Abstract
Background
Cytomegalovirus (CMV) pneumonia is a major cause of death in immunosuppressed patients. Despite the effective treatment with ganciclovir (GCV) and other antiviral agents, the mortality rate remains between 30% to 50%. Recently, the anti-malarial drug artesunate (ART) wasfound to exhibit significant anti-viral activity. Here, we examined the effects of ART on human cytomegalovirus (HCMV) infection and human embryonic lung fibroblast (HELF) proliferation in vitro.
Methods
HELFs infected with the GFP-expressing Towne-BAC strain of HCMV were divided into three treatment groups: Group I, cells treated with ART for 1.5 h before HCMV inoculation; Group II, cells infected with HCMV that was pre-treated with ART for 1.5 h before HCMV inoculation; Group III, cells that were treated with ART at 1.5 h post-HCMV inoculation. GFP expression was observed daily by fluorescence microscopy, and the number of GFP-positive cells in each experimental group was recorded at 4-5 days post-infection. At 10 days post-infection, the viability of cells in each group was recorded. GCV treatment was used as a control.
Results
While no significant effects on cytotoxicity, cell viability, viral infection rates, or antiviral activity were observed upon treatment of Group I or II cells with GCV or low levels of ART, the ART-treated Group III population exhibited significantly reduced rates of infection at drug concentrations higher than 12.5 µM. Similarly, we observed a GCV concentration-dependent reduction in the viral infection rate in Group III cells. Notably, ART-treated, but not GCV-treated, cells also exhibited decreased proliferation. The 50% cytostatic concentrations (CC50) and the half maximal inhibitory concentrations (IC50) of ART and GCV were 54.382 µM and 12.679 µM, and 3.76 M and 14.479 µM, respectively.
Conclusions
In addition to its robust antiviral activity, ART inhibits proliferation of HCMV-infected lung fibroblasts, making it a potential next-generation drug for CMV pneumonia treatment and for reducing fibroproliferation and fibrosis in these patients.
Keywords: Human cytomegalovirus (HCMV), pneumonia, artesunate (ART), lung fibroblast
Introduction
Cytomegalovirus (CMV) pneumonia is one of the major causes of death in patients with AIDS and in recipients of hematopoietic stem cell transplantation (HSCT) or solid organ transplantation. Notably, the incidence of CMV pneumonia is approximately 10-30% after HSCT and 20-70% after solid organ transplantation (1-4), and prior to the discovery of anti-CMV drugs, the mortality rate of this disease was greater than 90% (5). Currently however, even after standard treatment with anti-viral drugs such as ganciclovir (GCV), cidofovir (CDV), and foscarnet (FOS), the mortality rate of CMV pneumonia remains as high as 30-50%. This treatment failure could potentially be the result of the emergence of drug-resistant strains that are induced by the long-term use of these drugs, or to the limited application of these compounds owing to their toxic effects on the bone marrow and kidneys. Alternatively, it is conceivable that anti-viral therapy alone may not be sufficient to completely control CMV pneumonia (6,7).
On progression of CMV pneumonia to acute respiratory distress syndrome (ARDS), the mortality rate increases even further. The pathology of ARDS progresses from the acute exudative phase, to the fibroproliferative phase, and subsequently, to the fibrotic phase. These three phases overlap with one another, and both fibroproliferation and fibrosis are manifestations of the late stage reconstruction of the lung tissue. In cases of pulmonary fibrosis, the mortality rate can reach up to 57%, which is markedly higher than that associated with patients lacking fibrosis (8), and the pulmonary functions and exercise capacity of survivors are often significantly impaired (9,10). Because the abnormal proliferation of lung fibroblasts and the excessive secretion of collagen greatly contribute to the development of fibrotic tissue (11), the inhibition of lung fibroblast proliferation may comprise an effective means for treating and preventing CMV pneumonia and pulmonary fibrosis.
Artemisinin, first isolated from the traditional Chinese medicinal herb Artemisia annua in 1972, is a natural drug that exhibits potent antimalarial activity and low levels of toxicity to the host (12). Furthermore, this compound was shown to exert robust anti-viral effects on laboratory strains, clinically isolates, and drug-resistant strains of human CMV (HCMV) (13,14). Meanwhile, the semi-synthetic artemisinin derivative artesunate (ART) is associated with even higher levels of antimalarial activity than artemisinin, and was shown to also possess prominent anti-tumor, anti-arrhythmic, and antiviral activities (15); however, the effects of this compound on cells infected with HCMV have yet to be fully examined. In this study, we attempted to use human embryonic lung fibroblasts (HELFs) infected with a recombinant HCMV Towne-BAC strain as an experimental model to study the in vitro effects of ART on HCMV infection and on cell proliferation, so as to explore for potential experimental basis for the clinical application of ART for treatment of CMV pneumonia.
