Abstract
Objective
Given the critical role of behavior in preventing and treating chronic diseases, it is important to accelerate the development of behavioral treatments that can improve chronic disease prevention and outcomes. Findings from basic behavioral and social science research hold great promise for addressing behaviorally-based clinical health problems, yet there is currently no established pathway for translating fundamental behavioral science discoveries into health-related treatments ready for Phase III efficacy testing. This article provides a systematic framework for guiding efforts to translate basic behavioral science findings into behavioral treatments for preventing and treating chronic illness.
Methods
The ORBIT model for behavioral treatment development is described as involving a flexible and progressive process, pre-specified clinically significant milestones for forward movement, and return to earlier stages for refinement and optimization.
Results
This article presents the background and rationale for the ORBIT model, a summary of key questions for each phase, a selection of study designs and methodologies well-suited to answering these questions, and pre-specified milestones for forward or backward movement across phases.
Conclusions
The ORBIT model provides a progressive, clinically-relevant approach to increasing the number of evidence-based behavioral treatments available to prevent and treat chronic diseases.
Keywords: Health behavior, Behavioral intervention development, Translation of basic behavioral research into behavioral treatments, Behavioral study designs and methods, Prevention and management of chronic disease
INTRODUCTION
It is widely acknowledged that behavior plays a central role in the prevention and management of chronic disease. An estimated 40% of premature deaths are attributable to preventable behavioral factors such as smoking, unhealthy dietary intake, and sedentary lifestyle (Mokdad, Marks, Stroup, & Gerberding, 2004; Schroeder, 2007), all of which have been linked to major chronic illnesses such as cardiovascular disease, cancer, and diabetes (Chakravarty, Hubert, Krishnan, Bruce, Lingala & Fries, 2012; Yoon, Bastian, Anderson, Collins & Jaffe, 2014). Unhealthy lifestyles adversely affect health and well-being and increase the cost of health care, an increasingly important consideration given the aging of the U.S. population. Chronic diseases currently account for 85% of health care spending (Anderson, 2010), and the number of Americans who have at least one chronic disease is expected to rise from 145 million in 2009 to 157 million by 2020 (Anderson, 2010).
Engaging in health-promoting behaviors can reduce or delay the onset of chronic disease and disability (Paganini-Hill, Kawas & Corrada, 2011). Interventions that encourage healthy lifestyle behaviors, grounded in fundamental behavioral research and developed incrementally over time, have proven highly effective in reducing weight, blood pressure, cholesterol levels, and incidence of diabetes, among other clinically important outcomes (Appel, Clark, Yeh, Wang, Coughlin, Daumit, Miller, Dalcin et al., 2011; Appel, Champagne, Harsha, Cooper, Obarzanek, Elmer, Stevens, Vollmer, et al., 2003; Epstein, Paluch, Roemmich & Beecher, 2007; Knowler, Barrett-Connor, Fowler, Hamman, Lachin, Walker, Nathan, Diabetes Prevention Program Research Group, 2002; Wadden, Volger, Sarwer, Vetter, Tsai, Berkowitz, Kumanyika, Schmitz et al., 2011). Given the potential for evidence-based behavioral treatments to address many of the costly health problems facing an aging population, accelerating their development and optimization is a public health priority.
Translation I research in which “bench” findings are applied to the “bedside” is uncommon in the behavioral arena. Advances in understanding fundamental human processes such as motivation, emotion, cognition, self-regulation, decision-making, stress, and social networks are not being optimally applied to pressing behavioral health problems. Promising behavioral treatments “in the pipeline” are often abandoned, rather than refined, if they fail early tests. In some cases, treatments that achieve success in early studies are not pushed toward rigorous testing in Phase III efficacy trials, while others are tested in Phase III trials prematurely, without undergoing adequate development and refinement in early-phase studies. This contrasts with the biomedical arena which benefits from industry support for early translational research and has a well-defined process for developing a new drug or device, starting with basic science research and progressing to the FDA-required Phase III efficacy trial.
In light of the importance of behavioral treatment development, the National Institutes of Health Office of Behavioral and Social Sciences Research (OBSSR), in collaboration with the National Heart, Lung, and Blood Institute (NHLBI), the National Cancer Institute (NCI), the National Institute of Diabetes, Digestive and Kidney Diseases (NIDDK), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and investigators from the Obesity-Related Behavioral Intervention Trials (ORBIT) consortium organized a cross-disciplinary conference of experts in fields related to the development and early testing of behavioral health-related treatments. An important goal of this conference was to propose guidelines for developing behavioral treatments to prevent or manage chronic diseases, proceeding from the identification of innovative approaches to solving important clinical problems based on basic behavioral science theory and research, to the design and preliminary testing of promising treatments, through readiness to conduct a Phase III efficacy trial. Conference participants included members of the ORBIT consortium, all of whom are currently developing obesity treatments, NIH Program Officers, and experts in the discoveries, designs, methodologies, and infrastructures needed to develop and test innovative, health-related behavioral treatments (conference information can be found at http://www.nihorbit.org/ORBIT%20Content/Workshops%20and%20Conferences.aspx?PageView=Shared).
This paper presents the ORBIT model for developing behavioral treatments to prevent and/or manage chronic disease. The model was informed by the process of drug development and by previous efforts to define the behavioral treatment development process. It reflects a comprehensive synthesis of the recommendations made by Workshop participants as further refined by NIH collaborators and ORBIT consortium members.
THE ORBIT MODEL FOR BEHAVIORAL TREATMENT DEVELOPMENT
The Process of Drug Development
The development of a new drug is guided by regulatory requirements and typically sponsored by Industry (Friedman, Furburg, & DeMets, 1998; Lipsky & Sharp, 2001). It begins with preclinical basic science studies in animal or in vitro models to understand the disease process. Phase I and II studies turn to humans and focus on small numbers of highly-selected subjects. Phase I “dose-finding” studies determine the maximally tolerated dose of a candidate drug, while Phase II studies determine the impact of this maximally tolerated dose on significant biologic activity. If the drug has minimal biologic impact, it is re-examined in Phase I and/or preclinical studies or abandoned. If the drug has the expected impact, a pilot study is often conducted to determine the feasibility and acceptability of a trial protocol in preparation for the Phase III efficacy trial, where the drug’s impact on clinically meaningful endpoints such as disease onset, progression and mortality is evaluated (Friedman, Furburg, & DeMets, 1998; Lipsky & Sharp, 2001). Demonstration of benefit of the drug on clinically significant outcomes in Phase III efficacy trials is key to Food and Drug Administration (FDA) approval and introduction of the drug into the commercial market.
