Abstract
Medication nonadherence is a significant health care issue requiring regular behavioral treatment. Lack of sufficient health care resources and patient/family time commitment for weekly treatment are primary barriers to receiving appropriate self-management support. We describe the methodology of the Telehealth Enhancement of Adherence to Medication (TEAM) trial for medication nonadherence in pediatric inflammatory bowel disease (IBD). For this trial, participants 11–18 years of age will be recruited from seven pediatric hospitals and will complete an initial 4-week run in to assess adherence to a daily medication. Those who take less than 90% of their prescribed medication will be randomized. A total of 194 patients with IBD will be randomized to either a telehealth behavioral treatment (TBT) arm or education only (EO) arm. All treatment will be delivered via telehealth video conferencing. Patients will be assessed at baseline, post-treatment, 3-, 6-, and 12-months. We anticipate that participants in the TBT arm will demonstrate a statistically significant improvement at post-treatment and 3-, 6-, and 12-month follow-up compared to participants in the EO arm for both medication adherence and secondary outcomes (i.e., disease severity, patient quality of life, and health care utilization). If efficacious, the TEAM intervention could be disseminated broadly and reduce health care access barriers so that patients could receive much needed self-management intervention.
Keywords: Telehealth, Pediatrics, Inflammatory Bowel Disease, Behavioral, Adherence
1. Introduction
Nonadherence to medication is a critical clinical care issue with an estimated cost of $100–300 billion annually1,2. Across chronic illness populations, nonadherence is associated with increased disease morbidity3–8, poorer quality of life and psychological functioning9–11, higher risk of mortality12–14, and greater health care utilization15,16. Published reports indicate that as many as 50% of children6, and 65–88% of adolescents17,18, are nonadherent to treatment, increasing the risk of complications substantially.
Although nonadherence to medical treatment is common across all pediatric chronic conditions, adolescents with Crohn’s disease, ulcerative colitis, and indeterminate colitis, collectively known as Inflammatory Bowel Disease (IBD), are among the highest at risk for nonadherence, with nonadherence prevalence rates reported as high as 88%18. IBD is characterized by gastrointestinal inflammation leading to unpredictable periods of disease exacerbation (e.g., abdominal pain, diarrhea, bloody stools, delayed growth) and remission19, and affects approximately 71 of 100,000 individuals20 (mean age of diagnosis in pediatrics = 15 years)21. Treatment may involve multiple oral medications (e.g., mesalamine, immunomodulators, corticosteroids)22, often with varying dosing schedules and undesirable side effects (e.g., facial hair growth, emotional symptoms, acne, weight gain).
Reasons for nonadherence in adolescents with IBD are largely behavioral and include forgetting, being too busy, interference of the medication with an activity, and being away from home4,23–25. Multicomponent behavioral interventions have demonstrated efficacy at improving adherence in the IBD population26–29, with one study reporting a 25% improvement in mesalamine adherence27. Despite preliminary efficacy of such interventions, many who would benefit from treatment do not receive it due to time and distance barriers. At our clinic, the average IBD patient must travel over 20 miles/30 minutes each way to receive care. Most behavioral interventions necessitate 1 to 2 hour-long sessions on a weekly/bimonthly basis, representing a significant time investment that many families may not be able to make due to other obligations (e.g., work, school) as well as the cost associated with lost work and travel expenses.
Because many families who need treatment are not receiving it, researchers and clinicians must initiate innovative strategies to make behavioral treatments for nonadherence more accessible. Use of technology, such as video conferencing, serves as one medium through which these barriers can be overcome. By delivering adherence interventions via teleconferencing, families are able to receive treatment directly from the comfort and privacy of their own home. Technologically-based means of communication play an integral role in the lives of adolescents. Using technology to deliver adherence interventions to youth across the country may result in the advent of more generalizable, cost-efficient, and acceptable treatments. This is the first known large scale multisite national RCT using telehealth to deliver a behavioral treatment specifically for nonadherence to treatment.
2. Objectives of the TEAM trial
The Telehealth Enhancement of Adherence to Medication (TEAM) trial is a multi-site randomized control clinical trial funded by the National Institute of Child Health and Human Development (NICHD) at the National Institutes of Health (NIH). TEAM will aim to overcome accessibility barriers by delivering a face-to-face multicomponent behavioral intervention via an online/telehealth format (i.e., Skype™). Building on our pilot work using telehealth technology29, the effect of a telehealth behavioral treatment (TBT) will be compared to an education only (EO) intervention among adolescents found to be nonadherent with traditional IBD treatment. We anticipate that participants in the TBT arm will demonstrate statistically significant improvements at post-treatment and 3-, 6-, and 12-month follow-up compared to participants in the EO arm for both medication adherence and secondary outcomes (i.e., disease severity, patient quality of life, and health care utilization).
Many of the behavioral strategies used in addressing nonadherence in the TEAM Study can also be applied to other chronic illness populations. For example, “forgetting” is a common barrier reported across youth with chronic conditions. Interventions to address this barrier (e.g., improved organizational skills, reminder systems) can be readily applied across youth with different chronic conditions. Thus, although this study focuses specifically on a population known to be among the highest risk for nonadherence, strategies and lessons learned from this trial can be generalized to the development of intervention protocols in other chronic conditions.
3. Study design
3.1 Overview
Participants will be recruited from seven pediatric hospitals. Approximately 305 participants will be enrolled in the study, with an estimated 194 randomized to receive either the TBT or EO intervention. Eligibility for randomization to treatment will depend on adherence data collected at the conclusion of a 4-week run-in period (see Figure 1 for an overview of study design). Participants who complete the run-in phase with an adherence rate equal to or above 90% (according to pill count) will be finished with study participation (total time commitment: 4 weeks). Those taking less than 90% of doses will continue on to randomization to either the TBT or EO intervention for a total time commitment of 15 months (4 week run-in + baseline assessment + 2 month intervention + post-treatment, 3-, 6- and 12-month follow-up assessments). The 90% cut point, rather than 80%, was selected to provide a stricter test of the adherence intervention since there are no data to support therapeutic efficacy at 80% adherence for most medications including those of concern in this RCT. The study protocol was approved by the governing institutional review board at all seven study sites.