Materials and methods
Cell culturing
The seventh-generation HELF cells were purchased from Cellbio Co Ltd, Shanghai, China. The HELF cell line was cultivated in Dul-becco’s modified Eagle’s medium (DMEM) (Gibco, USA) containing 10% fetal bovine serum, 100 units (U)/mL penicillin, and 100 μg/mL streptomycin; after they grew into monolayer, maintenance culture of the HELF cells were performed in DMEM containing 2% fetal calf serum, 100 U/mL penicillin, and 100 μg/mL streptomycin.
HCMV viral strain
The HCMV Towne-BAC strain (16) was used for all experiments. This strain of virus exhibits a 9-KB deletion from the dispensable unique short (US) region (US1 to US12), and instead, contains bacterial artificial chromosome sequences and a GFP expression cassette (17,18). The virus was routinely propagated and titered.
Antimicrobial compounds
A stock solution of ART (Guilin Pharmaceutical Co., Shanghai, China) was prepared by reconstituting the lyophilized ART powder to a concentration of 156 mM in sterile water. Cell maintenance medium lacking antibiotics was then used to dilute the ART to the appropriate concentrations for use in each experiment. Similarly, the GCV stock solution was generated by reconstituting the lyophilized powder (Kawin Technology Beijing, China) to 200 mM in saline, and then diluted to the appropriate concentrations in antibiotic-free maintenance medium.
Determination of CMV titers
HELFs were collected during logarithmic growth by 0.25% trypsin (GIBCO, USA) digestion, counted, and diluted in PBS to 1×105 cells/mL. Approximately 1×104 cells (100 µL of the cell suspension) were then seeded into each well of a 96-well plate, and incubated overnight at 37 °C with 5% CO2 until a monolayer was formed. The virus stock was serially 10-fold diluted to a concentration of 10−8 (10-1, 10-2, 10-3, 10-4, 10-5, 10-6, 10-7, and 10-8), and each dilution was performed in duplicate (i.e., two wells, each). HELF monolayers were infected with each viral suspension (100 µL/well), respectively, and incubated at 37 °C with 5% CO2 for 1.5 h to allow for viral adsorption. After discarding the culture supernatants and washing the cells three times with PBS, 200 µL maintenance medium was added to each well. Meanwhile, the uninfected control cells were diluted and cultured at 37 °C in a 5% CO2 incubator. The growth of the HCMV-inoculated cells was observed daily, and the number of GFP-positive cells was counted and recorded on day 4-5 post-infection. Viral titers were calculated in PFU (plaque forming units) using the following formula: PFU = (average number of GFP-positive cells × the dilution factor)/the volume of the inoculum (mL). Meanwhile, MOIs were calculated by dividing the PFU by the total number of cells to be infected.
Cytotoxicity testing
HELFs were collected during logarithmic growth by 0.25% trypsin digestion, counted, and diluted to 1×105 cells/mL. Approximately 1×104 cells (100 µL of the cell suspension) were then seeded into each well of a 96-well plate and incubated overnight at 37 °C with 5% CO2 to allow for monolayer formation. ART and GCV were diluted to the appropriate concentrations (400, 200, 100, 50, 25, 12.5, 6.25 and 3.125 µM) in cell maintenance medium lacking antibiotics, and 200 µL of each solution was then added to HELF monolayers. Each treatment was performed in triplicate. Meanwhile, untreated cells were used as a control. MTT assay analysis was then used to monitor the cytotoxic effects of each treatment on the HELFs until the progression of the toxicity stopped (about 4-5 days later). For MTT assays, 20 µL of the MTT solution (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; 5 mg/mL) was added to each well, and cells were incubated at 37 °C with 5% CO2 for 4 h until the formation of visible formazan crystals within cells. After discarding the dye solution, 100 µL of DMSO was added, and the cells were subjected to low-speed oscillation for 10-15 min until the crystals were fully dissolved. The OD490 of each well was then measured in a microplate reader, and cell viability and cytopathic effects were calculated using the following formula: cell viability (%) = (OD490 of experimental wells/OD490 of control wells) ×100%.
