Abstract
Background
Crizotinib has been associated with intracranial disease control in anaplastic lymphoma kinase (ALK)-positive non-small-cell lung cancer (NSCLC) patients with brain metastases. Continued crizotinib treatment has also been used for prolonged disease control in patients experiencing isolated central nervous system (CNS) failure. However, there are few studies of crizotinib efficacy in ALK-positive Chinese patients. Thus, we retrospectively investigated the clinical efficacy of crizotinib in Chinese ALK-positive NSCLC patients with brain metastases at baseline, and evaluated the clinical benefit of continuing crizotinib beyond CNS failure.
Methods
A total of 120 advanced ALK-positive NSCLC patients treated with crizotinib were enrolled with 38 having brain metastases at baseline. The objective response rate (ORR) and progression-free survival (PFS) were compared between patients with and without brain metastases at baseline. A subset of patients who developed CNS failure continued crizotinib treatment beyond progressive disease (PD), and the second PFS from the time of the first progression was also evaluated.
Results
The ORR of crizotinib was similar between patients with and without brain metastases at baseline (68.4% vs. 69.5%, P=0.904). However, the patients without brain metastases at baseline experienced a longer median PFS [10.0 months, 95% confidence interval (CI), 7.6-12.5 vs. 7.0 months, 95% CI, 6.4-7.6; P=0.021]. Among 88 patients with PD defined Response Evaluation Criteria in Solid Tumors (RECIST), 33 developed CNS failure. A total of 24 patients who developed CNS failure continued crizotinib treatment beyond PD, and they achieved a second median PFS of 6.3 months (95% CI, 2.9-9.7).
Conclusions
Chinese ALK-positive NSCLC patients with brain metastases achieved a similar response to crizotinib and significantly shorter PFS compared to those without brain metastases at baseline. Continuous administration of crizotinib beyond PD in patients developing CNS failure appeared to be a valid treatment strategy.
Keywords: Non-small-cell lung cancer (NSCLC), anaplastic lymphoma kinase (ALK) rearrangement, brain metastases, crizotinib, central nervous system (CNS) failure
Introduction
Lung cancer is the leading cause of cancer-related death in the People’s Republic of China according to the annual report on status of cancer in China in 2011 (1). Over the last decade, personalized medicine based on patients’ genetic profile has significantly changed the treatment of lung cancer. Anaplastic lymphoma kinase (ALK) rearrangement, discovered by Soda et al. in 2007, has been validated as a well-established therapeutic target in non-small-cell lung cancer (NSCLC) (2). In Chinese NSCLC patients, the prevalence of ALK rearrangement was reported to be 3.3-11.6% (3-5). Crizotinib was the first ALK tyrosine kinase inhibitor (TKI) to undergo clinical development in ALK-positive NSCLC and has had remarkable efficacy in this group (6-10). Crizotinib was approved in 2013 by the Chinese Food and Drug Administration for advanced ALK-positive NSCLC.
Brain metastases are most frequently associated with advanced lung cancer and in advanced ALK-positive NSCLC patients, 25-35% developed brain metastases (9,11). Previous studies indicated that crizotinib poorly penetrated the blood-brain barrier, which may restrict its anticancer effect in tumors with brain metastases (12). However, a recent analysis determined that crizotinib exhibited favorable clinical response in ALK-positive NSCLC patients with brain metastases (13). Unfortunately, after an initial clinical benefit acquired resistance inevitably occurs, and central nervous system (CNS) is a common site of progression in ALK-positive patients treated with crizotinib (12,14,15). In clinical practice, molecular-targeted therapy is increasingly being continued beyond progression, especially in patients with CNS failure or oligo-progressive disease (PD) (15). Ou et al. described the survival benefit of continuing crizotinib treatment beyond progression in 120 patients with advanced ALK-positive NSCLC (16). In addition, in oncogene-addicted NSCLC patients developing CNS failure or oligo-PD, the use of local ablative therapy and continuation of the targeted agent could provide an additional 6 months of disease control (15).
Although the clinical efficacy of crizotinib in ALK-positive NSCLC patients with brain metastases has been reported, the experience in ALK-positive Chinese patients remains limited. Thus, we carried out a retrospective analysis to investigate the clinical efficacy of crizotinib in ALK-positive Chinese NSCLC patients with brain metastases at baseline. Additionally, we intended to evaluate the clinical benefit of continuing crizotinib treatment in ALK-positive patients developing CNS failure.
