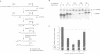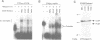Abstract
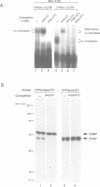
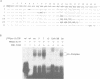
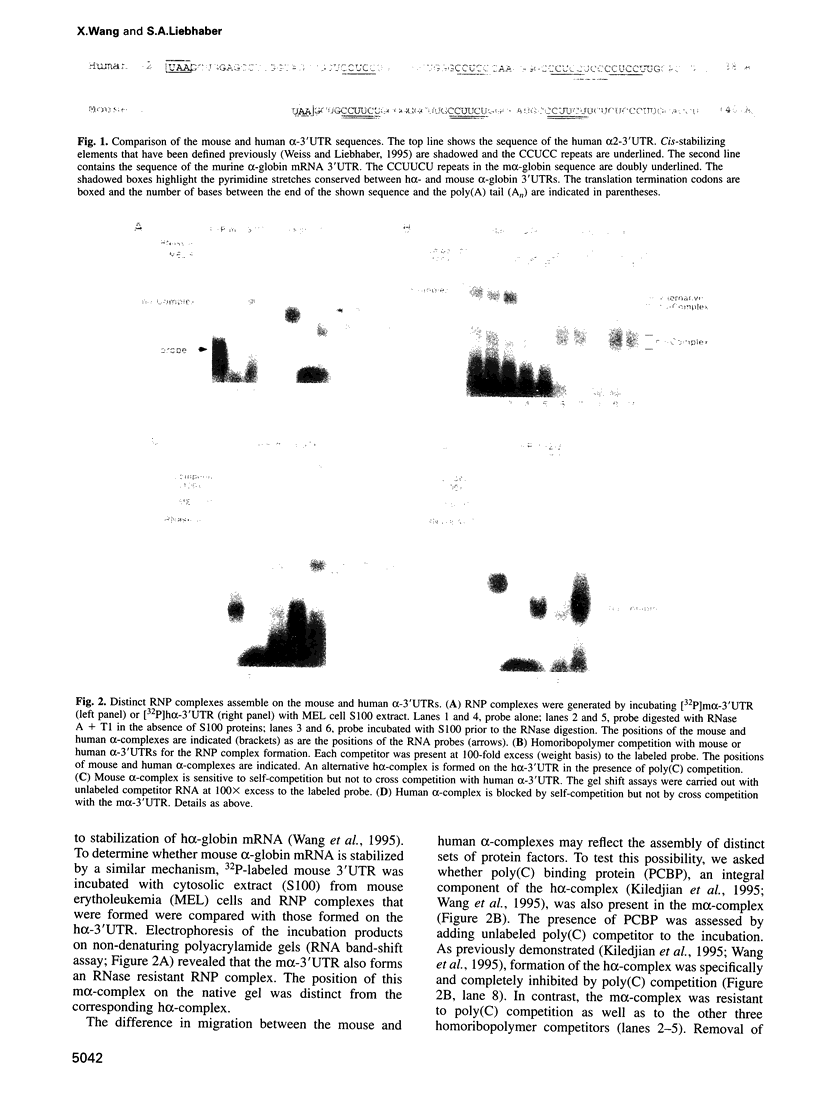
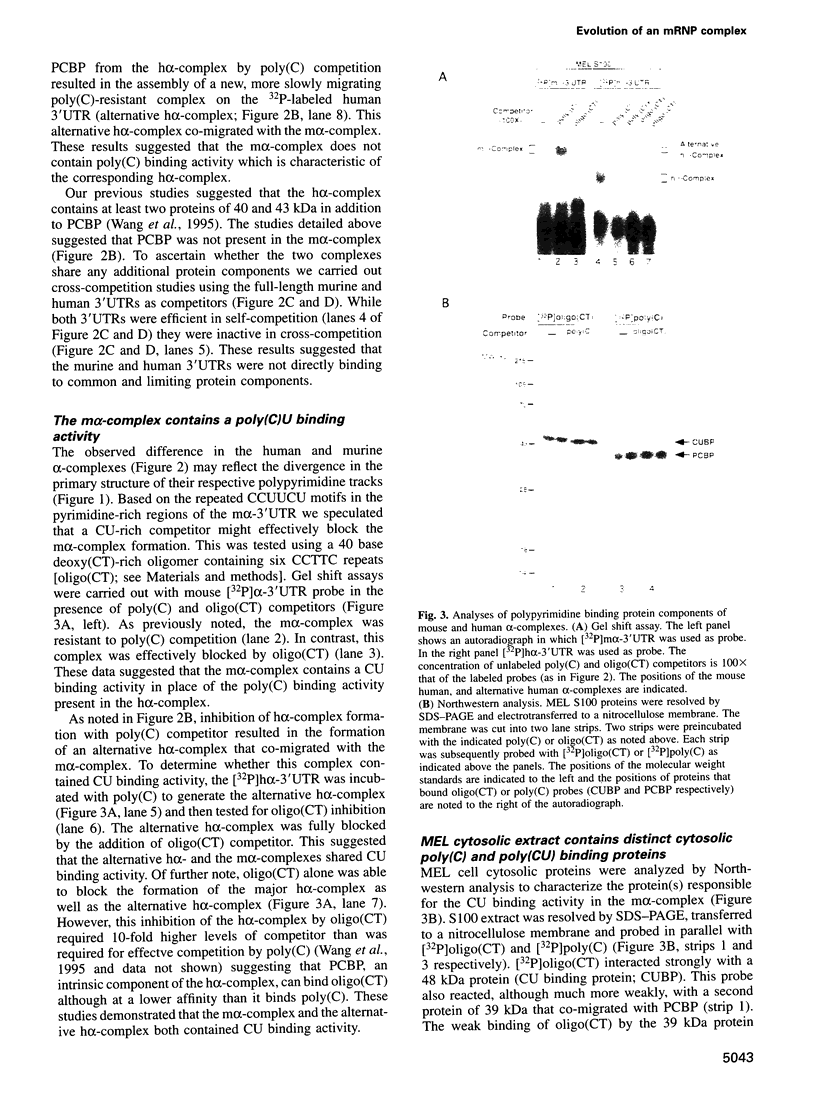
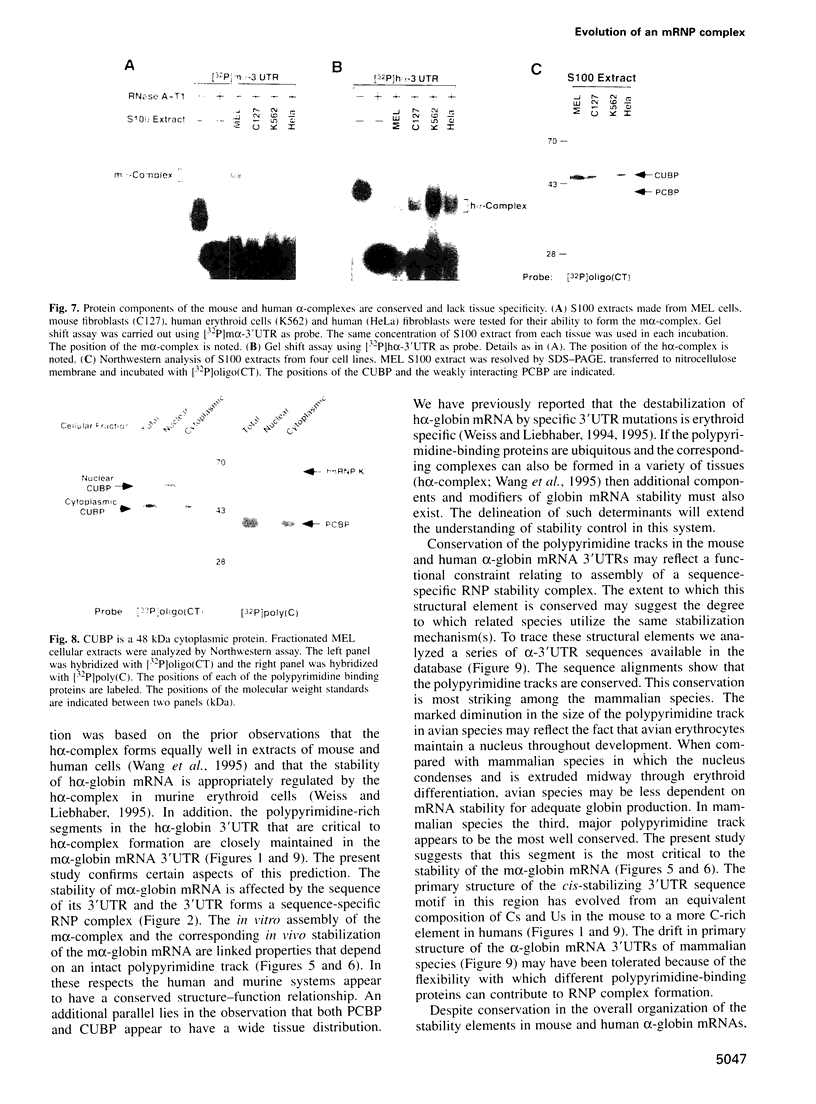
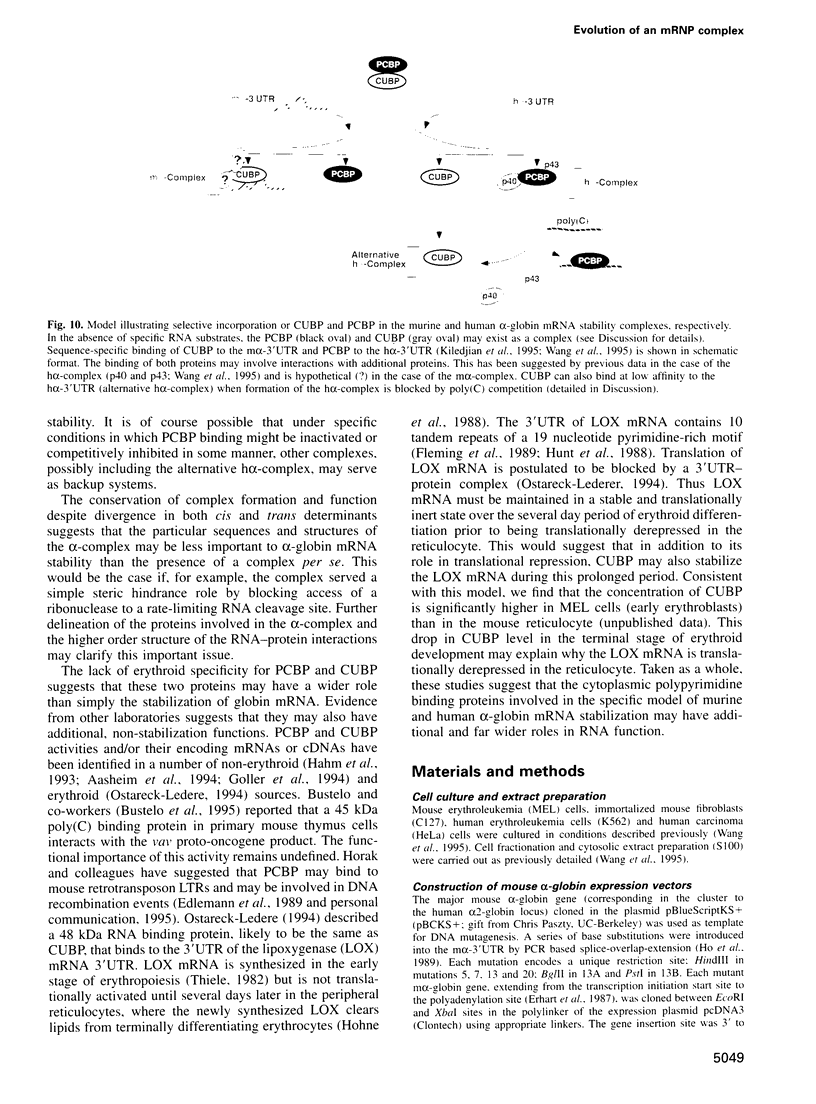
RNA-protein (RNP) complexes play significant roles in the fate and expression of mRNAs. The prolonged half-life of human alpha-globin mRNA, a major determinant of normal erythroid differentiation, is dependent on the assembly of a sequence-specific 3'-untranslated region (3'UTR) RNP (alpha-complex). We demonstrate that the stability of murine alpha-globin mRNA is controlled by a parallel mechanism. Unexpectedly, however, the respective 3'UTR RNP complexes that stabilize the h(alpha)- and m(alpha)-globin mRNAs differ in structure. While the cis determinants in both species are encoded in polypyrimidine tracks, the human determinant is C-rich (CCUCC motif) while the mouse alpha-3'UTR consists of an equal distribution of Cs and Us (CCUUCU motif). The protein components of the corresponding human and murine alpha-complexes differ in a complementary manner: the previously described 39 kDa poly(C) binding protein (PCBP) present in the human alpha-complex is replaced in the mouse alpha-complex by a 48 kDa cytoplasmic poly(CU) binding protein (CUBP). These results reveal that drift in the primary sequences of the alpha-globin mRNA 3'UTR polypyrimidine tracks in a comparison between mouse and human is paralleled by an alteration in the composition of the corresponding trans-acting components. Surprisingly, these structurally distinct complexes appear to perform the identical function of stabilizing the corresponding alpha-globin mRNAs.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aasheim H. C., Loukianova T., Deggerdal A., Smeland E. B. Tissue specific expression and cDNA structure of a human transcript encoding a nucleic acid binding [oligo(dC)] protein related to the pre-mRNA binding protein K. Nucleic Acids Res. 1994 Mar 25;22(6):959–964. doi: 10.1093/nar/22.6.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Voloch Z., Bastos R., Levy S. Biosynthesis and stability of globin mRNA in cultured erythroleukemic Friend cells. Cell. 1976 Aug;8(4):495–503. doi: 10.1016/0092-8674(76)90217-8. [DOI] [PubMed] [Google Scholar]
