Graphical abstract

Keywords: Biginelli adducts, Antiproliferative activity cancer, Calcium channel, Antimicrobial activity, Antioxidants
Abstract
Since the disclosure of Biginelli reaction by the chemist Pietro Biginelli, functionalized 3,4-dihydropyrimidin-2(1H)-ones/thiones (DHPMs) have emerged as prototypes for the design of compounds with a broad variety of biological activities. This mini-review describes over 100 Biginelli adducts demonstrated to be promising anticancer, inhibitors of calcium channel, anti-inflammatory, antimicrobial and antioxidant agents. Thus, this compilation presents the most notable in vitro and in vivo results for such fascinating class of organic compounds.
Introduction
The year 1891 was a milestone for the discovery of a new class of heterocycle molecules named Biginelli adducts after the chemist Pietro Biginelli who first report the simple one-pot process that furnish organic compounds of this kind [1]. The multicomponent reaction that provides Biginelli adducts, also known as 3,4-dihydropyrimidin-2(1H)-ones/thiones (DHPMs; Fig. 1), involves the reaction of 1,3-dicarbonyl compounds with aldehydes and (thio)urea [2]. Three main mechanisms have been proposed for the Biginelli reaction, but this subject is still under debate in the literature. Detailed information on these three mechanisms is addressed elsewhere [3]. Variation of all three building blocks has broadened the molecular diversity of DHPMs with wide variety biological activities. Indeed, a series of pharmacological properties of DHPMs have been reported, which include antiviral, antitumor, anti-inflammatory, antibacterial, antifungal, anti-epileptic, antimalarial, antileishmanial, among others. The next topics will cover for some of the most notable Biginelli adducts reported as anticancer, calcium channel inhibitors, anti-inflammatory, antimicrobial and antioxidant agents since the listed pharmacological properties are some of the most investigated for DHPMs.
Fig. 1.

Basic structure of Biginelli adducts.
Anticancer activity
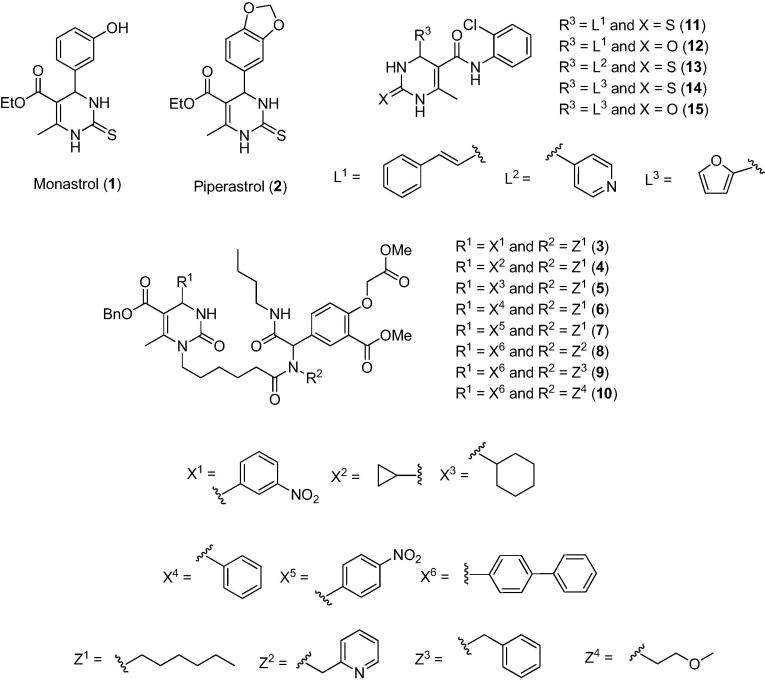
Biginelli adducts are promising compounds for the treatment of cancers in which monastrol (1) is the most studied with this regard (Fig. 2). The first work that explored the effect of monastrol on cancer cells was reported in 1999 [4]. Monastrol was found to interrupt mitosis by inhibiting the motor activity of the kinesin Eg5, a protein involved in spindle bipolarity formation [5].
Fig. 2.
Example of Biginelli adducts that possess antiproliferative activity against cancer cells.
Since then, monastrol has been used as an inspiration for the design of new anticancer agents. Out of eleven monastrol analogues synthesized, the Biginelli adduct 2 was identified as a potent anticancer agent based on the concentration of this adduct necessary to inhibit cell growth by 50% (EC50, IC50 or GI50) as it follows: MCF-7 breast (1.9 μg mL−1), 786-0 kidney (2.0 μg mL−1), HT-29 colon (2.5 μg mL−1), UACC.62 melanoma (6.0 μg mL−1) and OVCAR03 ovarian (6.6 μg mL−1) cancer cells [5].
Compounds 3–10 (Fig. 2) were described as some of the most effective pyrimidinone-peptoid hybrids against SK-BR-3 breast cancer cells, exhibiting GI50 values in the range of 6.0–8.8 μM [6].
Biginelli adducts bearing cinnamoyl (11 and 12), pyridin-4-yl (13) or furan-2-yl (14 and 15) groups (Fig. 2) showed significant cytotoxic effects against the MCF-7 breast cancer cell line, in which at concentration of 50 μg mL−1 prevented cell growth by at least 70% [7].
Biginelli adducts-amide derivatives such as 16 and 17 (Fig. 3) exhibited moderate antiproliferative activity against HepG2 epithelial carcinoma in which the IC50 value for both compounds was ca. 120 μg mL−1 [8]. On the other hand, the derivatives 17 and 18 showed IC50 values of around 190 μg mL−1 against HeLa hepatocellular carcinoma cells [8].
Fig. 3.
Other examples of Biginelli adducts well-known for their ability to inhibit cancer cells growth.