Materials and methods
Patients
From June 2008 to March 2015, a total of 120 patients with advanced ALK-positive NSCLC treated with crizotinib at the Guangdong Lung Cancer Institute (GLCI) were enrolled in this study. All patients received crizotinib at an initial dose of 250 mg twice daily with appropriate dosing modification as needed. ALK rearrangement was detected before initiation of crizotinib. All tissues used for ALK rearrangement assay were obtained from the GLCI tissue bank and informed consent was obtained from each patient for genetic analyses before any biopsy was performed. Patients’ clinical data were extracted from electronic medical records at the GLCI. This study was approved by the Institutional Review Board of Guangdong General Hospital (GGH).
Study design
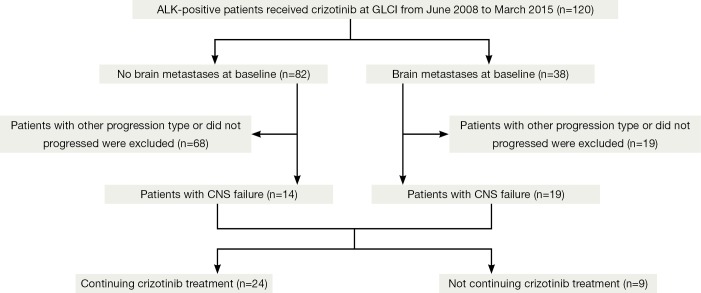
Patients were categorized into two groups as with (n=82) or without (n=38) brain metastases at baseline (Figure 1) prior to crizotinib treatment. We divided patients with brain metastases into two subgroups based on the local treatment history for brain metastases before initiation of crizotinib. In this analysis, continued use of crizotinib was allowed for all patients if the physician believed the patient could still achieve clinical benefit. CNS failure was defined as progression of pre-existing or development of new intracranial lesions in the presence of good disease control elsewhere in the body. For patients with CNS failure, the second progression-free survival (PFS) benefiting from crizotinib continuation beyond PD was measured.
Figure 1.
Study flow chart. CNS, central nervous system.
Analysis of ALK rearrangements
ALK rearrangement was detected by either fluorescence in situ hybridization (FISH), rapid amplification of cDNA ends-coupled polymerase chain reaction (PCR) and sequencing or immunohistochemistry (IHC). FISH detection was performed on unstained 4-μm-thick formalin-fixed, paraffin-embedded tissue slides using the Vysis LSI ALK Break Apart FISH Probe (Abbott Molecular, USA) according to the manufacturer’s instructions. Only tumors with >15% of tumor cells having a positive break-apart FISH signal were considered to have ALK rearrangements. RACE-coupled PCR and sequencing was conducted as reported previously (3).
Measurements of brain metastases and response evaluation
Brain imaging at baseline was required for patients enrolled in clinical trials, and if brain metastases existed, follow-up brain imaging would be required. For patients in clinical practice, brain imaging at baseline was carried out only if patients had symptoms or signs of brain metastases. Local treatment strategy for brain metastases included whole brain radiotherapy (WBRT), stereotactic radiosurgery (SRS) and surgical resection. IHC staining was also conducted on 4-μm-thick formalin-fixed, paraffin-embedded sections using Ventana anti-ALK (D5F3) rabbit monoclonal primary antibody (Roche Diagnostics, Germany). Detection was performed according to the manufacturers’ protocol. Each sample was matched with a rabbit monoclonal IgG negative control. The presence of strong granular cytoplasmic staining in the tumor cells (any percentage of positive tumor cells) was deemed as ALK-positive, while the absence of strong cytoplasmic staining in the tumor cells indicated that they were ALK-negative.
Measurements of brain metastases and response evaluation
Brain imaging at baseline was required for patients enrolled in the clinical trial, and when brain metastases were found, follow-up brain imaging was completed. For patients in clinical practice, brain imaging at baseline was carried out only if patients had symptoms or signs of brain metastases. The local treatment strategy for brain metastases included WBRT, SRS and surgical resection. The radiographic response to crizotinib treatment was evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST) (17,18). The objective tumor response was evaluated every 6-8 weeks. Additional assessments were performed based on clinical circumstances.
Statistical analysis
PFS was defined as the time from commencement of crizotinib treatment to first documentation of PD or death from any cause, and censored at the date of last radiological assessment (when carried out). The second PFS was calculated from the time of first documentation of PD until second radiological disease progression or symptom deterioration. Overall survival (OS) was measured from the date of metastatic NSCLC diagnosis to death resulting from any cause and censored at the last follow-up date. The last follow up was on April 23, 2015.