Previous Efforts to Define a Process for Behavioral Intervention Development
Since the development of behavioral treatments that target physical health outcomes has not been regulated by any governmental agency, it has not been guided by any widely recognized and agreed-upon process. A number of prior efforts describing behavioral treatment development include: Green’s (1974) PRECEDE/PROCEED model for health program planning; Greenwald & Cullen’s (1985) 5-phase cancer control model; Flay’s 8-stage health promotion model; the National Institute of Drug Abuse’s (NIDA) Stage Model (Carroll & Onken, 2005; Rounsaville, Carroll, & Onken, 2001); the EVOLVE mixed-methods model (Peterson, Czajkowski, Charlson, Link, Wells, Isen, Mancuso, Allegrante, et al., 2013); the Medical Research Council’s framework for complex interventions (Craig, Dieppe, Macintyre, Michie, Nazareth, & Petticrew, 2008; Craig, Dieppe, Macintyre, Michie, Nazareth, & Petticrew, www.mrc.ac.uk/complexinterventionsguidance, 2008); and Intervention Mapping (Bartholomew, Parcel, Kok, Gottlieb & Fernandez, 2011).
The ORBIT model, first introduced in several recent NIH initiatives (National Heart, Lung, and Blood Institute, 2008; Office of Behavioral and Social Sciences Research, 2010), differs from many of these earlier approaches in several ways. First, it focuses exclusively on the early, pre-efficacy phases of behavioral treatment development. This makes it possible to integrate the ORBIT framework with those frameworks that outline steps beyond the pre-efficacy phase (e.g., as NIDA’s Stage Model, the MRC model or Intervention Mapping). Second, it was developed for use with a broad array of chronic diseases, unlike some of the earlier models which were developed for specific disorders such as cancer, drug abuse or mental illness. Third, it intentionally uses terminology from the drug development model to enhance comprehension by medical gatekeepers who most commonly manage chronic diseases with drug therapy.
Overview
Figure 1 presents the ORBIT model. Critical features include a flexible and iterative progressive process, pre-specified clinically significant milestones for forward movement, and return to an earlier phase for refinement in the event of suboptimal results. Although its primary focus is on pre-efficacy development and testing, later phases of research are included to show that optimization of treatments can occur even for those that have reached the efficacy or effectiveness stage. Table 1 presents a summary of key questions of relevance for each phase, a selection of designs and methodologies that are well-suited to answering these questions, and pre-specified milestones for forward or backward movement across phases.
Figure 1.
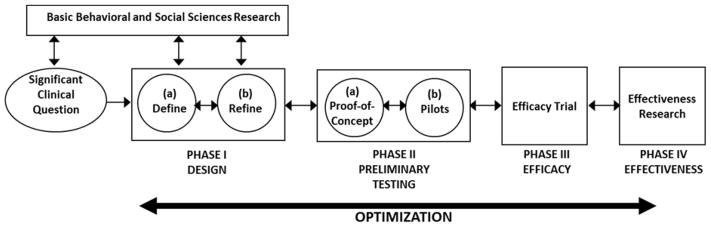
The ORBIT Model for Behavioral Treatment Development
Table 1.
Goals, methods, spirit of inquiry, and milestones, by phase, in the ORBIT model
| PHASE | CENTRAL GOAL | SPECIFIC GOALS | COMMON METHODS | SPIRIT OF INQUIRY | MILESTONE |
|---|---|---|---|---|---|
|
| |||||
| Phase Ia: Define | Define basic elements | Develop hypothesized pathway by which behavioral treatment can solve important clinical problem Provide scientific basis for behavioral milestones Provide basic behavioral/social science basis for treatment components and their respective treatment targets Identify appropriate subjects Assess safety, tolerability, acceptability |
Review of clinical guidelines, meta-analyses, clinical trials, basic behavioral/social science findings, epidemiologic studies. Experiments Time Series ABA designs N-of-1 trials Ecological observational studies Formative qualitative research:
Evidentiary studies |
Creativity Discovery Flexibility Experimentation Hypothesis formulation |
Candidate treatment components, methods, and milestones are defined. |
|
| |||||
| Phase Ib: Refine | Refine for strength and efficiency | Identify independent and interactive treatment components Determine needed dose/duration Determine mode of delivery Determine need for tailoring for:
If needed, optimize treatment using basic behavioral/social science findings Assess safety, tolerability, acceptability |
Factorial/fractional factorial designs Adaptive designs Experiments Time series Adaptive treatments Formative qualitative research:
Signal detection methods |
Efficiency Tailoring Clinical relevance Sensitivity to patient Optimization Flexibility |
Satisfaction that the treatment package is complete and the fixed protocol is ready for testing |
|
| |||||
| Phase IIa: Proof-of-Concept | Clinically significant signal | Prepare the Treatment Manual and procedures for fidelity monitoring. Determine whether the treatment can achieve a clinically significant signal on the behavioral risk factor |
Quasi-experimental, treatment-only design | Test of a fixed protocol | A clinically significant impact on the behavioral target |
|
| |||||
| Phase IIb: Pilot Testing | Clinically significant signal over noise | Replication of clinically significant signal in treated Clinically significant signal over noise Adequacy of control group:
|
Randomized designs | Randomized test of a fixed protocol Systematic study of control group |
Empirical support that:
|
| Feasibility Pilot | Feasibility of efficacy trial protocol | Prepare the Manual of Operations for trial protocol Determine logistical feasibility of trial protocol for investigators and participants Provide estimates needed for design of efficacy trial:
|
Qualitative and quantitative studies of feasibility & acceptability | Acceptability to target population Performance across multiple sites Preparation for efficacy trial
|
Empirical support that the trial protocol is logistically feasible Empirically derived estimates available for the design of an efficacy trial |
Identification of a Significant Clinical Question
Coller (2008) has observed that one of the key skills necessary for a successful translational effort is the ability to articulate a health need or clinical question requiring a solution “with the precision of a basic science hypothesis.” Thus, the process of behavioral treatment development is informed by the identification of a clinical question or problem for which a behavioral treatment could provide a solution. For example, the clinical problem could be that there is no current treatment for a specific condition, a current treatment is not potent or durable enough to change an important clinical outcome, a current treatment is effective but adherence to it is poor, or it produces too many side effects for routine use in clinical practice.
The hypothesis that change in a behavioral risk factor could solve a clinical problem is one of the entry points for behavioral treatment development (depicted in Figure 1 by the arrow going from “Significant Clinical Question” to Phase I studies). The initial explicit identification of the clinical problem does several things. It encourages investigators to (1) set sights on the Phase III efficacy trial which will test the benefit of the behavioral treatment on an outcome that is meaningful in clinical practice; (2) consider early on the primary behavioral, clinical, or biomedical endpoints in that efficacy trial; and (3) commit to achieving a sufficiently potent level of behavioral change to achieve meaningful change on the ultimate biomedical or clinical outcome.
