in MG luminal epithelial cells from Mn-treated rats compared to the saline controls at PND 30. Supporting this, Western blotting showed that Mn exposure resulted in increased (p < 0.05) phosphorylation of the mitogenic signaling protein ERK1/2 in epithelial enriched protein MG lysates at PND 30 (Figure 4(a) and (b)), but protein expression for phosphorylated Akt (Figure 4(c) and (d)), ERα, AP2α, and the tumor suppressor p53 was not altered (Figure 5(a) and (c) to (e)).
Figure 3.
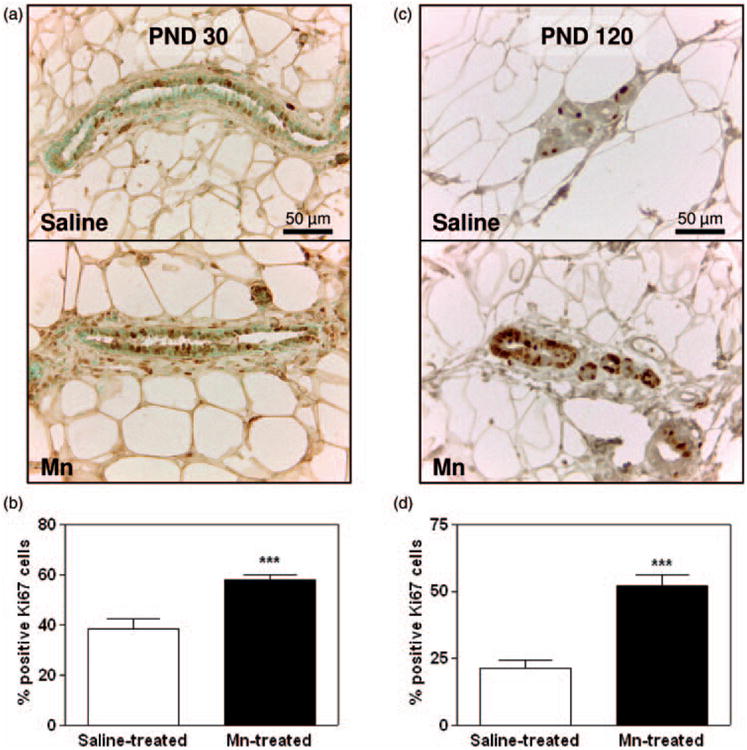
Effects of Mn on MG proliferation at PND 30 and 120. (a) Representative images showing Ki67 staining in the #4 abdominal MG from saline control (top panel) and Mn-treated (bottom panel) rats at PND 30. Note the increased dark nuclear staining in MG luminal epithelial cells from the Mn-treated females compared to saline controls. (b) Cell counts depicting the increased mean (±SEM) percentage (%) of cells at PND 30 that stained positive for Ki67 in MGs from Mn-treated animals compared to the saline controls. (c) Representative images showing Ki67 staining in the #4 abdominal MG from saline control (top panel) and Mn-treated (bottom panel) rats at PND 120. (d) Cell counts depicting the increased mean (±SEM) percentage (%) of cells at PND 120 that stained positive for Ki67 in MGs from Mn-treated animals compared to the saline controls. Bars represent 15 images per group (three serial sections from each of the five animals per group) in panel b and 30 images per group (five serial sections from each of the six animals per group) in panel d. * = p <0.05; ** = p <0.01; *** = p <0.001 (A color version of this figure is available in the online journal.)
Figure 4.
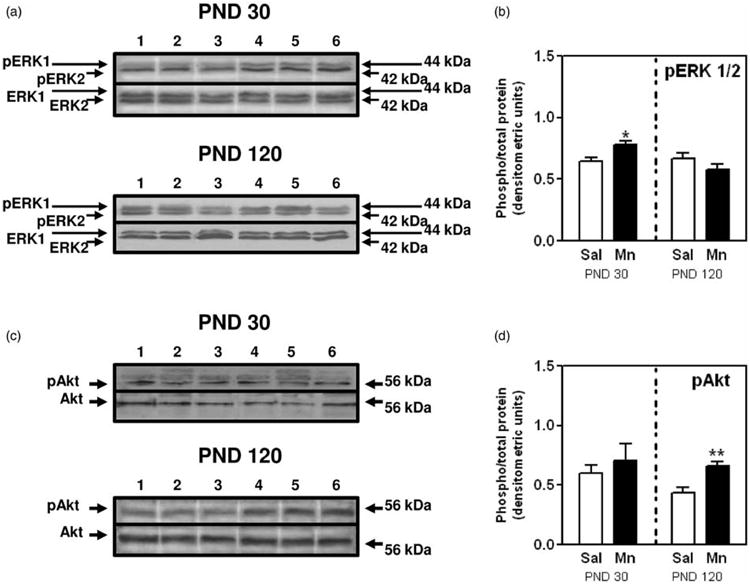
Effects of Mn on MG protein phosphorylation of ERK1/2 and Akt. (a) Representative immunoblot illustrating the protein expression levels of phosphorylated ERK1/2 (pERK 1 and pERK 2) and ERK (ERK 1 and ERK 2) in MGs from saline (lanes 1–3) and Mn-treated (lanes 4–6) animals at PND 30 (top) and 120 (bottom). (b) Bar graph depicting the changes in the mean (±SEM) protein expression of pERK1/2 relative to ERK1/2 expression in MGsfrom Mn-treated animals at PND 30 and 120. (c) Representative immunoblot illustrating the protein expression levels of phosphorylated Akt (pAkt) and Akt (Akt) in MGs from saline (lanes 1–3) and Mn-treated (lanes 4–6) animals at PND 30 (top) and 120 (bottom). (d) Bar graph showing the mean (±SEM) protein expression of pAkt relative to Akt expression in MGs from Mn-treated animals at PND 30 and 120. Bars represent an N of 4-5 (PND 30) and 7 (PND 120) animals per group. * = p < 0.05; ** = p < 0.01
Figure 5.
