Abstract
Video-assisted thoracoscopic surgery (VATS) is currently a better choice than thoracotomy for lung resection, and then single-port VATS has been increasingly applied in clinical settings with the improvements in both endoscopic instruments and surgical skills. Our center began to perform single-port VATS lobectomy in May 2014 and had performed all sort of lung resection in 168 patients till December 2014, including wedge resection, routine lobectomy, sleeve lobectomy, segmentectomy and pneumonectomy. All these procedures were successfully performed without any severe complication. We believe the single-port VATS lung resection is a safe and feasible procedure after surgery practice.
Keywords: Single-port, video-assisted thoracoscopic surgery (VATS), lobectomy, segmentectomy
Developments in single-port video-assisted thoracoscopic surgery (VATS)
The single-port VATS was initially reported by Migliore et al., who applied this technique in the diagnosis and treatment of noncomplex pleural disease (1).With the advances in thoracoscopic equipments and surgical skills, this technique has gradually applied in routine VATS and even for the surgical treatments of more complicated thoracic diseases. In 2011, Gonzalez et al. reported the single-port VATS lobectomy based on their extensive experiences (2). With the constant improvement of thoracoscopic techniques and development of surgical instruments, more and more thoracic surgeons have performed the single-port VATS lung resection. This technique has become a major developmental trend in minimally invasive surgery.
Single-port VATS in Fujian Medical University Union Hospital
In 2004, the single-port VATS was initially applied for pleural biopsy in our center. Subsequently, it was applied for thoracic sympathectomy, pleurodesis, and resection of pulmonary bullae. Since May 2014, it has been applied for the radical resection of lung cancer and then for more complicated surgeries such as segmentectomy and bronchoplastic procedures. In October 2014, sleeve lobectomy and pneumonectomy by single-port VATS were successfully performed in our center. Currently, the single-port VATS technique has been applied for almost all the traditional VATS lung surgeries including wedge resection, routine lobectomy, bronchoplasty, sleeve lobectomy, segmentectomy, and pneumonectomy.
Implementation of single-port VATS in Fujian Medical University Union Hospital (Tables 1,2,3, Figure 1)
Table 1. Implementation of single-port VATS in Fujian Medical University Union Hospital from May to December in 2014 (n=168).
