Graphical abstract

Keywords: Formazans, Biological activity, Tetrazolium salts, azo-hydrazones, Heterocycles
Abstract
This review provides an up to date information about the diverse pharmaceutical activities of formazans. The bibliography includes 97 references which have been published during the period from 1980 to 2013. The covered biological activities of the title compounds include antioxidant, anticonvulsant, therapeutic, anthelmintic, anti-tubercular, antiviral, anti-inflammatory, anticancer, anti-HIV, antimicrobial, antiparkinsonian, cardiovascular and antiproliferative activities.
Introduction
Formazans, of the general formula 1 (Chart 1), are an important and distinct class of organic compounds. Their chemistry has attracted the interest of many research groups due to their wide biological and industrial applications as well as their utility in analytical chemistry and synthesis of heterocyclic compounds. At present, there are several review articles and books devoted to the synthesis, physical properties and chemical reactions of formazans [1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16].
Chart 1.
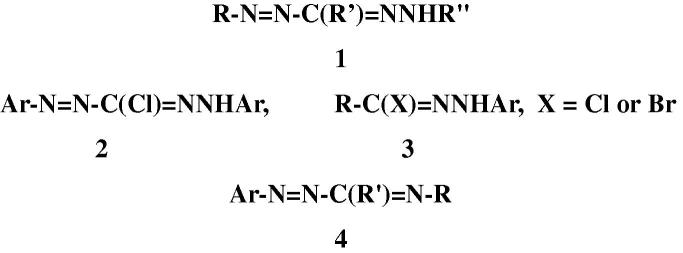
In contrast to these comprehensive literature reports on the chemistry of formazans, there has been no survey of their biological activities hitherto although they have been found to possess wide spectrum of biological activities such as antiviral, antimicrobial, anti-inflammatory, antifungal, anticancer, anti HIV. Based on these findings and in continuation of our studies of the chemistry of both 3-chloro-1, 5-diarylformazans 2 [17], [18], [19], [20], [21] and the related hydrazonoyl halides 3 [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35] (Chart 1), it was thought necessary to present this review. Our objective is to shed light on the recent developments in biological activities of various functionalized formazans 1 (Chart 1). Only recent reports that have been published during the period from 1980 to 2013 are covered. Compounds of type 4 (Chart 1), which were erroneously named, in some articles [36], [37], [38], [39], [40] as formazans, will not be included in this review as they are amidrazone derivatives and not formazans.
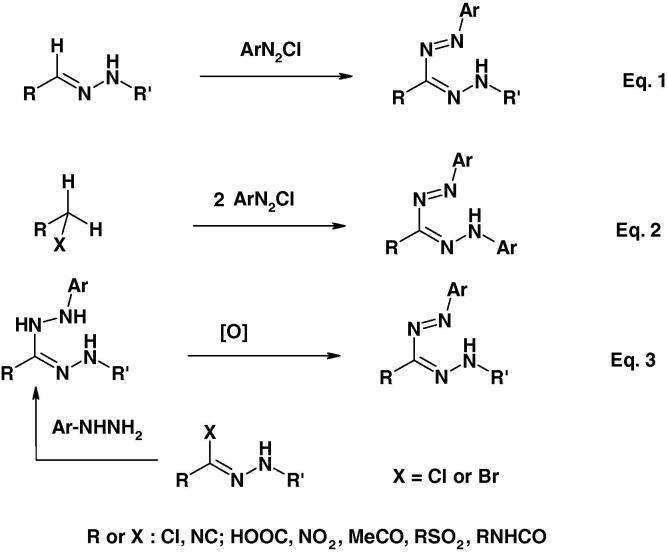
At present there are three synthetic strategies for the synthesis of formazans (Scheme 1) [2], [3]. The first one involves coupling of aldehyde hydrazones with diazonium salts (Eq. (1)). This strategy has the advantage of being able to synthesize symmetrical and asymmetrical formazans. The second strategy depends on coupling of active methylene compounds each with two molar equivalents of diazonium salts (Eq. (2)). This method proved useful for the synthesis of only symmetrical formazans. The third method involves oxidation of the corresponding hydrazidines (Eq. (3)) are usually prepared via reaction of hydrazonoyl halides with the appropriate hydrazine derivatives. Most of the formazans, whose biological activities are covered in this review, were prepared by the first method.