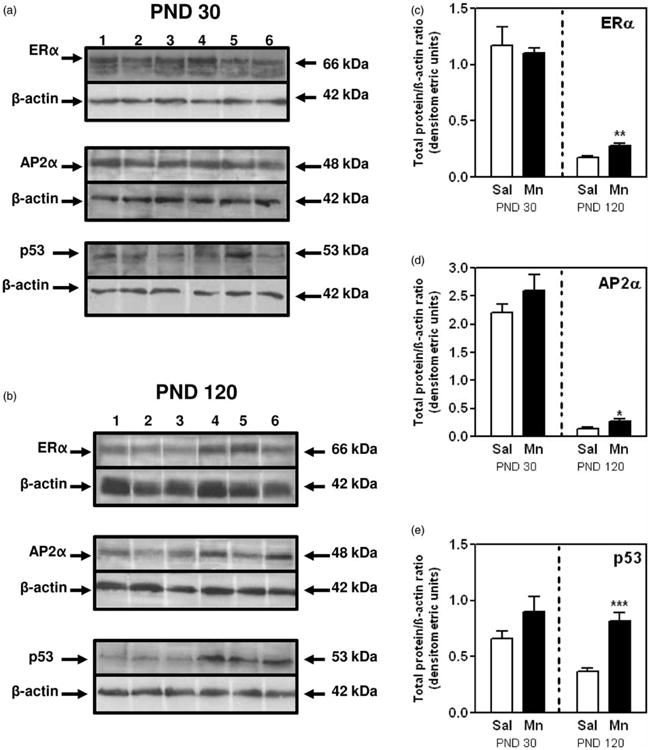
Effects of Mn on MG protein expression of ERa, AP2a, and p53 at PND30 and 120. (a) Representative immunoblots showing expression of ERα, AP2α, and p53 proteins in MGs from saline (lanes 1–3) and Mn-treated (lanes 4–6) rats at PNDs 30. (b) Representative immunoblots showing expression of ERα, AP2α, and p53 proteins in MGs from saline (lanes 1–3) and Mn-treated (lanes 4–6) rats at PNDs 120. (c) Depicts the quantitation of mean (±SEM) ERα protein levels in MGs from all animals at PNDs 30 and 120. (d) Depicts the quantitation of mean (±SEM) AP2α protein levels in MGs from all animals at PNDs 30 and 120. (e) Depicts the quantitation of mean (±SEM) p53 protein levels in MGs from all animals at PNDs 30 and 120. Bars represent an N of 4–5 (PND 30) and 6–7 (PND 120) animals per group. * = p < 0.05; ** = p < 0.01;*** = p < 0.001
Effects of Mn on MG ductal proliferation and protein expression at PND 120
The increase in luminal epithelial proliferation observed at PND 30 was sustained at PND 120—90 days after the last dose of Mn was administered. Specifically, Figure 3(c) shows representative IHC images with a greater intensity of nuclear Ki67 staining in abdominal MGs from Mn-exposed animals compared to the saline controls at PND 120. This is further demonstrated by a more than twofold increase (p < 0.001) in the mean percentage of MG luminal epithelial cells staining positive for Ki67 in Mn-treated rats (Figure 3(d)). It is also important to note the decrease in the number of proliferating cells in adult glands collected from saline control animals during diestrus at PND 120 (21%; Figure 3(d)), compared to the percentage of Ki67 positive cells in developing glands collected at PND 30 (38%; Figure 3(b)). Similarly, there was a marked decrease in cell signaling protein expression levels in MGs from diestrus animals at PND 120; thus, signifying maturation of the adult gland (Figures 4 and 5). However, there was a significant alteration in the expression levels of MG cell signaling proteins in the Mn-treated group compared to PND 30. Western blotting showed that Mn exposure resulted in increased (p < 0.01) phosphorylation of the cell signaling protein Akt (pAkt) in epithelial enriched MG lysates at PND 120 (Figure 4(c) and (d)), whereas expression of pERK1/2 was unaltered (Figure 4(a) and (b)). Furthermore, the levels of expression for ERα, AP2α, and p53 proteins in epithelial enriched MG lysates were significantly elevated (p < 0.05 to p<0.001) in the MGs of Mn-treated compared to control animals at PND 120 (Figure 5(b) to (e)). These changes in MG signaling were not observed at PND 30 (Figures 4 and 5).
Effects of Mn on MG hyperplasia at PND 120
Given the persistent epithelial cell proliferation noted between PND 30 and 120 due to prepubertal Mn treatments, H&E stained sections of the #4 abdominal MGs from PND 120 saline control (Figure 6(a)) and Mn-treated rats (Figure 6(b) to (f)) were assessed for aberrant growth. Strikingly, 40% of the MGs from the Mn-treated rats (two out five animals) exhibited dense irregular interlobular connective tissue and reactive stroma (Figure 6(b) and (d)), and 20% of the glands (one of five animals) had observable areas of intra-luminal focal hyperplasia (Figure 6(c) to (f)) comprised of disorganized epithelial growth (Figure 5(c) and (d)) and epithelial filling of ducts (Figure 6(e) and (f)). These observations were confirmed on multiple histological serial sections (four to five per animal) of ducts and never seen in the saline control animals at PND 120 or in either group at PND 30. Furthermore, the serum E2 levels from both control and Mn-treated rats were not different during diestrus at PND 120 (Figure 1(b)).