Application of Basic Behavioral and Social Sciences Research
Basic behavioral and social science research can foster hypotheses about the drivers of a behavioral risk factor. Treatment development would then proceed with the translation of these drivers of behavior into treatment targets, an identification of clinical methods by which these targets can be altered, and the translation of targets into quantifiable process measures to assess the success of the treatment. For example, basic behavioral science has shown that mobilization of executive function can serve as a “brake” on impulsive eating (Fagundo, de la Torre, Jimenez-Murcia, Aguera, Granero, Tarrega, Botella, Banos, et al., 2012). Thus, it is reasonable to hypothesize that improvement in executive function would reduce impulsive eating. Treatment development would then involve identifying specific executive function skills, such as planning and problem-solving; designing a training program to teach these skills; and developing process measures to assess them.
The reciprocal connection between basic behavioral and social sciences research and early phases of behavioral treatment development is depicted in Figure 1 by bi-directional arrows. While a clinical problem can foster a search for relevant basic science guidance, basic science discoveries can inspire an application of that process to a pressing clinical problem. Various independent basic science findings can justify inclusion of several candidate treatment components, each with their own treatment targets, clinical methods, and process measures. Basic science can inform the dose and method of delivery of a behavioral treatment. If a treatment approaches, but does not achieve, clinically significant milestones, avenues for optimization may also be informed by basic science findings.
Phase I: Design
The goal of Phase I is to design the essential features of a behavioral treatment or to adapt an existing treatment. The hallmark is that the protocols are fluid, permitting ongoing adjustments in response to evolving findings. While initial tests may be on a small number of highly selected participants, milestones for judging success of the treatment should be ambitious, providing a needed cushion for subsequent testing in more diverse samples.
Phase Ia: Define
Purpose and Goals
In Phase Ia, the scientific foundation and the basic elements of the behavioral treatment are defined. Among the goals are the development of a hypothesized pathway by which a behavioral treatment could solve a clinical problem, specification of clinically significant milestones on the behavioral risk factor being targeted, identification of appropriate subjects, definition of potential treatment components, and elucidation of basic behavioral and social sciences support for potential treatment components and their respective treatment targets.
Milestones for judging the success of a treatment on the behavioral risk factor are guided by the clinical problem. A priori specification of these milestones prevents “moving the target” after the data are in. Basing milestones on clinical significance avoids the problem of erroneously assuming that a treatment or treatment component is successful if it produces a small benefit that is statistically significant but not clinically meaningful. Milestones can be identified from epidemiological, experimental, or clinical studies which link the behavioral factor to accepted biomedical risk factors, surrogate markers, subclinical disease, or clinical events. Finding a cutpoint on a behavioral measure where biomedical risk increases is suggestive of a level of change in the behavioral factor that is clinically relevant. This may be an easier task for established behavioral risk factors such as smoking, alcohol/drug abuse, diets high in saturated fat or sodium, physical inactivity, stress, depression, and low social support, all of which have an evidence base to support their links to physical health. However, in the case of novel behavioral risk factors where such an evidence base may not exist, Phase Ia experimental studies examining covariation between the behavioral factor and a relevant biomedical risk factor would be justified. For example, weight loss of 3 – 5% in overweight and obese adults with cardiovascular disease (CVD) risk factors can be deemed a clinically significant change since it has been shown to result in clinically meaningful cardiovascular and metabolic benefits (Jensen, Ryan, Apovian, Loria, Ard, Millen, Comuzzie, Nonas, et al., 2013). Were these data not available, Phase Ia experimental studies examining the covariation between level of weight loss and a cardiovascular risk factor, such as blood pressure, might be needed.
Study Designs and Methods
A range of designs and methods are appropriate for Phase Ia studies. Systematic reviews, meta-analyses, and epidemiologic studies can be used to identify clinically significant problems, endpoints, milestones, and links between behavioral risk factors and clinical endpoints. Taxonomies and “mapping” strategies that characterize behavioral interventions and their components can help in selecting treatment elements (Michie, van Stralen, & West, 2011; Bartholomew, Parcel, Kok, & Gottlieb, 2011). Experimental and observational studies in the laboratory and field enable the researcher to identify potential treatment components and the respective biological, psychophysiological, social, environmental, and behavioral targets needed to accomplish change in them and, in turn, in the behavioral risk factor. Ethnographic observation and interviews, focus groups, and other qualitative research methods can engage the community of participants in the development of user-centered strategies and help identify attitudes, norms, and values that can affect an intervention’s acceptability and feasibililty (Peterson, Czajkowski, Charlson, Link, Wells, Isen, Mancuso, Allegrante, et al., 2013; Strolla, Gans, & Risica, 2006). Observational techniques such as behavioral event modeling (Wansink, 2010) and use of ecological momentary assessment (Shiffman, Stone, & Hufford, 2008) permit the study of treatment effects in naturalistic settings, leading not only to the identification of intervention targets but also of critical time points for intervention delivery. Time series and ABA designs, involving multiple baseline and treatment observations in small numbers of individuals (Ridenour, Pineo, Molina, & Lich, 2013), allow researchers to identify early indications of potential benefit. New analytic techniques for use with small samples, including Bayesian analyses of data from N-of-1 trials, may be useful in Phase Ia studies (Zucker, Ruthazer, & Schmid, 2010). Phase Ia studies are consistent with “evidentiary studies” (Stevens, Taber, Murray, & Ward, 2007), a term used to describe a series of small, inexpensive experiments or observational studies of the impact of potential treatment components on process variables.
Milestones
Movement to Phase Ib is warranted once the hypothesized pathway by which the proposed treatment translates into benefit on the behavioral risk factor is formulated, potential candidates for treatment components have been assembled, intervention targets for each component have been delineated, and milestones for determining clinically significant change on the behavioral risk factor have been defined.
Phase Ib: Refine
Purpose and Goals
The goal of Phase Ib is to refine the treatment to promote efficiency while at the same time retaining sufficient strength to promote clinically significant change. Candidate components are examined together for the first time, and those that are essential and show independent effects on the behavioral risk factor are identified. Serial presentation of independent components might be studied. Practical aspects of the intervention such as mode of delivery, agent of delivery, frequency of contact, and duration of contacts are studied to identify the most efficient ways to achieve clinical targets. In cases of variable response to a single protocol, the development of adaptive treatments with tailoring for special population subgroups or for differential response to treatment could be considered. Phase Ib studies are ideally suited to the adaptation of an existing treatment to new conditions, such as a new patient population, new settings or context, or new mode of delivery.