The χ2 or Fisher’s exact tests were used to compare qualitative data. The Kaplan-Meier method was used to estimate survival and differences among patient groups analyzed by the log-rank test. A Cox regression model was used to calculate hazard ratio (HR) and its 95% confidence interval (CI). Statistical analysis was performed using SPSS version 22.0 software (IBM, Armonk, NY, US). All statistical tests were two-sided and P<0.05 was deemed to indicate statistical significance.
Results
Clinical characteristics
The clinical characteristics of the 120 patients at baseline were similar between patients with or without brain metastases (Table 1). All patients were of Chinese ethnicity. The median age of all the patients was 48 years and the majority of patients (78.3%) were never-smokers. The most common histologic subtype was adenocarcinoma (96.7%). A total of 45 (37.5%) patients received crizotinib as a first-line treatment.
Table 1. Clinical characteristics of patients at baseline.
| Characteristic | Total (n=120), n (%) | No BM at baseline (n=82), n (%) | BM at baseline (n=38), n (%) | P |
|---|---|---|---|---|
| Age (years) | ||||
| Median | 48 | 48 | 47 | |
| Range | 23-76 | 23-76 | 28-73 | |
| Age category | 0.741 | |||
| <65 years | 109 (90.8) | 75 (91.5) | 34 (89.5) | |
| ≥65 years | 11 (9.2) | 7 (8.5) | 4 (10.5) | |
| Sex | 0.901 | |||
| Male | 61 (50.8) | 42 (51.2) | 19 (50.0) | |
| Female | 59 (49.2) | 40 (48.8) | 19 (50.0) | |
| Smoking history | 0.124 | |||
| Never-smoker | 94 (78.3) | 61 (74.4) | 33 (86.8) | |
| Ever-smoker | 26 (21.7) | 21 (25.6) | 5 (13.2) | |
| ECOG status | 0.580 | |||
| 0 | 8 (6.7) | 7 (8.5) | 1 (2.6) | |
| 1 | 102 (85.0) | 68 (82.9) | 34 (89.5) | |
| 2 | 8 (6.7) | 6 (7.3) | 2 (5.3) | |
| 3 | 2 (1.7) | 1 (1.2) | 1 (2.6) | |
| Histology | 1 | |||
| Adenocarcinoma | 116 (96.7) | 79 (96.3) | 37 (97.4) | |
| Non-adenocarcinoma | 4 (3.3) | 3 (3.7) | 1 (2.6) | |
| Line of crizotinib therapy | 0.919 | |||
| First | 45 (37.5) | 31 (37.8) | 14 (36.8) | |
| Second and further | 75 (62.5) | 51 (62.2) | 24 (63.2) | |
| Proportion of trial patients | 0.833 | |||
| Trial | 68 (56.7) | 47 (57.3) | 21 (55.3) | |
| Non-trial | 52 (43.3) | 35 (42.7) | 17 (44.7) |
BM, brain metastases; ECOG status, Eastern Cooperative performance status.
Efficacy of crizotinib treatment
All 120 patients were available for crizotinib response evaluation (Table 2). The overall objective response rate (ORR) was 69.2%. The ORR of crizotinib in patients without brain metastases at baseline was similar to that in those with brain metastases at baseline (69.5% vs. 68.4%, P=0.904).
Table 2. Efficacy of crizotinib treatment.
| Efficacy | All patients (n=120) | No baseline BM (n=82) | Baseline BM (n=38) | P |
|---|---|---|---|---|
| Tumor response, n (%) | ||||
| CR | 0 (0) | 0 (0) | 0 (0) | |
| PR | 83 (69.2) | 57 (69.5) | 26 (68.4) | |
| SD | 25 (20.8) | 15 (18.3) | 10 (26.3) | |
| PD | 12 (10.0) | 10 (12.2) | 2 (5.3) | |
| ORR, % | 69.2 | 69.5 | 68.4 | 0.904 |
| DCR, % | 90 | 87.8 | 94.7 | 0.335 |
| PFS, median (95% CI) (months) | 8.5 (6.8-10.1) | 10.0 (7.6-12.5) | 7.0 (6.4-7.6) | 0.021 |
| OS from the time of stage IV diagnosis, median (95% CI) (months) | 34.9 (28.4-41.5) | 34.2 (26.7-41.6) | 35.5 (24.4-46.7) | 0.861 |
CR, complete response; DCR, disease control rate; PD, progressive disease; PFS, progression-free survival; PR, partial response; SD, stable disease; ORR, objective response rate; OS, overall survival.