Figure 6.
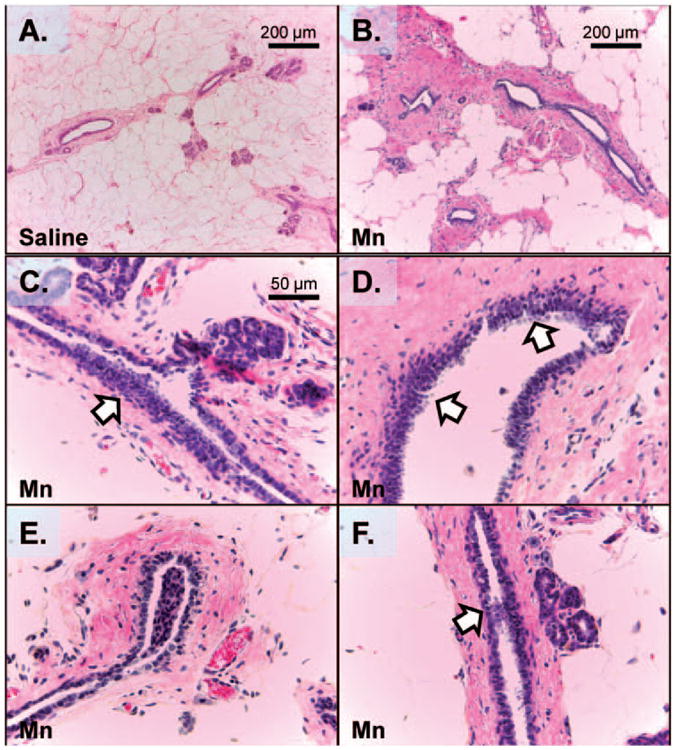
Mn causes aberrant MG growth at 120 days of age. This figure shows representative H&E images of MG ducts at 120 days of age in saline (a) and Mn-treated (b–f) rats. Prepubertal exposure to Mn resulted in reactive stroma (b and d), intra-luminal focal hyperplasia (arrows; c and d), and MG ductal filling (arrows; e and f). None of these observations were noted in saline control animals at PND 120 or in either group at PND 30 (not shown). Images taken from an N of 5 animals per group. Scale bar for panels c–f is located in panel c (A color version of this figure is available in the online journal.)
Discussion
While it has been established that Mn is required for normal physiological processes, no study to date has suggested that a timely, modest elevation may alter MG developmental process that increase the glands susceptibility to tumorigenesis. This is important given the element is prevalent in the environment, as is found in food, water, soil, and air. Some places have higher levels of Mn than others and certain cultures consume greater amounts of the element in their diet. Elevated levels of Mn have been reported in infants and children18,19,38,39 and both have been identified as being more sensitive to excess Mn exposure.16,18,19 Thus, this combination of environmental influences, especially in infants and children which are particularly vulnerable, may result in elevated Mn exposure that could potentially be harmful. Importantly, the 10 mg/kg daily supplemental dose of Mn used in this study did not exceed daily intake amounts previously set by the U.S. National Research Council. Therefore, we have shown that exposure to elevated yet realistic daily amounts of Mn, during a critical developmental window, predisposes the MG to premalignant transformation.
Our data suggest that Mn, through elevated precocious levels of E2, accelerates prepubertal MG development. In this regard, morphological assessments confirmed that Mn-treated females showed significant decreases in the number of undifferentiated epithelial structures (TEBs) with significant increases in differentiated structures (ABs, Lob1s, and TDs). These observations were apparent in the most actively growing region of the developing MG.35 Importantly, the observed morphological changes at PND 30 were associated with a twofold increase in serum E2 levels in Mn-treated animals. It has been well established that receptor-dependent E2 signaling is necessary for epithelial differentiation during pubertal MG development.40–43 Conversely, studies have shown elevated levels of estrogen or estrogenic compounds will advance growth of the gland.40,44–47 Prior to first ovulation, prepubertal development is marked by TEB-mediated ductal elongation regulated by reproductive hormones and locally acting growth factors.48,49 After pubertal onset, the MG is highly hormone dependent primarily due to the establishment of cycling levels of E2 and progesterone necessary to complete alveolar differentiation (AB and Lob1 structures) of the virgin gland.50 The fact that we observed a higher presence of postovulatory structures at PND 30 suggests that the precociously elevated levels of E2 induced by Mn are capable of promoting growth of the prepubertal MG. Supporting this, similar MG morphological structures have been observed in prepubertal rats exposed to the environmental endocrine disruptor diethylstilbestrol, a synthetic estrogen.47 While our data does not eliminate the possibility that Mn may directly influence MG proliferation, this scenario is unlikely. First, we observed no differences in Mn accumulation in the MGs of controls compared to Mn-treated animals at PND 30. Furthermore, our previous studies have established that Mn accumulates in the hypothalamus and is capable of inducing prepubertal GnRH regulated increases in levels of serum LH and E2; hence, resulting in precocious pubertal onset.20 In addition, MnCl2 at 1.0 μM has been shown to be only 9% as efficient as 17-β estradiol in activating E2 receptor-dependent transcriptional activity in the Michigan Cancer Foundation (MCF-7) human breast cancer cell line.51 Therefore, these data collectively suggest that Mn advances virgin MG development indirectly by centrally activating the hypothalamic–pituitary–ovarian axis.