Study Designs and Methods
Factorial and fractional factorial designs (Chakraborty, Collins, Strecher, & Murphy, 2009) are ideal for determining independent treatment components or combinations of components, optimum methods of delivery, and optimum dose of treatment. Small single case or case series studies can be used to evaluate dose, safety, tolerability, and impact of introduction of new treatment components (Dallery & Raiff, 2014; Kazdin, 1982). The more rigorous adaptive designs provide the methodology for determining the impact of serial introduction of multiple treatment components (Lei, Nahum-Shani, Lynch, Oslin, & Murphy, 2012). Modeling techniques derived from engineering and control systems theory can simulate intervention effects within complex systems to determine how to achieve maximum benefit (Navarro-Barrientos, Rivera, & Collins, 2011). Analytic procedures drawn from engineering can quantify the patterns of intervention effects over time to determine the appropriate timing for evaluating the effects of the treatment on a behavioral risk factor (Navarro-Barrientos, Rivera, & Collins, 2011). An adaptive treatment, rather than a “one size fits all” treatment, could be developed to feature tailoring based upon pre-specified decision rules for participant characteristics or response to treatment (Lei, Nahum-Shani, Lynch, Oslin, & Murphy, 2012). Signal detection methods can be useful for identification of subgroups for which tailoring of treatment could optimize their outcomes (Palmer & Hadley, 2005). Qualitative methods evaluate the acceptability to the target population and help refine the content and delivery of the intervention package (Strolla, Gans, & Risica, 2006).
Milestones
The essential milestone for moving to Phase II is the confidence of the investigator that the treatment package is complete, includes its essential components offered in an efficient way, is safe and acceptable to the population of interest, and it is plausible that the treatment will have a clinically significant benefit on the behavioral risk factor. Evidence of these achievements signals that the treatment protocol is ready to undergo a “proof-of-concept” test.
Phase II: Preliminary Testing
The goal of Phase II studies is to test the ability of a fixed treatment package to produce a clinically significant improvement on a behavioral risk factor.
The hallmark of Phase II studies is that the intervention protocol is fixed and clearly articulated in a treatment manual. Two types of testing can be conducted, often serially. A preliminary, proof-of-concept test is a cost-effective way to determine if a treatment package can achieve benefit on a clinically significant target in a small, select sample. If it does, it justifies going forward with more rigorous pilot testing using a randomized design and larger, more representative samples. Sequential testing, beginning with a small proof-of-concept test, avoids wasting the resources needed for the more ambitious randomized design on a treatment that cannot hit clinically significant targets under optimum conditions.
Phase IIa: Proof-of-Concept
Purpose and Goals
The main goal of the proof-of-concept study is to determine, in an efficient way, whether or not the treatment merits more rigorous and costly testing using a randomized design. This is done by determining if the treatment package that emerges from Phase I studies can produce a clinically significant improvement on the behavioral risk factor under ideal conditions. Because the protocol is fixed, a Treatment Manual can be written that promotes quality assurance, including materials for training, ongoing supervision, and fidelity monitoring of interventionists.
Study Designs and Methods
Quasi-experimental, within-subjects designs where subjects act as their own controls in a pre-post treatment comparison are ideal. The sample size can be small since clinical, not statistical, benefit is sought and sample size calculations are unnecessary. The sample can be selected from accessible subjects, rather than be representative, because this initial test will determine only whether the treatment merits more rigorous testing. The focus of Phase IIa studies is deliberately placed on the treatment and its ability to produce clinically significant change on a behavioral risk factor. This is in contrast to Phase IIb concerns with determining the source of a treatment effect (e.g., separation of treatment effects from effects due to the passage of time) and with choosing the appropriate control group, in itself a complex undertaking (Mohr, Spring, Freedland, Beckner, Arean, Hollon, Ockene, & Kaplan, 2009).
Milestones
The essential milestone for moving to Phase IIb is that the treatment achieved a clinically significant change in the behavioral risk factor. If an ambitious clinical criterion was approached, but not achieved, optimization using qualitative inquiry could justify forward movement to Phase IIb. If the clinical criterion was not even approached, the researchers could return to Phase I studies or decide if it is justified to abandon the treatment.
Phase IIb: Pilot Testing
Purpose and Goals
Pilot studies have a variety of purposes and can take many forms, but the proposed model includetwo general types: pilot studies and feasibility pilot studies. Pilot studies are natural follow-ups to a positive proof-of-concept study. Their main goal is to determine whether a clinically significant benefit on the behavioral risk factor can be achieved in a larger, more representative sample and whether this benefit is above and beyond the passage of time and non-specific effects that could occur in a control group. There are many choices for a control group, ranging from a less stringent usual care control to a more stringent attention control (Freedland, Mohr, Davidson, & Schwartz, 2011; Kraemer, Mintz, Noda, & Tinklenberg, 2006). At minimum, all control group designs control for the passage of time by determining whether the subject would have achieved benefit over time without treatment. The specific choice of the type of control should be based upon the clinical question of interest. For example, some questions focus on whether or not the treatment can do better than usual care. Other questions focus on whether or not the treatment can do better than non-specific attention, whether it can outperform an existing treatment, or equal the performance with less burden and cost. Once the decision about the appropriate control is made, systematic study of the control group’s properties is needed (Mohr, Spring, Freedland, Beckner, Arean, Hollon, Ockene, & Kaplan, 2009). Phase IIb is an ideal time to study the control group’s credibility, dropout rate, and influence on the behavioral risk factor.
Feasibility pilot studies serve as preparation for the Phase III efficacy trial. Their key purpose is to assess the feasibility of the trial protocol and provide estimates needed to design the efficacy trial. Is the trial protocol feasible and acceptable to the investigators and to the participants? Is there differential drop-out from the randomized arms? What is the screening to enrollment ratios overall and at each of the recruiting sites? Although a feasibility pilot study produces an unstable estimate of the effect size on the primary endpoint of the Phase III efficacy trial (Kraemer, Mintz, Noda & Tinklenberg, 2006), it should be considered along with accumulated effect sizes from similar past trials to determine the sample size needed for the efficacy trial.
Study Designs and Methods
Most types of pilot studies use a randomized design. Systematic evaluation of a variety of control groups can proceed using fractional factorial designs. Feasibility pilot studies draw on qualitative methods, such as focus groups and ethnographic interviews, to assess participant and staff acceptance of, and preference for, all aspects of the protocol including methods and measures, treatment and control conditions and procedures, and quality assurance protocols. The findings can facilitate protocol refinement and simplification in light of direct experience.
Milestones
Phase IIb data showing that a behavioral treatment produces a clinically significant signal on the behavioral risk factor in patients with the specified clinical problem, while the chosen control does not, supports moving to a Phase III efficacy trial. Empirical support for the feasibility of the trial protocol includes a conservative and feasible recruitment plan, an estimate of drop-out rates from both treated and control conditions, and an estimate of the probable range of effect sizes that will be attained on the proposed primary clinical endpoint in the efficacy trial.
Application of the ORBIT Model
The ORBIT model proposes a set of studies that form a chain of evidence that signals readiness for an efficacy trial. This chain of evidence can be translated into a pathway by which the treatment is hypothesized to work (Figure 2). The link between A and B demonstrates that the specific targets of the treatment can be implemented by the subjects. The link between A and C shows that the treatment is powerful enough to produce clinically significant improvement in the behavioral risk factor of interest. A fully-powered determination of the causal links between A and D or E would occur in an eventual efficacy trial.
Figure 2.