Anti-HCMV activity testing
Approximately 1×104 HELFs suspended in 100 µL were seeded into each well of a 96-well plate and cultured overnight until a monolayer was formed. ART and GCV were serially diluted to the appropriate concentrations (100, 50, 25, 12.5, 6.25, 3.125 and 0 µM) in cell maintenance medium. Likewise, the HCMV Towne-BAC strain was diluted to an MOI (multiplicity of infection) of 0.25 in cell maintenance medium. Cells were divided into three treatment groups to test the three possible mechanisms of the drug’s antiviral activity: inhibition of infection (Group I), direct inactivation of the virus (Group II), and inhibition of cell proliferation (Group III). Each treatment was performed in duplicate (i.e., two wells were tested per drug dilution), and GCV alone (200 µM) was used as a blank control. The cells in Group I were treated with 100 µL of medium containing the different concentrations of ART or GCV for 1.5 h prior to HCMV infection. The supernatants were then discarded, and the cells were inoculated with HCMV at an MOI of 0.25 (100 µL total volume), and incubated at 37 °C for 1.5 h to allow for viral adsorption. The supernatant was then removed and replaced with maintenance medium. For Group II, the viral suspensions (MOI of 0.25; 100 µL) were incubated with 100 µL of culture medium containing the distinct concentrations of ART or GCV for 1.5 h at 37 °C, and then added to HELF monolayers and incubated at 37 °C for an additional 1.5 h to allow for adsorption. Subsequently, the solution was replaced with maintenance medium. Lastly, the cells in Group III were exposed to the HCMV Towne-BAC strain at an MOI of 0.25 (100 µL) for 1.5 h to allow for viral adsorption. After discarding the supernatants, the cells were washed once with maintenance medium and then treated with 100 µL of medium containing different concentrations of ART or GCV, respectively. The growth of the HCMV-infected cell populations was examined daily and the number of GFP-positive cells was counted in each group at 4-5 days post-infection. The viability of the cells in each experimental group was then assessed at 10 days post-infection by MTT assay analysis using the following formula: Infection rate (%) = (fluorescence intensity of HCMV-infected cells treated with ART or GCV/fluorescence intensity of untreated HCMV-infected cells) ×100%.
Statistical analysis
All data were processed with PASW Statistics for Windows V18.0 (SPSS Inc., Chicago, USA). Numerical data were presented as mean ± standard deviation (SD). The measurement data of two samples were determined by t test. One-way analysis of variance was used for pairwise comparison of multiple samples. According to the calculated rates of cell viability and virus inhibition, 50% cytostatic concentration (CC50) and half maximal inhibitory concentration (IC50) of the drugs, respectively, were computed with Probit regression. P values less than 0.05 were considered statistically significant.
Results
Viral titers of the HCMV Towne strain +
The titer of the Towne strain was 8×105 PFU/mL.
Cytotoxicity of ART
ART exhibited no significant cytotoxic effects on uninfected HELFs at concentrations lower than 12.5 µM (i.e., 6.25 and 3.125 µM). However, as the drug concentration and the reaction time increased, there was a concurrent increase in cytotoxicity that was indicated by decreased cell numbers, decreased cell growth, larger spaces between individual cells, increased numbers of granules, and increased numbers of necrotic cells. Furthermore, Probit regression analysis indicated that the 50% cytostatic concentration (CC50) for ART was 54.38 µM. In contrast, GCV treatment resulted in no significant cytotoxic effects on uninfected HELFs, and the CC50 was 3.76 M.