Figure 1.
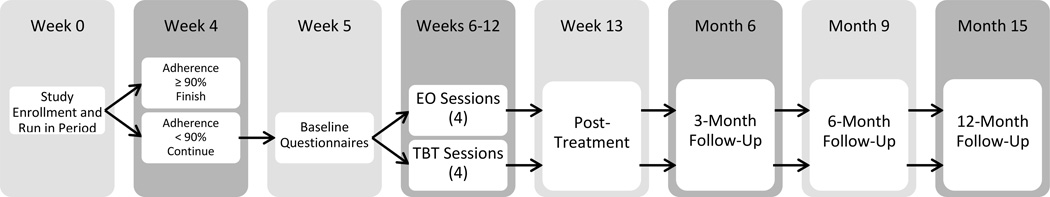
Overview of TEAM Study design
3.2 Inclusion Criteria
Adolescents between the ages of 11 and 18 years who have been diagnosed with IBD will be eligible for the study. Participants must be prescribed a daily oral immunomodulator (e.g., 6-MP/azathioprine, methotrexate) and/or a 5-ASA (e.g., Pentasa, Asacol) in pill form so that adherence to medication can be electronically monitored. As caregiver and adolescent interaction is needed for the intervention portion of the study, the adolescent will be required to live at home. All participating adolescents must have at least one caregiver consistently completing study visits along with the adolescent. A secondary caregiver will also be allowed to participate in the Skype™ intervention sessions, provided informed consent is obtained prior to these sessions. Additionally, both the caregiver and adolescent must be fluent in English.
3.3 Exclusion Criteria
Families will be excluded from the study if the adolescent or caregiver has been diagnosed with a pervasive developmental disorder or a serious mental illness, such as schizophrenia or bipolar disorder. Medical chart reviews will be conducted by study personnel at each site to verify eligibility for the study.
3.4 Recruitment and retention
Recruitment will be conducted over the course of approximately 40 months at the seven participating pediatric hospitals: Cincinnati Children’s Hospital Medical Center (CCHMC), Connecticut Children’s Medical Center (CCMC), Children’s Hospital of Philadelphia (CHOP), Nationwide Children’s Hospital (NCH), Children’s Hospital of Pittsburgh (CHP), UCSF Benioff Children’s Hospital (UCSF), and Children’s Mercy Hospital (CMH). Such large scale collaboration between prominent pediatric institutions to address a critical clinical care issue represents a positive move toward improving children’s health on a national-level and is a significant improvement over single-site, regionally-bound research which has dominated pediatric behavioral intervention research. CCHMC will be the lead coordinating site, and each site will have a site-specific Principal Investigator (PI) and at least one study coordinator. Designated study personnel across all sites will be responsible for identifying eligible patients via medical chart review or direct physician referral.
Recruitment will occur via telephone or in person during the patient’s regularly scheduled gastroenterology clinic visit or infliximab infusion. For patients recruited via telephone, coordinators at each study site will send recruitment letters which will give a brief explanation of the study, PI names, and a local opt out telephone number that families can call if they do not wish to be contacted. Two weeks after letters are sent, families that have not opted out of the study will be contacted and provided with an in-depth description of the TEAM trial. Families interested in participating will be mailed consent/assent forms to review over the telephone with their site coordinator. Completed forms will be returned to the site coordinator, who will then upload them to a secure website, along with family demographic information, for CCHMC coordinators to download and initiate participation. Referrals by gastroenterologists will be screened for eligibility based on previously specified study inclusion/exclusion criteria. If ineligible, the gastroenterologist will be notified so an appropriate referral can be made.
Once enrolled, participant progress will be monitored and managed by study staff at CCHMC. Compensation in the form of debit cards will be mailed to families immediately upon completion of each study visit, and rates will increase as families progress through the study to promote study retention. Adolescents and caregivers will each receive $20 for completing the baseline assessment, each of the four intervention sessions, and the post-treatment assessment. Compensation for completing the 3-, 6-, and 12-month follow up visits will be $25, $30, and $35 per person, respectively. In addition, the study interventionist will maintain phone and email contact with families throughout the intervention, as outlined in the manualized treatment protocol, to promote study retention. This includes regularly scheduled contact outlined in the session manual as well as communication with families to reschedule any missed sessions.
3.5. Randomization
Participants will be randomized to treatment if: 1) they are less than 90% adherent to their prescribed oral medication during the 4-week run in, and 2) complete the online baseline assessment. Upon meeting these two criteria, a request containing the patient name and study site will be sent by the CCHMC study coordinator to the divisional data core, who will manage the randomization process. Overall randomization to TBT or EO will be 1:1. A stratified (by site) block randomization designed by the study’s biostatistician will be used, with each enrollment site’s randomization plan including randomly generated blocks of size 2 and 4. The divisional data core will use this plan to randomize each patient to an intervention arm. They will report the treatment assignment condition to the lead interventionist, who oversees the intervention portion of the trial.
3.6. Blinding
All study investigators, coordinators, and treating gastroenterologists, including the PI, and those involved in the collection of outcomes data, will be blinded to treatment condition. Only the divisional data core and study interventionists will be aware of treatment assignment.
3.7. Assessment and outcome measures
Web-based assessments will occur at baseline, post-treatment, and 3-, 6-, and 12-month follow-up. Telephone interviews will also be conducted at each time point to gather pill count information and obtain disease severity. Health care utilization data will be collected via medical chart review for 15 months prior to, and after, the date of enrollment.
Medication adherence
Electronic monitoring
Participants will be given a Medication Event Monitoring System (MEMS®) 6 Trackcap for each prescribed immunomodulator and/or 5-ASA. MEMS tracks adherence to medication by recording the time and date of bottle openings via a microchip embedded in the cap. Participants will use their MEMS bottles during the run in period and, if randomized, they will continue to use it throughout the entire 15 month enrollment period. Data will be downloaded from MEMS caps during regularly scheduled clinic visits at each enrollment site and exported to a computer for review and statistical analysis. Although the frequency of regularly scheduled clinic visits depends on a number of factors (e.g., patient health status, provider judgment), the microchip embedded within in each MEMS cap is capable of storing several years of data. Thus, if a patient misses a scheduled appointment, data will remain accessible in the future. The patient’s daily adherence percentage will be the primary outcome variable.