- Beelman C. A., Parker R. Degradation of mRNA in eukaryotes. Cell. 1995 Apr 21;81(2):179–183. doi: 10.1016/0092-8674(95)90326-7. [DOI] [PubMed] [Google Scholar]
- Binder R., Horowitz J. A., Basilion J. P., Koeller D. M., Klausner R. D., Harford J. B. Evidence that the pathway of transferrin receptor mRNA degradation involves an endonucleolytic cleavage within the 3' UTR and does not involve poly(A) tail shortening. EMBO J. 1994 Apr 15;13(8):1969–1980. doi: 10.1002/j.1460-2075.1994.tb06466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohjanen P. R., Petryniak B., June C. H., Thompson C. B., Lindsten T. An inducible cytoplasmic factor (AU-B) binds selectively to AUUUA multimers in the 3' untranslated region of lymphokine mRNA. Mol Cell Biol. 1991 Jun;11(6):3288–3295. doi: 10.1128/mcb.11.6.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd C. G., Dreyfuss G. Conserved structures and diversity of functions of RNA-binding proteins. Science. 1994 Jul 29;265(5172):615–621. doi: 10.1126/science.8036511. [DOI] [PubMed] [Google Scholar]
- Bustelo X. R., Suen K. L., Michael W. M., Dreyfuss G., Barbacid M. Association of the vav proto-oncogene product with poly(rC)-specific RNA-binding proteins. Mol Cell Biol. 1995 Mar;15(3):1324–1332. doi: 10.1128/mcb.15.3.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey J. L., Koeller D. M., Ramin V. C., Klausner R. D., Harford J. B. Iron regulation of transferrin receptor mRNA levels requires iron-responsive elements and a rapid turnover determinant in the 3' untranslated region of the mRNA. EMBO J. 1989 Dec 1;8(12):3693–3699. doi: 10.1002/j.1460-2075.1989.tb08544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. Y., Shyu A. B. AU-rich elements: characterization and importance in mRNA degradation. Trends Biochem Sci. 1995 Nov;20(11):465–470. doi: 10.1016/s0968-0004(00)89102-1. [DOI] [PubMed] [Google Scholar]