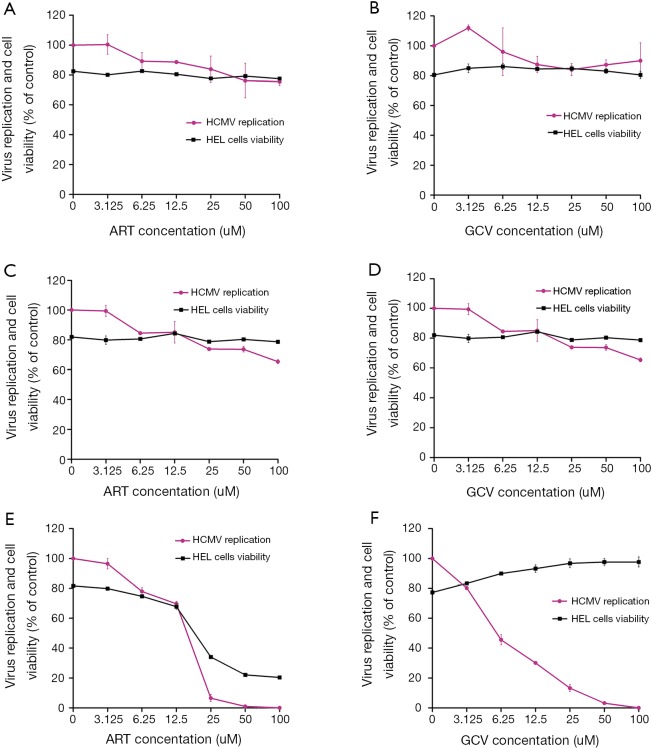
Anti-HCMV effects of ART under different treatment conditions (Figure 1)
Figure 1.
Comparison of the anti-human cytomegalovirus (HCMV) activities of artesunate (ART) and ganciclovir (GCV) and their effects on cell proliferation. The effects of ART and GCV on human embryonic lung fibroblasts (HELFs) infected with the HCMV Towne-BAC strain were examined. Three distinct drug treatment conditions were assessed: Group I (infection blocking) comprised cells that were pre-treated with ART or GCV for 1.5 h prior to viral inoculation (A,B); Group II (direct inactivation) comprised cells infected with HCMV that was pre-treated with ART or GCV for 1.5 h prior to infection (C,D); Group III (inhibition of proliferation) comprised cells that were inoculated with the virus, and then treated with ART or GCV at 1.5 h post-viral adsorption (E,F). Viral GFP expression was examined daily using an inverted microscope. At 4-5 days post-infection, the number of GFP-positive cells was counted in each experimental group, and the optical density at 490 nm (OD490) of each well was determined using a microplate reader. All measurements were performed in duplicate (mean values ± standard deviations).
As expected, GFP fluorescence was observed in the HCMV-infected cells of the experimental groups, but not in the cells of the control group. Meanwhile, the Group I (Figure 1A) and Group II (Figure 1C) cell populations treated with ART exhibited low levels of cytotoxicity and little to no change in cell viability compared to the control group (P>0.05). Additionally, ART treatment failed to significantly reduce viral infection rates or to exert antiviral activity in these cell populations at the concentrations tested. Similar results were observed in the Group I (Figure 1B) and Group II (Figure 1D) cells treated with GCV (P>0.05). In contrast, we observed a concentration-dependent reduction in the rate of HCMV infection, but no significant effect on cell viability, in Group III cells treated with GCV (Figure 1F), indicating that the antiviral effect of this compound occurred after viral adsorption. Notably, however, there were significant reductions in both cell viability and viral infection rates in the Group III (P<0.05) (Figure 1E) cells treated with ART at concentrations greater than 12.5 µM. According to the enumeration of GFP-positive cells, the half maximal inhibitory concentration (IC50) values of ART and GCV were 12.679 and 14.479 µM, respectively.
Discussion
Ever since Efferth and colleagues (14) initially characterized the anti-HCMV activity of ART in 2002, there have been multiple studies that have obtained similar findings (13,19-22). Meanwhile, Arav-Boger et al. recently reported that artemisinin dimers are up to 500-fold more effective in inhibiting HCMV replication in vitro than are artemisinin, ART, and other artemisinin-derived monomers (21,23). However, these studies failed to detect a concurrent inhibitory effect on the proliferation of lung fibroblasts during HCMV infection. Indeed, a reduction in lactate dehydrogenase (LDH) activity, which is used as a marker of cell death, was not observed in cells treated with 3.7 and 50 μM of ART (14,24), indicating that the application of ART at concentrations sufficient for antiviral activity does not results in significant toxicity to cultured cells (HELFs and human foreskin fibroblasts in these studies). In the current study, we utilized HELF cells infected with the recombinant HCMV Towne-BAC virus strain as an experimental model to examine the antiviral activity of ART in vitro. Furthermore, we employed MTT assay analyses to examine the influence of ART on the proliferation of these cells. Our results demonstrate that at concentrations greater than 12.5 µM, ART significantly inhibits the proliferation of HELF cells.
