Abstract
Objective
In the 7th edition of the TNM classification of malignant tumors, the prognosis for pT4-M1a stage IV lung cancer is better than for stage pIIIB. Subgroups of lung cancer patients who underwent incomplete resection (R1/R2) have a favorable prognosis. This study compares the prognosis between cases of invisible local residual disease and intrathoracic disseminated pT4-M1aIV.
Methods
Patient characteristics and histological and molecular profiles were retrospectively collected for lung cancer patients who underwent resection intended to be curative but were accidentally incomplete. All patients were divided into either a local residual group or an intrathoracic disseminated pT4M1a group. Progression-free survival (PFS) and overall survival (OS) were evaluated by Kaplan-Meier and Cox regression models.
Results
In total, 1,483 consecutive lung cancer patients receiving thoracotomies at Guangdong Lung Cancer Institute were retrospectively analyzed. Fifty-eight patients receiving incomplete resections (R1/R2) were enrolled, including 38 patients with local residual cancer (2.6% of all patients) and 20 patients with disseminated pM1a (1.3%). Patient characteristics, and histological and molecular profiles of the two groups were different. Compared to the local residual group, the disseminated pT4-M1a group contained more females (P=0.002), more patients younger than 60 years of age (P=0.028), more non-smokers (P=0.037), more adenocarcinomas (20/20 vs. 20/38, P<0.001), more adenocarcinomas with lepidic pattern (11/20 vs. 4/38, P<0.001), higher carcinoembryonic antigen (CEA) levels (P=0.06), higher epidermal growth factor receptor (EGFR) mutation rates (16/20 vs. 7/38, P<0.001), a higher R2/R1 resection ratio (P=0.013), a higher advanced stage IV/IIIB ratio (P<0.001), but fewer lymph node metastases (P=0.013). Median PFS for the local residual and disseminated pT4-M1a groups was 9.0 and 18.0 months, respectively [95% confidence interval (CI), 5.285-16.715; P =0.099]. Median OS was 15.0 and 45.0 months, respectively (95% CI, 18.972-39.028; P=0.001). Cox regression analysis revealed that group (local residual vs. disseminated pT4-M1a) was the only independent prognostic factor (P=0.044) for OS.
Conclusions
Accidental invisible intrathoracic disseminated pT4-M1a may be a distinct lung cancer subtype with a favorable prognosis. The prolonged PFS and OS might reflect the natural history of this distinct subtype, together with a favorable response to EGFR tyrosine kinase inhibitors (EGFR-TKI). For asymptomatic and slow-growing accidental pT4-M1a disease, the role of a wait-and-see strategy and the appropriate timing of systemic treatment require further investigation.
Keywords: Lung cancer, incomplete resection, accidental invisible pT4-M1a, prognosis
Introduction
Lung cancer is the leading cause of cancer-related mortality throughout the world (1). Over the past 20 years, the incidence of lung cancer in China has increased significantly. Non-small cell lung cancer (NSCLC) accounts for 85% of lung cancer cases. Surgical resection is associated with increased long-term survival for patients with early or even some locally advanced NSCLC (2,3). TNM staging is one of the most important clinical prognostic factors. Nevertheless, in the 7th edition of the TNM classification of malignant tumors, there appears to be no good explanation for why the prognosis of stage pT4-M1a stage IV lung cancer is better than pIIIB. Median overall survival (OS) and 5-year survival with stage IV was superior to stage IIIB (17 vs. 13 months, 13% vs. 7%) (4). With the application of high-resolution CT (HRCT) or PET/CT, the proportion of incomplete resections (R1/R2) of lung cancer is being gradually reduced. However, inconsistencies between preoperative imaging and surgical findings inevitably lead to exploratory thoracotomies, invisible local residual disease, and intrathoracic disseminated pT4-M1a (5). In clinical practice, some patients undergoing incomplete resection have a more favorable prognosis than would be expected (5). Some papers have reported that the prognosis of patients diagnosed with malignant pleural disease (pM1a) at thoracotomy is relatively favorable (6,7), although the related prognostic factors and the specific mechanism remain unclear (7,8). This study compares the prognosis between invisible local residual disease and intrathoracic disseminated pT4-M1a after incomplete resection, and indicates that the latter might represent a distinct subset of lung cancers with a favorable prognosis.