- Chen C. Y., Shyu A. B. Selective degradation of early-response-gene mRNAs: functional analyses of sequence features of the AU-rich elements. Mol Cell Biol. 1994 Dec;14(12):8471–8482. doi: 10.1128/mcb.14.12.8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg J. B., Weatherall D. J., Milner P. F. Haemoglobin Constant Spring--a chain termination mutant? Nature. 1971 Dec 10;234(5328):337–340. doi: 10.1038/234337a0. [DOI] [PubMed] [Google Scholar]
- Curtis D., Lehmann R., Zamore P. D. Translational regulation in development. Cell. 1995 Apr 21;81(2):171–178. doi: 10.1016/0092-8674(95)90325-9. [DOI] [PubMed] [Google Scholar]
- Decker C. J., Parker R. Diversity of cytoplasmic functions for the 3' untranslated region of eukaryotic transcripts. Curr Opin Cell Biol. 1995 Jun;7(3):386–392. doi: 10.1016/0955-0674(95)80094-8. [DOI] [PubMed] [Google Scholar]
- Dreyfuss G., Matunis M. J., Piñol-Roma S., Burd C. G. hnRNP proteins and the biogenesis of mRNA. Annu Rev Biochem. 1993;62:289–321. doi: 10.1146/annurev.bi.62.070193.001445. [DOI] [PubMed] [Google Scholar]
- Edelmann W., Kröger B., Goller M., Horak I. A recombination hotspot in the LTR of a mouse retrotransposon identified in an in vitro system. Cell. 1989 Jun 16;57(6):937–946. doi: 10.1016/0092-8674(89)90332-2. [DOI] [PubMed] [Google Scholar]
- Erhart M. A., Piller K., Weaver S. Polymorphism and gene conversion in mouse alpha-globin haplotypes. Genetics. 1987 Mar;115(3):511–519. doi: 10.1093/genetics/115.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C. A., Wickens M. Poly(A) removal during oocyte maturation: a default reaction selectively prevented by specific sequences in the 3' UTR of certain maternal mRNAs. Genes Dev. 1990 Dec;4(12B):2287–2298. doi: 10.1101/gad.4.12b.2287. [DOI] [PubMed] [Google Scholar]
- Gavis E. R., Lehmann R. Localization of nanos RNA controls embryonic polarity. Cell. 1992 Oct 16;71(2):301–313. doi: 10.1016/0092-8674(92)90358-j. [DOI] [PubMed] [Google Scholar]
- Gavis E. R., Lehmann R. Translational regulation of nanos by RNA localization. Nature. 1994 May 26;369(6478):315–318. doi: 10.1038/369315a0. [DOI] [PubMed] [Google Scholar]
- Goller M., Funke B., Gehe-Becker C., Kröger B., Lottspeich F., Horak I. Murine protein which binds preferentially to oligo-C-rich single-stranded nucleic acids. Nucleic Acids Res. 1994 May 25;22(10):1885–1889. doi: 10.1093/nar/22.10.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens M., Lee K. Y., Liebhaber S. A., Kan Y. W. Globin structural mutant alpha 125Leu leads to Pro is a novel cause of alpha-thalassaemia. Nature. 1982 Apr 29;296(5860):864–865. doi: 10.1038/296864a0. [DOI] [PubMed] [Google Scholar]