Scheme 1.
The general structural formulas of the various formazans covered in this review together with their biological activities are indicated in Table 1.
Table 1.
The general structural formulas of the various formazans covered in this review together with their biological activities which have been evaluated.
| No. | General structure | Their screened biological activities |
|---|---|---|
| I | Ar—C( NNHAr′)—N N—Ar″ | Anti-oxidant, anticonvulsant, antiviral. anti-inflammatory Antimicrobial, antiparkinsonian, analgesic |
| II | Ar—C( NNHAr′)—N N—Het | Anticonvulsant, antiviral. antimicrobial, antiparkinsonian, antiproliferative |
| III | Het—C( NNHAr′)—N N—Ar″ | Antiviral |
| IV | Ar—C( NNH—CO—Ar′)—N N—Ar″ | Anthelmintic, anti-inflammatory, anticancer, anti-HIV, antimicrobial, analgesic |
| V | Ar—C( NNH—CO—Het)—N N—Ar″ | Anti-oxidant, Antitubercular, Antimicrobial |
| VI | Het—C( NNH—CO—Ar′)—N N—Ar″ | Antiviral, anti-inflammatory |
| VII | Het—C( NNH—CO—Ar′)—N N—Het′ | Antimicrobial |
| VIII | Ar—C( NNH—R)—N N—Ar″ | Anti-inflammatory antimicrobial, antiparkinsonian, cardiovascular |
| IX | Ar—C( NN—Het)—N N—Ar″ | Antiviral, anti-inflammatory, antimicrobial |
Pharmacological activities
A survey of literature reveals that many formazans have been reported to possess wide spectrum of biological activities. In the following, a complete coverage of the recently reported pharmacological applications of various formazan derivatives is presented. In such a coverage, the formazans, that have been screened for some biological activities and found to be inactive, are also pointed out.
Markers of cell vitality and antioxidant activity
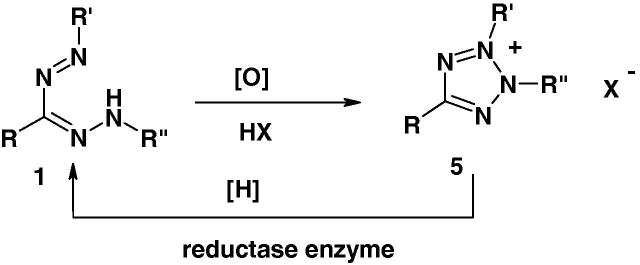
Formazans are colored compounds because of the π–π* and n–π* electronic transitions of the azo-hydrazone chromophore. Oxidation of such compounds results in their conversion into colorless tetrazolium salts 5 (Scheme 2) [41]. Among the various oxidants used for the oxidation of formazans 1 to the tetrazolium salts 5 are mercuric oxide, nitric acid, isoamyl nitrite, N-bromo succinimide, potassium permanganate, lead tetraacetate and t-butyl hypochlorite. When a tetrazolium salt is given to a living organism, it is reduced back to the corresponding colored formazan by the reductase enzyme in the living cells and stains tissues depending upon the viability of the organism. Accordingly, tetrazolium–formazan systems are classified as markers of vitality [42], [43].
Scheme 2.
Formazans are used for screening of anticancer drugs, determination of activity of tumor cell and sperm viability [42], [43], [44], [45], [46], [47]. They are also used in Brucella-ring test in milk [48] and to investigate dehydrogenase activity inhibition of a soil bacterium caused by soil contaminated with Cu, Pb and As [49].
At present, the reduction of tetrazolium salts to formazans is widely exploited as indicator of reducing systems with applications in chemistry and biology [36], [50]. For example, it is applied to natural products drug discovery for Leishmania [51], the assay of lactate dehydrogenase [52] and as indicator of viability in respiring bacteria [53]. Also, they have been used in developing method of yeast cells permeabilization in succinate dehydrogenase activity assay in situ which can be used for different industrial Saccharomyces cerevisiae strains [54].