Model of pathway by which a behavioral treatment is hypothesized to improve a clinical outcome. CS
 = Clinically Significant Change
= Clinically Significant Change
The value of articulating a hypothesized pathway, and collecting measurements for each of its links, lies in the ability to more precisely interpret the results of Phase III trials (Powell, Calvin, Mendes de Leon, Richardson, Grady, Flynn, Rucker-Whitaker, Janssen, et al., 2008). If the trial produces positive results, evidence specifically supporting each link of the chain strengthens the assertion that the observed effects were due to hypothesized pathways. Evidence collected for each link of the chain in negative trials can help determine whether null findings were due to a failure of the hypothesis that change in the behavioral risk factor is causally related to change in the biomedical outcome or a failure of the treatment to affect its targets.
Use of the ORBIT model is illustrated by applying it to the development of a behavioral treatment to prevent diabetes in insulin resistant patients. Clinical guidelines promote lifestyle change as the first step in treatment of insulin resistant patients (Handelsman, Mechanick, Blonde, Gruberger, Bloomgarden, Bray, Dagogo-Jack, Davidson, et al., 2011), but few validated lifestyle interventions are available. The scenario below shows how the model could be implemented to develop and test such an intervention.
Using the ORBIT model, a Phase Ia review of epidemiological studies and clinical trials provides empirical support for the clinical significance of a 30% reduction in diabetes over standard drug treatment (Hamman, Wing, Edelstein, Lachin, Bray, Delahanty, Hoskin, Kriska, et al., 2006), a 5% weight loss as a biomedical target (Hamman, Wing, Edelstein, Lachin, Bray, Delahanty, Hoskin, Kriska, et al., 2006), and both dietary changes (e.g., a 5% reduction in percent fat) and increases in physical activity (e.g., 150 minutes/week of moderately vigorous physical activity) as behavioral targets (Hamman, Wing, Edelstein, Lachin, Bray, Delahanty, Hoskin, Kriska, et al., 2006). Phase Ia investigation of basic science studies shows both theoretical and empirical support for the value of daily self-monitoring on reducing percent fat in the diet and increasing physical activity.
A Phase Ia test of daily self-monitoring in a small N time series of selected patients shows that patients will self-monitor both their dietary intake and their physical activity, as evidenced by their willingness to transmit data from daily food diaries and accelerometers. However, although the goals of a 5% reduction in fat and physical activity target of 150 minutes/week are achieved, they are not sustained over time. Based on further review of basic science studies suggesting that tailored feedback potentiates the effects of self-monitoring, internet-delivered tailored feedback is added to the Phase Ia small N time series study and receipt of the feedback is measured by frequency of patient log-ins to receive feedback. With this addition, sustained achievement of the diet and physical activity milestones is obtained.
A subsequent Phase Ib fractional factorial study determines that daily self-monitoring and tailored feedback together are more potent than is either one alone in achieving the 5% weight loss (biomedical) target. The combined findings suggest that a treatment with two components is needed and the effects are robust enough to move to Phase II studies, at which point a Treatment Manual would be written and a treatment-only “proof-of-concept” study is run on a small number of accessible patients. If the dietary, physical activity and weight loss milestones are achieved, this justifies a more robust pilot test in a larger, more diverse sample of patients using a randomized design. Such a series of studies make it possible to articulate the hypothesized pathway by which the proposed treatment may improve the biomedical or clinical outcome (Figure 3).
Figure 3.
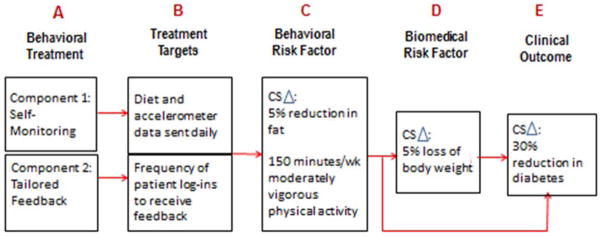
Illustration of a pathway by which a weight loss treatment is hypothesized to reduce diabetes (CS
 = Clinically Significant Change)
= Clinically Significant Change)
All of the studies presented in this illustration could be conducted by a single investigator, by a single team of investigators, or by a group of investigators who are, at best, loosely connected. An essential assumption of the ORBIT model is that research efficiency and quality are enhanced when a collaborative team of investigators works to integrate diverse studies into a chain of evidence that justifies a Phase III trial.
SUMMARY AND CONCLUSIONS
Given the vital role of behavior change in delaying or mitigating the effects of costly chronic diseases, effective and durable behavioral treatments are urgently needed. The ORBIT model was developed to identify the most productive ways to accomplish this. In doing so it provides guidance on the process of treatment development, but does not propose any specific content. It suggests that use of a progressive, transdisciplinary framework to facilitate the translation of basic behavioral science findings to clinical application will strengthen behavioral treatments, encourage their testing in rigorous efficacy and effectiveness trials, promote success, and foster dissemination into clinical practice.
Currently, all of the elements needed to go from ideas to the efficacy trial exist, but the pathway for doing so is not well-defined. Without the early discovery and innovation that is characteristic of basic science, the potential for behavioral treatments to be robust enough to produce clinically significant results is undercut. Without a progressive approach to treatment development that embraces a variety of designs and methods, there is a risk that promising treatments will be abandoned prematurely because they fail tests that are too stringent for an evolving understanding of their fundamental elements. Without input from clinical practice, treatments that are judged to be successful because they achieve statistical significance may produce results that are too small to be clinically meaningful. Without a consistent framework to support progression toward the behavioral efficacy trial, the ability of behavioral and social science researchers to provide the type of evidence that is meaningful to clinical gatekeepers and third-party payers is compromised.
The ORBIT model stitches together these existing crucial elements in the behavioral sciences in several ways. First, it pushes behavioral and clinical investigators to set sights on the efficacy trial, identifies the chain of evidence needed to support such a trial, and outlines the careful, progressive program of research that can provide this evidence.
Second, the ORBIT model encourages a transdisciplinary approach. Crosstalk between basic and applied behavioral and social scientists can foster innovative approaches to common problems, leading to novel methods for motivating patients to stick with early changes, sustaining change by mobilizing social networks, or maximizing executive function as a way to minimize impulsivity. Crosstalk between clinicians in medical practice and applied behavioral scientists can increase the significance of the questions posed and identify targets that have meaning in clinical practice. Such crosstalk can facilitate the evaluation of a behavioral treatment’s viability as an alternative or adjunctive treatment, its side effect profile and effects on quality of life, and its potential cost-effectiveness relative to existing treatments.
Third, the ORBIT model advocates for flexibility in both the designs and methodologies used for treatment development. In the early phases, it encourages investigators to draw on clinical acumen and creativity to “tweak” treatments in response to ongoing results as treatments unfold. This type of work can and should be clearly separated from the testing of fixed protocols. Currently, most behavioral treatments are tested in the same way using randomized designs with attention control groups, fixed protocols, and blinding to outcomes until follow-up is complete. There is a strong rationale for such methods, but when used universally, particularly in the early phases of treatment development, their use can undercut creativity, impede discovery, and produce weak treatments.