| Procedures | Number |
|---|---|
| Wedge resection | 43 |
| Segmentectomy | 28 |
| Routine lobectomy | 93 |
| Sleeve lobectomy | 3 |
| Pneumonectomy | 1 |
VATS, video-assisted thoracoscopic surgery.
Table 2. Implementation of single-port VATS lobectomy and mediastinal lymph node dissection for non-small cell lung cancer in Fujian Medical University Union Hospital from May to December in 2014 (n=82).
| Procedures | Outcomes |
|---|---|
| Tumor location | |
| Right upper lobe | 31 |
| Right middle lobe | 7 |
| Right lower lobe | 16 |
| Right middle & lower lobe | 2 |
| Left upper lobe | 15 |
| Left lower lobe | 11 |
| Pathologic type | |
| Minimally invasive adenocarcinoma | 14 |
| Invasive adenocarcinoma | 56 |
| Squamous cell carcinoma | 8 |
| Adenosquamous carcinoma | 2 |
| Carcinoid | 1 |
| Mucoepidermoid carcinoma | 1 |
| Tumor size (cm) | 2.1±1.3 |
| Tumor stage | |
| 0 | 1 |
| Ia | 39 |
| Ib | 17 |
| IIa | 10 |
| IIb | 9 |
| IIIa | 6 |
| Operation time (mins) | 173.5±36.3 |
| Intraoperative blood loss (mL) | 90.0±54.6 |
| Total number of lymph node dissection | 22.4±9.4 |
| Intrapulmonary lymph nodes | 8.2±4.1 |
| Mediastinal lymph nodes | 14.3±7.6 |
| Post-operative chest drainage duration (days) | 4.3±1.6 |
| Post-operative hospital stay (days) | 7.4±4.3 |
VATS, video-assisted thoracoscopic surgery.
Table 3. Implementation of single-port VATS segmentectomy in Fujian Medical University Union Hospital from May to December in 2014 (n=28).
| Procedures | Outcomes |
|---|---|
| Range of surgical resection | |
| Right lung | 11 |
| S1 | 2 |
| S2 | 3 |
| S3 | 1 |
| S1 + S2 | 1 |
| S6 | 3 |
| S7 + S8 + S9 + S10 | 1 |
| Left lung | 17 |
| S1 | 1 |
| S2 | 2 |
| S1 + S2 | 1 |
| S1 + S2 + S3 | 6 |
| S3 + S4 + S5 | 1 |
| S4 + S5 | 2 |
| S6 | 1 |
| S7 + S8 | 1 |
| S7 + S8 + S9 + S10 | 2 |
VATS, video-assisted thoracoscopic surgery.
Figure 1.
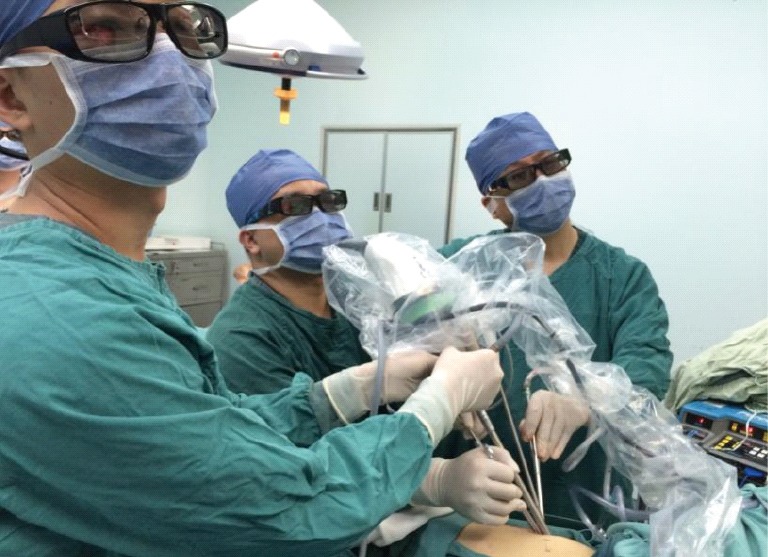
Operative scene.
Operative position during single-port VATS: placed in a 90° lateral decubitus position on the healthy side
See Figure 2.
Figure 2.
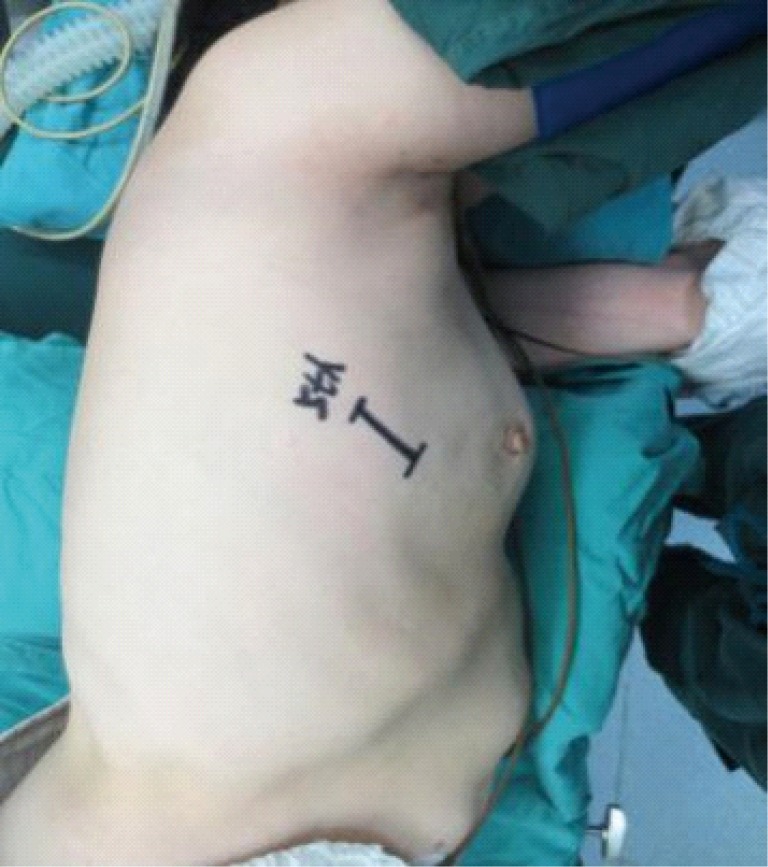
Operative position.
Design of the incision
The surgical incision (3.0-4.5 cm in length) was created at the 4th or 5th intercostal space at the anterior axillary line, depending on the patient’s height and weight as well as the lesion location. In most cases, the incision was made at the 5th intercostal space (Figure 3).
Figure 3.
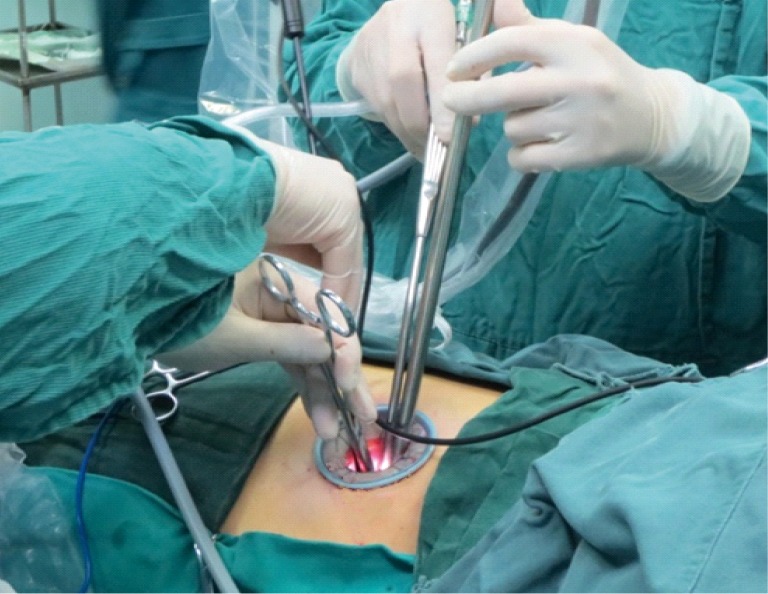
Surgical incision.
Surgery video
Single-port VATS lobectomy
Right upper lobectomy (Figure 4)
Figure 4.