The noted increase in differentiated structures present in the prepubertal MG (PND 30) of Mn-treated animals directly correlated with a significant increase in luminal epithelial cell proliferation. This corresponded with increased pERK1/2 signaling in MGs from Mn-treated animals. This could account, in part, for their robust proliferative MG growth. Interestingly, we observed that luminal epithelial cell proliferation was also significantly increased in adult MGs from Mn-treated animals at PND 120. As expected, proliferation in control animals at this time point had decreased. This suggests that Mn-induced estrogen regulated prepubertal MG growth modified cellular turnover, resulting in sustained proliferative growth between PND 30 and 120. Importantly, this event is indicative of early stages of MG tumorigenesis.52 Studies hypothesize that a precocious elevation in estrogens, associated with centrally mediated early pubertal onset, plays a pivotal role in reorganization of tissue interactions within the MG resulting in altered cell fate.53–55 While the mechanisms involved are unclear, we observed a change from increased pERK1/2 protein expression in MGS from Mn-treated animals at PND 30 to increased pAkt protein expression in proliferative adult glands on PND 120. Akt, an effector of the phosphatidylinositol 3-kinase pathway, is known for regulating pro-survival and growth-promoting signaling. Furthermore, Akt is associated with invasive breast cancer and pAkt status has been proposed as a biomarker for predicting therapeutic response to treating breast cancer. Interestingly, ligand-dependent ERα extranuclear signaling has been shown to cause proliferation of MCF-7 cells via Src kinase activation of PI3-K triggering Akt upregulation of cyclin D1.56 Therefore, Mn stimulated precocious MG development may result in altered cell signaling pathways regulating apoptosis, a common regulatory event found in breast cancer.57
The Mn-induced advancement of E2-mediated MG development resulted in MG deregulation as a consequence of amplified proliferative growth in adult glands at PND 120. This resulted in reactive stroma, disorganized MG luminal epithelial growth, and epithelial filling of ducts in postpubertal MGs from Mn-exposed animals. These histological observations are known indicators of hyperplasic MG growth. These observations were surprising given these animals had not been exposed to Mn for 90 days and serum E2 levels in these animals were similar to controls. However, this can be clarified by the noted change in expression levels of ERa in the adult gland. While it is widely known that ligand-dependent ERα activation and expression plays a major role in promoting both normal and malignant breast epithelial cell proliferation, the mechanisms are different. In both the developing and normal adult virgin MG, estrogen-mediated epithelial cell proliferation occurs in ERα negative (ERα–) cells, through a complex paracrine mechanism regulated by ERa positive (ERα+) cells.58–60 In the normal adult virgin MG, estrogen-mediated epithelial cell proliferation, while not as robust, also occurs in ERα– cells, but primarily due to ligand-dependent downregulation of ERα expression in cells that have entered the cell cycle.26 Conversely, in ERα+breast cancer, proliferative tumor cells are ERα+.27 Similarly, we observed that ERα protein expression, although not different, was considerably higher in prepubertal MGs (PND 30) in both groups (indicative of a developing gland) compared to their respective adult virgin MGs (PND 120). However, there was a significant upregulation in ERα protein expression in adult MGs from Mn-treated rats compared to controls on PND120. This noted difference was associated with increased cellular proliferation and hyperplasic MG growth in these animals. We suggest that earlier prepubertal exposure to E2, as a result of Mn, alters postpubertal E2 signaling due to a higher presence of ER+ cells that directly promote ligand dependent proliferation. This creates a postpubertal gland that is more responsive to normal cyclic levels of E2 that subsequently lead to aberrant growth as a consequence of cumulative endogenous stimulation.
Supporting our hyperplasic phenotype generated by prepubertal Mn supplementation, we noted increased expression levels of cell cycle regulatory proteins known to be expressed in ERα + clinical breast cancer.61–63 We observed a significant increase in protein expression of the transcription factor AP2α and the tumor suppressor p53 in adult glands of Mn-treated animals at PND 120–actions that were observed with increased protein expression of ERα. The simultaneous expression of these known cell cycle regulators in proliferating glands is not surprising. Nuclear expression of AP2α has been shown to positively associate with ERα expression in clinical breast cancer63 and ERα positive human breast cancers express wild-type p53.61,62,64 P53 plays a major role in sensing cell stress and responding to DNA damage, suggesting the p53 induction that we observed at PND 120 could be an attempt to regulate the aberrant cellular proliferation of the MG between PND 30 and 120. AP2α has been shown to directly interact with p53 to regulate gene expression controlling cell growth.65,66 Stabach et al.67 showed that increasing levels of AP2α can alter the stability of p53 limiting the proteins ability to mediate cell cycle regulation. Therefore, the noted increase in AP2α at PND120 may alter the ability of these proteins to regulate cell proliferation. Furthermore, the increased expression of ERα at PND120 could be a consequence of p53 induction. Shirley et al.68 showed that p53 increases transcription of the ER gene promoter in MCF-7 cells, establishing a functional link between p53 expression and the growth-promoting action of ERα in breast cancer progression. Collectively, the combined elevated expression levels of ERα, AP2α, and p53 at PND 120 suggests that these cells are in early stages of malignant transformation.