Among the three treatment protocols assessed in this study, the Group I protocol was designed to examine the ability of ART and GCV to prevent viral infection. Meanwhile, Group II was designed to assess the direct killing effect of the drugs on the virus, and Group III was utilized to examine the therapeutic effects of the drugs on HCMV-infected cells. No significant antiviral activities were observed in the Group I and Group II cells treated with either ART or GCV. Conversely, the viral infection rate of the Group III cells decreased significantly after treatment with ART at concentrations greater than 12.5 µM. Similarly, there was a concentration-dependent reduction in the viral infection rate of Group III cells treated with GCV. These findings indicate that ART and GCV possess similar levels of antiviral activity, and that ART exerts its antiviral effects only after viral adsorption, in vitro. However, while neither GCV treatment nor treatment with low concentrations of ART significantly effected Group I or Group II HELF cell proliferation and viability, Group III cells treated with ART, but not GCV, exhibited significant reductions in cell proliferation, as well as significantly reduced levels of cell viability, at concentrations above 12.5 µM. Indeed, we observed an ART concentration- and reaction time-dependent increase in cytotoxic characteristics, which were indicated by reduced cell numbers, decreased cell growth, larger spaces between individual cells, increased numbers of granules, and elevated cell necrosis. Since the abnormal proliferation of lung fibroblasts plays important roles in the formation of pulmonary fibrosis, we propose that ART could be used to effectively inhibit both pulmonary fibrosis and viral activity. As such, the inclusion of ART for the treatment of HCMV pneumonia would provide additional advantages over the use of GCV or other anti-HCMV medications alone.
In this study, a preliminary investigation of the antiviral and anti-pulmonary fibrotic activity of ART was carried out at a cellular level in vitro. While the mechanisms by which ART exerts its anti-HCMV and anti-proliferative activities require further investigation, it is speculated that ART may inhibit the central regulatory process of HCMV replication within infected cells. In previous studies, ART was found to markedly inhibit the protein expression of NF-kB and Sp1, which play critical roles in the initiation of the early viral replication stage of HCMV (14,25). Meanwhile, whether the mechanism by which ART mediates its anti-fibrotic activity is similar to the mechanism of its anti-tumor activity, which is associated with cell apoptosis, is yet unclear.
In summary, we determined that, in addition to its strong antiviral activity, ART possesses an anti-pulmonary fibrotic function, and that this effect is observed at concentrations that are also relevant for antiviral therapy. Given its wide clinical application as an antimalarial drug with few side effects, as well as other advantages, including the absence of drug resistance, treatment effects against both HCMV infection and pulmonary fibrosis, and the fact that the pharmacological mechanism of this compound is distinct from those of currently used anti-HCMV drugs, we believe that ART comprises an attractive new therapeutic option for the treatment of HCMV pneumonia. Understandably, the findings of this experimental study were based largely on HCMV-infected cell models, such that the performance of ART in vivo remains to be elucidated. Future studies are needed to focus on ART in CMV pneumonic animal models regarding protective effects against HCMV infection and pulmonary fibrosis.
Acknowledgements
We thank Guangzhou Rida Biotech Co. for providing all instruments used in experimentation and technical support.