Recently, a rapid method was reported for detection of reduction of 2-hydroxy ketones. This method is based on enzymatic reduction of colorless 2,3,5-triphenyltetrazolium chloride to a red colored 1,3,5-triphenylformazan via ω-transaminases [55]. The enzymatic reductive amination of a wide range of various aliphatic, aliphatic–aromatic and aromatic–aromatic 2-hydroxy ketones was determined spectrophotometrically at λ 510 nm by the decrease in the red coloration due to substrate consumption.
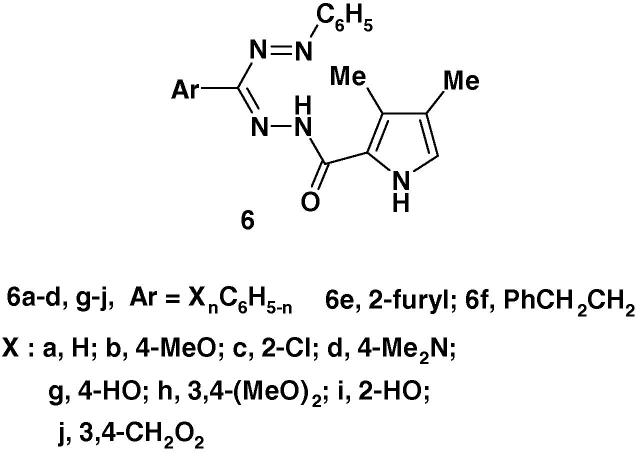
Also, the evaluation of the antioxidant effect of formazans 6a–j (Chart 2) was recently reported [56]. In that study, the formazans, as antioxidants, each reacts with the stable free radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) and converts it to 2,2-diphenyl-1-picrylhydrazine. The degree of decoloration indicates the scavenging potential of the compound. The results showed that compound 6g, while it shows good activity comparable with the standard compound namely ascorbic acid, the other compounds exhibited moderate to good activities.
