Abstract
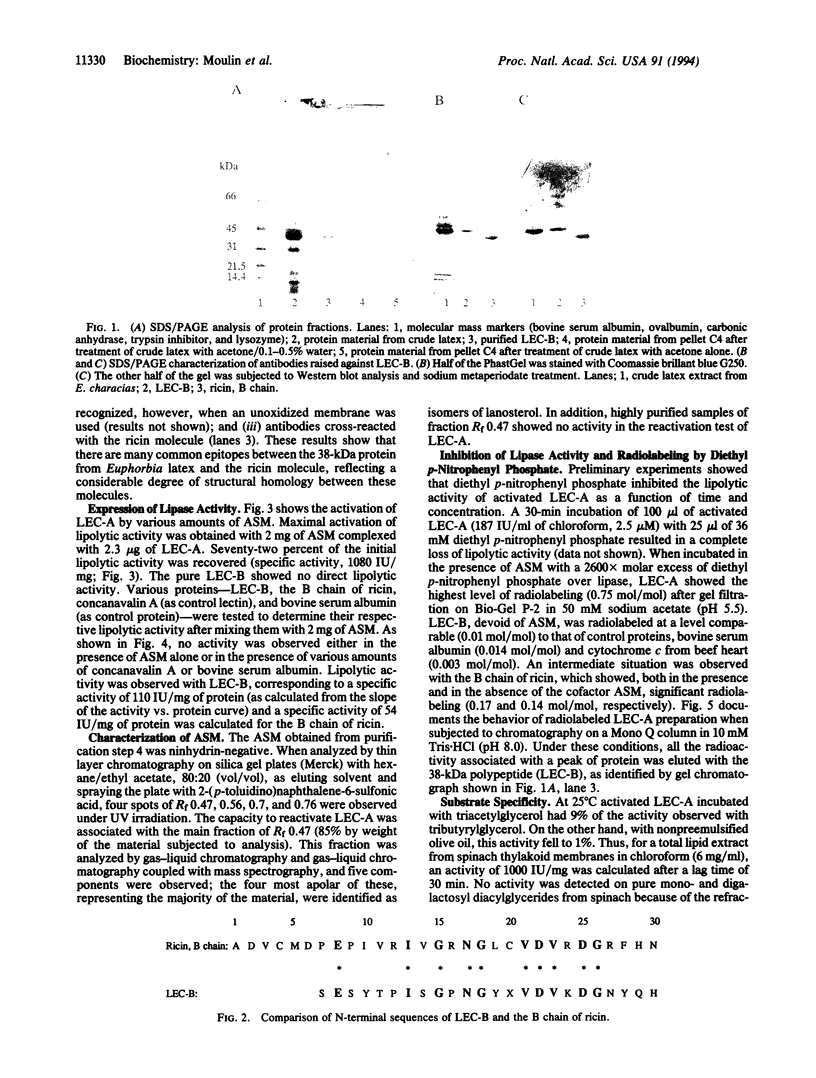
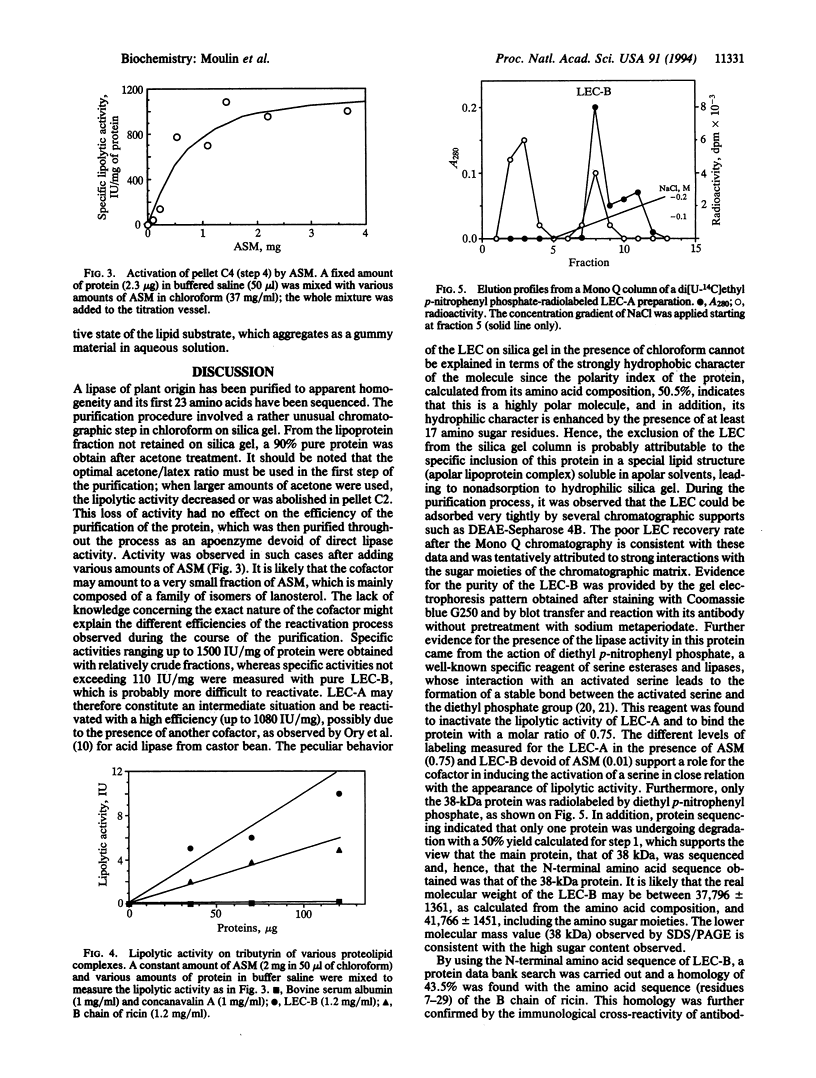
A lipase from the latex of Euphorbia characias was purified using a method involving extraction with apolar solvent and adsorption chromatography on silica gel. The lipase (specific activity, 1500 international units/mg of protein) was eluted from silica gel complexes with a lipid. The main protein fraction, which had a molecular mass of 38 kDa, was inactive when dissociated from the lipid fraction. When the lipid and protein fractions were reassociated, 72% of the lipolytic activity was recovered. This lipolytic activity was inhibited by diethyl p-nitrophenyl phosphate, which was shown to bind the lipase with a molar ratio of 0.75. High specific activities (1000 international units/mg) were measured for the lipase of E. characias on lipid extracts rich in galactosyl diacylglycerols. The apolipase was sequenced up to residue 23. The B chain of ricin has a strong homology (43.5%) with that sequence and cross-reacted with antibodies raised against the purified lipase from E. characias. The activity of the B chain of ricin was comparable (54 international units/mg) to that of the apolipase of E. characias (100 international units/mg) mixed with the same lipid cofactor complex. The primary structure (residues 68-72) of the B chain of ricin contains the lipase consensus sequence Gly-Xaa-Ser-Xaa-Gly. Its reactivity with diethyl p-nitrophenyl phosphate indicates the presence of an activated serine that, in addition to its well-documented lectin activity for galactosides, suggests that the B chain of ricin may be a galactosyl diacylglycerol lipase, closely analogous to the lipase from E. characias.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beevers H. Glyoxysomes of castor bean endosperm and their relation to gluconeogenesis. Ann N Y Acad Sci. 1969 Dec 19;168(2):313–324. doi: 10.1111/j.1749-6632.1969.tb43118.x. [DOI] [PubMed] [Google Scholar]