Patients and methods
Patients and data collection
We retrospectively reviewed lung cancer patients whose preoperative diagnosis was either clinical stage 1-2 or resectable stage 3a, and who underwent thoractomy at Guangdong Lung Cancer Institute between June 2007 and July 2013. All surgeries were curative in intention. Patients with incomplete resections were divided into a local residual group and an intrathoracic disseminated pT4-M1a group, based on intrathoracic findings. For each patient we collected the following data: age, gender, TNM stage, epidermal growth factor receptor (EGFR) mutation status, histology subtype, N stage, ratio of R1/R2 resection, level of carcinoembryonic antigen (CEA), expression of EGFR, vascular endothelial growth factor (VEGF), excision repair cross complementing 1 (ERCC1), and β-tubulin, progression-free survival (PFS), and OS.
Surgery and incomplete resection
Resection margins were routinely monitored by perioperative frozen section. Resection was classified as R1 if a microscopic tumor was found at the edge of the operative specimen and R2 if gross residual disease remained after surgical excision of the tumor. R2 resections usually involved a small amount of tumor remaining on a non-resectable structure, or cases where larger resections were deemed functionally unacceptable (9). Cases of invisible intrathoracic disseminated pT4-M1a were defined as early stage before surgery, but demonstrated either multiple separate tumor nodules in different lobes, ipsilateral to that of the primary tumor, or tumor with pleural nodules. All primary tumors and metastatic lesions were confirmed by pathology. Surgical decisions about performing sampling, sub-lobectomy or lobectomy, lymph node dissection, and fulguration of the parietal pleura were based on the surgeon’s extemporaneous judgment, as influenced by patient age, tumor size and number, location, lung function, and other characteristics.
Statistical considerations
We compared the local residual group to the disseminated pT4-M1a group in gender, smoking history, age, pathological type, EGFR mutation, CEA level, expression of EGFR and VEGF, and tumor stage. Chi-square or Fisher’s exact tests were used to compare qualitative data. PFS was defined as the time from commencement of surgery to the first documentation of progressive disease (PD) or to death from any cause. OS was calculated from commencement of surgery to the last visit or to death from any cause. The Kaplan-Meier method was used to estimate survival curves. Log-rank tests were used to compare survival curves between the two groups. Multivariate Cox proportional hazards regression was used to evaluate independent prognostic factors associated with OS. All statistical tests were two-sided, and values for P<0.05 were deemed to be significant. Statistical Package for Social Sciences (SPSS) software version 13.0 was used.
Results
Patient characteristics
In total, 1,483 consecutive patients with lung cancer underwent potentially curative surgery between June 2007 and July 2013. Of the 58 patients receiving incomplete resection (R1/R2), 38 (65.5%) had local residual disease, and 20 (34.5%) had disseminated pT4-M1a.
This cohort included 36 (62.1%) males and 22 (37.9%) females. Ages ranged from 34 to 83 years (median 58). In all, 19 patients (32.8%) were smokers, and 39 (67.2%) had no smoking history. There were 40 (69.0%) patients diagnosed with adenocarcinoma, and 18 (31.0%) with non-adenocarcinomas. All of the cases in the disseminated pT4-M1a group were adenocarcinomas (20/20, 100.0%). The local residual group included 20 adenocarcinomas (20/38, 52.6%), 15 (39.5%) squamous cell carcinomas, 1 sarcomatoid carcinoma, 1 small cell lung cancer, and 1 neuroendocrine carcinoma (P<0.001). Compared to the local residual group, the disseminated pT4-M1a group contained more females (P=0.002), more patients younger than 60 years of age (P=0.028), and more non-smokers (P=0.037). Patients with disseminated pT4-M1a had a lower proportion of lymph node metastases than did the local residual group (19.10 vs. 34.97, P<0.001). More stage IV disease (P<0.001) was found in the disseminated pT4-M1a group. The proportion of R1/R2 resections in the disseminated pT4M1a group and the local residual group was 0/20 and 12/26, respectively (P=0.013).