Single-port VATS right upper lobectomy (3). VATS, video-assisted thoracoscopic surgery. Available online: http://www.asvide.com/articles/554
Before the surgery, this patient was pathologically confirmed to be with invasive adenocarcinoma. Right upper lobectomy and mediastinal lymph node dissection were then performed. The steps of single-port right upper lobectomy were as follows: after thoracic cavity exploration, the pleura facing the right superior pulmonary vein was opened in front of hilum to expose the upper lung branch of the superior pulmonary vein and then dissociated the hilum. After the right upper lobe bronchus being exposed, removed the lymph nodes surrounding the bronchus. After a sufficiently long bronchus was dissociated, it was clamped with the linear stapler; since the inspection showed that the residual lungs were well dilated, the bronchus was then transected. Dissected the main pulmonary artery to expose its branches in the right upper lobe, which were also transected using the linear stapler. The upper lobe branch of the superior pulmonary vein was transected using the linear stapler. Finally, divided the hypoplastic pulmonary fissures using the ultrasonic scalpel.
Right middle lobectomy (Figure 5)
Figure 5.

Single-port VATS right middle lobectomy (4). VATS, video-assisted thoracoscopic surgery. Available online: http://www.asvide.com/articles/555
Non-peripheral pulmonary nodules were found in the right middle lobe of this patient. Since it was difficult to perform wedge resection in this case, right middle lobectomy was performed instead. After thoracic cavity exploration, the middle lobe branch of the right superior pulmonary vein was dissociated firstly, which was then transected after ligation with silk thread at the proximal end. The right middle pulmonary artery was exposed at the junction of interlobular fissures and then transected using the linear stapler. After the right middle lobe bronchus being dissociated, it was clamped with the linear stapler; since the inspection showed that the residual lungs were well dilated, the bronchus was then transected. Finally, divided the hypoplastic horizontal fissure using the linear stapler.
Right lower lobectomy (Figure 6)
Figure 6.

Single-port VATS right lower lobectomy (5). VATS, video-assisted thoracoscopic surgery. Available online: http://www.asvide.com/articles/556
The patient had right lower lung cancer, which was pathologically confirmed to be invasive adenocarcinoma after the pre-operative CT-guided lung puncture. Right lower lobectomy and mediastinal lymph node dissection were then performed. After thoracic cavity exploration, exposed the pulmonary artery at the junction of interlobular fissures. After the posterior section of the oblique fissure being sufficiently opened, dissected the arteries in the basal and dorsal segments of the right lower lobe and then transected them with the linear stapler. Divided the hypoplastic posterior section of the oblique fissure using the linear stapler. Dissociated the right inferior pulmonary vein, and then transected it with the linear stapler. After the right lower lobe bronchus was dissociated, it was clamped with the linear stapler; since the inspection showed that the residual lungs were well dilated, the bronchus was then transected.
Left upper lobectomy (Figure 7)
Figure 7.