- Haff L. A. Improved quantitative PCR using nested primers. PCR Methods Appl. 1994 Jun;3(6):332–337. doi: 10.1101/gr.3.6.332. [DOI] [PubMed] [Google Scholar]
- Hahm K., Kim G., Turck C. W., Smale S. T. Isolation of a murine gene encoding a nucleic acid-binding protein with homology to hnRNP K. Nucleic Acids Res. 1993 Aug 11;21(16):3894–3894. doi: 10.1093/nar/21.16.3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989 Apr 15;77(1):51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Hunt D. M., Higgs D. R., Winichagoon P., Clegg J. B., Weatherall D. J. Haemoglobin Constant Spring has an unstable alpha chain messenger RNA. Br J Haematol. 1982 Jul;51(3):405–413. doi: 10.1111/j.1365-2141.1982.tb02796.x. [DOI] [PubMed] [Google Scholar]
- Hämäläinen L., Oikarinen J., Kivirikko K. I. Synthesis and degradation of type I procollagen mRNAs in cultured human skin fibroblasts and the effect of cortisol. J Biol Chem. 1985 Jan 25;260(2):720–725. [PubMed] [Google Scholar]
- Höhne M., Thiele B. J., Prehn S., Giessmann E., Nack B., Rapoport S. M. Activation of translationally inactive lipoxygenase mRNP particles from rabbit reticulocytes. Biomed Biochim Acta. 1988;47(1):75–78. [PubMed] [Google Scholar]
- Izaurralde E., Mattaj I. W. RNA export. Cell. 1995 Apr 21;81(2):153–159. doi: 10.1016/0092-8674(95)90323-2. [DOI] [PubMed] [Google Scholar]
- Jackson R. J. Cytoplasmic regulation of mRNA function: the importance of the 3' untranslated region. Cell. 1993 Jul 16;74(1):9–14. doi: 10.1016/0092-8674(93)90290-7. [DOI] [PubMed] [Google Scholar]
- Katz D. A., Theodorakis N. G., Cleveland D. W., Lindsten T., Thompson C. B. AU-A, an RNA-binding activity distinct from hnRNP A1, is selective for AUUUA repeats and shuttles between the nucleus and the cytoplasm. Nucleic Acids Res. 1994 Jan 25;22(2):238–246. doi: 10.1093/nar/22.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller W. No end yet to messenger RNA 3' processing! Cell. 1995 Jun 16;81(6):829–832. doi: 10.1016/0092-8674(95)90001-2. [DOI] [PubMed] [Google Scholar]