Other monastrol (1) analogues were synthesized and tested against cancer cell lines of different histological origins [9]. Twelve Biginelli adducts (19–30; Fig. 3) were more potent than monastrol (GI50 in the range of 4.0–29.6 μg mL−1) against one or more of the seven cancer cell lines studied (Table 1) [9]. Notably, compound 19 was determined to be over 90- and 10-fold more potent than monastrol (1) against U251 glioma cells and NCI-ADR/RES multiple drug-resistant ovarian cancer cells, respectively (Table 1). Compound 20 was found to be almost 90-fold more potent than monastrol against NCI-ADR/RES multiple drug-resistant ovarian cancer cells while the GI50 value for 21 is about 30-fold lower than that of monastrol toward U251 cells (Table 1). The results also indicate that six Biginelli adducts present GI50 values at least 5-fold lower than those of monastrol against some of the following cancer cells: U251 glioma, NCI-ADR/RES multiple drug-resistant ovarian, 786 renal, NCI-H460 non-small lung, PC-3 prostate, OVCAR-03 ovarian and HT-29 colon cancer (Table 1).
Table 1.
Potency (in folds) of Biginelli adducts relative to monastrol (1) with respect to the antiproliferative activity against cancer cells of different histological origins. Adapted from da Silva and coworkers [9].
| Biginelli adduct | U251 | NCI-ADR/RES | 786-0 | NCI-H460 | PC-3 | OVCAR-03 | HT-29 |
|---|---|---|---|---|---|---|---|
| 19 | 96.0 | 10.6 | (–) | (–) | (–) | 9.0 | (–) |
| 20 | 5.0 | 88.8 | (–) | (–) | (–) | 8.4 | 1.0 |
| 21 | 31.0 | 1.0 | (–) | (–) | (–) | 7.5 | 1.0 |
| 22 | 1.0 | 7.0 | (–) | (–) | 1.0 | 14.3 | 1.0 |
| 23 | 1.0 | 1.0 | 1.0 | 3.0 | 11.0 | 6.5 | 1.0 |
| 24 | 5.7 | 7.0 | (–) | (–) | (–) | (–) | 1.0 |
| 25 | 1.0 | 1.0 | (–) | (–) | (–) | 11.0 | 1.0 |
| 26 | 4.7 | 2.0 | (–) | (–) | (–) | 2.4 | 1.0 |
| 27 | (–) | 4.0 | (–) | (–) | (–) | (–) | 1.0 |
| 28 | 3.0 | 8.0 | 1.5 | 3.0 | (–) | 1.4 | 3.0 |
| 29 | 4.0 | (–) | (–) | (–) | (–) | 1.7 | 1.7 |
| 30 | 1.0 | 6.0 | (–) | (–) | 3.0 | (–) | 1.0 |
GI50 values for monastrol were in range of 4.0–29.6 μg mL−1[9]. (–) Indicates that the Biginelli adduct was less potent than monastrol (1). U251, glioma cells; NCI-ADR/RES, multiple drug-resistant ovarian cancer cells; 786, renal cancer cells; NCI-H460, non-small lung cancer cells; PC-3, prostate cancer cells; OVCAR-03, ovarian cancer cells and HT-29, colon cancer cells.
Morphological alterations in MCF-7 breast cancer cells that culminated in the death of over 80% cells were observed after 72 h of treatment with the Biginelli adducts 31 (dimethylenastron) to 33 (Fig. 3) at concentrations in the range of 400 μM to 1 mM. Such compounds showed minute toxic effects against fibroblast healthy cells [10].
Calcium channel inhibition
Dihydropyridines such as nifedipine were introduced to the market in 1975 for the treatment of cardiovascular diseases (hypertension, cardiac arrhythmias and angina) due to the ability to inhibit calcium channels [11]. After the discovery of this drug several analogues, including Biginelli adducts, were synthesized to verify the potential to block calcium channels.
A structure–activity relationship study with Biginelli adducts was reported in 1990 with respect to the ability to target calcium channels [12,13]. It was determined that thio-adducts were the most potent Biginelli compounds in comparison with oxo- and aza-analogues [12,13]. In vitro assays revealed that the adduct bearing a nitro group at ortho-position of aromatic ring was more effective antihypertensive compound than that containing CF3 or Cl as substituent (Fig. 4) [13]. Interestingly, the presence of an isopropyl ester group at C5 improved the Biginelli adduct potency by 10- and 60-fold in comparison with the effect of the ones bearing an ethyl ester or methyl ester group at the same carbon, respectively [13]. Although compounds bearing substituents at N3 are potent calcium channel blockers in vitro, their antihypertensive properties are lost in in vivo experiments as a result of metabolization by rats [13]. Additionally, oxo-analogues were found to be more stable as homogenates from rat liver did not present metabolites derived from such these compounds [13]. Finally, the stereocenter at C4 also plays a key role in the activity of such Biginelli adducts toward calcium channel; the (R)-enantiomer (34a; Fig. 4) is 750-fold more potent vasorelaxant agent than the corresponding (S)-enantiomer (34b; Fig. 4) [13]. Atwal and coworkers then substituted the acyl at N3 for a carbamoyl group to check whether such structural changes would affect the inhibition of calcium channel by Biginelli adducts related to 34 [14]. The best compounds (35–39; Fig. 4) tested in vitro exhibited IC50 values of 3, 12, 13, 16 and 60 nM, respectively. Thus, it was concluded that the presence of substituents at carbamoyl group influenced compounds potency as it follows: benzyl group > hydrogen, methyl or ethyl group > isopropyl group [14]. Compounds bearing 1-(phenylmethyl)-4-piperidinyl carbamate at N3 were described as the most promising calcium channel blockers in in vivo experiments, in which the presence of CF3 at ortho-position of aromatic ring enhanced compounds effect when compared to the ones bearing nitro group [15]. Additionally, fluorine at para-position of benzyl moiety prevented the Biginelli adduct from metabolization by rat cells and conferred much higher potency than that of the reference drug amlodipine. Again, in vitro experiments demonstrated that the (R)-enantiomer (40a; Fig. 4) is much more potent than the corresponding (S)-enantiomer (40b; Fig. 4), since the former exhibits an IC50 value of 15 nM while the IC50 value for the latter is determined to be higher than 1000 nM [15].