Chart 2.
Anticonvulsant activity
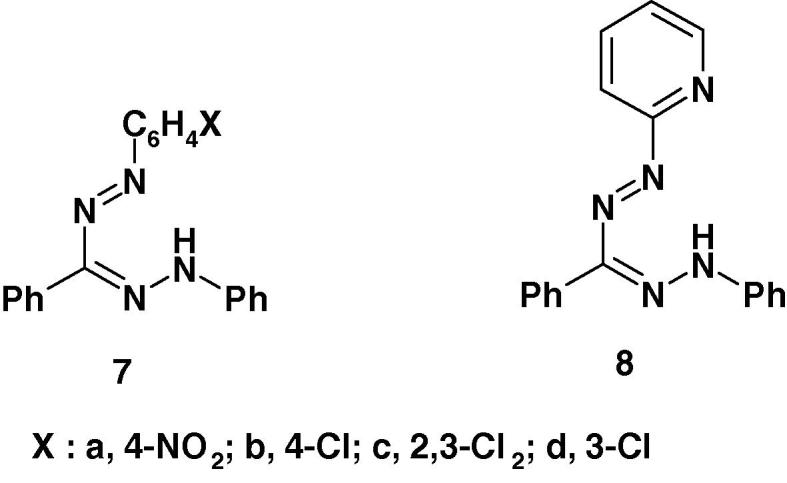
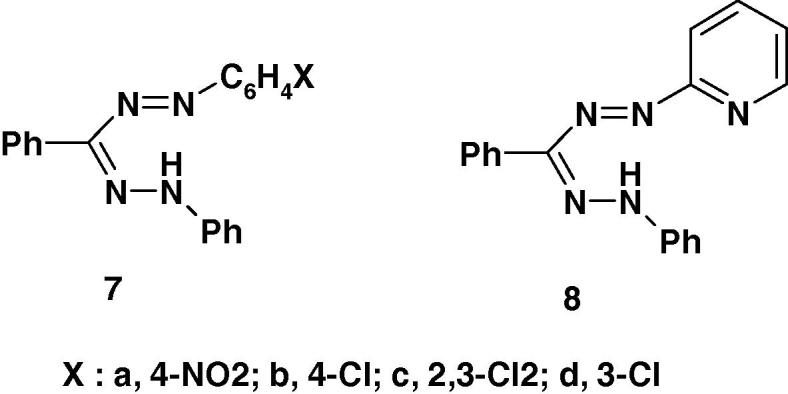
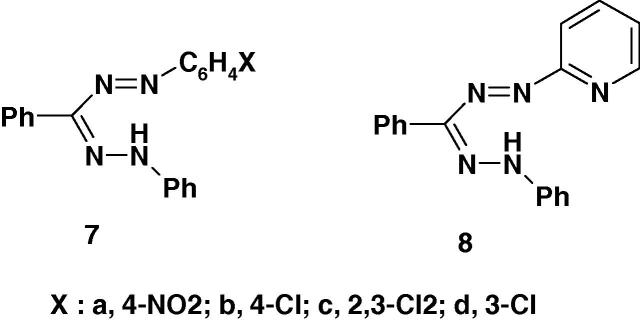
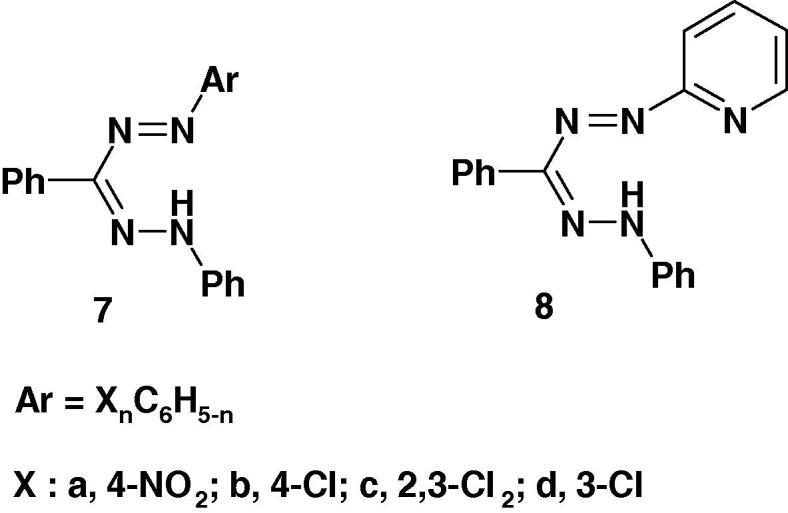
Several formazans were reported to show promising anticonvulsant and therapeutic activities [57], [58]. For example, Mariappan et al. [58] reported the results of screening of the formazan derivatives 7a–d and 8 (Chart 3a) for their anticonvulsant activity by maximum electroshock induced seizures (MES) method using diazepam, as a standard. Compound 8 was the most active among the studied compounds. The other compounds 7a–d were found to be moderate or less active. None of the studied compounds showed neurotoxicity [58]. From the SAR point of view, the formazan 8 derived from 2-aminopyridine displayed profound anticonvulsant activity.
Chart 3a.
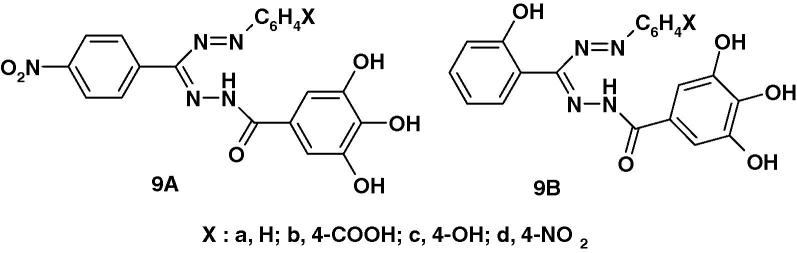