Fourth, the ORBIT model provides a language for conveying the significance of a small, single study, which can be especially important in relation to evaluations of grants and papers. Small, early phase studies run the risk of being judged to be not very important. However, if they are conceptualized as crucial steps in a program of research that is directed toward efficacy and effectiveness trials to answer pressing clinical problems, their value becomes more apparent.
There are several things that the ORBIT model is not. First, it is not prescriptive or restrictive. Rather, it identifies a flexible strategy for collecting a chain of evidence to enhance confidence that a promising behavioral treatment is ready for Phase III testing. The entire series of studies in the model could be conducted by a single investigator, a team, or, to save money and time, by integrating findings from multiple but unrelated investigators. By analogy, to build a house one could do everything alone or could serve in the role of an architect who creates the design but relies upon a team for construction, painting, plumbing, and electricity.
Second, the ORBIT model does not require that treatment development proceed precisely as specified in the framework, starting with Phase Ia and ending with Phase IIb. In many cases, a behavioral treatment already exists and the task is simply to adapt it to a new clinical problem, a new patient population, and/or a new outcome. In this case, the model could be used to decide what additional testing is needed. A small proof-of-concept study in a new patient population may show, for example, that results are promising but suboptimal, suggesting moving back to Phase Ib for refinement that could optimize results. In other cases, a new behavioral treatment may be so innovative and compelling that development is unnecessary and immediate testing in a feasibility pilot is warranted. The value of the ORBIT model lies in the provision of a framework that can facilitate decision-making in light of various outcomes.
Third, the ORBIT model does not require evidence to be accrued for each “link” in the chain. For example, where well-established evidence exists linking a behavioral or biomedical risk factor to a clinical outcome (e.g., evidence of association between smoking and heart or lung disease), evidence from an efficacy trial that a behavioral treatment affects that behavioral or biomedical risk factor (e.g., smoking) may be sufficient to influence clinical practice. In other cases, evidence of an intervention’s effect on a treatment target may be adequate for proceeding to a clinical endpoint trial without evidence of the intervention’s effect on a behavioral risk factor. An example is the use of biofeedback to treat chronic pain. Research using fMRI has shown that biofeedback can directly alter neural activity in specific brain regions and these changes can affect chronic clinical pain (deCharms, Maeda, Glover, Ludlow, Pauly, Soneji, Gabrieli, & Mackey, 2005). Here, one could move from evidence of an effect on the treatment target of brain activity (B) to a test of the efficacy of biofeedback on a clinical outcome (severity of pain) without evidence of the treatment’s effect on an intervening behavioral risk factor (C).
Fourth, the ORBIT model does not require mechanisms and moderators to be included in all phases of behavioral treatment development. While understanding the mechanisms through which an intervention produces its effects can be useful in developing better-targeted interventions, it is not critical to understand mechanisms of action in order to demonstrate an intervention’s effectiveness (Blumenthal, Babyak, Wei, O’Connor, Waugh, Eisenstein, Mark, Sherwood, et al., 2002). In the ORBIT model, physiological mechanisms by which the behavioral risk factor translates into biomedical risk (referred to as biologic plausibility in epidemiology) can be focused on in early Phase Ia studies to help justify the choice of a behavioral risk factor and its cutpoint for clinical significance. Moreover, moderators (also called a priori subgroups in clinical trial methodology) can be studied in Phase Ib with the goal of adapting treatments so they are maximally powerful across all patients at risk. This can and should occur before the fixed protocol is tested in Phase II.
In summary, the ORBIT model is a systematic framework for guiding efforts to link behavioral solutions to clinical problems. Discoveries in the basic behavioral and social sciences hold great promise for addressing clinical health problems, yet there is no commonly established way to translate these findings into behavioral treatments. In the absence of industry support and regulatory guidelines, it is important for the behavioral science community to articulate and adopt models of behavioral treatment development that can facilitate the application of viable behavioral solutions to pressing health problems. Past models have emphasized the value of a systematic, phased approach to behavioral treatment development, and the recent emergence of evidence-based behavioral medicine (Davidson, Goldstein, Kaplan, Kaufmann, Knatterud, Orleans, Spring, Trudeau, & Whitlock, 2002) provides clear standards for rigor. The ORBIT model complements and extends these efforts in proposing a systematic, well-defined process by which the investigator can achieve these rigorous standards.
Transparency of milestones for success in later phases of interventional research has been made possible by the required registration of trials in clinicaltrials.gov. An infrastructure to allow similar transparency in the earlier phases of behavioral treatment development is needed and perhaps can be facilitated by use of the ORBIT framework. Finally, since the ORBIT model focuses exclusively on the early phases of behavioral treatment development, further evolution of the model to deal with later phases of translation is needed to achieve the ultimate goal of improving the utilization of proven behavioral treatments in clinical and community settings.
Currently, a window of opportunity exists in which demand is growing for primary and secondary prevention strategies that can reduce the burden of costly chronic diseases which primarily have behavioral causes. This creates the need for a larger pool of effective behavioral treatments available for use in clinical care than currently exist. To take advantage of this opportunity, it is essential that investigators systematically build the rationale for the need for a behavioral efficacy trial, conduct that efficacy trial, and, by so doing, build the evidence base for the value of behavioral treatments in clinical practice using the language of medicine and third-party payers. Creating a robust evidence base for the value of behavioral treatments in clinical care has enormous potential to expand choices for patients, improve quality of care, prevent or delay disease and disability, and, most urgently, lower health care costs not only for individual patients but for society as a whole.
Supplementary Material
Acknowledgments
The Obesity-Related Behavioral Intervention Trials (ORBIT) consortium, consisting of seven research projects and a Resource and Coordination Unit (RCU), was supported by cooperative agreement grants from the National Institutes of Health. In particular, the National Heart, Lung, and Blood Institute funded grants # U01 HL097843, U01 HL097894 and U01 HL097973. The National Heart, Lung, and Blood Institute and the Eunice Kennedy Shriver National Institute of Child Health and Human Development together co-funded grants # U01 HL097889 and U01 HL097839. The National Cancer Institute funded grant # U01 CA150387. The National Institute of Diabetes and Digestive and Kidney Diseases funded grant # U01 DK088380. The NIH Office of Behavioral and Social Science Research funded the RCU at Northwestern University to facilitate collaboration across the seven funded research projects. Preparation of this manuscript was also supported by the National Institute on Aging, the American Federation for Aging Research, the John A. Hartford Foundation, and the Atlantic Philanthropies under Grant # K23AG042869. The authors would like to acknowledge key feedback and suggestions made by Peter Kaufmann, Ph.D., Rena Wing, Ph.D., and Leonard Epstein, Ph.D., during earlier phases of this manuscript’s development.