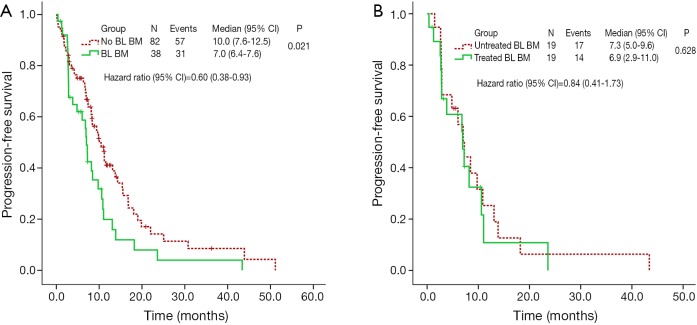
All of the patients were available for survival analysis. Median PFS was 8.5 months (95% CI, 6.8-10.1) for the whole cohort. Patients without brain metastases at baseline experienced a longer median PFS of 10.0 months (95% CI, 7.6-12.5) than that of 7.0 months (95% CI, 6.4-7.6; P=0.021) in patients with brain metastases at baseline (Figure 2A). We analyzed the correlation between the PFS and clinical characteristics, including age, gender, smoking history, histologic subtype, ECOG status, brain metastases status at baseline, and treatment-line of crizotinib usage. Multivariate Cox regression analysis indicated that the patients without brain metastases at baseline had a lower risk of disease progression (HR =0.60; 95% CI, 0.38-0.93, P=0.023).
Figure 2.
PFS of patients undergoing crizotinib treatment. (A) PFS of crizotinib in patients with or without BL BM; (B) PFS of crizotinib in patients with untreated and treated brain metastases. PFS, progression-free survival; BL BM, brain metastases at baseline.
By April 23, 2015, 40.8% (49/120) of patients had died. The median OS was 34.9 months (95% CI, 28.4-41.5) in all study subjects. There was no significant difference in OS between the patients with and without brain metastases at baseline (35.5 months, 95% CI, 24.4-46.7 vs. 34.2 months, 95% CI, 26.7-41.6, P=0.861).
Patients with brain metastases at baseline and response to crizotinib treatment
At baseline, a total of 38 patients had brain metastases, of whom 19 (50%) did not receive local treatment for brain metastases, and 19 (50%) received local intervention for brain metastases (Table 3). Among patients with previously untreated brain metastases, 18 (94.7%) were asymptomatic. Only 1 (5.3%) with symptomatic brain metastases received crizotinib without local treatment for brain metastases since this patient refused to undergo WBRT. Of 19 patients who received local treatment, 10 (52.6%) received WBRT, 5 (26.3%) received SRS, 3 (15.8%) underwent surgical resection, and 1 (5.3%) received both local surgical resection and WBRT.
Table 3. Efficacy of crizotinib treatment in patients with brain metastases at baseline.
| Variables | Previously untreated for BM (n=19) | Previous treated for BM (n=19) | P |
|---|---|---|---|
| Symptomatic, n (%) | <0.001 | ||
| Yes | 1 (5.3) | 14 (73.7) | |
| No | 18 (94.7) | 5 (26.3) | |
| Tumor response, n (%) | |||
| CR | 0 (0) | 0 (0) | |
| PR | 14 (73.7) | 12 (63.2) | |
| SD | 4 (21.1) | 6 (31.6) | |
| PD | 1 (5.3) | 1 (5.3) | |
| ORR, % | 73.7 | 63.2 | 0.485 |
| DCR, % | 94.7 | 94.7 | 1.000 |
| PFS, median (95% CI) (month) | 7.3 (5.0-9.6) | 6.9 (2.9-11.0) | 0.628 |
| OS from the time of stage IV diagnosis, median (95% CI) (months) | 39.6 (NR) | 29.2 (19.2-39.1) | 0.496 |
| 2-year OS probability (95% CI) | 64% (0.40-0.88) | 68% (0.44-0.92) | |
| 3-year OS probability (95% CI) (%) | 64% (0.40-0.88) | 37% (0.08-0.66) |
CR, complete response; DCR, disease control rate; PD, progressive disease; PFS, progression-free survival; PR, partial response; SD, stable disease; ORR, objective response rate; OS, overall survival.
There was no significant difference in ORR between the two subgroups (73.7% vs. 63.2%, χ2=0.487, P=0.485). In patients with previously untreated brain metastases, the median PFS was 7.3 months (95% CI, 5.0-9.6), which was comparable with that of patients treated for brain metastases (6.9 months, 95% CI, 2.9-11.0; P=0.628) (Figure 2B).