Epidemiological data show that early pubertal onset results in a 10–20% increase in developing breast cancer,4,5,7 which is a significant concern, as recent studies have shown that puberty in girls is occurring at an earlier age69,70 with no identifiable cause in over 95% of the cases. Elucidating how early pubertal events contribute to breast cancer risk has been complicated. This is, in part, due to the difficulty in identifying factors that augment normal neuroendocrine pathways that regulate pubertal onset. In addition, there is a lack of in vivo models that closely mimics the complex hormone milieu that accompanies this stage of development. We previously established that Mn acts centrally to replicate the hormonal milieu that regulates puberty except that these events are advanced20,71 resulting in Mn-induced precocious puberty. Using this model, we have revealed an altered outcome within the adult virgin gland, in that 20% of the Mn-treated animals showed MG hyper-plasia. These findings are significant because we have identified Mn as potential causal link between environmental/life style induced early pubertal onset and breast cancer risk.
In conclusion, we clearly show that prepubertal exposure to elevated but environmentally relevant levels of Mn results in a peripubertal increase in serum E2, which is associated with precocious MG development. These pubertal changes lead to increased expression of ERα and associated proteins resulting in sustained MG proliferation and hyperplasia in some of the adults. This study shows that Mn exposure during juvenile developmental can stimulate pubertal MG growth rendering it more conducive to tumorigenesis. We suggest that the normally beneficial effect of Mn at puberty can be harmful if an individual is exposed to elevated but low amounts of the metal at earlier ages of life.
Acknowledgments
This work was supported by National Institute of Environmental Health Sciences–award number ESO13143 (WLD) and National Institute of General Medical Sciences–award number 1R25GM100866 (RKD). The authors would like to thank Dr Frank Dirrigl for assisting in the utilization of the Leica DM500 microscope. The content of this publication is the responsibility of the authors and does not represent the views of the NIH.
Footnotes
Author contributions: WLD conceived and RKD participated in the design of the study; all authors participated in conducting the experiment, analysis of data, and review of the manuscript; RKD and WLD did the majority of the writing of this manuscript, but all authors contributed in this regard.
References
- 1.Yorifuji T, Tsuda T, Doi H, Grandjean P. Cancer excess after arsenic exposure from contaminated milk powder. Environ Health Prev Med. 2011;16:164–70. doi: 10.1007/s12199-010-0182-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohn BA, Wolff MS, Cirillo PM, Sholtz RI. DDT and breast cancer in young women: New data on the significance of age at exposure. Environ Health Perspect. 2007;115:1406–14. doi: 10.1289/ehp.10260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cory-Slechta DA, Virgolini MB, Rossi-George A, Thiruchelvam M, Lisek R, Weston D. Lifetime consequences of combined maternal lead and stress. Basic Clin Pharmacol Toxicol. 2008;102:218–7. doi: 10.1111/j.1742-7843.2007.00189.x. [DOI] [PubMed] [Google Scholar]
- 4.Berkey CS, Frazier AL, Gardner JD, Colditz GA. Adolescence and breast carcinoma risk. Cancer. 1999;85:2400–9. doi: 10.1002/(sici)1097-0142(19990601)85:11<2400::aid-cncr15>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 5.Kelsey JL, Gammon MD, John EM. Reproductive factors and breast cancer. Epidemiol Rev. 1993;15:36–47. doi: 10.1093/oxfordjournals.epirev.a036115. [DOI] [PubMed] [Google Scholar]
- 6.Kvale G. Reproductive factors in breast cancer epidemiology. Acta Oncol. 1992;31:187–94. doi: 10.3109/02841869209088901. [DOI] [PubMed] [Google Scholar]
- 7.Titus-Ernstoff L, Longnecker MP, Newcomb PA, Dain B, Greenberg ER, Mittendorf R, Stampfer M, Willet W. Menstrual factors in relation to breast cancer risk. Cancer Epidemiol Biomarkers Prev. 1998;7:783–9. [PubMed] [Google Scholar]
- 8.Bernstein L. Epidemiology of endocrine-related risk factors for breast cancer. J Mammary Gland Biol Neoplasia. 2002;7:3–15. doi: 10.1023/a:1015714305420. [DOI] [PubMed] [Google Scholar]
- 9.Henderson BE, Ross RK, Pike MC. Toward the primary prevention of cancer. Science. 1991;254:1131–8. doi: 10.1126/science.1957166. [DOI] [PubMed] [Google Scholar]
- 10.Erikson KM, Syversen T, Aschner JL, Aschner M. Interactions between excessive manganese exposures and dietary iron-deficiency in neuro-degeneration. Environ Toxicol Pharmacol. 2005;19:415–21. doi: 10.1016/j.etap.2004.12.053. [DOI] [PubMed] [Google Scholar]
- 11.Wedler FC. Biological significance of manganese in mammalian systems. Prog Med Chem. 1993;30:89–133. doi: 10.1016/s0079-6468(08)70376-x. [DOI] [PubMed] [Google Scholar]