Funding: This study was funded by the Guangdong Provincial Science and Technology Projects (2008B030301080 and 2011B080701062) and Guangdong Natural Science Foundation (S2012010008623).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Moon SM, Sung H, Kim MN, et al. Diagnostic yield of the cytomegalovirus (CMV) antigenemia assay and clinical features in solid organ transplant recipients and hematopoietic stem cell transplant recipients with CMV pneumonia. Transpl Infect Dis 2012;14:192-7. [DOI] [PubMed] [Google Scholar]
- 2.Travi G, Pergam SA. Cytomegalovirus pneumonia in hematopoietic stem cell recipients. J Intensive Care Med 2014;29:200-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leruez-Ville M, Ouachée M, Delarue R, et al. Monitoring cytomegalovirus infection in adult and pediatric bone marrow transplant recipients by a real-time PCR assay performed with blood plasma. J Clin Microbiol 2003;41:2040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ison MG, Fishman JA. Cytomegalovirus pneumonia in transplant recipients. Clin Chest Med 2005;26:691-705, viii. [DOI] [PubMed] [Google Scholar]
- 5.Morris DJ. Opportunities for diagnosing cytomegalovirus in pulmonary infections. Thorax 1995;50:3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lurain NS, Chou S. Antiviral drug resistance of human cytomegalovirus. Clin Microbiol Rev 2010;23:689-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Razonable RR. Immune-based therapies for cytomegalovirus infection. Immunotherapy 2010;2:117-30. [DOI] [PubMed] [Google Scholar]
- 8.Martin C, Papazian L, Payan MJ, et al. Pulmonary fibrosis correlates with outcome in adult respiratory distress syndrome. A study in mechanically ventilated patients. Chest 1995;107:196-200. [DOI] [PubMed] [Google Scholar]
- 9.Marshall RP, Bellingan G, Webb S, et al. Fibroproliferation occurs early in the acute respiratory distress syndrome and impacts on outcome. Am J Respir Crit Care Med 2000;162:1783-8. [DOI] [PubMed] [Google Scholar]
- 10.Weiss CH, Budinger GR, Mutlu GM, et al. Proteasomal regulation of pulmonary fibrosis. Proc Am Thorac Soc 2010;7:77-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Selman M, Thannickal VJ, Pardo A, et al. Idiopathic pulmonary fibrosis: pathogenesis and therapeutic approaches. Drugs 2004;64:405-30. [DOI] [PubMed] [Google Scholar]
- 12.Yan HX, Zhang LF, Zhang SQ. Pharmacokinetics and drug interaction of artemisinin-like antimalarials drug. Chinese Remedies and Clinics 2006;6:645-7. (in Chinese) [Google Scholar]
- 13.Kaptein SJ, Efferth T, Leis M, et al. The anti-malaria drug artesunate inhibits replication of cytomegalovirus in vitro and in vivo. Antiviral Res 2006;69:60-9. [DOI] [PubMed] [Google Scholar]
- 14.Efferth T, Marschall M, Wang X, et al. Antiviral activity of artesunate towards wild-type, recombinant, and ganciclovir-resistant human cytomegaloviruses. J Mol Med (Berl) 2002;80:233-42. [DOI] [PubMed] [Google Scholar]
- 15.Ho WE, Peh HY, Chan TK, et al. Artemisinins: pharmacological actions beyond anti-malarial. Pharmacol Ther 2014;142:126-39. [DOI] [PubMed] [Google Scholar]
- 16.Marchini A, Liu H, Zhu H. Human cytomegalovirus with IE-2 (UL122) deleted fails to express early lytic genes. J Virol 2001;75:1870-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marschall M, Freitag M, Weiler S, et al. Recombinant green fluorescent protein-expressing human cytomegalovirus as a tool for screening antiviral agents. Antimicrob Agents Chemother 2000;44:1588-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rechter S, König T, Auerochs S, et al. Antiviral activity of Arthrospira-derived spirulan-like substances. Antiviral Res 2006;72:197-206. [DOI] [PubMed] [Google Scholar]
- 19.Gantt S, Huang ML, Magaret A, et al. An artesunate-containing antimalarial treatment regimen did not suppress cytomegalovirus viremia. J Clin Virol 2013;58:276-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chou S, Marousek G, Auerochs S, et al. The unique antiviral activity of artesunate is broadly effective against human cytomegaloviruses including therapy-resistant mutants. Antiviral Res 2011;92:364-8. [DOI] [PubMed] [Google Scholar]
- 21.Arav-Boger R, He R, Chiou CJ, et al. Artemisinin-derived dimers have greatly improved anti-cytomegalovirus activity compared to artemisinin monomers. PLoS One 2010;5:e10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shapira MY, Resnick IB, Chou S, et al. Artesunate as a potent antiviral agent in a patient with late drug-resistant cytomegalovirus infection after hematopoietic stem cell transplantation. Clin Infect Dis 2008;46:1455-7. [DOI] [PubMed] [Google Scholar]
- 23.He R, Park K, Cai H, et al. Artemisinin-derived dimer diphenyl phosphate is an irreversible inhibitor of human cytomegalovirus replication. Antimicrob Agents Chemother 2012;56:3508-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schnepf N, Corvo J, Pors MJ, Mazeron MC. Antiviral activity of ganciclovir and artesunate towards human cytomegalovirus in astrocytoma cells. Antiviral Res 2011;89:186-8. [DOI] [PubMed] [Google Scholar]
- 25.Efferth T, Romero MR, Wolf DG, et al. The antiviral activities of artemisinin and artesunate. Clin Infect Dis 2008;47:804-11. [DOI] [PubMed] [Google Scholar]