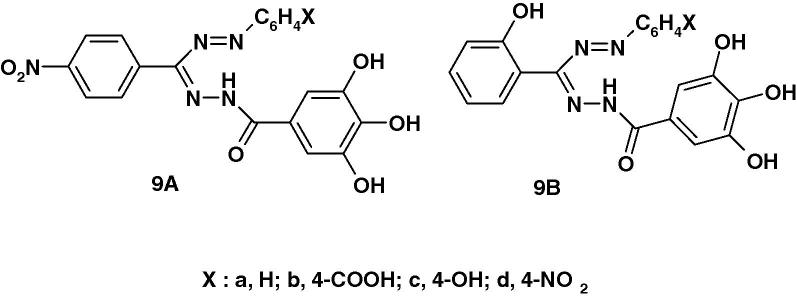
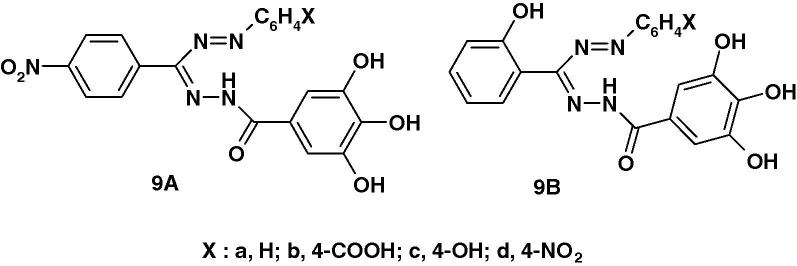
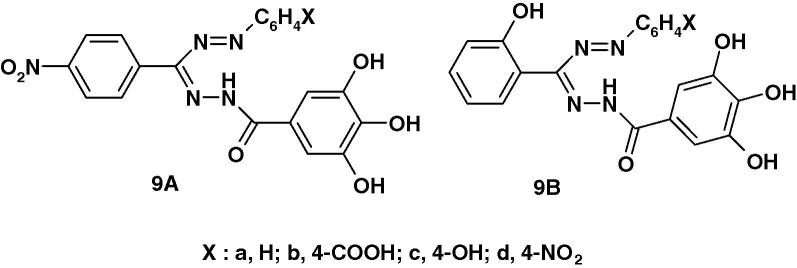
Very recently, Kumara Prasad et al. [59] reported the synthesis of two series of formazans 9A and 9B (Chart 4a) derived from gallic acid and screened them for their anticonvulsant activity by maximum electroshock method (MES). The reported results indicate that compounds 9Ab and 9Ad exhibited 55.04% and 58.73% protection as compared with the standard phenytoin. The other compounds were found less active.
Chart 4a.
Anthelmintic activity
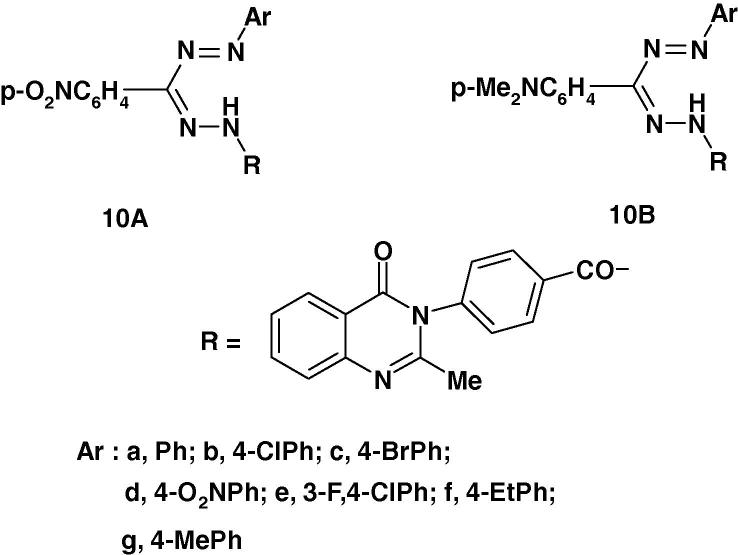
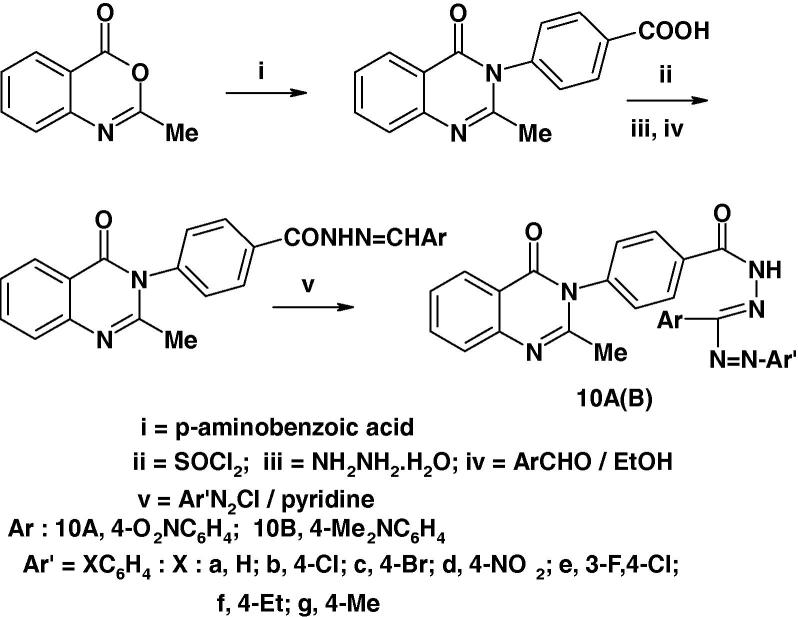
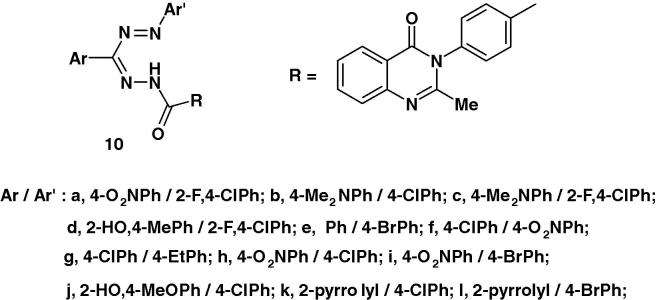
Nadendla et al. [60] prepared a series of formazans of type 10 (Chart 5) and screened their anthelmintic activity using the earthworms Pheretima posthuma and albendazole. Piperazine citrate was used as reference standard. The results of such screening revealed that compounds 10a–d possess significant anthelmintic activity. The other compounds exhibited moderate activity.