Fig. 4.
Example of Biginelli adducts that exhibit inhibitory effect on calcium channels.
The thio-Biginelli adducts 41 and 42 were determined to be relaxant agents as effective as the reference drug nicardipine (inhibition of stimulus by 35.5 ± 4.2%) on KCl-stimulated lamb carotid strips when used at 100 μM [16]. Compounds 41 and 42 present a Cl atom as substituent at meta- and para-position, respectively (Fig. 4). The relaxant effect of the thio-Biginelli adduct 43 (Fig. 4) on KCl-stimulated contractions in rat thoracic aorta was comparable to that of nicardipine (inhibition of stimulus by 20.5 ± 2.9%) [17]. Other oxo-Biginelli adducts were investigated for the calcium channel blockage-dependent relaxant effect on KCl-stimulated lamb carotid strips. Compounds containing Br, CH3 or CF3 at ortho-position or Br at meta-position in aromatic ring (44–47; Fig. 4) at 1 μM were either as potent or as more potent than nicardipine that at the same concentration was able to inhibit the stimulus by 2.5 ± 1.8% [18].
The acetylated thio-Biginelli adduct derivative 48 (Fig. 4) effectively caused the relaxation of KCl-stimulated guinea pig ileum as attested by its value of negative log molar concentration of antagonist required to reduce the response of agonist by 50% (PA2 = 6.06) in relation to the reference drug verapamil [19].
Anti-inflammatory activity
Inflammation process can be characterized by five phases that may or may not occur simultaneously, named pain, heat, redness, swelling and ultimately loss of function. They comprise a defensive body response to invasion of a foreign material. Acute inflammation can cause several damages in tissues or organs. The anti-inflammatory potential of a certain molecule can be investigated by various means, such as the analgesic effect using paw edema as model, the inhibition of proinflammatory cytokines (e.g. tumor necrosis factor (TNF-α) and interleukin 6 (IL-6)) [20], the effect on prostaglandin E2 and/or hialuronidase, nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) and transient receptor A1 (TRPA1), among others [20–24].
Biginelli adducts have received great attention with respect to their potential as anti-inflammatory agents. Based on the duration of action and percentage of inflammation inhibition on Albino rats paw edema, the propanoic acid derivatives thio-adducts (49–53; Fig. 5) were found to be the most promising anti-inflammatory compounds when compared to diclofenac, a reference drug [25]. The Biginelli derivative 54, which bears a 1,3,4-oxadiazol-2-yl moiety (Fig. 5), controls inflammation process by inhibiting the carrageenan-induced rat paw edema by 75% after 3 h of treatment, an effect comparable to that exhibited by diclofenac [26].
Fig. 5.
Example of Biginelli adducts that exhibit anti-inflammatory effect.
The potential of the thio-analogue Biginelli adduct 55 (Fig. 5) to inhibit the production of proinflammatory cytokines in LPS-induced human monocytic leukemia cells (THP-1) was addressed [20]. The production of TNF-α and IL-6 in THP-1 cells in the presence of compound 55 at 10 μM was 78% and 96% lower than that of cells incubated in the absence of this Biginelli adduct, respectively. Under the same experimental conditions, dexamethasone (reference drug at 1 μM) inhibit TNF-α and IL-6 production by 71% and 84%, respectively [20].
Chronic inflammation is known to be associated with increased activity of hyaluronidases, enzymes that catalyzes the degradation of hyaluronic acid [27,28]. Based on this, Gireesh and coworkers performed molecular docking studies using some Biginelli adducts and related derivatives to identify compounds with potential to inhibit hyaluronidase [24]. Indeed, in vitro assays confirmed that 100 μg of compounds 56–59 (Fig. 5) was able to inhibit the activity of hyaluronidase (3–5 units) in the range from 89% to 100%. Similar results were achieved when compounds 56–59 were substituted for indomethacin, a reference drug [24].
The anti-inflammatory properties of Biginelli adducts 60–62 (Fig. 5) were attested by their capacity to inhibit NO production in LPS-activated microglia at IC50 values ranging from 41.3 to 67.3 μM [29]. Compound 60 was also the most potent among these Biginelli adducts in the inhibition of prostaglandin E2 (PGE2) production and iNOS and COX-2 genes expression. Additionally, 60 negatively affected the production of TNFα and interleukin-1 β (IL-1β) [23].
Biginelli adducts bearing meta-substituents have been described as very promising anti-inflammatory agents in studies carried out with human embryonic kidney 293 cell lines (HEK293) overexpressing the transient receptor potential A1 (TRPA1) either from human or rat [22]. Thus, compounds 63a-b and 64a-b (Fig. 5) were able to inhibit both human and rat TRPA1 at concentrations ranging from 4 to 75 nM. The R isomers (63b and 64b), however, were identified as the most potent inhibitors acting on rat TRPA1 at IC50 values as low as 4 and 12 nM, respectively, while the IC50 for the corresponding S isomers (63c and 64c; Fig. 5) were found to be higher than 10,000 nM [22].
Antibacterial activity
Biginelli compounds bearing a 1,3-diarylpyrazole moiety (65–68; Fig. 6) exhibited minimal inhibition concentration (MIC) of 20 ng mL−1, 20 ng mL−1, 250 ng mL−1 and 125 ng mL−1 against the Mycobacterium tuberculosis H37Rv (MTB H37Rv), respectively [30,31]. The effect of 65 and 66 on normal kidney-derived African green monkey cells (VERO line) was assessed, revealing that both Biginelli adducts are highly selective to MTB H37Rv (selectivity index >500) [30]. Other 16 Biginelli adducts (69–74; Fig. 6) were found to be as potent as or more potent than the reference drugs ethambutol (MIC = 7.6 μM) and ciprofloxacin (MIC = 9.4 μM) against MTB H37Rv. The MIC values for compounds 69–74 ranged from 3.4 to 76.2 μM [32].