- 12.Gray L, Jr, Laskey JW. Multivariate analysis of the effects of manganese on the reproductive physiology and behavior of the male house mouse. J Toxicol Environ Health. 1980;6:861–7. doi: 10.1080/15287398009529904. [DOI] [PubMed] [Google Scholar]
- 13.Laskey JW, Rehnberg GL, Hein JF, Carter SD. Effects of chronic manganese (Mn3O4) exposure on selected reproductive parameters in rats. J Toxicol Environ Health. 1982;9:677–87. doi: 10.1080/15287398209530195. [DOI] [PubMed] [Google Scholar]
- 14.Boyer CB, Shaw JH, Phillips PH. Studies on manganese deficiency in the rat. J Biol Chem. 1942;143:417–25. [Google Scholar]
- 15.Smith SE, Medlicott M, Ellis GH. Manganese deficiency in the rabbit. Arch Biochem Biophys. 1944;4:281–9. [Google Scholar]
- 16.US Environmental Protection Agency (EPA) Health effects support document for manganese. Report-822-R-03-003. 2003 [Google Scholar]
- 17.Lonnerdal B. Effects of milk and milk components on calcium, magnesium, and trace element absorption during infancy. Physiol Rev. 1997;77:643–69. doi: 10.1152/physrev.1997.77.3.643. [DOI] [PubMed] [Google Scholar]
- 18.Stastny D, Vogel RS, Picciano MF. Manganese intake and serum manganese concentration of human milk-fed and formula-fed infants. Am J Clin Nutr. 1984;39:872–8. doi: 10.1093/ajcn/39.6.872. [DOI] [PubMed] [Google Scholar]
- 19.Cockell KA, Bonacci G, Belonje B. Manganese content of soy or rice beverages is high in comparison to infant formulas. J Am Coll Nutr. 2004;23:124–30. doi: 10.1080/07315724.2004.10719352. [DOI] [PubMed] [Google Scholar]
- 20.Pine M, Lee B, Dearth R, Hiney JK, Dees WL. Manganese acts centrally to stimulate luteinizing hormone secretion: a potential influence on female pubertal development. Toxicol Sci. 2005;85:880–5. doi: 10.1093/toxsci/kfi134. [DOI] [PubMed] [Google Scholar]
- 21.Srivastava VK, Hiney JK, Dees WL. Early life manganese exposure upregulates tumor-associated genes in the hypothalamus of female rats: Relationship to manganese-induced precocious puberty. Toxicol Sci. 2013;136:373–81. doi: 10.1093/toxsci/kft195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee B, Pine M, Johnson L, Rettori V, Hiney JK, Dees WL. Manganese acts centrally to activate reproductive hormone secretion and pubertal development in male rats. Reprod Toxicol. 2006;22:580–5. doi: 10.1016/j.reprotox.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 23.Silberstein GB, Van Horn K, Shyamala G, Daniel CW. Essential role of endogenous estrogen in directly stimulating mammary growth demonstrated by implants containing pure antiestrogens. Endocrinology. 1994;134:84–90. doi: 10.1210/endo.134.1.8275973. [DOI] [PubMed] [Google Scholar]
- 24.Bocchinfuso WP, Lindzey JK, Hewitt SC, Clark JA, Myers PH, Cooper R, Korach KS. Induction of mammary gland development in estrogen receptor-alpha knockout mice. Endocrinology. 2000;141:2982–94. doi: 10.1210/endo.141.8.7609. [DOI] [PubMed] [Google Scholar]
- 25.Anderson E, Clarke RB, Howell A. Estrogen responsiveness and control of normal human breast proliferation. J Mammary Gland Biol Neoplasia. 1998;3:23–35. doi: 10.1023/a:1018718117113. [DOI] [PubMed] [Google Scholar]
- 26.Reid G, Hubner MR, Metivier R, Brand H, Denger S, Manu D, Beaudouin J, Ellenberg J, Gannon F. Cyclic, proteasome-mediated turnover of unliganded and liganded ERalpha on responsive promoters is an integral feature of estrogen signaling. Mol Cell. 2003;11:695–707. doi: 10.1016/s1097-2765(03)00090-x. [DOI] [PubMed] [Google Scholar]
- 27.Clarke RB, Howell A, Potten CS, Anderson E. Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res. 1997;57:4987–91. [PubMed] [Google Scholar]
- 28.Wang TT, Phang JM. Effects of estrogen on apoptotic pathways in human breast cancer cell line MCF-7. Cancer Res. 1995;55:2487–9. [PubMed] [Google Scholar]
- 29.Huseby RA, Maloney TM, McGrath CM. Evidence for a direct growth-stimulating effect of estradiol on human MCF-7 cells in vivo. Cancer Res. 1984;44:2654–9. [PubMed] [Google Scholar]
- 30.Shafie SM, Liotta LA. Formation of metastasis by human breast carcinoma cells (MCF-7) in nude mice. Cancer Lett. 1980;11:81–7. doi: 10.1016/0304-3835(80)90097-x. [DOI] [PubMed] [Google Scholar]
- 31.Herynk MH, Fuqua SA. Estrogen receptor mutations in human disease. Endocr Rev. 2004;25:869–98. doi: 10.1210/er.2003-0010. [DOI] [PubMed] [Google Scholar]
- 32.Dees WL, Skelley CW. Effects of ethanol during the onset of female puberty. Neuroendocrinology. 1990;51:64–9. doi: 10.1159/000125317. [DOI] [PubMed] [Google Scholar]
- 33.Srivastava V, Hiney JK, Nyberg CL, Dees WL. Effect of ethanol on the synthesis of insulin-like growth factor 1 (IGF-1) and the IGF-1 receptor in late prepubertal female rats: a correlation with serum IGF-1. Alcohol Clin Exp Res. 1995;19:1467–73. doi: 10.1111/j.1530-0277.1995.tb01009.x. [DOI] [PubMed] [Google Scholar]