Primary tumor removals were performed in 20 cases of pT4M1a, which included 9 pulmonary wedge resections and 11 lobectomies. Two patients had systematic mediastinal lymphadenectomy, nine patients had lymph nodal sampling, and no lymph nodes were removed in the remaining nine cases. The demographic data for all patients are summarized in Table 1.
Table 1. Patient characteristics.
| Characteristics | N=58 (%) | Local residual, n=38 (%) | Disseminated pT4M1a, n=20 (%) | P value |
|---|---|---|---|---|
| Sex | 0.002 | |||
| Male | 36 (62.1) | 29 (76.3) | 7 (35.0) | |
| Female | 22 (37.9) | 9 (23.7) | 13 (65.0) | |
| Age, years | 0.028 | |||
| ≤60 | 32 (55.2) | 17 (44.7) | 15 (75.0) | |
| >60 | 26 (44.8) | 21 (55.3) | 5 (25.0) | |
| Smoking | 0.037 | |||
| Yes | 19 (32.8) | 16 (42.1) | 3 (15.0) | |
| No | 39 (67.2) | 22 (57.9) | 17 (85.0) | |
| Resection | 0.013 | |||
| R1 | 12 (20.7) | 12 (31.6) | 0 | |
| R2 | 46 (79.3) | 26 (68.4) | 20 (100.0) | |
| Pathologic type | <0.001 | |||
| ADC | 40 (69.0) | 20 (52.6) | 20 (100.0) | |
| Non-ADC | 18 (31.0) | 18 (47.4) | 0 | |
| N status | 0.001 | |||
| N0 | 24 (41.4) | 9 (23.7) | 15 (75.0) | |
| N1 | 9 (15.5) | 7 (18.4) | 2 (10.0) | |
| N2 | 24 (41.4) | 21 (55.3) | 3 (15.0) | |
| N3 | 1 (1.7) | 1 (2.6) | 0 | |
| Systemic treatment | 0.005 | |||
| Chemo only | 39 (67.2) | 29 (76.3) | 10 (50.0) | |
| TKI | 10 (17.2) | 2 (5.3) | 8 (40.0) | |
| None | 9 (15.5) | 7 (18.4) | 2 (10.0) | |
| TNM stage | <0.001 | |||
| IA/IB | 4 (6.9) | 4 (10.5) | 0 | |
| IIA/IIB | 2 (3.4) | 2 (5.3) | 0 | |
| IIIA | 15 (25.9) | 15 (39.5) | 0 | |
| IIIB | 14 (24.1) | 13 (34.2) | 1 (5.0) | |
| IV | 23 (39.7) | 4 (10.5) | 19 (95.0) |
ADC, adenocarcinoma; TKI, tyrosine kinase inhibitor.
Pathology and biomarkers
The disseminated pT4-M1a group included more adenocarcinomas with a lepidic pattern (11/20 vs. 4/38, P<0.001), and a higher proportion of EGFR mutations (16/20, 80.0%) compared to the local residual group (7/38, 18.4%, P<0.001). The level of CEA in the two groups was 34.21 and 25.57, respectively (P=0.06). The expression of EGFR in the disseminated and local residual groups was 22.70 and 33.08, respectively (P=0.022). In addition, the expression of VEGF in the two groups was 26.53 and 31.07, respectively (P=0.314). Distinctions in pathology and biomarkers between the two groups are summarized in Table 2.
Table 2. Pathology and molecular markers.
| Definition | Local residual | Disseminated pT4-M1a | P value |
|---|---|---|---|
| ADC with lepidic pattern | 4/38 | 11/20 | <0.001 |
| Level of CEA | 4.17 (0.49-601.00) | 12.47 (0.58-286.63) | 0.060 |
| EGFR mutation/WT | 7/29 | 16/2 | <0.001 |
| EGFR (−/+/++/+++) | (2/6/6/12) | (4/4/4/7) | 0.022 |
| VEGF (−/+/++/+++) | (5/11/5/6) | (0/10/6/3) | 0.314 |
| ERCC1 (−/+/++/+++) | (5/13/1/8) | (4/8/5/2) | 0.070 |
| β-tubulin (−/+/++/+++) | (6/3/4/14) | (3/4/5/7) | 0.055 |
ADC, adenocarcinoma; CEA, carcinoembryonic antigen; EGFR, epidermal growth factor receptor; WT, wild type; VEGF, vascular endothelial growth factor; ERCC1, excision repair cross complementing 1.