Chart 5.
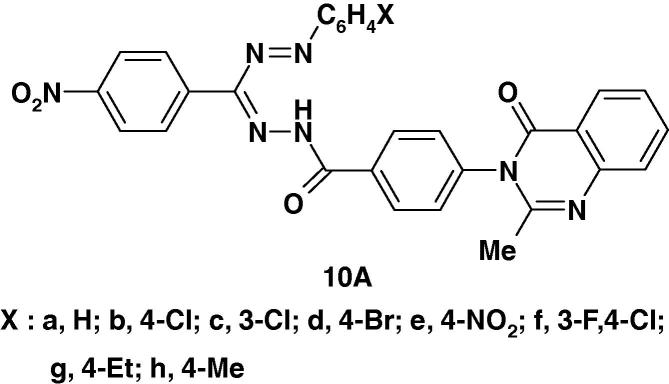
In a recent paper, Babu and Nadendla [36] reported the synthesis of two other series of formazans namely 10A, B (Chart 6a) and testing their anthelmintic activity on earth worms using piperazine citrate as standard drug. The reported results revealed that compounds 10Aa, 10Ab, 10Ad and 10Ae exhibited equipotent activity compared with the standard drug while compounds 10Ac and 10Bf exhibited moderate activity.
Chart 6a.
Antitubercular activity
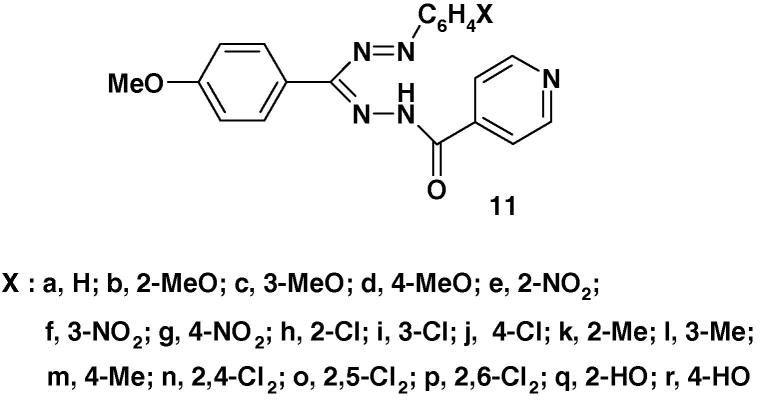
1-Isonicotinoyl-3-(4-methoxyphenyl)-5-(substituted phenyl)-formazans (11a–r) (Chart 7) were tested for their in vitro anti-tubercular activity against Mycobacterium tuberculosis H37Rv using the BACTEC 460 radiometric system. The results of such a study indicated that compounds 11n and 11p showed the highest antitubercular activity and exhibited >90% inhibition at lower concentration [61].