- Kiledjian M., Wang X., Liebhaber S. A. Identification of two KH domain proteins in the alpha-globin mRNP stability complex. EMBO J. 1995 Sep 1;14(17):4357–4364. doi: 10.1002/j.1460-2075.1995.tb00110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Ha J., Kerr K., Macdonald P. M. Translational regulation of oskar mRNA by bruno, an ovarian RNA-binding protein, is essential. Cell. 1995 May 5;81(3):403–412. doi: 10.1016/0092-8674(95)90393-3. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Rouault T. A., Harford J. B. Regulating the fate of mRNA: the control of cellular iron metabolism. Cell. 1993 Jan 15;72(1):19–28. doi: 10.1016/0092-8674(93)90046-s. [DOI] [PubMed] [Google Scholar]
- Krowczynska A., Yenofsky R., Brawerman G. Regulation of messenger RNA stability in mouse erythroleukemia cells. J Mol Biol. 1985 Jan 20;181(2):231–239. doi: 10.1016/0022-2836(85)90087-7. [DOI] [PubMed] [Google Scholar]
- Lagnado C. A., Brown C. Y., Goodall G. J. AUUUA is not sufficient to promote poly(A) shortening and degradation of an mRNA: the functional sequence within AU-rich elements may be UUAUUUA(U/A)(U/A). Mol Cell Biol. 1994 Dec;14(12):7984–7995. doi: 10.1128/mcb.14.12.7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. A., Beebe D. C. Messenger RNA stabilization in chicken lens development: a reexamination. Dev Biol. 1991 Jul;146(1):239–241. doi: 10.1016/0012-1606(91)90463-d. [DOI] [PubMed] [Google Scholar]
- Liebhaber S. A., Kan Y. W. Differentiation of the mRNA transcripts originating from the alpha 1- and alpha 2-globin loci in normals and alpha-thalassemics. J Clin Invest. 1981 Aug;68(2):439–446. doi: 10.1172/JCI110273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malter J. S. Identification of an AUUUA-specific messenger RNA binding protein. Science. 1989 Nov 3;246(4930):664–666. doi: 10.1126/science.2814487. [DOI] [PubMed] [Google Scholar]
- Matunis M. J., Michael W. M., Dreyfuss G. Characterization and primary structure of the poly(C)-binding heterogeneous nuclear ribonucleoprotein complex K protein. Mol Cell Biol. 1992 Jan;12(1):164–171. doi: 10.1128/mcb.12.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mättä A., Ekholm E., Penttinen R. P. Effect of the 3'-untranslated region on the expression levels and mRNA stability of alpha 1(I) collagen gene. Biochim Biophys Acta. 1995 Feb 21;1260(3):294–300. doi: 10.1016/0167-4781(94)00207-j. [DOI] [PubMed] [Google Scholar]