Survival analysis
The last follow-up recorded was January 20, 2015. The median PFS for the local residual and disseminated groups was 9.0 and 18.0 months, respectively [95% confidence interval (CI), 5.285-16.715; P=0.099]. The median OS was 15.0 and 45.0 months, respectively (95% CI, 18.972-39.028, P=0.001). The difference in OS between the two groups was statistically significant (Figures 1 and 2).
Figure 1.
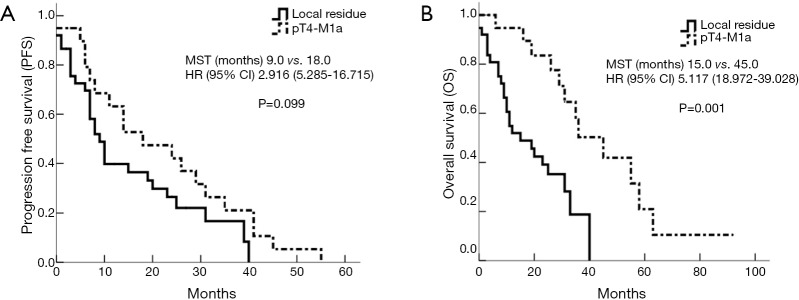
Survival analysis between local residual and accidental invisible disseminated pT4-M1a. MST, median survival time; HR, hazard ratio; CI, confidence interval.
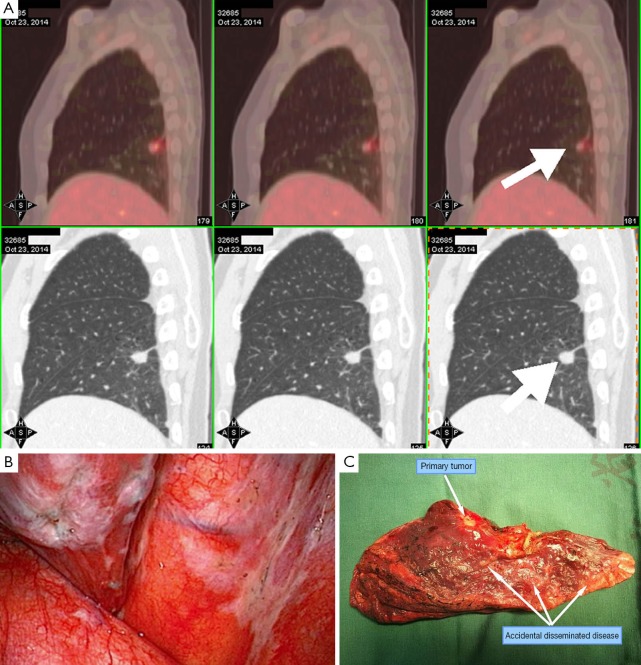
Figure 2.
Accidental invisible intrathoracic disseminated pT4-M1a. (A) Image before surgery, diagnosed as early stage; (B) intrathoracic surgical finding of parietal pleura; (C) resected lobe with disseminated disease.
Cox regression
Initially, group, gender, age, pathological type, EGFR mutation, CEA level, N stage, and expression of EGFR and VEGF were selected for univariate analysis. Then we conducted multivariate Cox regression analysis, based on the variables screened through univariate analysis. Multivariate analysis revealed that group (local residual vs. disseminated pT4-M1a) was the only independent prognostic factor (P=0.044) for OS (Table 3).