Footnotes
The views expressed in this article are those of the authors and do not necessarily reflect the view of the National Institutes of Health (NIH) or the US Department of Health and Human Services.
Contributor Information
Susan M. Czajkowski, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD
Lynda H. Powell, Rush University Medical Center, Chicago, IL
Nancy Adler, University of California, San Francisco, CA
Sylvie Naar-King, Wayne State University, Detroit, MI
Kim D. Reynolds, Claremont Graduate University, Claremont, CA
Christine M. Hunter, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD
Barbara Laraia, University of California, Berkeley, CA
Deborah H. Olster, Office of Behavioral and Social Sciences Research, National Institutes of Health, Bethesda, MD.
Frank M. Perna, National Cancer Institute, National Institutes of Health, Bethesda, MD
Janey C. Peterson, Weill Medical College of Cornell University, New York, NY.
Elissa Epel, University of California, San Francisco, CA
Josephine E. Boyington, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD
Mary E. Charlson, Weill Medical College of Cornell University, New York, NY
References
- Anderson G. Chronic care: Making the case for ongoing care. Princeton, NJ: Robert Wood Johnson Foundation; 2010. Available at www.rwjf.org/pr/product.jsp?id=50968. [Google Scholar]
- Appel LJ, Clark JM, Yeh HC, Wang NY, Coughlin JW, Daumit G, Miller ER, Dalcin A, Jerome GJ, Geller S, Noronha G, Pozefsky T, Charleston J, Reynolds JB, Durking N, Rubin RR, Louise TA, Brancati FL. Comparative Effectiveness of Weight-Loss Interventions in Clinical Practice. New Engl J Med. 2011;365(20):1959–68. doi: 10.1056/NEJMoa1108660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel LJ, Champagne CM, Harsha DW, Cooper LS, Obarzanek E, Elmer PJ, Stevens VJ, Vollmer WM, et al. Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA. 2003;289(16):2083–93. doi: 10.1001/jama.289.16.2083. [DOI] [PubMed] [Google Scholar]
- Bartholomew L, Parcel G, Kok G, Gottlieb G. Planning health promotion programs: Intervention mapping. San Francisco: Jossey-Bass; 2011. [Google Scholar]
- Blumenthal JA, Babyak M, Wei J, O’Connor C, Waugh R, Eisenstein E, Mark D, Sherwood A, et al. Usefulness of psychosocial treatment of mental stress-induced myocardial ischemia in men. Am J Cardiol. 2002;89(2):164–8. doi: 10.1016/s0002-9149(01)02194-4. http://dx.doi.org/10.1016/S0002-9149(01)02194-4. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Onken LS. Behavioral therapies for drug abuse. Am J Psychiatry. 2005;162:1452–60. doi: 10.1176/appi.ajp.162.8.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Somerville LH, Gotlib IH, Ayduk O, Franklin NT, Askren MK, Jonides J, Berman MG, et al. Behavioral and neural correlates of delay of gratification 40 years later. Proc Nat Acad of Sciences. 2011;108(36):14998–5003. doi: 10.1073/pnas.1108561108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty B, Collins LM, Strecher V, Murphy SA. Developing multicomponent interventions using fractional factorial designs. Statistics in Medicine. 2009;28:2687–708. doi: 10.1002/sim.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarty EF, Hubert HB, Krishnan E, Bruce BB, Lingala VB, Fries JF. Lifestyle risk factors predict disability and death in healthy aging adults. Am J Med. 2012;125(2):190–7. doi: 10.1016/j.amjmed.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller BS. Translational research: Forging a new cultural identity. Mt Sinai J Med. 2008;5(5):478–87. doi: 10.1002/msj.20064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008;337:a1655. doi: 10.1136/bmj.a1655. http://dx.doi.org/10.1136/bmj.a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: New guidance. www.mrc.ac.uk/complexinterventionsguidance.
- Dallery J, Raiff BR. Optimizing behavioral health interventions with single-case designs: from development to dissemination. Behav Med Pract Policy Res. 2014 doi: 10.1007/s13142-014-0258-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson KW, Goldstein M, Kaplan RM, Kaufmann PG, Knatterud GL, Orleans CT, Spring B, Trudeau KJ, Whitlock EP. Evidence-based behavioral medicine: what is it and how do we achieve it? Ann Behav Med. 2003;26(3):161–71. doi: 10.1207/S15324796ABM2603_01. [DOI] [PubMed] [Google Scholar]
- Debiec J, LeDoux JE. Noradrenergic signaling in the amygdala contributes to the reconsolidation of fear memory: Treatment implications for PTSD. Ann NY Acad Sci. 2006;1071:521–4. doi: 10.1196/annals.1364.056. [DOI] [PubMed] [Google Scholar]
- deCharms RC, Maeda F, Glover GH, Ludlow D, Pauly JM, Soneji D, Gabrieli JD, Mackey SC. Control over brain activation and pain learned by using real-time functional MRI. Proc Natl Acad Sci USA. 2005;102:18626–31. doi: 10.1073/pnas.0505210102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Paluch RA, Roemmich JN, Beecher MD. Family-Based Obesity Treatment, Then and Now: Twenty-Five Years of Pediatric Obesity Treatment. Health Psychol. 2007;26(4):381–391. doi: 10.1037/0278-6133.26.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagundo AB, de la Torre R, Jiménez-Murcia S, Agüera Z, Granero R, Tárrega S, Botella C, Baños R, et al. Executive Functions Profile in Extreme Eating/Weight Conditions: From Anorexia Nervosa to Obesity. PLoS ONE. 2012;7(8):e43382. doi: 10.1371/journal.pone.0043382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flay BR. Efficacy and Effectiveness Trials (and Other Phases of Research) in the Development of Health Promotion Programs. Prev Med. 1986;15:451–74. doi: 10.1016/0091-7435(86)90024-1. http://dx.doi.org/10.1016/0091-7435(86)90024-1. [DOI] [PubMed] [Google Scholar]
- Freedland KE, Mohr DC, Davidson KW, Schwartz JE. Usual and Unusual Care: Existing Practice Control Groups In Randomized Controlled Trials of Behavioral Interventions. Psychosom Med. 2011;73(4):323–35. doi: 10.1097/PSY.0b013e318218e1fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman LM, Furberg CD, DeMets DL. Fundamentals of Clinical Trials. 3. New York: Springer-Verlag; 1998. [Google Scholar]
- Green LW. Toward cost-benefit evaluations of health education: some concepts, methods, and examples. Health Education Monographs. 1974;2(Suppl 2):34–64. [Google Scholar]
- Greenwald P, Cullen JW. The new emphasis in cancer control. J Natl Cancer Inst. 1985;74(3):543–51. [PubMed] [Google Scholar]
- Handelsman Y, Mechanick JI, Blonde L, Gruberger G, Bloomgarden ZT, Bray GA, Dagogo-Jack S, Davidson JA, et al. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for Developing a Diabetes Mellitus Comprehensive Care Plan. Endocr Pract. 2011;17(Suppl 2):1–53. doi: 10.4158/ep.17.s2.1. [DOI] [PubMed] [Google Scholar]