Fig. 6.
Example of Biginelli adducts that exhibit antimicrobial activity.
Compounds 75 and 76, containing a nitro group and fluorine at para-position, respectively, exhibited MIC values of 12.5 μg mL−1 (for the former) and 12.5–25.0 μg mL−1 (for the latter) against Escherichia coli, Klebsiella pneumonia, Pseudomonas aeruginosa, Salmonella typhi and Staphylococcus aureus, which make these compounds more potent than ciprofloxacin [33]. Biginelli adducts bearing a 1,3-dihydro-2H-indol-2-one core showed moderate antibacterial activities (62.5–250.0 μg mL−1) against Bacillus subtilis (MTCC-441), E. coli (MTCC-443), K. pneumonia (MTCC-109), P. aeruginosa (MTCC-1688), S. typhi (MTCC-98), S. aureus (MTCC-96) and Staphylococcus pyogenus (MTCC-442) [34].
Antiviral activity
Kim and coworkers showed the potential of some Biginelli adducts as agents for preventing human immunodeficiency virus HIV-1 replication [35,36]. Notably, compounds 77–82 (Fig. 6) compromised the HIV-1 replication in CEMx174-LTR-GFP cells (clone CG8) by 50% when employed at concentrations lower than 90 nM. At the same experimental conditions, the reference drug nevirapine exhibited an EC50 value of 150 nM [35,36]. The (S)-enantiomer was determined to be more potent than the corresponding (R)-enantiomer with respect to the antiviral activity. Indeed, it was shown that (S)-77 is at least 26-fold more potent than (R)-77 [35,36].
The potential of the Biginelli-type pyrimidines 83 (IC50 = 1.8 μM) and 84 (IC50 = 0.9 μM) against herpes simplex virus (HSV-KOS strain) was shown elsewhere (Fig. 6) [37]. Notably, the analogue 84 exhibited negligible toxicity toward the mammalian cells tested indicating its selectivity to the studied virus. A time-of-addition study was then performed with 84 revealing that the administration of such compound to cells 2 and 4 h post inoculation was sufficient to negatively affect virus replication. The lack of inhibition of virus adhesion and/or entry to the cells suggests that compound 84 inhibits virus replication in late stages [37].
Antifungal activity
Fungi have emerged worldwide as some of the most frequent causes of healthcare-associated infections. Invasive fungal infections can be life-threatening and the number of antifungal agents currently available in the market is very limited [38].
Although Biginelli adducts have been poorly explored with respect to the antifungal activity, some examples of promising compounds are described in the literature. Eleven Biginelli-type pyrimido[4,5-d]pyrimidine-2,5-diones were described as potential anti-Aspergillus niger and anti-Candida albicans agents, exhibiting MIC values raging from 11 to 57 μg mL−1 [39]. The most active compounds (85–88; Fig. 6) showed MIC values near to or lower than 20 μg mL−1 in comparison with the reference antifungal clotrimazole, whose MIC values against A. niger and C. albicans were 20 and 25 μg mL−1, respectively. Thus, analogues bearing withdrawing groups, with exception of 4-Cl substituent, were the most active against A. niger and C. albicans [39].
The Biginelli adducts 75 and 76 (Fig. 6) efficiently inhibited the growth of C. albicans, Aspergillus flavus, Rhizopus sp. and Mucor sp. as attested by the MIC values in the range of 12.5–25 μg mL−1, being most of the time more potent than amphotericin B (MIC = 25–50 μg mL−1) [33].
According to Rajanarendar and coworkers [40], isaxole Biginelli adducts are promising antifungal agents against A. niger, Chrysosporium tropicum, Rhizopusoryzae, Fusarium moniliformae and Curvularia lunata. When tested at 100 μg mL−1, compounds 89 and 90 (Fig. 6) were able to induce the formation of a zone of fungal growth inhibition from 60 mm to 65 mm against the strains tested, which confers to these compounds higher potency in comparison with clotrimazole (inhibition zone of up to 35 mm) [40]. Studies of formation of zones of fungal growth inhibition were also carried out with C. albicans and Aspergillus parasiticus and the adducts 91–93 (Fig. 6) [41]. An average zone of inhibition of 16.5 mm was verified in cultures of C. albicans in the presence of Biginelli adducts at 10 μg mL−1, while clotrimazole triggered the formation of a 21 mm-inhibition zone. As for A. parasiticus, the inhibition zone in the presence of compounds 91–93 and clotrimazole (all at 10 μg mL−1) were, respectively, 13 mm, 17 mm, 18 mm and 22 mm [41].
Antioxidant activity
Oxygen and nitrogen reactive species (ROS and RNS, respectively) are ubiquitous in nature being a result of electron escape from electron transport chain (present in mitochondria and chloroplast). The overproduction of ROS and/or RNS can be deleterious to cells if the cellular antioxidant system is not able to efficiently restore the normal levels, which can ultimately cause pathologies [9,42].
The first report on the antioxidant properties of Biginelli adducts was published in 2006 in a study that investigated the potential of such molecules to prevent ROS formation and lipid peroxidation in male adult albino Wistar rats [42]. The Biginelli adducts 94 and 95 (Fig. 7) restored the lipid hydroperoxide to normal levels in liver cells when administered at 200 μM. These results indicate that the presence of a nitro group on aromatic ring is not mandatory for adduct 95 preventing lipid peroxidation. Compounds 94 and 96 (Fig. 7) were found to be more efficient than the corresponding nitro-analogues 95 and 97 (Fig. 7) to prevent the overproduction of ROS [42].
Fig. 7.
Example of Biginelli adducts with ability to scavenge oxygen and/or nitrogen reactive species.