Table 3. Cox regression for overall survival.
| Variable | N=58 (%) | Overall survival |
||
|---|---|---|---|---|
| Odds ratio | 95% CI | P value | ||
| Univariate analysis | ||||
| Group | 3.677 | 1.595-8.476 | 0.002 | |
| Local residue | 38 (65.5) | |||
| Disseminated pM1a | 20 (34.5) | |||
| Gender | 1.590 | 0.794-3.183 | 0.191 | |
| Male | 36 (62.1) | |||
| Female | 22 (37.9) | |||
| Age, years | 0.488 | 0.242-0.986 | 0.046 | |
| ≤60 | 32 (55.2) | |||
| >60 | 26 (44.8) | |||
| EGFR mutation | 0.884 | 0.203-3.844 | 0.869 | |
| Yes | 23 (39.7) | |||
| No | 31 (53.4) | |||
| unknown | 4 (6.9) | |||
| Pathologic type | 0.402 | 0.200-0.808 | 0.011 | |
| ADC | 40 (69.0) | |||
| Non-ADC | 18 (31.0) | |||
| N status | 0.118 | 0.276-1.157 | 0.565 | |
| N0 | 24 (41.4) | |||
| N1 | 9 (15.5) | |||
| N2 | 24 (41.4) | |||
| N3 | 1 (1.7) | |||
| Multivariate analysis | ||||
| Group | 2.746 | 1.028-7.340 | 0.044 | |
| Local residual | 38 (65.5) | |||
| Disseminated pT4M1a | 20 (34.5) | |||
CI, confidence interval; EGFR, epidermal growth factor receptor; ADC, adenocarcinoma.
Discussion
Traditionally, residual disease at a resection margin is characterized as microscopic (R1) or macroscopic (R2) during exploratory thoracotomy for disseminated disease. Hofmann (10) reviewed 596 cases of radical surgery of lung cancer, in which 26 patients (4.4%) showed R1 and 12 (2.0%) showed R2 residual disease. The 5-year survival rate for patients with R1 resection was 14%, close to that for stage IIIA. Most patients with R2 resection live less than 1 year. In accordance with the definitions of complete and incomplete resection in lung cancer surgery, the prognosis for R1 resection is superior to R2 resection (11). Theoretically, the prognosis of local residual disease (not stage IV) is better than disseminated pT4-M1a (stage IV) on exploratory thoracotomy. However, the present study suggests that, between types of incomplete resection, the median and overall PFS and OS of disseminated pT4-M1a stage IV was better than that of local residual disease. This may explain the better prognosis of stage pIV over stage pIIIB noted in the 7th edition of TNM staging classifications for lung cancer.
Compared to the local residual group, the disseminated pT4-M1a group contained more females, more younger patients, more non-smokers, more adenocarcinomas (especially of lepidic pattern), higher CEA levels, a higher EGFR mutation rate, a higher R2/R1 resection ratio, fewer lymph node metastases, a higher stage IV/IIIB ratio, and longer PFS and OS. Exploratory thoracotomies found, in addition to the primary tumor, 1-10 mm diameter miliary nodules distributed on the surface of the visceral pleura, parietal pleura, pericardium, or diaphragm, visualized by perioperative imaging (Figure 2). Mediastinal lymph node metastasis and extra-pulmonary metastasis of this distinct form of the disease were late events, and were rare at the time of thoracotomy. The natural history of the disease was one of slow growth. Responses to EGFR tyrosine kinase inhibitors (EGFR-TKI) after progression were favorable (12). For local residual disease, surgical time was relatively longer and the complication rate was higher. Postoperative radiotherapy induced immediate and long-term side effects, which could partly explain the prognostic difference between the two groups (13).