Pill count
Pill count data will also be collected for the monitored medication(s). Information collected will include dose, most recent refill date, quantity of refill, and number of pills remaining. Pill count data will be the primary adherence report during the 4-week run in and will be used to determine eligibility for randomization (i.e., adherence rate less than 90%). This was chosen because remote MEMS data downloads are not available. Downloading of these data require in-person visits and most patient clinic visits will not coincide with the study randomization timeline. Thus, to minimize family burden, an alternative, reliable and objective method of assessing adherence (e.g., pill counts)30 was chosen to determine eligibility. Pill counts will be conducted by parents with study personnel via telephone. Pill count adherence rates will be calculated using the following formula: doses consumed ÷ doses prescribed × 100. Pill count data will be collected at all assessment time points and will serve as secondary adherence data in the unlikely event that MEMS data are not available due to equipment malfunction or participant loss of MEMS bottles.
Disease severity
Disease severity will be assessed via telephone interview at each assessment time point using either the Partial Harvey Bradshaw Index31 (PHBI; for patients with Crohn’s Disease) or Pediatric Ulcerative Colitis Activity Index32 (PUCAI; for patients with ulcerative colitis or indeterminate colitis). Questions vary by measure; however, items generally address abdominal pain, frequency and consistency of stools, limitation in activity, and overall well-being. The PUCAI assess patient symptoms within the last 2 days whereas the PHBI records symptomology within the past week. Higher scores on both measures denote more active disease. Both measures are well-validated and used reliably in IBD research (r=.81–.86 and r=.91 for PHBI and PUCAI respectively31–33).
Health care utilization
All health care utilization data will be collected by study staff; no data will be completed by participants. Medical charts will be reviewed by study staff at each enrollment site to determine health care utilization for the 15 month period prior to, and directly following, enrollment. Chart review variables will include number of IBD-related hospital admissions, emergency department visits, gastroenterology outpatient visits, psychology visits, laboratory visits, infliximab infusions, telephone encounters, medication refill requests, surgeries, and other necessary referral services due to the IBD diagnosis (e.g., ophthalmology, urology).
Patient-reported health-related quality of life
The IMPACT-III questionnaire will be completed online by patients at each time point to assess patient health-related quality of life (HRQOL). This 35-item self-report of HRQOL asks patients to rate the extent he/she is affected by a particular IBD issue (e.g., stomach pain) on a 5-point Likert scale. Total scores range from 35 to 175 and higher scores denote a higher quality of life. The measure has demonstrated good reliability (α = .90, test-retest estimates = .9034) and validity in current research.
In addition to these outcome measures, patients and caregivers will also complete behavioral measures to assess psychosocial functioning, barriers to adherence, problem solving, and treatment responsibility. These measures will assess aspects of patient and family functioning that often correlate with medication nonadherence and may help identify mechanisms of change in the intervention employed in the TEAM trial.
Behavior Assessment System for Children-Second Edition, Parent Rating Scale (BASC-2-PRS)35
The BASC-2-PRS is a widely used inventory to assess and identify children and adolescents (ages 2–18; different forms for developmental levels) with emotional disturbances and behavioral disorders. The BASC measures externalizing, internalizing, and school problems, adaptive skills, and other problems. This measure has adequate internal consistency (.80 and .90 with adolescents) and test-retest reliability (.80’s to .90’s over a 1-month period).
Brief Symptom Inventory (BSI)36
This parent self-report measure consists of 53 items that assess psychological functioning and distress. Symptoms are categorized into 9 symptom scales: Somatization, Obsessive-Compulsive, Interpersonal Sensitivity, Depression, Anxiety, Hostility, Phobic Anxiety, Paranoid Ideation, and Psychoticism. Each item is rated on a 5-point rating scale with higher scores meaning higher symptom severity. Cronbach’s alpha37 reliability ranges from .71 to .8536.
Parent and Adolescent Medication Barriers Scale (PMBS and AMBS)38
This patient-report (17-item) and caregiver-report (16-item) measure assesses perceived barriers to treatment adherence. Respondents rate the extent to which he/she is affected by each item (i.e., barrier) addressed. Both patient- and caregiver-report forms have demonstrated good reliability (α = .86 for patient, α = .87 for caregiver).
Social Problem-Solving Inventory-Revised Short (SPSI-RS)39
This parent and patient self-report is a 25-item measure assesses 5 different dimensions of social-problem solving: Positive Problem Orientation (PPO), Negative Problem Orientation (NPO), Rational Problem Solving (RPS), and Impulsivity/Carelessness Style (ICS), and Avoidance Style (AS). Greater problem solving ability is indicated by higher scores on the PPO and RPS and lower scores on the NPO, AS, and ICS. The SPSI-R also generates an overall problem solving ability score, with higher scores indicating more adaptive problem solving abilities. The SPSI-RS has good reliability and validity39.
Allocation of Treatment Responsibility (ATR)40
The ATR is an 18-item assessment of treatment responsibility for patient- and caregiver-report. The measure has 3 subscales to assess responsibility for oral medication, clinic visits, and laboratory visits. The oral medication and clinic visits subscales will be used for this study. Respondents rate each question on a four-point Likert scale with a higher score denoting higher responsibility. Both patient- and caregiver-report forms have demonstrated good reliability (α = .91 for patient, α = for caregiver).