The potency of the thio-adducts 98 and 99 (87.5%; Fig. 7) to scavenge hydroxyl radicals was comparable to that of the reference antioxidant quercetol (92.3%) when all compounds were used at 100 μM [43]. The thio-adducts 22 (Fig. 3) and 100 (Fig. 7) exhibit IC50 values of 10 μM and 76 μM, respectively, regarding the scavenging of 2,2-diphenyl-1-picrylhydrazyl (DPPH) radicals [44]. Also, compounds 22 and 100 at 300 μM diminished, at similar extents, the lipid hydroperoxide levels in homogenates of cerebral cortex from rats [44].
The adduct 101 (Fig. 7) effectively scavenged DPPH radicals exhibiting an IC50 value of 0.6 mg mL−1, while the IC50 value for gallic acid (a known radical scavenger) was 0.8 μg mL−1 [45].
A series of Biginelli adducts were tested by da Silva and coworkers to compare the ability of thio- and oxo-derivatives to scavenge RNS and ROS [9]. Compounds 19, 21, 25 (Fig. 3) and 102 (Fig. 7) were determined to be the most promising RNS scavengers among the tested adducts, as they showed IC50 values of 20.3, 29.7, 23.3 and 24.2 μM, respectively, while resveratrol exhibited an IC50 of 34.4 μM in reactions containing DPPH 100 μM. As for ROS scavenging, the IC50 values for 19, 21, 25 and 102 and resveratrol toward O2− were 33.0, 25.7, 122.3, 78.0, 121.4 μM, respectively [9].
Compounds 20 (Fig. 3) and 103–105 (Fig. 7) were demonstrated to be as efficient as gallic acid in the scavenging of DPPH at 40 μg mL−1 as the IC50 values for these adducts ranged from 2.1 to 5.0 μg mL−1 [46].
Concluding remarks
The diverse biological profile of Biginelli adducts brought perspectives for the development of novel drugs to improve human and animal health. Here, we compiled the effect of over 100 Biginelli adducts on cancer cells, calcium channels, inflammation, microorganisms (bacteria, viruses and fungi) and ROS and RNS scavenging. Some progress has been made with respect to the mechanism of action by which monastrol (1) and related molecules trigger the inhibition of cancer cells growth. However, the mechanisms of action of Biginelli adducts that lead to the attenuation and/or prevention of other pathologies are still incipient. Therefore, advances in this matter will certainly contribute to the rational design of more efficient and selective calcium channel inhibitor, anti-inflammatory, antimicrobial and antioxidant agents based on Biginelli adducts core.
Conflict of interest
The authors have declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Acknowledgments
This work was financially supported, in part, by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG). AdF and LVM are recipients of research fellowships from CNPq.
Biographies

Ângelo de Fátima received his PhD degree in Science in 2005 from the State University of Campinas (SP, Brazil). He is currently Associate Professor of the Department of Chemistry at the Federal University of Minas Gerais (MG, Brazil). Dr. de Fátima is the coordinator of the Network for the Development of Novel Urease Inhibitors (www.redniu.org) and Group of Studies on Organic and Biological Chemistry. His research interests include the synthesis of molecules with biological, functional profile and the evaluation of their activities against cancer cells, fungi, bacteria and virus of clinical interest.

Taniris Cafiero Braga was born in 1990. She earned her BSc. degree in Chemistry in 2013 at the Federal University of Minas Gerais (MG, Brazil) when she joined the Graduation Program in Chemistry to start her Master studies under the mentoring of Dr. de Fátima. Her research interests are in the field of Organic and Medicinal Chemistry.

Leonardo da Silva Neto is Pharmacist and received his MSc. degree in Chemistry in 2011 from the Federal University of Minas Gerais (MG, Brazil). He is currently a PhD student at the same University developing research under the mentoring of Dr. de Fátima. MSc. Silva Neto research interests are focused on the synthesis of calix[n]arenes and H2S-releasing compounds and their biological profiles.

Bruna Silva Terra was born in 1988. She earned her BSc. degree in Pharmacy in 2011 at the State University of Londrina (PR, Brazil). She received her MSc. degree in Chemistry in 2013 from the Federal University of Minas Gerais (MG, Brazil). She is currently performing her PhD studies in Chemistry under the mentoring of Dr. de Fátima. Her research interests are in the field of Organic and Medicinal Chemistry.

Breno Germano de Freitas Oliveira was born in 1990. He earned his BSc. degree in Chemistry in 2014 at the Federal University of Minas Gerais (MG, Brazil). Afterward he joined Dr. de Fátima’s group to perform his Master studies in Organic Chemistry. His research interests are in the fields of Organic Synthesis and Biological Chemistry.

Daniel Leite da Silva received his BSc. and MSc. in Chemistry in 2009 and 2011 from the Federal University of Viçosa (MG, Brazil) and Federal University of Minas Gerais (MG, Brazil), respectively. He is currently performing his PhD studies in Chemistry under the mentoring of Dr. de Fátima. His research interests are focused on the synthesis and biological activity of Biginelli adducts.