Single-port VATS left upper lobectomy (6). VATS, video-assisted thoracoscopic surgery. Available online: http://www.asvide.com/articles/557
The patient was found to be with a peripheral nodule in the left upper lobe. At first, wedge resection was performed using the linear stapler to remove the nodule, which was intra-operatively confirmed to be invasive adenocarcinoma by frozen pathology. Then, radical resection of left upper lobe was performed. The inferior pulmonary ligament was dissociated till the inferior pulmonary vein level. The superior pulmonary vein was showed in front of the hilum and then lifted with silk thread. After the hilum being released, dissected the interlobular fissure to expose the pulmonary artery trunk. Divided the posterior section of the oblique fissure using the linear stapler. After the trisegemental branches of the left pulmonary artery being exposed, transected them using the linear stapler, followed by the transection of the left superior pulmonary vein. Transected the lingular artery branches using the ultrasonic scalpel after its proximal end being ligated with silk thread. Divided the anterior section of the oblique fissure using the linear stapler. After the upper left lobe bronchus being dissociated, it was clamped with the linear stapler. Since the inspection showed that the residual lungs were well dilated, the bronchus was then transected.
Left lower lobectomy (Figure 8)
Figure 8.

Single-port VATS left lower lobectomy (7). VATS, video-assisted thoracoscopic surgery. Available online: http://www.asvide.com/articles/558
In a patient with peripheral pulmonary nodule in his left lower lobe, wedge resection and then left lower lobectomy and mediastinal lymph node dissection were performed, during which the nodule was confirmed to be invasive adenocarcinoma by frozen pathology. The steps of single-port left lower lobectomy were as follows: the inferior pulmonary ligament was dissociated till the inferior pulmonary vein level. Dissociated the inferior pulmonary vein. Dissected the interlobular fissure to expose the pulmonary artery, during which the pulmonary artery should be carefully protected. Dissected the hypoplastic posterior section of the oblique fissure with the ultrasonic scalpel and dissociated the arteries in the basal and dorsal segments. Transected the left inferior pulmonary vein with the linear stapler and then transected the arteries of the basal and dorsal segments. After the left lower lobe bronchus being dissociated, it was clamped with the linear stapler. Since the inspection showed that the residual lungs were well dilated, the bronchus was then transected.
Right upper lobe bronchial sleeve lobectomy (Figure 9)
Figure 9.

Single-port VATS right upper lobe bronchial sleeve lobectomy (8). VATS, video-assisted thoracoscopic surgery. Available online: http://www.asvide.com/articles/559
In a patient with central non-small cell lung cancer (NSCLC), bronchoscopy showed that the tumor had invaded the right main bronchus. Right upper lung bronchial sleeve lobectomy was then performed. This video mainly introduced the single-port VATS bronchial sleeve lobectomy. After the thoracic cavity exploration, transected the right superior pulmonary vein and artery with the linear stapler, followed by the dissection of mediastinal lymph nodes, transection of azygos vein, and finally bronchial sleeve resection. The right main bronchus that had been invaded by the tumor underwent the sleeve resection. Since the intra-operative frozen section analysis for the two bronchial stumps showed negative results, bronchial anastomosis was further performed. Continuously suturing using a single 3-0 prolene suture (a 4-quadrant suturing technique) was applied. With the first suture made in the inner side of the bronchial wall and knotted outside the bronchus, a continuous suture was made on one quarter of the circumference on both sides and drawn to reduce the tension, followed by further continuous sutures to cover the entire circumference, with a final knot at the outer side of the wall to complete the anastomosis. Leak testing was conducted following the anastomosis, in which no leakage was detected up to an airway pressure of 30 cmH2O (2.94 kPa).
Single-port VATS segmentectomy
Right upper lobe apical segmentectomy (S1) (Figure 10)
Figure 10.

Single-port VATS right upper lobe apical segmentectomy (S1) (9). VATS, video-assisted thoracoscopic surgery. Available online: http://www.asvide.com/articles/560
Firstly, the right superior pulmonary vein was dissociated towards the distal end, and the apical segment of the vein was identified. Meanwhile, the artery of the apical segment was dissociated. Since the vein and artery of the apical segment were very close to each other, they were transected together with the same linear stapler. Then, pulled downward the right upper lobe to dissociate the apical segmental bronchus. The apical segmental bronchus was clamped with vessel smooth forceps, then the lungs were dilated to confirm the border of the apical segmental lung tissues. Divided the bronchus using the linear stapler. Finally, removed the apical segment using the linear stapler along its border.
Right upper lobe anterior segmentectomy (S3) (Figure 11)
Figure 11.