4. Intervention
4.1. Interventionists
Interventions in the TEAM trial will be delivered by post-doctoral fellows, psychology interns/residents, and master’s-level graduate students who have a background in child health/pediatric psychology and behavioral approaches to health promotion. All interventionists will undergo a structured training program which includes: 1) a broad overview of study aims and organization, 2) educational readings relevant to the study population and selected treatment approach (e.g., physiology and medical treatment of pediatric IBD, nonadherence in pediatric IBD, problem solving training), 3) orientation to the technology used in session and in data collection, 4) listening to complete audio session recordings for a prior TBT and EO participant, and 5) role playing the entire manualized TEAM intervention (TBT and EO) with the study’s lead interventionist serving as a mock participant. Mock sessions will place a heavy emphasis on understanding the medical management of IBD, psychosocial concerns of IBD in adolescence, and successfully applying the problem-solving approach to help families overcome barriers to adherence. All interventionists will be assigned both TBT and EO families across treatment sites and participate in weekly supervision with the lead interventionist to monitor participant progress, challenges in treatment, and to plan upcoming sessions. One interventionist will be assigned per family and all treatment sessions will be digitally recorded.
Each quarter, 20% of newly completed cases will be randomly selected for fidelity checks using trained coders who will be unaware of study aims and hypotheses. Fidelity checklists have been created for each treatment session and condition. In addition to documenting whether certain aspects of the protocol were delivered as planned, coders will be asked to document any potential cases of treatment cross-contamination (e.g., interventionist delivers an aspect of the TBT intervention to a family randomized to EO). Any deviation from the study protocol will result in remedial training of the interventionist.
4.2. Study sessions and use of Skype™
Participants will complete four study sessions, each approximately 2 weeks apart; a self-guided session delivered via the study website (session 1) and three interventionist-lead sessions delivered via Skype™ videoconferencing software (sessions 2–4). An overview of session content is provided in Table 1.
Table 1.
Overview of Telehealth Behavioral Treatment (TBT) and Education Only (EO) session content
| Week | TBT | EO |
|---|---|---|
| 6 | Session 1: IBD education delivered via TEAM website | |
| 7 | Skype™ technology testing | Skype™ technology testing |
| 8 | Session 2: Parental monitoring of adherence, introduction to adherence monitoring, use of problem solving approach to overcome barriers to adherence | Session 2: Review of adolescent’s specific medication regimen. Tailored discussion of medication regimen including mechanism of action within the body and potential side effects |
| 9 | Telephone check in of family’s progress | Follow-up email of previous session materials and links to related educational materials |
| 10 | Session 3: Functional analysis of family’s specific barriers to adherence, discussion of organizational tools to improve adherence, problem solving of one adherence barrier | Session 3: Discussion of local and national resources available to patients with IBD via the Crohn’s and Colitis Foundation of America |
| 11 | Telephone check in of family’s progress | Follow-up email of previous session materials and links to related educational materials |
| 12 | Session 4: Transition of IBD treatment responsibility from the parent to the adolescent, maintenance of adherence gains, problem solving one future barrier to adherence | Session 4: Education on the role of healthy lifestyle habits (i.e., nutrition, hydration, sleep, and exercise) as part of a comprehensive IBD management approach |
In session 1, IBD education, will be delivered to both TBT and EO families via the TEAM website. The educational materials on this website were designed by clinicians and patients at CCHMC to provide patients and families with IBD information that has been developmentally-tailored to adolescents. Topics covered include: 1) what is IBD?, 2) what are the symptoms of IBD and how is it diagnosed?, 3) how is IBD treated?, 4) what are potential complications of IBD?, 5) nutritional approaches to managing IBD, and 6) general management of IBD (e.g., at school, taking medicine, communicating with your doctor). Each section contains embedded links that provide additional information on the materials presented, videos of adolescent patients with IBD sharing their personal stories with regard to IBD (i.e., diagnosis, treatment, and day-to-day management), and a quiz to assess participant mastery of the material covered.
TEAM sessions 2–4 will be conducted via Skype™, a free video-conferencing software program. These sessions will be led by trained interventionists and contain treatment arm-specific material. To facilitate telehealth sessions, families will be provided with a free webcam as well as instructions for downloading and using Skype™. A test trial of Skype™ will be conducted 1 week prior to the start of session 2 to familiarize families with the technology and troubleshoot any difficulties. For families in which use of Skype™ is not possible due to limited access to a computer or a reliable internet connection, sessions will be conducted over the telephone.
4.3. Telehealth Behavioral Treatment (TBT) Intervention
TBT sessions 2–4 will last approximately 60–90 minutes and will begin by reviewing the family’s most recent adherence behavior. While session content will vary slightly across sessions based on each family’s individual barriers to adherence (see Table 1), all TBT sessions will place a heavy emphasis on applying a structured problem solving approach41 to overcome barriers to adherence. This approach is ideally suited to target nonadherence in this population as the majority of reasons for nonadherence to IBD treatment are behavioral in nature (i.e., forgetting). Other reasons for nonadherence (e.g., medication side-effects) will also be addressed as they occur and may involve helping the family problem-solve ways to overcome the barrier on their own or in collaboration with their medical team.
At the end of each TBT session, families will develop a behavioral contract which outlines: 1) the barrier to adherence being targeted, 2) the family’s adherence goal, 3) the plan the family selected to overcome that barrier and reach their goal, 4) individual responsibilities to make this plan successful, and 5) the mutually agreed upon reward that the adolescent will receive if they reach their adherence goal (see Figure 2 for a sample). Families will be instructed to follow this contract for the next week and to track their level of success in implementing the plan and improving their adherence. Families will receive a telephone call from their interventionist approximately 1 week after their problem solving session. The purpose of this call is to check in on the family’s progress, provide reinforcement for their effort, and re-implement problem solving if the family has been unsuccessful in reaching their goal.
Figure 2.

Sample Adherence Contract following a TBT session
4.4. Education Only (EO) Intervention
EO sessions will last approximately 20–30 minutes and will focus on providing education information regarding IBD management and relevant local and national resources for patients with IBD. Educational material will be customized to the patient’s current IBD regimen (session 2), geographic location (session 3), and current lifestyle habits (session 4). Families will receive an email from their interventionist approximately 1 week after their session which summarizes prior session content and provides them with additional web-based resources relevant to the session content.