- 34.Reilly MP, Saca JC, Hamilton A, Solano RF, Rivera JR, Whitehouse-Innis W, Parsons JG, Dearth RK. Prepubertal exposure to arsenic(III) suppresses circulating insulin-like growth factor-1 (IGF-1) delaying sexual maturation in female rats. Reprod Toxicol. 2013 Sep 30; doi: 10.1016/j.reprotox.2013.09.005. [Epub ahead of print] – in press. doi:10.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Russo J, Russo IH. Susceptibility of the mammary gland to carcinogenesis. II. Pregnancy interruption as a risk factor in tumor incidence. Am J Pathol. 1980;100:497–512. [PMC free article] [PubMed] [Google Scholar]
- 36.da Silva WGP, Campos RC, Miekeley N. A simple digestion procedure for the determination of cadmium, copper, molybdenum, and vanadium in plants for the determination of cadmium, copper, molybdenum and vanadium in plants by graphite furnace atomic absorption spectrometry and mass inductively coupled plasma spectrometry. Anal Lett. 1998;31:1061–70. [Google Scholar]
- 37.National Research Council (NCR) Subcommittee on the 10th edition of the recommended dietary allowances, food and nutrition board, commission on life sciences. 10th. Washington, DC: National Academy Press; 1989. [Google Scholar]
- 38.Pihl RO, Parkes M. Hair element content in learning disabled children. Science. 1977;198:204–6. doi: 10.1126/science.905825. [DOI] [PubMed] [Google Scholar]
- 39.Fell JM, Reynolds AP, Meadows N, Khan K, Long SG, Quaghebeur G, Taylor WJ, Mila PJ. Manganese toxicity in children receiving long-term parenteral nutrition. Lancet. 1996;347:1218–21. doi: 10.1016/s0140-6736(96)90735-7. [DOI] [PubMed] [Google Scholar]
- 40.Jones LA, Bern HA. Cervicovaginal and mammary gland abnormalities in BALB/cCrgl mice treated neonatally with progesterone and estrogen, alone or in combination. Cancer Res. 1979;39:2560–7. [PubMed] [Google Scholar]
- 41.Nandi S. Endocrine control of mammary gland development and function in the C3H/He Crgl mouse. J Natl Cancer Inst. 1958;21:1039–63. [PubMed] [Google Scholar]
- 42.Mallepell S, Krust A, Chambon P, Brisken C. Paracrine signaling through the epithelial estrogen receptor alpha is required for proliferation and morphogenesis in the mammary gland. Proc Natl Acad Sci USA. 2006;103:2196–201. doi: 10.1073/pnas.0510974103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Russo J, Russo IH. Development pattern of human breast and susceptibility to carcinogenesis. Eur J Cancer Prev. 1993;2:85–100. [PubMed] [Google Scholar]
- 44.Cotroneo MS, Wang J, Fritz WA, Eltoum IE, Lamartiniere CA. Genistein action in the prepubertal mammary gland in a chemoprevention model. Carcinogenesis. 2002;23:1467–74. doi: 10.1093/carcin/23.9.1467. [DOI] [PubMed] [Google Scholar]
- 45.Bern HA, Mills KT, Jones LA. Critical period for neonatal estrogen exposure in occurrence of mammary gland abnormalities in adult mice. Proc Soc Exp Biol Med. 1983;172:239–42. doi: 10.3181/00379727-172-41552. [DOI] [PubMed] [Google Scholar]
- 46.Bern HA, Edery M, Mills KT, Kohrman AF, Mori T, Larson L. Long-term alterations in histology and steroid receptor levels of the genital tract and mammary gland following neonatal exposure of female BALB/cCrgl mice to various doses of diethylstilbestrol. Cancer Res. 1987;47:4165–72. [PubMed] [Google Scholar]
- 47.Brown NM, Lamartiniere CA. Xenoestrogens alter mammary gland differentiation and cell proliferation in the rat. Environ Health Perspect. 1995;103:708–13. doi: 10.1289/ehp.95103708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kleinberg DL. Role of IGF-I in normal mammary development. Breast Cancer Res Treat. 1998;47:201–8. doi: 10.1023/a:1005998832636. [DOI] [PubMed] [Google Scholar]
- 49.Kleinberg DL, Feldman M, Ruan W. IGF-I: An essential factor in terminal end bud formation and ductal morphogenesis. J Mammary Gland Biol Neoplasia. 2000;5:7–17. doi: 10.1023/a:1009507030633. [DOI] [PubMed] [Google Scholar]
- 50.Brisken C. Hormonal control of alveolar development and its implications for breast carcinogenesis. J Mammary Gland Biol Neoplasia. 2002;7:39–48. doi: 10.1023/a:1015718406329. [DOI] [PubMed] [Google Scholar]
- 51.Choe SY, Kim SJ, Kim HG, Lee JH, Choi Y, Lee H, Kim Y. Evaluation of estrogenicity of major heavy metals. Sci Total Environ. 2003;312:15–21. doi: 10.1016/S0048-9697(03)00190-6. [DOI] [PubMed] [Google Scholar]
- 52.Shilkaitis A, Green A, Steele V, Lubet R, Kelloff G, Christov K. Neoplastic transformation of mammary epithelial cells in rats is associated with decreased apoptotic cell death. Carcinogenesis. 2000;21:227–33. doi: 10.1093/carcin/21.2.227. [DOI] [PubMed] [Google Scholar]
- 53.Berstein L, Tsyrlina E, Poroshina T, Bychkova N, Kalinina N, Gamajunova V, Vasiyev D, Kovalenko I. Switching (overtargeting) of estrogen effects and its potential role in hormonal carcinogenesis. Neoplasma. 2002;49:21–5. [PubMed] [Google Scholar]