Luzia Valentina Modolo received her PhD degree in Functional and Molecular Biology in 2004 from the State University of Campinas (SP, Brazil). She is currently the Head of the Department of Botany at the Federal University of Minas Gerais (MG, Brazil). Dr. Modolo is also the coordinator of the Network for the Development of Novel Urease Inhibitors (www.redniu.org) and Group of Studies on Plant Biochemistry (www.gebioplan.com). Her research interests include the signalling processes coordinated in plant tissues in response to environmental stress, plant nutrition and plant secondary metabolism.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.(a) Biginelli P. Intorno ad uramidi aldeidiche dell’etere acetilacetico. Gazz Chim Ital. 1891;21:455–461. [Google Scholar]; (b) Biginelli P. Intorno ad uramidi aldeidiche dell’etere acetilacetico. II. Gazz Chim Ital. 1891;21:497–500. [Google Scholar]; (c) Biginelli P. Ueber Aldehyduramide des Acetessigäthers. Ber Dtsch Chem Ges. 1891;24:1317–1319. [Google Scholar]; (d) Biginelli P. Ueber Aldehyduramide des Acetessigäthers. II. Ber Dtsch Chem Ges. 1891;24:2962–2967. [Google Scholar]; (e) Biginelli P. Aldehyde-urea derivatives of aceto- and oxaloacetic acids. Gazz Chim Ital. 1893;23:360–413. [Google Scholar]
- 2.Kappe C.O. Biologically active dihydropyrimidones of the Biginelli-type – a literature survey. Eur J Med Chem. 2000;35(12):1043–1062. doi: 10.1016/s0223-5234(00)01189-2. [DOI] [PubMed] [Google Scholar]
- 3.(a) de Fátima A., Terra B.S., Silva-Neto L., Braga T.C. Organocatalyzed Biginelli reactions: a greener chemical approach for the synthesis of biologically active 3,4-dihydropyrimidin-2(1H)-ones/thiones. In: Brahmachari G., editor. Green synthetic approaches for biologically relevant heterocycles. 1st ed. Elsevier Science Publishing Co. Inc.; 2014. pp. 317–337. [chapter 12] [Google Scholar]; (b) Alvim H.G.O., Lima T.B., de Oliveira A.L., de Oliveira H.C.B., Silva F.M., Gozzo F.C. Facts, presumptions, and myths on the solvent-free and catalyst-free Biginelli reaction. What is catalysis for? J Org Chem. 2014;79(8):3383–3397. doi: 10.1021/jo5001498. [DOI] [PubMed] [Google Scholar]; (c) Tron G.C., Minassi A., Appendino G. Pietro Biginelli: the man behind the reaction. Eur J Org Chem. 2011;2011(28):5541–5550. [Google Scholar]; (d) Papeo G., Pulici M. Italian chemists’ contributions to named reactions in organic synthesis: an historical perspective. Molecules. 2013;18(9):10870–10900. doi: 10.3390/molecules180910870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mayer T.U., Kapoor T.M., Haggarty S.J., King R.W., Schreiber S.L., Mitchison T.J. Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science. 1999;286(5441):971–974. doi: 10.1126/science.286.5441.971. [DOI] [PubMed] [Google Scholar]
- 5.(a) Russowsky D., Canto R.F.S., Sanches S.A.A., D’oca M.G.M., de Fátima A., Pilli R.A. Synthesis and differential antiproliferative activity of Biginelli compounds against cancer cell lines: monastrol, oxo-monastrol and oxygenated analogues. Bioorg Chem. 2006;34(4):173–182. doi: 10.1016/j.bioorg.2006.04.003. [DOI] [PubMed] [Google Scholar]; (b) Prokopcová H., Dallinger D., Uray G., Kaan H.Y.K., Ulaganathan V., Kozielski F. Structure-activity relationships and molecular docking of novel dihydropyrimidine-based mitotic Eg5 inhibitors. ChemMedChem. 2010;5(10):1760–1769. doi: 10.1002/cmdc.201000252. [DOI] [PubMed] [Google Scholar]
- 6.Wright C.M., Chovatiya R.J., Jameson N.E., Turner D.M., Zhu G., Werner S. Pyrimidinone-peptoid hybrid molecules with distinct effects on molecular chaperone function and cell proliferation. Bioorg Med Chem. 2008;16(6):3291–3301. doi: 10.1016/j.bmc.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar B.R.P., Sankar G., Baig R.B.N., Chandrashekaram S. Novel Biginelli dihydropyrimidines with potential anticancer activity: a parallel synthesis and CoMSIA study. Eur J Med Chem. 2009;44(10):4192–4198. doi: 10.1016/j.ejmech.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 8.Soumyanarayanan U., Bhat V.G., Kar S.S., Mathew J.A. Monastrol mimic Biginelli dihydropyrimidinone derivatives: synthesis, cytotoxicity screening against HepG2 and HeLa cell lines and molecular modeling study. Org Med Chem Lett. 2012;2(23):1–11. doi: 10.1186/2191-2858-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.da Silva D.L., Reis F.S., Muniz D.R., Ruiz A.L.T.G., Carvalho J.E., Sabino A.A. Free radical scavenging and antiproliferative properties of Biginelli adducts. Bioorg Med Chem. 2012;20:2645–2650. doi: 10.1016/j.bmc.2012.02.036. [DOI] [PubMed] [Google Scholar]
- 10.Ramos L.M., Guido B.C., Nobrega C.C., Corrêa J.R., Silva R.G., de Oliveira H.C.B. The Biginelli reaction with an imidazolium-tagged recyclable iron catalyst: kinetics, mechanism, and antitumoral activity. Chem Eur J. 2013;19:4156–4168. doi: 10.1002/chem.201204314. [DOI] [PubMed] [Google Scholar]
- 11.Janis R.A., Silver P.J., Triggle D.J. Drug action and cellular calcium regulation. Adv Drug Res. 1987;16:309–591. [Google Scholar]