Single-port VATS right upper lobe anterior segmentectomy (S3) (10). VATS, video-assisted thoracoscopic surgery. Available online: http://www.asvide.com/articles/561
The patient was found to be with a benign lesion in his anterior segment of the right upper lobe. The horizontal fissure was poorly developed. Firstly, the horizontal fissure was opened with a linear stapler. Then, the right superior pulmonary vein was dissociated towards the distal end. After each branch of the pulmonary vein being dissociated and confirmed, transected the anterior segmental branch using the linear stapler. Transected the anterior branch of the right pulmonary artery with the linear stapler after dissociating it carefully. Then, pulled backwards the right upper lung to dissociate the anterior segmental bronchus along the deep surface. The anterior segmental bronchus was clamped using vessel smooth forceps, then the lungs were dilated to confirm the borders of the anterior segmental lung tissues. Divided the bronchus using the linear stapler. Finally, removed the lung tissue along the borders of the anterior segment.
Right upper lobe apicoposterior segmentectomy (S1 + S2) (Figure 12)
Figure 12.

Single-port VATS right upper lobe apicoposterior segmentectomy (S1 + S2) (11). VATS, video-assisted thoracoscopic surgery. Available online: http://www.asvide.com/articles/562
Firstly, the posterior mediastinal pleura was opened and the posterior oblique fissure was divided using electric hook, followed by the dissociation and transection of the artery and vein of the posterior segment. Then the apical segmental artery was dissociated and then transected with the linear stapler. The right upper lobe bronchus was dissociated from the posterior side, and then the bronchus of the apicoposterior segment was dissociated. The bronchus of the apicoposterior segment was clamped with vessel smooth forceps, then the lungs were dilated to confirm the borders of the apicoposterior segmental lung tissues. Divided the bronchus of the apicoposterior segment using the linear stapler. Then, the vein of the apical segment was dissociated from the anterior side, ligated with a 1-0 silk suture, and finally transected using the ultrasonic scalpel. Finally, removed the apicoposterior segment using the linear stapler along its borders.
Right lower lobe dorsal segmentectomy (S6) (Figure 13)
Figure 13.

Single-port VATS right lower lobe dorsal segmentectomy (S6) (12). VATS, video-assisted thoracoscopic surgery. Available online: http://www.asvide.com/articles/563
The patient was found to be with multiple nodules in the right lung. There was a 10 mm nodule at the dorsal segment of the right lower lobe. Since wedge resection was not suitable, resection of the dorsal segment was performed. Opened the posterior oblique fissure and the posterior mediastinal pleura to dissociate the artery of the dorsal segment along the pulmonary artery trunk. The artery of the dorsal segment was then transected using the linear stapler. Pulled downwards the right lower lobe to dissociate the dorsal segmental bronchus. Searching for the segmental bronchus was often interfered by its surrounding lymph nodes (station 13). Therefore, removal of the station 13 lymph node could make the dissociation of the segmental bronchus easier. Divided the dorsal segmental bronchus using the linear stapler. Before the transection of the dorsal segmental bronchus, the lungs were dilated to confirm the borders of the dorsal segmental lung tissues. Then, transected the dorsal segmental vein using the linear stapler. Finally, removed the dorsal segment using the linear stapler along its borders.
Right lower lobe basal segmentectomy (S7 + S8 + S9 + S10) (Figure 14)
Figure 14.

Single-port VATS right lower lobe basal segmentectomy (S7 + S8 + S9 + S10) (13). VATS, video-assisted thoracoscopic surgery. Available online: http://www.asvide.com/articles/564
The patient had a ground glass opacity at his basal segment of the right lower lobe. The tumor sized about 10 mm and could not be removed by wedge resection. Firstly, dissociated the vein and artery of the basal segment, and then transected them using the linear stapler. Pulled backwards the lower right lung to dissociate the basal segmental bronchus. The basal segmental bronchus was clamped with vessel smooth forceps, then the lungs were dilated to confirm the borders of the basal segmental lung tissues. Divided the basal segmental bronchus using the linear stapler. Finally, removed the lung tissue along the borders of the basal segment.
Left upper lobe posterior segmentectomy (S2) (Figure 15)
Figure 15.

Single-port VATS left upper lobe posterior segmentectomy (S2) (14). VATS, video-assisted thoracoscopic surgery. Available online: http://www.asvide.com/articles/565
The patient had a ground glass opacity at the posterior segment of the left upper lobe. Firstly, opened the posterior oblique fissure to dissociate the posterior segmental artery. This artery was ligated with a 1-0 silk suture and transected using the ultrasonic scalpel. Then, dissociated the posterior segmental bronchus. Clamped it with vessel smooth forceps, then the lungs were dilated to confirm the borders of the posterior segmental lung tissues. Divided the posterior segmental bronchus using the linear stapler. Finally, removed the lung tissues with the linear stapler along the borders of posterior segment.
Left upper lobe apicoposterior segmentectomy (S1 + S2) (Figure 16)
Figure 16.