Incidence of CNS progression and benefit from continuation of crizotinib
Among 82 patients without brain metastases at baseline, 57 had progression at the time of the analysis, and 14 (24.6%, 14/57) developed CNS failure. Of the 38 patients with brain metastases at baseline, 31 progressed and 61.3% (19/31) of these patients developed CNS failure. There was no significant difference of CNS failure rate between patients with or without brain metastases at baseline (χ2=11.6, P=0.001).
In total, 33 patients experienced CNS failure, and 24 (72.7%) continued crizotinib treatment beyond PD. Most of them (19/24) received local CNS treatment (WBRT or SRS) followed by continuation of crizotinib treatment, whereas five directly continued crizotinib treatment beyond PD without receiving any local treatment for CNS failure. In the 24 patients, the second median PFS was 6.3 months (95% CI, 2.9-9.7). In the remaining nine patients who did not continue crizotinib treatment, one received LDK378 in a clinical trial (19), one was treated with gefitinib treatment, and seven received supportive care alone.
Discussion
This is the first retrospective study focusing on Chinese ALK-positive NSCLC patients with brain metastases treated with crizotinib. Our data revealed that ALK-positive NSCLC patients with brain metastases at baseline could achieve clinical response from crizotinib treatment, but their PFS was significantly shorter than those without brain metastases at baseline (P=0.021). Notably, the clinical benefit of crizotinib in patients with untreated brain metastases at baseline was similar to treated populations (P=0.628), suggesting that WBRT could be postponed in patients with asymptomatic brain metastases at baseline. This study also suggested that continuation of crizotinib could extend disease control for about 6 months in patients who developed CNS failure after crizotinib treatment. Continuous administration of crizotinib beyond PD in patients developed CNS failure appeared to be a valid treatment strategy.
Costa et al. recently reported the clinical response of intracranial metastases to crizotinib treatment, and their results showed that the systemic PFS was similar for patients with or without brain metastases at baseline (13). This difference could be explained by the composition of patients included in the study, as this was the first study to include only Chinese ALK-positive patients. We noted that the ORR of crizotinib in Chinese ALK-positive patients was higher than that in non-Asian patients (20), which suggested that there might be a slight difference in crizotinib efficacy between Chinese and non-Asian patients. Another possible reason for this difference was the sample size of 120 compared to 888 as this was a single institutional retrospective study with a smaller sample size than previous multi-center studies. However, the present study was still useful because it provided a better understanding of the actual efficacy of crizotinib in Chinese ALK-positive patients.
Jackman et al. proposed that patients who experience isolated CNS failure might not have systemic acquired resistance to TKI therapy (21). Thus, the management of patients with isolated CNS failure should be distinguished from that of patients with systemic progression. In addition, previous reports have showed that continuous administration of TKI following local treatment after CNS failure appeared to be a valid treatment option (15,22). This treatment paradigm is now recommended by National Comprehensive Cancer Network guidelines and our study confirmed that this treatment strategy was suitable for Chinese ALK-positive patients.
We noted that patients with brain metastases at baseline have a high risk of developing CNS failure under crizotinib treatment than those without brain metastases at baseline (24.6% vs. 61.3%, P=0.001). This phenomenon was consistent with a recent study (70% vs. 20%) (13), although the mechanism mediating the high incidence of CNS failure in patients with metastases at baseline needs to be investigated further. We hypothesized that ALK-positive NSCLC may be a heterogeneous disease. For ALK-positive patients with brain metastases at baseline, the aberrantly activated ALK may increase the predisposition of cancer cells to invade the CNS. Thus, this subgroup of patients was highly susceptible to development of CNS failure.
There are several limitations of this study. First, this was a single institutional retrospective study. Larger multi-institutional randomized trials are required to validate the efficacy of crizotinib in patients with brain metastases at baseline. In addition, in this study, FISH, RACE-PCR and IHC were combined in detecting ALK rearrangements and the guidelines for the ALK rearrangement assay in China are different from those in the US and EU. All three methods were recommended by a consensus opinion of Chinese experts and have been approved as companion diagnostic tests by the Chinese Food and Drug Administration (23). Furthermore, this study only included a small sample size of patients who developed CNS failure and the OS was immature for analysis. Therefore, we did not compare the OS between patients who continued crizotinib treatment beyond PD and those who did not. Further investigations in a larger number of patients are needed to determine whether this strategy is beneficial.