- Nishioka Y., Leder P. The complete sequence of a chromosomal mouse alpha--globin gene reveals elements conserved throughout vertebrate evolution. Cell. 1979 Nov;18(3):875–882. doi: 10.1016/0092-8674(79)90139-9. [DOI] [PubMed] [Google Scholar]
- Ostareck-Lederer A., Ostareck D. H., Standart N., Thiele B. J. Translation of 15-lipoxygenase mRNA is inhibited by a protein that binds to a repeated sequence in the 3' untranslated region. EMBO J. 1994 Mar 15;13(6):1476–1481. doi: 10.1002/j.1460-2075.1994.tb06402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J., Pizarro A. Human beta and delta globin messenger RNAs turn over at different rates. J Mol Biol. 1983 Jul 5;167(3):607–617. doi: 10.1016/s0022-2836(83)80101-6. [DOI] [PubMed] [Google Scholar]
- Sachs A. B. Messenger RNA degradation in eukaryotes. Cell. 1993 Aug 13;74(3):413–421. doi: 10.1016/0092-8674(93)80043-e. [DOI] [PubMed] [Google Scholar]
- Samaniego F., Chin J., Iwai K., Rouault T. A., Klausner R. D. Molecular characterization of a second iron-responsive element binding protein, iron regulatory protein 2. Structure, function, and post-translational regulation. J Biol Chem. 1994 Dec 9;269(49):30904–30910. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A. Split genes and RNA splicing. Cell. 1994 Jun 17;77(6):805–815. doi: 10.1016/0092-8674(94)90130-9. [DOI] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Sheets M. D., Fox C. A., Hunt T., Vande Woude G., Wickens M. The 3'-untranslated regions of c-mos and cyclin mRNAs stimulate translation by regulating cytoplasmic polyadenylation. Genes Dev. 1994 Apr 15;8(8):926–938. doi: 10.1101/gad.8.8.926. [DOI] [PubMed] [Google Scholar]
- Siomi H., Matunis M. J., Michael W. M., Dreyfuss G. The pre-mRNA binding K protein contains a novel evolutionarily conserved motif. Nucleic Acids Res. 1993 Mar 11;21(5):1193–1198. doi: 10.1093/nar/21.5.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Johnston D. The intracellular localization of messenger RNAs. Cell. 1995 Apr 21;81(2):161–170. doi: 10.1016/0092-8674(95)90324-0. [DOI] [PubMed] [Google Scholar]