Single-port VATS left upper lobe apicoposterior segmentectomy (S1 + S2) (15). VATS, video-assisted thoracoscopic surgery. Available online: http://www.asvide.com/articles/566
Firstly, dissociated left superior pulmonary vein toward its distal end, and then the apical and posterior segmental branches were confirmed. Divided the posterior oblique fissure, dissociated the arteries of the apicoposterior segment and then transected the arteries and veins of the apicoposterior segment using the linear stapler. The left upper lobe was pulled forwards, and then the bronchus of the apicoposterior segment were dissociated. The bronchus of the apicoposterior segment was clamped with vessel smooth forceps, then the lungs were dilated to confirm the borders of the apicoposterior segmental lung tissues. Divided the bronchus at the apicoposterior segment using the linear stapler. Finally, removed the lung tissues of the apicoposterior segment using the linear stapler.
Left upper lobe trisegmentectomy (S1 + S2 + S3) (Figure 17)
Figure 17.

Single-port VATS left upper lobe trisegmentectomy (S1 + S2 + S3) (16). VATS, video-assisted thoracoscopic surgery. Available online: http://www.asvide.com/articles/567
Firstly, opened the anterior and posterior mediastinal pleura to dissociate the veins, then the arteries of the trisegment. Transected the veins of the trisegment using the linear stapler. The posterior segmental artery was ligated with a 1-0 silk suture and transected with the ultrasonic scalpel, and the remaining arteries were handled using the linear stapler. Then the bronchus of the trisegment was transected with the linear stapler. Finally, removed the lung tissues of the trisegment along their borders. During the surgery, the lung tissues of the proper segment were confirmed using two methods: method A—the trisegmental bronchus was clamped firstly before lung dilation; thus, the undilated lung tissue was the proper segment; method B—the lung was dilated firstly, followed by the clamping of the trisegmental bronchus, thus, the unclapsed lung tissue was the trisegment.
Left upper lobe lingular segmentectomy (S4 + S5) (Figure 18)
Figure 18.

Single-port VATS left upper lobe lingular segmentectomy (S4 + S5) (17). VATS, video-assisted thoracoscopic surgery. Available online: http://www.asvide.com/articles/568
At first, opened the oblique fissure to expose the pulmonary artery trunk. However, no lingular segmental artery was found. Further dissection showed the variation of the lingular artery, which arose from the proximal end of the left pulmonary artery and then entered the lingular segmental lung tissue after passing the frontal side of the left upper lobe bronchus. Then, the lingular segmental bronchus, vein, and artery were handled one by one. Finally, removed the lung tissues of the lingular segment along its borders.
Left lower lobe anterior medial basal segmentectomy (S7 + S8) (Figure 19)
Figure 19.

Single-port VATS left lower lobe anterior medial basal segmentectomy (S7 + S8) (18). VATS, video-assisted thoracoscopic surgery. Available online: http://www.asvide.com/articles/569
Firstly, opened the anterior oblique fissure to dissociate the anterior medial basal segmental artery, which was then transected using the linear stapler. After being exposed, the anterior medial basal segmental bronchus was clamped with vessel smooth forceps, then the lungs were dilated to confirm the borders of the anterior medial basal segmental lung tissues. Divided the anterior medial basal segmental bronchus using the linear stapler. Dissociated the left inferior pulmonary vein to the distal end to identify its branches. The anterior medial basal segmental vein was then transected using the linear stapler. Finally, removed the lung tissue of the anterior medial basal segment along its borders.
Single-port VATS pneumonectomy (Figure 20)
Figure 20.