- Capaldi R. A., Vanderkooi G. The low polarity of many membrane proteins. Proc Natl Acad Sci U S A. 1972 Apr;69(4):930–932. doi: 10.1073/pnas.69.4.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARTLEY B. S., KILBY B. A. The reaction of p-nitrophenyl esters with chymotrypsin and insulin. Biochem J. 1954 Feb;56(2):288–297. doi: 10.1042/bj0560288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maylié M. F., Charles M., Desnuelle P. Action of organophosphates and sulfonyl halides on porcine pancreatic lipase. Biochim Biophys Acta. 1972 Jul 13;276(1):162–175. doi: 10.1016/0005-2744(72)90017-4. [DOI] [PubMed] [Google Scholar]
- Moreau H., Moulin A., Gargouri Y., Noël J. P., Verger R. Inactivation of gastric and pancreatic lipases by diethyl p-nitrophenyl phosphate. Biochemistry. 1991 Jan 29;30(4):1037–1041. doi: 10.1021/bi00218a022. [DOI] [PubMed] [Google Scholar]
- Moulin A., Giordani R., Teissère M., Piéroni G. Purification d'une lipase dans le latex d'Euphorbia characias par une méthode d'extraction en solvant apolaire. C R Acad Sci III. 1992;314(8):337–342. [PubMed] [Google Scholar]
- Muto S., Beevers H. Lipase Activities in Castor Bean Endosperm during Germination. Plant Physiol. 1974 Jul;54(1):23–28. doi: 10.1104/pp.54.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L., Blaustein J. The interaction of Ricinus communis agglutinin with normal and tumor cell surfaces. Biochim Biophys Acta. 1972 May 9;266(2):543–547. doi: 10.1016/0005-2736(72)90109-5. [DOI] [PubMed] [Google Scholar]
- ORY R. L., BARKER R. H., BOUDREAUX G. J. NATURE OF THE COFACTOR FOR THE ACID LIPASE OF RICINUS COMMUNIS. Biochemistry. 1964 Dec;3:2013–2016. doi: 10.1021/bi00900a040. [DOI] [PubMed] [Google Scholar]
- Ory R. L., Kircher H. W., Altschul A. M. Separation of a heat-stable protein activator for the castor bean lipase. Biochim Biophys Acta. 1967 Oct 23;147(2):200–207. doi: 10.1016/0005-2795(67)90399-6. [DOI] [PubMed] [Google Scholar]
- Puigserver A. Further characterization of subunit III of bovine procarboxypeptidase A-S6 as a non activatable zymogen. Biochim Biophys Acta. 1976 Jul 8;438(2):514–521. doi: 10.1016/0005-2744(76)90267-9. [DOI] [PubMed] [Google Scholar]
- Wilkinson M., Iacobucci G. A., Myers D. V. 5-Methyltryptophan: an internal strandard for tryptophan determination by ion-exchange chromatography. Anal Biochem. 1976 Feb;70(2):470–478. doi: 10.1016/0003-2697(76)90472-3. [DOI] [PubMed] [Google Scholar]