Conclusions
Chinese ALK-positive NSCLC patients with brain metastases achieved a similar response to crizotinib and significantly shorter PFS, compared to those without baseline brain metastases. CNS was a common site of progression for crizotinib treatment, especially in patients with brain metastases at baseline. Continuous administration of crizotinib beyond PD in patients who developed CNS failure appeared to be a valid treatment strategy.
Acknowledgements
Funding: This study was supported by the following (I) Guangdong Provincial Key Laboratory of Lung Cancer Translational Medicine (grant no 2012A061400006); (II) special fund for research in the public interest from National Health and Family Planning Commission of People’s Republic of China (grant no 201402031); and (III) research fund from Guangzhou Science and Technology Bureau (grant no 2011Y2-00014).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Chen W, Zheng R, Zeng H, et al. Annual report on status of cancer in China, 2011. Chin J Cancer Res 2015;27:2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561-6. [DOI] [PubMed] [Google Scholar]
- 3.Zhang X, Zhang S, Yang X, et al. Fusion of EML4 and ALK is associated with development of lung adenocarcinomas lacking EGFR and KRAS mutations and is correlated with ALK expression. Mol Cancer 2010;9:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.An SJ, Chen ZH, Su J, et al. Identification of enriched driver gene alterations in subgroups of non-small cell lung cancer patients based on histology and smoking status. PLoS One 2012;7:e40109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y, Li Y, Yang T, et al. Clinical significance of EML4-ALK fusion gene and association with EGFR and KRAS gene mutations in 208 Chinese patients with non-small cell lung cancer. PLoS One 2013;8:e52093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363:1693-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camidge DR, Bang YJ, Kwak EL, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol 2012;13:1011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim DW, Ahn MJ, Shi Y, et al. Results of a global phase II study with crizotinib in advanced ALK-positive non-small cell lung cancer (NSCLC). J Clin Oncol 2012;30:abstr 7533.
- 9.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385-94. [DOI] [PubMed] [Google Scholar]
- 10.Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [DOI] [PubMed] [Google Scholar]
- 11.Doebele RC, Lu X, Sumey C, et al. Oncogene status predicts patterns of metastatic spread in treatment-naive nonsmall cell lung cancer. Cancer 2012;118:4502-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costa DB, Kobayashi S, Pandya SS, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol 2011;29:e443-5. [DOI] [PubMed] [Google Scholar]
- 13.Costa DB, Shaw AT, Ou SH, et al. Clinical Experience With Crizotinib in Patients With Advanced ALK-Rearranged Non-Small-Cell Lung Cancer and Brain Metastases. J Clin Oncol 2015;33:1881-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maillet D, Martel-Lafay I, Arpin D, et al. Ineffectiveness of crizotinib on brain metastases in two cases of lung adenocarcinoma with EML4-ALK rearrangement. J Thorac Oncol 2013;8:e30-1. [DOI] [PubMed] [Google Scholar]
- 15.Weickhardt AJ, Scheier B, Burke JM, et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol 2012;7:1807-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ou SH, Janne PA, Bartlett CH, et al. Clinical benefit of continuing ALK inhibition with crizotinib beyond initial disease progression in patients with advanced ALK-positive NSCLC. Ann Oncol 2014;25:415-22. [DOI] [PubMed] [Google Scholar]
- 17.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205-16. [DOI] [PubMed] [Google Scholar]
- 18.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [DOI] [PubMed] [Google Scholar]
- 19.LDK378 in Adult Chinese Patients With ALK-rearranged (ALK-positive) Advanced Non-small Cell Lung Cancer (NSCLC) Previously Treated With Crizotinib. Available online: http://www.clinicaltrials.gov/ct2/show/NCT02040870
- 20.Niu FY, Wu YL. Personalized treatment strategies for non-small- cell lung cancer in Chinese patients: the role of crizotinib. Onco Targets Ther 2015;8:999-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackman D, Pao W, Riely GJ, et al. Clinical definition of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. J Clin Oncol 2010;28:357-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shukuya T, Takahashi T, Naito T, et al. Continuous EGFR-TKI administration following radiotherapy for non-small cell lung cancer patients with isolated CNS failure. Lung Cancer 2011;74:457-61. [DOI] [PubMed] [Google Scholar]
- 23.Zhang XC, Lu S, Zhang L, et al. The Chinese expert consensus opinion of the diagnosis of ALK-positive NSCLC (2013 version). Chin J Pathol 2013;42:402-6. (in Chinese). [DOI] [PubMed] [Google Scholar]