Single-port VATS pneumonectomy (19). VATS, video-assisted thoracoscopic surgery. Available online: http://www.asvide.com/articles/570
The patient with bronchiectasis in the whole left lung was admitted to our hospital due to recurrent hemoptysis. Left pneumonectomy was then performed. Firstly, opened the anterior and posterior mediastinal pleura to thoroughly dissociate the left pulmonary artery and vein and the left main bronchus. Afterwards, the left inferior pulmonary vein, left superior pulmonary vein, left pulmonary artery trunk, and left main bronchus were transected using the linear stapler one by one.
Discussion
Along with the advances in thoracoscopic techniques, VATS instruments, and minimally invasive concepts, the number of incisions made during VATS has decreased from 3 or 4 to 1. The application of the single-port VATS in more complicated procedures including lobectomy (2,20), segmentectomy (21), pneumonectomy (22), sleeve lobectomy (23), and pulmonary artery angioplasty (24) have also been reported. Compared with the conventional triple-port complete VATS, the single-port VATS no longer use the auxiliary port at the posterior axillary line and the observation port at the midaxillary line. The 4th or 5th intercostal space at the anterior axillary line is relatively wide, with fewer muscle layers at the chest wall; therefore, it has high elasticity, and bleeding at this site can be easily stopped. Incision at this area will cause milder injury and pain and thus has fewer impacts on the patient’s feelings and activities. During the single-port VATS, however, both the operational instruments and endoscope pass in and out from the same port and therefore may interfere with each other; in particular, it is difficult to expose the lesions located at the dorsal side or near the diaphragm, making the operations more challenging. Thus, the beginners should operate via multiple ports firstly before using a single port. In our center, the VATS teams had rich experiences in performing three-port VATS lobectomy (25,26), which laid a solid foundation for the adoption of the single-port VATS.
Our recommendations include:
Incision selection should be based on a specific lesion. The incision should be a bit far away from the lesion, so as to avoid the mutual interference among the instruments. Typically, the incision is often located at the 5th intercostal space; for patients with a chunky body, the 4th intercostal space will be more feasible;
The surgical instruments should be thin and long, with multiple joints or cornered design, so as to save the incision space to avoid the mutual interference among surgical instruments and the “arrow effect” during the maneuvers; also, the use of such instruments is helpful to lower the operational difficulty, increase the surgical smoothness, and shorten the surgical duration;
A close cooperation between the camera-holding assistant and the operator is particularly important. Typically, the camera-holding assistant stands opposite to the operator, maintaining the thoracoscope over the incision. Such a position decreases the body interference between the camera-holding assistant and the operator, and leaves the majority of the incision space to the operator. Since the single-port VATS is performed via the sagittal plane and through the tail-head approach, the eyes and hands are used at the same level during the operation; thus, an observation from tail to head should be avoided. The impacts of the quality of camera-holding on the VATS can be reflected in multiple facets including the comfort of the operator, the smoothness of the surgery, the operational duration, the surgical viewing, and the operational safety. A qualified assistant must have flexible camera-holding skills, be familiar with the detailed surgical procedures, understand the operational habits of the operator, and have rich teamwork experiences;
During the operation, vascular injury due to excessive extraction should be avoided. Meanwhile, the surgical field should be adequately exposed, and forced insertion of the stapler should be avoided. Due to the surgical angles, the vessels (in particular the two upper lung veins) should be thoroughly dissociated to ensure a safe transection;
For patients who are scheduled for segmentectomy, the lesion should be repeatedly located before and during the surgery. In our center, the lesion is located by lung CT three-dimensional reconstruction or by thin-slice CT scan before surgery in combination with finger palpation during the surgery;
In our earlier practices, we routinely placed a chest tube for the resection of the middle or lower lobes; for the resection of the upper lobes, two chest tubes were placed along the surgical incision. Now we are using a modified method: a drainage tube is placed along the surgical incision, and then an Abel drainage tube is placed by acupunture at the 8th or 9th intercostal space along the posterior axillary line. This new method can shorten the chest tube duration and reduce pulmonary complications. Also, the Abel drainage tube is more comfortable for the patient and can adequately drain the pleural fluids; it can be indwelled for a longer period of time and thus reduce the rate of chest tube replacement.