- 12.Atwal K.S., Rovnyak G.C., Schwartz J., Moreland S., Hedberg A., Gougoutas J.Z. Dihydropyrimidine calcium channel blockers: 2-heterosubstituted 4-aryl-1,4-dihydro-6-methyl-5-pyrimidinecarboxylic acid esters as potent mimics of dihydropyridines. J Med Chem. 1990;33(9):1510–1515. doi: 10.1021/jm00167a035. [DOI] [PubMed] [Google Scholar]
- 13.Atwal K.S., Rovnyak G.C., Kimball S.D., Floyd D.M., Moreland S., Swanson B.N. Dihydropyrimidine calcium channel blockers. 2. 3-Substituted-4-aryl-1,4-dihydro-6-methyl-5-pyrimidinecarboxylic acid esters as potent mimics of dihydropyridines. J Med Chem. 1990;33(9):2629–2635. doi: 10.1021/jm00171a044. [DOI] [PubMed] [Google Scholar]
- 14.Atwal K.S., Swanson B.N., Unger S.E., Floyd D.M., Moreland S., Hedberg A. Dihydropyrimidine calcium channel blockers. 3. 3-Carbamoyl-4-aryl-1,2,3,4-tetrahydro-6-methyl-5-pyrimidinecarboxylic acid esters as orally effective antihypertensive agents. J Med Chem. 1991;34(2):806–811. doi: 10.1021/jm00106a048. [DOI] [PubMed] [Google Scholar]
- 15.Rovnyak G.C., Atwal K.S., Hedberg A., Kimball S.D., Moreland S., Gougoutas J.Z. Dihydropyrimidine calcium channel blockers. 4. Basic 3-substituted-4-aryl-1,4-dihydropyrimidine-5-carboxylic acid esters. Potent antihypertensive agents. J Med Chem. 1992;35:3254–3263. doi: 10.1021/jm00095a023. [DOI] [PubMed] [Google Scholar]
- 16.Yarim M., Sara S., Ertan M., Sultan F., Erol K. Synthesis, enantioseparation and pharmacological activity of 4-aryl-7,7-dimethyl-5-oxo-l,2,3,4,5,6,7,8-octahydroquinazoline-2-thiones. Arzneimittel-Forsch. 2002;52(1):27–33. doi: 10.1055/s-0031-1299852. [DOI] [PubMed] [Google Scholar]
- 17.Zorkun I.S., Saraç S., Çelebib S., Erol K. Synthesis of 4-aryl-3,4-dihydropyrimidin-2(1H)-thione derivatives as potential calcium channel blockers. Bioorg Med Chem. 2006;14(24):8582–8589. doi: 10.1016/j.bmc.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 18.Saraç S., Çiftçi M., Zorkun I.S., Tunç Ö., Erol K. Studies on the synthesis and biological activity of 6-ethyl-4-aryl-5-methoxycarbonyl-3,4-dihydropyrimidin-2(1H)-ones. Arzneimittel-Forsch. 2007;57(3):137–142. doi: 10.1055/s-0031-1296596. [DOI] [PubMed] [Google Scholar]
- 19.Sati B., Sati H., Nargund L.V.G., Khaidem S., Bhatt P.C., Saklani S. Synthesis of acetylated dihydropyrimidine analogues under solvent free conditions and their evaluation as calcium channel blockers. Orient J Chem. 2012;28(2):1055–1059. [Google Scholar]
- 20.Tale R.H., Rodge A.H., Hatnapure G.D., Keche A.P., Patil K.M., Pawar R.P. The synthesis, anti-inflammatory and antimicrobial activity evaluation of novel thioanalogs of 3,4-dihydrotyopyrimidin-2(1H)-one derivatives of N-aryl urea. Med Chem Res. 2012;21:4252–4260. [Google Scholar]
- 21.Chikhale R.V., Bhole R.P., Khedekar P.B., Bhusari K.P. Synthesis and pharmacological investigation of 3-(substituted 1-phenylthanone)-4-(substitudedphenyl)-1,2,3,4-tetrahydropyrimidine-5-carboxylates. Eur J Med Chem. 2009;44(9):3645–3653. doi: 10.1016/j.ejmech.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 22.Gijsen H.J.M., Berhelot D., Cleyn M.A.J.D., Geuens I., Bröne B., Mercken M. Tricyclic 3,4-dihydropyrimidine-2-thione derivatives as potent TRPA1 antagonists. Bioorg Med Chem Lett. 2012;22(2):797–800. doi: 10.1016/j.bmcl.2011.12.068. [DOI] [PubMed] [Google Scholar]
- 23.Kwon O.W., Moon E., Chari M.A., Kim T.W., Kim A.J., Lee P. A substituted 3,4-dihydropyrimidinone derivative (compound D22) prevents inflammation mediated neurotoxicity; role in microbial activation in BV-2 cells. Bioorg Med Chem Lett. 2012;22(16):5199–5203. doi: 10.1016/j.bmcl.2012.06.082. [DOI] [PubMed] [Google Scholar]
- 24.Gireesh T., Kamble R.R., Kattimani P.P., Dorababu A., Manikantha M., Hoskeri J.H. Synthesis of sydnone substituted Biginelli derivatives as hyaluronidase inhibitors. Arch Pharm Chem Life Sci. 2013;346(9):645–653. doi: 10.1002/ardp.201300118. [DOI] [PubMed] [Google Scholar]
- 25.Mokale S.N., Shinde S.S., Elgire R.D., Sangshetti J.N., Shinde D.B. Synthesis and anti-inflammatory activity of some 3-(4,6-disubtituted-2-thioxo-1,2,3,4-tetrahydropyrimindin-5-yl) propanoic acid derivatives. Bioorg Med Chem Lett. 2010;20:4424–4426. doi: 10.1016/j.bmcl.2010.06.058. [DOI] [PubMed] [Google Scholar]
- 26.Mishra K.M., Gupta A.K., Negi S. Anti-inflammatory activity of some new dihydropyrimidines derivatives. Int J Pharm Sci Res. 2010;1(8):92–95. [Google Scholar]
- 27.Tammi R., Ripellino J.A., Margolis R.U., Tammi M. Localization of epidermal hyaluronic acid using the hyaluronate binding region of cartilage proteoglycan as a specific probe. J Invest Dermatol. 1988;90(3):412–414. doi: 10.1111/1523-1747.ep12456530. [DOI] [PubMed] [Google Scholar]
- 28.Foschi D., Castoldi L., Radaelli E., Abelli P., Calderini G., Mariscotti C. Hyaluronic acid prevents oxygen free radical damage to granulation tissue: a study in rats. Int J Tissue React. 1990;12(6):333–339. [PubMed] [Google Scholar]