Chart 7.
Antiviral activity
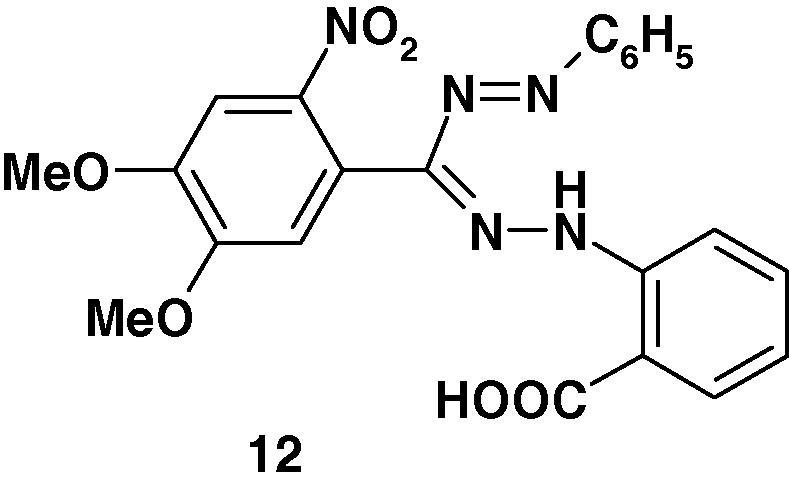
Some formazans possess antiviral activity [62], [63]. For example, 1-(o-Carboxyphenyl)-3-(3′.4′-dimethoxy-6′-nitrophenyl)-5-phenylformazan 12 (Chart 8), which was prepared by coupling of benzenediazonium chloride with the corresponding aldehyde N-(2-carboxyphenyl)hydrazone, was reported to exhibit 100% protection against the Ranikhet disease virus [62].
Chart 8.
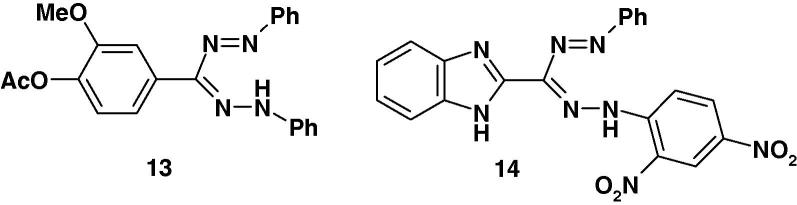
Misra and Dhar [63] synthesized the two formazan derivatives 13 and 14 (Chart 9) by coupling of the corresponding hydrazones each with benzene diazonium salt and screened them for their antiviral activity against vaccinia virus and Ranikhet disease virus in a stationary culture of chorioallantoic membrane of chick embryo. The results revealed that the two compounds were found to exhibit significant activity 87% and 83%, respectively against the Ranikhet disease virus. However no activity against vaccinia virus could be observed [63].
Chart 9.
Also, Mukerjee et al. [64] prepared the formazan derivatives 15a–o (Chart 10) by coupling of 3-nitro-anisaldehyde N-4-nitrophenylhydrazone with the appropriate aryldiazonium salts and evaluated their antiviral activity against Ranikhet disease virus (RDV) in chick embryos (WLH). The results of the study of structure activity relationship (SAR) revealed that structure variations in the aryl moiety have a high effect on their activity. For example, compound 15c with p-bromo substituent showed 50% protection against RDV as compared with compound 15a with the unsubstituted ring, whereas 4-nitro derivative 15e was found to be inactive. Also, introduction of carboxyl group in 2- or 4-position as in formazans 15f and 15h, respectively enhances the antiviral activity where the corresponding formazan esters 15i–l exhibit no marked antiviral activity.
Chart 10.