4.5. Data Safety Monitoring Board
A Data Safety Monitoring Board (DSMB) has been created to maintain safety and regulatory compliance across sites. The DSMB is comprised of three prominent experts in their respective fields and includes a pediatric psychologist, biostatistician, and gastroenterologist. Members of the DSMB will review study reports during biannual meetings with the PI and CCHMC study coordinators. Reports will include enrollment and randomization numbers per site, withdrawal rates, reasons for withdrawal, adverse events, and overall progress toward study objectives. No interim analyses will be conducted. Adverse events and protocol deviations will be promptly reported to CCHMC, reviewed by the PI, and reported to the IRB, if necessary.
5. Data Management & Analyses
5.1. Data Management
All data will be managed by the divisional data core at CCHMC. The data core is comprised of a database programmer, data manager, and application developer who will develop a study-specific website for data collection and patient education. Questionnaire data will be entered by patients and caregivers directly into the secured study website, and pill count and disease severity data will be entered manually by trained study staff at CCHMC. Data entered into the study website will be backed up nightly on a secure server. Chart reviews conducted by study coordinators at other sites will be transferred to CCHMC via secure server. Study staff at CCHMC will review data and utilize a double entry system to ensure accuracy of data. At minimum, the data core will run monthly quality reports to maintain reliability across all databases.
5.2. Power Analyses and Sample Size Considerations
Sample size calculation was based on our prior RCT studies using the proposed behavioral intervention to improve treatment adherence in pediatric IBD, in which we have observed a 25% increase in medication adherence and medium effect sizes (d = 0.57). We anticipate a modest (i.e., 5%) increase in adherence for patients in the EO condition due to education and attention intervention. A total of 194 children will be needed for this study (97 children/arm × 2 arms). This sample size estimate is predicated upon a two-group repeated measures analysis of variance test with five observation periods, a difference in adherence of 20% attributable to TBT at the 12-month evaluation (effect size = 2.33 OR), R = 0.70 (autocorrelation), and 90% power (α = 0.05). Although the study is sufficiently powered at 77 children/arm, this estimate allows for a very liberal 20% attrition rate (97 × 0.80 = 77.6 children/arm) across the 12-month study period.
5.3. Data Analytic Plan
Missing Data
Patterns of missingness will be evaluated for outcomes as well as covariates, for the group as a whole as well as each treatment group, individually, in an effort to uncover any patterns among the data. Imputation procedures will be handled in accord with recommendations outlined in Little and Rubin42. The linear mixed effects models described below are quite capable of accommodating unbalanced designs.
Preliminary Data Analyses
Descriptive statistics will be computed for all relevant variables in the data set, including measures of central tendency, variability, and association, where appropriate. Preliminary analyses will include evaluating the distributional properties of key outcomes overall, by adherence to medication type, by interventionist, and by observation period using graphical and numeric methods. In the event that the primary outcome, adherence rate, deviates substantially from normality and linear mixed effects models are deemed less appropriate, alternative transformational and modeling strategies will be considered.
Hypotheses Testing
Primary Aim analyses will consist of a regression-based 2-factor repeated measures analysis, considering post treatment, 3-, 6-, and 12-month monitoring as a nested effect. The primary outcome will be the electronically monitored adherence rate. Our testable covariate will be treatment arm (TBT, EO). A baseline measure of adherence will be included in the model as an influential covariate while a limited number of behavioral measures will be included as potential covariates (i.e., BASC parent- and self-report, BSI). A linear mixed-effects model is deemed most appropriate given its ability to handle repeated (daily) observations over a 12-month period within the context of unbalanced data structures while allowing for alternative time-series covariance structures. Significant differences between treatment arms will be evaluated at the nominal α = 0.05 level, immediately following initial treatment, and at the 3-, 6-, and 12-month follow-up evaluations specifically to examine stability of treatment effects over time. Sphericity will be evaluated as appropriate; residuals will be evaluated for normality, constant error variance, and independence. Semiparametric regression in the context of the previously described mixed model framework will also be considered should assumptions for the parametric model not be met. Once data are collected, appropriate basis functions will be chosen for analysis. The linearity assumption will be tested using a likelihood ratio test.
Secondary Aims hypotheses will be modeled and analyzed similarly to the Primary Aim; however, the outcomes of interest will vary by hypothesis: disease severity (H2), HRQOL (H3), and health care utilization (H4). The testable covariate in each case will be treatment arm (TBT, EO), after adjusting for significant behavioral covariates (i.e., BASC parent- and self-report and BSI). As with the Primary Aim, we will test the difference between arms at post-treatment and 3-, 6-, and 12-month follow up (α < 0.05). Difference in health care utilization between conditions at post-treatment is not expected due to the brief time span between baseline and post-treatment; thus, differences between groups on health care utilization will be examined only at 3-, 6-, and 12-month follow up. Within the context of these secondary analyses, selected mediators (patient/parent functioning, barriers to treatment, enhanced problem solving) will be included in the aforementioned Aim 2 models consistent with strategies outlined by Kraemer and colleagues43,44, Holmbeck45, and Baron and Kenny46. Specifically, analyses will be conducted using both direct and indirect effects with and without interaction terms involving the mediator and treatment variables to identify candidate mechanisms of influence.
6. Discussion
The TEAM trial is a multi-site randomized control clinical trial examining the efficacy of a multicomponent behavioral adherence intervention delivered via telehealth. Demonstrating the efficacy of telehealth approaches to treatment of nonadherence in pediatric conditions will be a critical step in overcoming accessibility barriers and reaching underserved populations. If evidence for efficacy is found, TEAM will give nonadherent patients, who would otherwise not receive behavioral treatment, the opportunity to learn and develop skills critical to effective daily medication management. Improved adherence may prevent future declines in psychosocial and medical functioning currently associated with nonadherence. This in turn, may improve patient quality of life and reduce health care expenditures and burden on the health care system. Indeed, we recently published a review demonstrating increased health care utilization among pediatric patients who were nonadherent to their medication regimen47.