In summary, all the single-port VATS procedures were successfully performed in our center. No perioperative severe complication or death was noted. Nevertheless, the middle- and long-term efficacies of these procedures for lung cancer still require evidences from further follow-up. In our view, the single-port VATS is safe and feasible, and thus warrants further clinical applications.
Acknowledgements
Funding: Project supported by Fujian Provincial Department of Science & Technology (Key Program, NO. 2015Y0018).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Migliore M. Efficacy and safety of single-trocar technique for minimally invasive surgery of the chest in the treatment of noncomplex pleural disease. J Thorac Cardiovasc Surg 2003;126:1618-23. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez D, Paradela M, Garcia J, et al. Single-port video-assisted thoracoscopic lobectomy. Interact Cardiovasc Thorac Surg 2011;12:514-5. [DOI] [PubMed] [Google Scholar]
- 3.Zhu Y, Xu G, Zheng B, et al. Single-port VATS right upper lobectomy. Asvide 2015;2:058. Available online: http://www.asvide.com/articles/554
- 4.Zhu Y, Xu G, Zheng B, et al. Single-port VATS right middle lobectomy. Asvide 2015;2:093. Available online: http://www.asvide.com/articles/555
- 5.Zhu Y, Xu G, Zheng B, et al. Single-port VATS right lower lobectomy. Asvide 2015;2:094. Available online: http://www.asvide.com/articles/556
- 6.Zhu Y, Xu G, Zheng B, et al. Single-port VATS left upper lobectomy. Asvide 2015;2:095. Available online: http://www.asvide.com/articles/557
- 7.Zhu Y, Xu G, Zheng B, et al. Single-port VATS left lower lobectomy. Asvide 2015;2:096. Available online: http://www.asvide.com/articles/558
- 8.Zhu Y, Xu G, Zheng B, et al. Single-port VATS right upper lobe bronchial sleeve lobectomy. Asvide 2015;2:097. Available online: http://www.asvide.com/articles/559
- 9.Zhu Y, Xu G, Zheng B, et al. Single-port VATS right upper lobe apical segmentectomy (S1). Asvide 2015;2:098. Available online: http://www.asvide.com/articles/560
- 10.Zhu Y, Xu G, Zheng B, et al. Single-port VATS right upper lobe anterior segmentectomy (S3). Asvide 2015;2:099. Available online: http://www.asvide.com/articles/561
- 11.Zhu Y, Xu G, Zheng B, et al. Single-port VATS right upper lobe apicoposterior segmentectomy (S1 + S2). Asvide 2015;2:100. Available online: http://www.asvide.com/articles/562
- 12.Zhu Y, Xu G, Zheng B, et al. Single-port VATS right lower lobe dorsal segmentectomy (S6). Asvide 2015;2:101. Available online: http://www.asvide.com/articles/563
- 13.Zhu Y, Xu G, Zheng B, et al. Single-port VATS right lower lobe basal segmentectomy (S7 + S8 + S9 + S10). Asvide 2015;2:102. Available online: http://www.asvide.com/articles/564
- 14.Zhu Y, Xu G, Zheng B, et al. Single-port VATS left upper lobe posterior segmentectomy (S2). Asvide 2015;2:103. Available online: http://www.asvide.com/articles/565
- 15.Zhu Y, Xu G, Zheng B, et al. Single-port VATS left upper lobe apicoposterior segmentectomy (S1 + S2). Asvide 2015;2:104. Available online: http://www.asvide.com/articles/566
- 16.Zhu Y, Xu G, Zheng B, et al. Single-port VATS left upper lobe trisegmentectomy (S1 + S2 + S3). Asvide 2015;2:105. Available online: http://www.asvide.com/articles/567
- 17.Zhu Y, Xu G, Zheng B, et al. Single-port VATS left upper lobe lingular segmentectomy (S4 + S5). Asvide 2015;2:106. Available online: http://www.asvide.com/articles/568
- 18.Zhu Y, Xu G, Zheng B, et al. Single-port VATS left lower lobe anterior medial basal segmentectomy (S7 + S8). Asvide 2015;2:107. Available online: http://www.asvide.com/articles/569
- 19.Zhu Y, Xu G, Zheng B, et al. Single-port VATS pneumonectomy. Asvide 2015;2:108. Available online: http://www.asvide.com/articles/570
- 20.Gonzalez-Rivas D, de la Torre M, Fernandez R, et al. Single-port video-assisted thoracoscopic left upper lobectomy. Interact Cardiovasc Thorac Surg 2011;13:539-41. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez-Rivas D, Paradela M, Fernandez R, et al. Uniportal video-assisted thoracoscopic lobectomy: two years of experience. Ann Thorac Surg 2013;95:426-32. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez-Rivas D, Fieira E, Mendez L, et al. Single-port video-assisted thoracoscopic anatomic segmentectomy and right upper lobectomy. Eur J Cardiothorac Surg 2012;42:e169-71. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez-Rivas D, de la Torre M, Fernandez R, et al. Video: Single-incision video-assisted thoracoscopic right pneumonectomy. Surg Endosc 2012;26:2078-9. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez-Rivas D, Delgado M, Fieira E, et al. Single-port video-assisted thoracoscopic lobectomy with pulmonary artery reconstruction. Interact Cardiovasc Thorac Surg 2013;17:889-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu W, Chen C, Lin R, et al. Complete video-assisted thoracoscopic surgery lobectomy; Report of 32 cases. J Fujian Med Univ 2010;44:49,46.
- 26.Xu G, Zheng W, Guo Z, et al. Complete video-assisted thoracoscopic surgery upper left bronchial sleeve lobectomy. J Thorac Dis 2013;5 Suppl 3:S298-300. [DOI] [PMC free article] [PubMed] [Google Scholar]