- 54.Hamilton AS, Mack TM. Puberty and genetic susceptibility to breast cancer in a case-control study in twins. N Engl J Med. 2003;348:2313–22. doi: 10.1056/NEJMoa021293. [DOI] [PubMed] [Google Scholar]
- 55.Li CI, Malone KE, Daling JR, Potter JD, Bernstein L, Marchbanks PA, Strom BL, Simon MS, Press MF, Ursin G, Burkman RT, Folger SG, Norman S, McDonald JA, Spirtas R. Timing of menarche and first fullterm birth in relation to breast cancer risk. Am J Epidemiol. 2008;167:230–9. doi: 10.1093/aje/kwm271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Castoria G, Migliaccio A, Bilancio A, Di Domenico M, de Falco A, Lombardi M, Fiorentino R, Varricchio L, Barone MV, Auricchio F. PI3-kinase in concert with Src promotes the S-phase entry of oestradiol-stimulated MCF-7 cells. EMBO J. 2001;20:6050–9. doi: 10.1093/emboj/20.21.6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dillon RL, White DE, Muller WJ. The phosphatidyl inositol 3-kinase signaling network: Implications for human breast cancer. Oncogene. 2007;26:1338–45. doi: 10.1038/sj.onc.1210202. [DOI] [PubMed] [Google Scholar]
- 58.Grimm SL, Seagroves TN, Kabotyanski EB, Hovey RC, Vonderhaar BK, Lydon JP, Miyoshi K, Hennighausen L, Ormandy CJ, Lee AV, Stull MA, Wood TL, Rose JM. Disruption of steroid and prolactin receptor patterning in the mammary gland correlates with a block in lobuloal-veolar development. Mol Endocrinol. 2002;16:2675–91. doi: 10.1210/me.2002-0239. [DOI] [PubMed] [Google Scholar]
- 59.Russo J, Ao X, Grill C, Russo IH. Pattern of distribution of cells positive for estrogen receptor alpha and progesterone receptor in relation to proliferating cells in the mammary gland. Breast Cancer Res Treat. 1999;53:217–27. doi: 10.1023/a:1006186719322. [DOI] [PubMed] [Google Scholar]
- 60.Seagroves TN, Lydon JP, Hovey RC, Vonderhaar BK, Rosen JM. C/EBPbeta (CCAAT/enhancer binding protein) controls cell fate determination during mammary gland development. Mol Endocrinol. 2000;14:359–68. doi: 10.1210/mend.14.3.0434. [DOI] [PubMed] [Google Scholar]
- 61.Bhargava V, Thor A, Deng G, Ljung BM, Moore DH, Waldman F, Benz C, Goodson W, Mayall B, Chew K. The association of p53 immunopositivity with tumor proliferation and other prognostic indicators in breast cancer. Mod Pathol. 1994;7:361–8. [PubMed] [Google Scholar]
- 62.Coles C, Condie A, Chetty U, Steel CM, Evans HJ, Prosser J. p53 mutations in breast cancer. Cancer Res. 1992;52:5291–8. [PubMed] [Google Scholar]
- 63.Turner BC, Zhang J, Gumbs AA, Maher MG, Kaplan L, Carter D, Glazer PM, Hurst HC, Haffty BG, Williams T. Expression of AP-2 transcription factors in human breast cancer correlates with the regulation of multiple growth factor signalling pathways. Cancer Res. 1998;58:5466–72. [PubMed] [Google Scholar]
- 64.Anderson WF, Jatoi I, Devesa SS. Distinct breast cancer incidence and prognostic patterns in the NCI's SEER program: Suggesting a possible link between etiology and outcome. Breast Cancer Res Treat. 2005;90:127–37. doi: 10.1007/s10549-004-3777-3. [DOI] [PubMed] [Google Scholar]
- 65.McPherson LA, Loktev AV, Weigel RJ. Tumor suppressor activity of AP2alpha mediated through a direct interaction with p53. J Biol Chem. 2002;277:45028–33. doi: 10.1074/jbc.M208924200. [DOI] [PubMed] [Google Scholar]
- 66.Wajapeyee N, Somasundaram K. Cell cycle arrest and apoptosis induction by activator protein 2alpha (AP-2alpha) and the role of p53 and p21WAF1/CIP1 in AP-2alpha-mediated growth inhibition. J Biol Chem. 2003;278:52093–101. doi: 10.1074/jbc.M305624200. [DOI] [PubMed] [Google Scholar]
- 67.Stabach PR, Thiyagarajan MM, Woodfield GW, Weigel RJ. AP2alpha alters the transcriptional activity and stability of p53. Oncogene. 2006;25:2148–59. doi: 10.1038/sj.onc.1209250. [DOI] [PubMed] [Google Scholar]
- 68.Shirley SH, Rundhaug JE, Tian J, Cullinan-Ammann N, Lambertz I, Conti CJ, Fuchs-Young R. Transcriptional regulation of estrogen receptor-alpha by p53 in human breast cancer cells. Cancer Res. 2009;69:3405–14. doi: 10.1158/0008-5472.CAN-08-3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Herman-Giddens ME, Slora EJ, Wasserman RC, Bourdony CJ, Bhapkar MV, Koch GG, Hasemeier CM. Secondary sexual characteristics and menses in young girls seen in office practice: A study from the Pediatric Research in Office Settings network. Pediatrics. 1997;99:505–12. doi: 10.1542/peds.99.4.505. [DOI] [PubMed] [Google Scholar]
- 70.Parent AS, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon JP. The timing of normal puberty and the age limits of sexual precocity: variations around the world secular trends, and changes after migration. Endocr Rev. 2003;24:668–93. doi: 10.1210/er.2002-0019. [DOI] [PubMed] [Google Scholar]
- 71.Lee B, Pine M, Johnson L, Rettori V, Hiney JK, Dees WL. Manganese acts centrally to activate reproductive hormone secretion and pubertal development in male rats. Reprod Toxicol. 2006;22:580–5. doi: 10.1016/j.reprotox.2006.03.011. [DOI] [PubMed] [Google Scholar]