Findings from the TEAM trial will have implications for both clinical practice and research. For this trial, we are using a web-based video conferencing service that is readily available and highly utilized by the general public; however, it is not HIPAA compliant, which may limit the extension of this exact protocol into practice or other research settings. Researchers interested in using video conferencing as a method of treatment delivery may want to consider using HIPAA compliant services, such as Citrix GoToMeeting, SecureVideo, or Secure Telehealth. Although such services provide greater protection and security, each would require patients and families to receive specific training on their use since they are not commonly used applications. This may require additional time and investment on the part of researchers and families. In addition, licensure across state lines and reimbursement for professional services remain as barriers to implementing telehealth services on a large scale currently. Nevertheless, many opportunities are available to treat patients with self-management difficulties within state lines, though they may have to be physically located at another clinical office (e.g., a colleague’s office) in order to secure reimbursement. Partnerships with multidisciplinary practices and hospitals will be critical to disseminating the application of telehealth approaches to treating nonadherence in the near future. As reimbursement practices continue to evolve in the next few years it will be important to have evidence from RCTs like the TEAM trial to provide an empirical base from which to argue for better reimbursement for telehealth services. This is also likely to be an economical approach to care as intervention personnel would not be needed locally as the intervention could be delivered from various locations to wherever it is needed.
With regard to research, the TEAM trial will inform the field on the potential of telehealth approaches to treating pediatric behavioral health problems. We will collect data on the use of the online education module with regard to time spent on the module, frequency of accessing the module, and topics covered. We will also learn and be able to share a considerable amount of information regarding the logistical aspects of this multisite trial that spans all time zones in the United States. Practical issues such as how to provide treatment from the east coast to the west coast at a convenient time for patients/families and how to quickly build rapport using virtual face-to-face interaction will be very informative to ongoing telehealth treatment outcome research efforts. Finally, our secondary outcomes assessment will allow us to better understand the impact of telehealth-delivered behavioral intervention to improve disease status, HRQOL, and health care utilization. A significant effect on any of those would represent the potential to have a substantial financial impact on health care as each of these outcomes is related to increased costs.
The TEAM trial faces several anticipated challenges. Each site has its own unique research infrastructure and resource needs, which helps determine how recruitment is handled. We are able to recruit patients at other study sites via telephone from the primary site in most cases, but this involves some challenges. First, after initial screening and contact from their local facility, patients and families receive a call from someone in a different city or state. Although they are prompted that this will happen, it’s often difficult to keep that in mind, so families may require some reminding about why they are receiving the call. Calls are also sometimes ignored due to unfamiliar phone numbers on caller ID. In addition, each site has multiple ongoing studies, which necessitates careful consideration of how each one impacts the others in terms of recruitment and potential impact on outcomes. Referrals from clinicians to the TEAM study are an attractive option since the resources to address nonadherence are not readily available at each site; however, this requires frequent communication to clinicians that referrals are an option for their patients.
Because patients and families are accustomed to in-person contact with clinicians, the lack thereof may impact participant recruitment and retention. In addition, the targeted patients we are recruiting for this trial are normally nonadherent, not only to treatment regimens, but often to medical appointments as well. While we have attempted to preemptively address this issue, the convenience of completing treatment sessions online from home may connote to patients a loosening of expectations for treatment session attendance.
As with any RCT, participants could potentially be dissatisfied with the random assignment to treatment arm they receive. Related to this is an issue that is specific to adherence trials. Some participants may simply not accept the findings from the run-in period. Many families believe they are significantly more adherent than objective data reveal. This is true for everyone’s estimates of their own health promoting behavior, not just patients. Due to this perception bias, some families are likely to not agree with the results of their run-in data, which will result in a subset of patients who are eligible for treatment not being randomized.
An overarching theme of the TEAM trial is to provide treatment to those who need it in a convenient manner. Convenience is addressed via the telehealth approach as well as having a focused and time-limited intervention consisting of four substantive sessions. Several issues may arise in a large trial with a treatment approach like this. First, the treatment might not be long enough/contain enough contacts to result in long-term acquisition of behavioral skills that result in better medication adherence. Second, the intervals between treatment sessions may not be long enough. That is, there may be enough sessions, but more time may be needed for skill acquisition. Finally, some families might experience significant psychosocial distress, such as depression, anxiety, divorce, economic hardship, etc., which may impact the extent to which patients are adherent to their medication regimens. However, these issues are not addressed in the TEAM trial’s intervention manual. We strove to have a focused treatment approach targeting nonadherence. While this is advantageous in targeting a specific set of behavioral factors, it is limited by not addressing the wide array of factors that may impact adherence.
7. Conclusions
Randomized controlled trials examining the efficacy of treatment for medication nonadherence in pediatric populations are rare despite the impact of nonadherence on patients and health care costs and the importance of building behavioral skills that promote optimal health in this developmental period. It is anticipated that improvements in adherence to medication regimens will result in improved overall health, better health-related quality of life, and decreased health care utilization. Further, improvement in self-management of adolescent patients is optimal because this age group is at a critical developmental period in which long-term health behavior habits are formed.
Use of innovative methods of care delivery in this trial should be both appealing to the target population (i.e., adolescents) and convenient for families, which is a significant barrier to receiving this type of treatment. Moreover, the benefit of using a telehealth approach is that geographical limitations are quite minimal. Indeed, the sites involved in this trial span the continental United States. If demonstrated to be efficacious, the TEAM intervention could theoretically be delivered across international borders.
Going forward, the existing policies pertaining to licensure reciprocity and reimbursement must be revisited as the development and application of technology in health care is advancing at a faster rate than health care insurance and law. Better use of existing resources, on-site or available virtually, will result in decreased health care costs and better comprehensive and preventive care that incorporates behavioral health skill training. With health care organizations beginning to be incentivized based on the health care outcomes of their patients (e.g., decreased readmission rates), improved behavioral health care that results in better disease management will be increasingly important.