- Hamman RF, Wing RR, Edelstein SL, Lachin JM, Bray GA, Delahanty L, Hoskin M, Kriska AM, et al. Effect of Weight Loss With Lifestyle Intervention on Risk of Diabetes. Diabetes Care. 2006;29(9):2102–7. doi: 10.2337/dc06-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MD, Ryan DH, Apovian CM, Loria CM, Ard JD, Millen BE, Comuzzie AG, Nonas CA, Donato KA, Pi-Sunyer X, Hu FB, Stevens J, Hubbard VS, Stevens VJ, Jakicic JM, Wadden TA, Kushner RF, Wolfe BM, Yanovski SZ. 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults. J Am Coll Cardiol. 2013 doi: 10.1016/j.jacc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- Kazdin A. Single-Case Research Designs: Methods for Clinical and Applied Settings. Oxford University Press; USA: 1982. Available at https://www.amazon.com/Single-Case-Research-Designs-Clinical-settings/dp/0195030206. [Google Scholar]
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer HC, Mintz J, Noda A, Tinklenberg J. Caution regarding the use of pilot studies to guide power calculations for study proposals. Arch Gen Psychiatry. 2006;63:484–9. doi: 10.1001/archpsyc.63.5.484. [DOI] [PubMed] [Google Scholar]
- Lei H, Nahum-Shani I, Lynch K, Oslin D, Murphy SA. A “SMART” design for building individualized treatment sequences. Annu Rev Clin Psychol. 2012;8:21–48. doi: 10.1146/annurev-clinpsy-032511-143152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsky MS, Sharp LK. From idea to market: the drug approval process. J Am Board Fam Pract. 2001;14(5):362–7. [PubMed] [Google Scholar]
- Michie S, van Stralen MM, West R. The behaviour change wheel: A new method for characterizing and designing behaviour change interventions. Implement Sci. 2011;6:42. doi: 10.1186/1748-5908-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr DC, Spring B, Freedland KE, Beckner V, Arean P, Hollon SD, Ockene J, Kaplan R. The selection and design of control conditions for randomized controlled trials of psychological interventions. Psychother Psychosom. 2009;78:275–84. doi: 10.1159/000228248. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291(10):1238–45. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- National Heart, Lung, and Blood Institute. 2008 Available at http://grants.nih.gov/grants/guide/rfa-files/RFA-HL-08-013.html.
- Navarro-Barrientos JE, Rivera DE, Collins LM. A dynamical systems model for understanding behavioral interventions and body composition change. Math Comput Model Dyn Syst. 2011;17(2):183–203. doi: 10.1080/13873954.2010.520409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office of Behavioral and Social Sciences Research. 2011 Available at http://grants.nih.gov/grants/guide/pa-files/PA-11-063.html.
- Paganini-Hill A, Kawas CH, Corrada MM. Activities and mortality in the elderly: The Leisure World cohort study. J Gerontol A Biol Sci Med Sci. 2011;66(5):559–67. doi: 10.1093/gerona/glq237. Epub 2011 Feb 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer CG, Hadley DW. Evaluating the impact of genetic counseling and testing with signal detection methods. J Genet Couns. 2005;14(1):17–27. doi: 10.1007/s10897-005-1497-4. [DOI] [PubMed] [Google Scholar]
- Peterson JC, Czajkowski S, Charlson ME, Link AR, Wells MT, Isen AM, Mancuso CA, Allegrante JP, et al. Translating basic behavioral and social science research to clinical application: The EVOLVE mixed methods approach. J Consult Clin Psychol. 2013;81(2):217–30. doi: 10.1037/a0029909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell LH, Calvin JE, Jr, Mendes de Leon CF, Richardson D, Grady KL, Flynn KJ, Rucker-Whitaker CS, Janssen I, et al. The Heart Failure Adherence and Retention Trial (HART): design and rationale. Am Heart J. 2008;156:452–60. doi: 10.1016/j.ahj.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridenour TA, Pineo TZ, Molina MM, Lich KH. Toward rigorous idiographic research in prevention science: comparison between three analytic strategies for testing preventive intervention in very small samples. Prev Sci. 2013;14:267–78. doi: 10.1007/s11121-012-0311-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rounsaville BJ, Carroll KM, Onken LS. A stage model of behavioral therapies research: getting started and moving on from Stage I. Clin Psychol Sci Pract. 2001;8:133–42. [Google Scholar]
- Schroeder SA. Shattuck Lecture. We can do better — improving the health of the American people. N Engl J Med. 2007;357:1221–8. doi: 10.1056/NEJMsa073350. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annu Rev Clin Psychol. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- Stevens J, Taber JR, Murray DM, Ward DS. Advances and controversies in the design of obesity prevention trials. Obesity. 2007;15:2163–70. doi: 10.1038/oby.2007.257. [DOI] [PubMed] [Google Scholar]
- Strolla LO, Gans KM, Risica PM. Using qualitative and quantitative formative research to develop tailored nutrition intervention materials for a diverse low-income audience. Health Educ Res. 2006;21(4):465–76. doi: 10.1093/her/cyh072. [DOI] [PubMed] [Google Scholar]
- Wadden TA, Volger S, Sarwer DB, Vetter ML, Tsai AG, Berkowitz RI, Kumanyika S, Schmitz KH, Diewald LK, Barg R, Chittams J, Moore RH. A Two-Year Randomized Trial of Obesity Treatment in Primary Care Practice. N Engl J Med. 2011;365:1969–1979. doi: 10.1056/NEJMoa1109220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wansink B. Designing Interventions that Stick: Behavioral Event Monitoring. Presented at NIH Workshop on “Translating Ideas into Interventions: The Process of Developing Behavorial Interventions; December 6–7, 2010; Bethesda, MD. 2010. Available at http://www.nihorbit.org/ORBIT%20Content/Workshops%20and%20Conferences.aspx?PageView=Shared. [Google Scholar]
- Wing RR, Lang W, Wadden TAa, Safford M, Knowler WC, Bertoni AG, Hill JO, Brancati FL, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34(7):1481–6. doi: 10.2337/dc10-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon PW, Bastian B, Anderson RN, Collins JL, Jaffe HW. Potentially preventable deaths from the five leading causes of death — United States, 2008–2010. Morbidity and Mortality Weekly Report (MMWR) 2014 May 2;63(17):369–374. [PMC free article] [PubMed] [Google Scholar]
- Zucker DR, Ruthazer R, Schmid CH. Individual (N-of-1) trials can be combined to give population comparative treatment effect estimates: methodologic considerations. J Clin Epidemiol. 2010;63:1312–23. doi: 10.1016/j.jclinepi.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