- Swanson M. S., Dreyfuss G. Classification and purification of proteins of heterogeneous nuclear ribonucleoprotein particles by RNA-binding specificities. Mol Cell Biol. 1988 May;8(5):2237–2241. doi: 10.1128/mcb.8.5.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele B. J., Andree H., Höhne M., Rapoport S. M. Lipoxygenase mRNA in rabbit reticulocytes. Its isolation, characterization and translational repression. Eur J Biochem. 1982 Dec;129(1):133–141. doi: 10.1111/j.1432-1033.1982.tb07031.x. [DOI] [PubMed] [Google Scholar]
- Volloch V., Housman D. Stability of globin mRNA in terminally differentiating murine erythroleukemia cells. Cell. 1981 Feb;23(2):509–514. doi: 10.1016/0092-8674(81)90146-x. [DOI] [PubMed] [Google Scholar]
- Wang X., Kiledjian M., Weiss I. M., Liebhaber S. A. Detection and characterization of a 3' untranslated region ribonucleoprotein complex associated with human alpha-globin mRNA stability. Mol Cell Biol. 1995 Mar;15(3):1769–1777. doi: 10.1128/mcb.15.3.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss I. M., Liebhaber S. A. Erythroid cell-specific determinants of alpha-globin mRNA stability. Mol Cell Biol. 1994 Dec;14(12):8123–8132. doi: 10.1128/mcb.14.12.8123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss I. M., Liebhaber S. A. Erythroid cell-specific mRNA stability elements in the alpha 2-globin 3' nontranslated region. Mol Cell Biol. 1995 May;15(5):2457–2465. doi: 10.1128/mcb.15.5.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens M. Messenger RNA. Springtime in the desert. Nature. 1993 May 27;363(6427):305–306. doi: 10.1038/363305a0. [DOI] [PubMed] [Google Scholar]
- Wisdom R., Lee W. The protein-coding region of c-myc mRNA contains a sequence that specifies rapid mRNA turnover and induction by protein synthesis inhibitors. Genes Dev. 1991 Feb;5(2):232–243. doi: 10.1101/gad.5.2.232. [DOI] [PubMed] [Google Scholar]
- Zhang W., Wagner B. J., Ehrenman K., Schaefer A. W., DeMaria C. T., Crater D., DeHaven K., Long L., Brewer G. Purification, characterization, and cDNA cloning of an AU-rich element RNA-binding protein, AUF1. Mol Cell Biol. 1993 Dec;13(12):7652–7665. doi: 10.1128/mcb.13.12.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]