Acknowledgment
Funding for this study was provided by the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01 HD067174). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berg JS, Dischler J, Wagner DJ, Raia JJ, Palmer-Shevlin N. Medication compliance: a healthcare problem. The Annals of pharmacotherapy. 1993;27(9 Suppl):S1–S24. [PubMed] [Google Scholar]
- 2.DiMatteo MR. The role of effective communication with children and their families in fostering adherence to pediatric regimens. Patient Educ Couns. 2004;55(3):339–344. doi: 10.1016/j.pec.2003.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Kane S, Huo D, Aikens J, Hanauer S. Medication nonadherence and the outcomes of patients with quiescent ulcerative colitis. The American Journal of Medicine. 2003;114(1):39–43. doi: 10.1016/s0002-9343(02)01383-9. [DOI] [PubMed] [Google Scholar]
- 4.Gray WN, Denson LA, Baldassano RN, Hommel KA. Treatment adherence in adolescents with inflammatory bowel disease: The collective impact of barriers to adherence and anxiety/depressive symptoms. Journal of Pediatric Pscyhology. 2012;37(3):282–291. doi: 10.1093/jpepsy/jsr092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rapoff MA. Adherence to pediatric medical regimens. New York: Kluwer Academic; 1999. [Google Scholar]
- 6.Rapoff MA, Barnard MU. Compliance with pediatric medical regimens. In: Cramer JA, Spilker B, editors. Patient compliance in medical practice and clinical trials. New York: Raven Press; 1991. pp. 73–98. [Google Scholar]
- 7.DiMatteo MR, Giordani PJ, Lepper HS, Croghan TW. Patient adherence and medical treatment outcomes: A meta-analysis. Medical care. 2002;40(9):794–811. doi: 10.1097/00005650-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Fredericks EM, Lopez MJ, Magee JC, Shieck V, Opipari-Arrigan L. Psychological functioning, nonadherence and health outcomes after pediatric liver transplantation. Am J Transplant. 2007;7(8):1974–1983. doi: 10.1111/j.1600-6143.2007.01878.x. [DOI] [PubMed] [Google Scholar]
- 9.Hommel KA, Franciosi J, Gray WN, Hente E, Ahrens A, Rothenberg ME. Behavioral functioning and treatment adherence in pediatric eosinophilic gastrointestinal disorders. Pediatric Allergy and Immunology. 2012;23:494–499. doi: 10.1111/j.1399-3038.2012.01297.x. [DOI] [PubMed] [Google Scholar]
- 10.Hommel KA, Davis CM, Baldassano RN. Medication adherence and quality of life in pediatric inflammatory bowel disease. J. Pediatr. Psychol. 2008;33(8):867–874. doi: 10.1093/jpepsy/jsn022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hovinga CA, Asato MR, Manjunath R, et al. Association of non-adherence to antiepileptic drugs and seizures, quality of life, and productivity: Survey of patients with epilepsy and physicians. Epilepsy Behav. 2008;13(2):316–322. doi: 10.1016/j.yebeh.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Berquist RK, Berquist WE, Esquivel CO, Cox KL, Wayman KI, Litt IF. Adolescent non-adherence: prevalence and consequences in liver transplant recipients. Pediatr Transplant. 2006;10(3):304–310. doi: 10.1111/j.1399-3046.2005.00451.x. [DOI] [PubMed] [Google Scholar]
- 13.Lurie S, Shemesh E, Sheiner PA, et al. Non-adherence in pediatric liver transplant recipients -- an assessment of risk factors and natural history. Pediatr Transplant. 2000;4(3):200–206. doi: 10.1034/j.1399-3046.2000.00110.x. [DOI] [PubMed] [Google Scholar]
- 14.Horwitz RI, Viscoli CM, Donaldson RM, et al. Treatment adherence and risk of death after a myocardial infarction. The Lancet. 1990;336(8714):542–545. doi: 10.1016/0140-6736(90)92095-y. [DOI] [PubMed] [Google Scholar]
- 15.Balkrishnan R, Rajagopalan R, Camacho FT, Huston SA, Murray F, Anderson RT. Predictors of medication adherence and associated health care costs in an older population with type 2 diabetes mellitus: al longitudinal cohort study. Clinical Therapeutics. 2003;25(11):2958–2971. doi: 10.1016/s0149-2918(03)80347-8. [DOI] [PubMed] [Google Scholar]
- 16.Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Medical care. 2005;43(6):521–530. doi: 10.1097/01.mlr.0000163641.86870.af. [DOI] [PubMed] [Google Scholar]
- 17.Logan DE, Zelikovsky N, Labay L, Spergel J. The Illness Management Survey: Identifying adolescents' perceptions of barriers to adherence. J Pediatr Psychol. 2003;28(6):383–392. doi: 10.1093/jpepsy/jsg028. [DOI] [PubMed] [Google Scholar]
- 18.Hommel KA, Davis CM, Baldassano RN. Objective versus subjective assessment of oral medication adherence in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2009;15(4):589–593. doi: 10.1002/ibd.20798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dubinsky M. Special issues in pediatric inflammatory bowel disease. World journal of gastroenterology : WJG. 2008;14(3):413–420. doi: 10.3748/wjg.14.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Auvin S, Molinie F, Gower-Rousseau C, et al. Incidence, clinical presentation and location at diagnosis of pediatric inflammatory bowel disease: a prospective population-based study in northern France (1988–1999) J Pediatr Gastroenterol Nutr. 2005;41(1):49–55. doi: 10.1097/01.mpg.0000162479.74277.86. [DOI] [PubMed] [Google Scholar]
- 21.Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn's disease and ulcerative colitis in the United States. Clin. Gastroenterol. 2007;5(12):1424–1429. doi: 10.1016/j.cgh.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 22.Zeisler B, Lerer T, Markowitz J, et al. Outcome following aminosalicylate therapy in children newly diagnosed as having ulcerative colitis. J Pediatr Gastroenterol Nutr. 2013;56(1):12–18. doi: 10.1097/MPG.0b013e31826ac41a. [DOI] [PubMed] [Google Scholar]