- 29.Donthabhakthuni S., Murugulla A.C., Murugulla P.C., Yeou K.S. Synthesis of 3,4-dihydropyrimidin-2-ones (DHPMs) using highly efficient recyclable silica supported rhodium chloride as heterogeneous catalyst and their anti-neuroinflammatory activity. Lett Drug Des Discov. 2012;9(10):962–966. [Google Scholar]
- 30.Trivedi A.R., Bhuva V.R., Dholariya B.H., Dodiya D.K., Kataria V.B., Shah V.H. Novel dihydropyrimidines as a potential new class of antitubercular agents. Bioorg Med Chem Lett. 2010;20(20):6100–6102. doi: 10.1016/j.bmcl.2010.08.046. [DOI] [PubMed] [Google Scholar]
- 31.Yadlapalli R.K., Chourasia O.P., Vemuri K., Sritharan M., Perali R.S. Synthesis and in vitro anticancer and antitubercular activity of diarylpyrazole ligated dihydropyrimidines possessing lipophilic carbamoyl group. Bioorg Med Chem Lett. 2012;22(8):2708–2711. doi: 10.1016/j.bmcl.2012.02.101. [DOI] [PubMed] [Google Scholar]
- 32.Raju B.C., Rao R.N., Suman P., Yogeeswari P., Sriram D., Shaik T.B. Synthesis, structure–activity relationship of novel substituted 4H-chromen-1,2,3,4-tetrahydropyrimidine-5 carboxylates as potential anti-mycobacterial and anticancer agents. Bioorg Med Chem Lett. 2011;21:2855–2859. doi: 10.1016/j.bmcl.2011.03.079. [DOI] [PubMed] [Google Scholar]
- 33.Chitra S., Devanathan D., Pandiarajan K. Synthesis and in vitro microbiological evaluation of novel 4-aryl-5-isopropoxycarbonyl-6-methyl-3,4-dihydropyrimidinones. Eur J Med Chem. 2010;45(1):367–371. doi: 10.1016/j.ejmech.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 34.Akhaja T.N., Raval J.P. 1,3-Dihydro-2H-indol-2-ones derivatives: design, synthesis, in vitro antibacterial, antifungal and antitubercular study. Eur J Med Chem. 2011;46(11):5573–5579. doi: 10.1016/j.ejmech.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 35.Kim J., Park C., Ok T., So W., Jo M., Seo M. Discovery of 3,4-dihydropyrimidin-2(1H)-ones with inhibitory activity against HIV-1 replication. Bioorg Med Chem Lett. 2012;22(5):2119–2124. doi: 10.1016/j.bmcl.2011.12.090. [DOI] [PubMed] [Google Scholar]
- 36.Kim J., Ok T., Park C., So W., Jo M., Kim Y. A novel 3,4-dihydropyrimidin-2(1H)-one: HIV-1 replication inhibitors with improved metabolic stability. Bioorg Med Chem Lett. 2012;22(7):2522–2526. doi: 10.1016/j.bmcl.2012.01.133. [DOI] [PubMed] [Google Scholar]
- 37.Zabihollahi R., Fassihi A., Aghasadeghi M.R., Memarian H.R., Soleimani M., Majidzadeh-A K. Inhibitory effect and structure-activity relationship of some Biginelli-type pyrimidines against HSV-1. Med Chem Res. 2013;22(3):1270–1276. [Google Scholar]
- 38.Oren I., Paul M. Up to date epidemiology, diagnosis and management of invasive fungal infections. Clin Microbiol Infec. 2014;20(S6):1–4. doi: 10.1111/1469-0691.12642. [DOI] [PubMed] [Google Scholar]
- 39.Sharma P., Rane N., Gurram V.K. Synthesis and QSAR studies of pyrimido[4,5-d]pyrimidine-2,5-dione derivatives as potential antimicrobial agents. Bioorg Med Chem Lett. 2004;14:4185–4190. doi: 10.1016/j.bmcl.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 40.Rajanarendar E., Reddy M.N., Murthy K.R., Reddy K.G., Raju S., Srinivas M. Synthesis, antimicrobial, and mosquito larvicidal activity of 1-aryl-4-methyl-3,6-bis-(5-methylisoxazol-3-yl)-2-thioxo-2,3,6,10b-tetrahydro-1H-pyrimido[5,4-c]quinolin-5-ones. Bioorg Med Chem Lett. 2010;20(20):6052–6055. doi: 10.1016/j.bmcl.2010.08.060. [DOI] [PubMed] [Google Scholar]
- 41.Beena K.P., Akelesh T. Synthesis and screening of some dihydropyrimidine derivatives as antimicrobial agents. Int Res J Pharm. 2012;3(9):303–304. [Google Scholar]
- 42.Stefani H.A., Oliveira C.B., Almeida R.B., Pereira C.M.P., Braga R.C., Cella R. Dihydropyrimidin-(2H)-ones obtained by ultrasound irradiation: a new class of potential antioxidant agents. Eur J Med Chem. 2006;41(4):513–518. doi: 10.1016/j.ejmech.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 43.Ismaili L., Nadaradjane A., Nicod L., Guyon C., Xicluna A., Robert J.F. Synthesis and antioxidant activity evaluation of new hexahydropyrimido[5,4-c]quinoline-2,5-diones and 2-thioxohexahydropyrimido[5,4-c]quinoline-5-ones obtained by Biginelli reaction in two steps. Eur J Med Chem. 2008;43(6):1270–1275. doi: 10.1016/j.ejmech.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 44.Vasconcelos A., Oliveira P.S., Ritter M., Freitag R.A., Romano R.L., Quina F.H. Antioxidant capacity and environmentally friendly synthesis of dihydropyrimidin-(2H)-ones promoted by naturally occurring organic acids. J Biochem Mol Toxicol. 2012;26(4):155–161. doi: 10.1002/jbt.20424. [DOI] [PubMed] [Google Scholar]
- 45.Mansouri M., Movahedian A., Rostami M., Fassihi A. Synthesis and antioxidant evaluation of 4-(furan-2-yl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate esters. Res Pharm Sci. 2012;7(4):257–264. [PMC free article] [PubMed] [Google Scholar]
- 46.Gangwar N., Kasana V.K. 3,4-Dihydropyrimidin-2(1H)-one derivatives: organocatalysed microwave assisted synthesis and evaluation of their antioxidant activity. Med Chem Res. 2012;21:4506–4511. [Google Scholar]