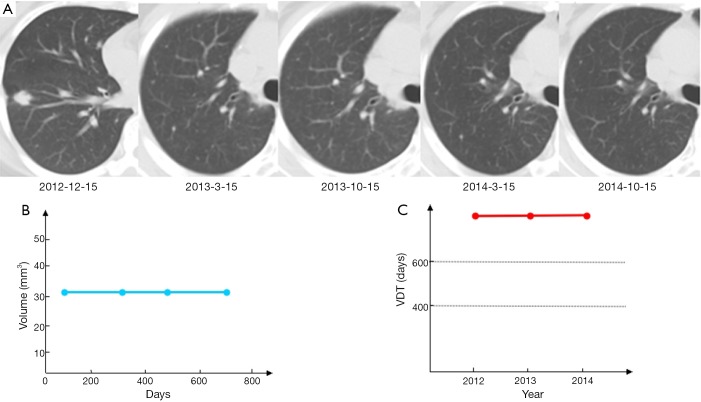
Veronesi et al. (14) found that slow-growing or indolent lung cancers comprised approximately 25% of incident cases, many of which may have represented overdiagnoses. In addition to early-stage cancers, this study indicated that slow-growing forms occur in lung cancers considered “advanced disease”. As shown in Figure 3, a 37-year-old female non-smoker was diagnosed with a right lower lobe lung cancer, cT1bN0M0 stage 1a, during a preoperative workup for uterine fibroid surgery. Video-assisted thoracic surgery (VATS) found multiple nodules (1-3 mm) distributed on the surface of the visceral and parietal pleurae. A right lower lobectomy and mediastinal lymph node dissection was performed, and the patient was ultimately diagnosed as pT4N0M1a stage IV. There were no visible lesions by CT scan and the patient was asymptomatic. After fully informing the patient, she was closely followed with no disease progression for 2 years, indicating that some pT4N0M1 lung cancers are slow growing and even indolent in terms of volume doubling time (VDT).
Figure 3.
Imaging follow-up for lung cancer patient with invisible T4-M1a. A 37-year-old female patient was diagnosed with accidental intrathoracic disseminated pT4-M1a, and received a right lower lobe resection on December 15, 2012. No disease progression was noted over 22 months, without systemic therapy. VDT, volume doubling time.
In fact, we hypothesize that rT4N0M1a (recurrence), accidental pT4N0M1a (intra-thoracic finding), and the cT4N0M1a (initially diagnosed as stage IV and receiving TKI therapy) represent the same disease, with common biological behaviors but in different phases. In patients with clinical early-stage and suspected T4-M1a, if a disseminated pT4-M1a were confirmed unexpectedly during surgery, what is the best treatment strategy? Just sampling, open and close? Wedge resection/lobectomy? Should lymph node dissection be performed? Several studies have found that limited surgery might be a wise choice (15-17), but there are no prospective randomized data. After incomplete resection, the standard of care for stage IV disease is chemotherapy or TKI treatment. However, for asymptomatic and slow-growing accidental pT4-M1a disease, the role of a wait-and-see strategy and the appropriate timing of systemic treatment need further exploration (Figure 4).
Figure 4.
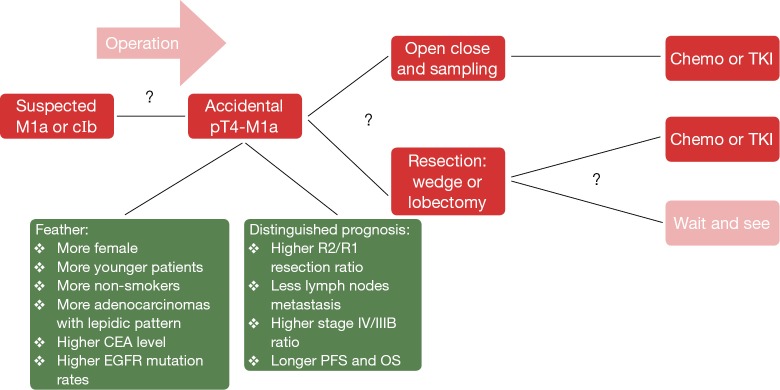
Treatment algorithm for invisible pT4M1a. CEA, carcinoembryonic antigen; EGFR, epidermal growth factor receptor; PFS, progression-free survival; OS, overall survival; TKI, tyrosine kinase inhibitor.
In conclusion, invisible intra-thoracic disseminated pT4-M1a may be a distinct lung cancer subtype, with a more favorable prognosis relative to local residual disease (18). The prolonged PFS and OS may be part of the natural history of this distinct subtype, as well as a favorable response to EGFR-TKI.