- 23.Ingerski LM, Baldassano RN, Denson LA, Hommel KA. Barriers to oral medication adherence for adolescents with inflammatory bowel disease. Journal of Pediatric Psychology. 2010;35(6):683–691. doi: 10.1093/jpepsy/jsp085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hommel KA, Baldassano RN. Brief report: Barriers to treatment adherence in pediatric inflammatory bowel disease. Journal of Pediatric Psychology. 2010;35(9):1005–1010. doi: 10.1093/jpepsy/jsp126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greenley RN, Stephens M, Doughty A, Raboin T, Kugathasan S. Barriers to adherence among adolescents with inflammatory bowel disease. Inflammatory bowel diseases. 2010;16(1):36–41. doi: 10.1002/ibd.20988. [DOI] [PubMed] [Google Scholar]
- 26.Hommel KA, Hente EA, Odell S, et al. Evaluation of a group-based behavioral intervention to promote adherence in adolescents with inflammatory bowel disease. European Journal of Gastroenterology and Hepatology. 2012;24(1):64–69. doi: 10.1097/MEG.0b013e32834d09f1. 10.1097/MEG.1090b1013e32834d32809f32831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hommel KA, Herzer M, Ingerski LM, Hente E, Denson LA. Individually-tailored treatment of medication nonadherence. J. Pediatr. Gastroenterol. Nutr. 2011;53(4):435–439. doi: 10.1097/MPG.0b013e3182203a91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stark LJ, Hommel KA, Mackner LM, et al. Randomized trial comparing two methods of increasing dietary calcium intake in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2005;40(4):501–507. doi: 10.1097/01.mpg.0000157913.32465.45. [DOI] [PubMed] [Google Scholar]
- 29.Hommel KA, Hente E, Herzer M, Ingerski LM, Denson LA. Telehealth behavioral treatment for medication non-adherence: A pilot and feasibility study. Eur. J. Gastroenterol. Hepatol. doi: 10.1097/MEG.0b013e32835c2a1b. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rapoff MA. Adherence to Pediatric Medical Regimens. 2nd ed. New York: Springer; 2010. [Google Scholar]
- 31.Hyams JS, Ferry GD, Mandel FS, et al. Development and validation of a pediatric Crohn's Disease Activity Index. J Pediatr Gastroenterol Nutr. 1991;12(4):439–447. [PubMed] [Google Scholar]
- 32.Turner D, Otley AR, Mack D, et al. Development, validation, and evaluation of a pediatric ulcerative colitis activity index: a prospective multicenter study. Gastroenterology. 2007;133(2):423–432. doi: 10.1053/j.gastro.2007.05.029. PMID: 17681163. [DOI] [PubMed] [Google Scholar]
- 33.Markowitz J, Grancher K, Kohn N, Lesser M, Daum F, Group TPMC. A multicenter trial of 6-mercaptopurine and prednisone in children with newly diagnosed Crohn's disease. Gastroenterology. 2000;119(4):895–902. doi: 10.1053/gast.2000.18144. [DOI] [PubMed] [Google Scholar]
- 34.Otley A, Smith C, Nicholas D, et al. The IMPACT questionnaire: A valid measure of health-related quality of life in pediatric inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 2002;35(4):557–563. doi: 10.1097/00005176-200210000-00018. [DOI] [PubMed] [Google Scholar]
- 35.Reynolds CR, Kamphaus RW. Behavior assessment system for children (BASC) Circle Pines, MN: AGS; 1992. [Google Scholar]
- 36.Derogatis LR. Brief Symptom Inventory: Administration, Scoring, and Procedures Manual. 4th Edition. Minneapolis, MN: NCS Pearson, Inc; 1993. 4 ed. [Google Scholar]
- 37.Cronbach L. Coefficient alpha and the internal structure of tests. Psychometrika. 1951;16:297–334. [Google Scholar]
- 38.Simons LE, Blount RL. Identifying barriers to medication adherence in adolescent transplant recipients. J. Pediatr. Psychol. 2007;32:831–844. doi: 10.1093/jpepsy/jsm030. [DOI] [PubMed] [Google Scholar]
- 39.D’Zurilla TJ, Nezu AM, Maydeu-Olivares A. Social Problem-Solving Inventory-Revised. Technical Manual. New York: Multi-Health Systems Inc; 2002. [Google Scholar]
- 40.Pai AL, Gray E, Kurivial K, Ross J, Schoborg D, Goebel J. The Allocation of Treatment Responsibility scale: a novel tool for assessing patient and caregiver management of pediatric medical treatment regimens. Pediatr. Transplant. 2010;14(8):993–999. doi: 10.1111/j.1399-3046.2010.01391.x. [DOI] [PubMed] [Google Scholar]
- 41.Robin AL, Foster SL. Negotiating parent-adolescent conflict: A behavioral-family system approach. New York: Guilford Press; 1989. [Google Scholar]
- 42.Little RJ, Rubin DB. Statistical Analysis with Missing Data. 2nd ed. Hoboken, NJ: Wiley; 2002. [Google Scholar]
- 43.Kraemer HC, Kiernan M, Essex M, Kupfer DJ. How and why criteria defining moderators and mediators differ between the Baron & Kenny and MacArthur approaches. Health Psychol. 2008;27(Suppl):101–108. doi: 10.1037/0278-6133.27.2(Suppl.).S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kraemer HC, Wilson T, Fairburn CG, Agras WS. Mediators and moderators of treatment effects in randomized clinical trials. Archives of General Psychiatry. 2002;59:877–883. doi: 10.1001/archpsyc.59.10.877. [DOI] [PubMed] [Google Scholar]
- 45.Holmbeck GN. Toward terminological, conceptual, and statistical clarity in the study of mediators and moderators: Examples from the child-clinical and pediatric psychology literatures. Journal of Consulting and Clinical Psychology. 1997;65(4):599–610. doi: 10.1037//0022-006x.65.4.599. [DOI] [PubMed] [Google Scholar]
- 46.Baron RM, Kenny DA. The moderator–mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 47.McGrady ME, Hommel KA. Medication adherence and health care utilization in pediatric chronic illness: A systematic review. Pediatrics. 2013 doi: 10.1542/peds.2013-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]