Acknowledgements
Funding: This work was supported by grants from the National Natural Science Foundation of China (Grant Nos. 81001031 and 81372285 to WZ Zhong); the Natural Science Foundation of Guangdong (Grant No. S2013010016354); the Guangdong Provincial Key Laboratory of Lung Cancer Translational Medicine (Grant No. 2012A061400006); the Special Fund for Research in the Public Interest from the National Health and Family Planning Commission of PRC (Grant No. 201402031); and the Research Fund of the Guangzhou Science and Technology Bureau (Grant No. 2011Y2-00014).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [DOI] [PubMed] [Google Scholar]
- 2.Zheng H, Hu XF, Jiang GN, et al. Define relative incomplete resection by highest mediastinal lymph node metastasis for non-small cell lung cancers: rationale based on prognosis analysis. Lung Cancer 2011;72:348-54. [DOI] [PubMed] [Google Scholar]
- 3.Verhagen AF, Schoenmakers MC, Barendregt W, et al. Completeness of lung cancer surgery: is mediastinal dissection common practice? Eur J Cardiothorac Surg 2012;41:834-8. [DOI] [PubMed] [Google Scholar]
- 4.Postmus PE, Brambilla E, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for revision of the M descriptors in the forthcoming (seventh) edition of the TNM classification of lung cancer. J Thorac Oncol 2007;2:686-93. [DOI] [PubMed] [Google Scholar]
- 5.Dall K, Ford C, Fisher R, et al. Is there a survival advantage of incomplete resection of non-small-cell lung cancer that is found to be unresectable at thoracotomy? Interact Cardiovasc Thorac Surg 2013;16:529-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohta Y, Tanaka Y, Hara T, et al. Clinicopathological and biological assessment of lung cancers with pleural dissemination. Ann Thorac Surg 2000;69:1025-9. [DOI] [PubMed] [Google Scholar]
- 7.Mordant P, Arame A, Foucault C, et al. Surgery for metastatic pleural extension of non-small-cell lung cancer. Eur J Cardiothorac Surg 2011;40:1444-9. [DOI] [PubMed] [Google Scholar]
- 8.Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [DOI] [PubMed] [Google Scholar]
- 9.Foucault C, Mordant P, Grand B, et al. Unexpected extensions of non-small-cell lung cancer diagnosed during surgery: revisiting exploratory thoracotomies and incomplete resections. Interact Cardiovasc Thorac Surg 2013;16:667-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hofmann HS, Taege C, Lautenschläger C, et al. Microscopic (R1) and macroscopic (R2) residual disease in patients with resected non-small cell lung cancer. Eur J Cardiothorac Surg 2002;21:606-10. [DOI] [PubMed] [Google Scholar]
- 11.Rami-Porta R, Wittekind C, Goldstraw P, et al. Complete resection in lung cancer surgery: proposed definition. Lung Cancer 2005;49:25-33. [DOI] [PubMed] [Google Scholar]
- 12.He YY, Zhang XC, Yang JJ, et al. Prognostic significance of genotype and number of metastatic sites in advanced non-small-cell lung cancer. Clin Lung Cancer 2014;15:441-7. [DOI] [PubMed] [Google Scholar]
- 13.Massard G, Doddoli C, Gasser B, et al. Prognostic implications of a positive bronchial resection margin. Eur J Cardiothorac Surg 2000;17:557-65. [DOI] [PubMed] [Google Scholar]
- 14.Veronesi G, Maisonneuve P, Bellomi M, et al. Estimating overdiagnosis in low-dose computed tomography screening for lung cancer: a cohort study. Ann Intern Med 2012;157:776-84. [DOI] [PubMed] [Google Scholar]
- 15.Okamoto T, Iwata T, Mizobuchi T, et al. Pulmonary resection for lung cancer with malignant pleural disease first detected at thoracotomy. Eur J Cardiothorac Surg 2012;41:25-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimizu J, Oda M, Morita K, et al. Comparison of pleuropneumonectomy and limited surgery for lung cancer with pleural dissemination. J Surg Oncol 1996;61:1-6. [DOI] [PubMed] [Google Scholar]
- 17.Fukuse T, Hirata T, Tanaka F, et al. The prognostic significance of malignant pleural effusion at the time of thoracotomy in patients with non-small cell lung cancer. Lung Cancer 2001;34:75-81. [DOI] [PubMed] [Google Scholar]
- 18.Ichinose Y, Tsuchiya R, Koike T, et al. The prognosis of patients with non-small cell lung cancer found to have carcinomatous pleuritis at thoracotomy. Surg Today 2000;30:1062-6. [DOI] [PubMed] [Google Scholar]