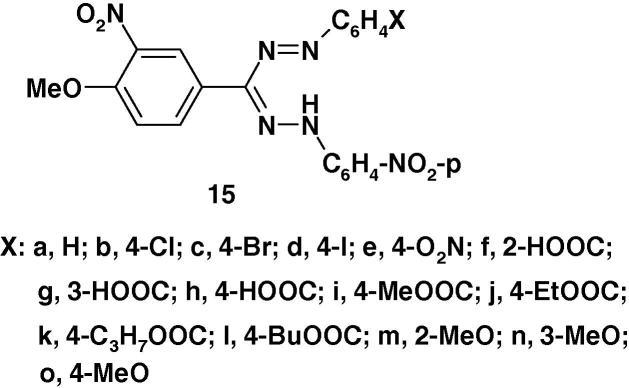
Tiwari et al. [65] screened the formazan derivatives 16 (Chart 11) for their antiviral activity against Ranikhet disease and vaccinia viruses. The results indicated that some of such formazans showed promising activity against one or both viruses in vitro [65].
Chart 11.
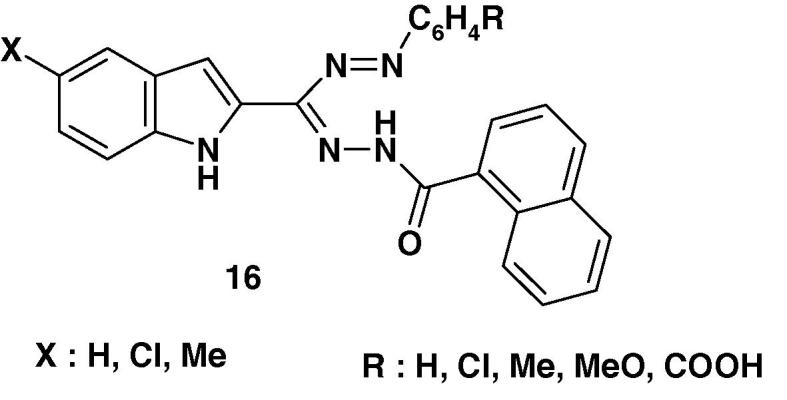
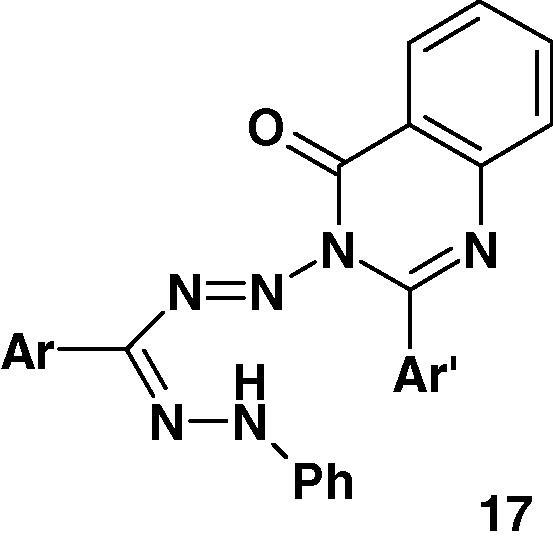
In addition, Pandy and Negi [66] reported the synthesis of a series of 1-(2′-aryl-4′-oxo(3H)quinazolyl)-3-aryl-5-phenyl-formazans 17 (Chart 12) and indicated that they exhibit mild activity against Vaccinia virus.
Chart 12.
Recently, the formazan derivatives 7 and 8 (Chart 3b) were tested for their antiviral activity against Japanese encephalitis virus [58]. The results showed that none of them was found effective.
Chart 3b.
Anti-inflammatory activity
Several formazans have been found to exhibit anti-inflammatory activity [67], [68], [69]. For example, in a recent article, Babu and Naderndia [70] reported the synthesis of two series of formazans 10A and 10B (Scheme 3) and the results of their anti-inflammatory screening. The results revealed that the compounds 10Ad, 10Ae, 10Ae and 10Bf afforded 67% protection against carrageenan induced edema equally with the standard drug diclofenac sodium under similar conditions while compounds 10Ab, 10Ac, 10Af, 10Ag, 10Bd and 10Bg exhibited 33% protection.
Scheme 3.
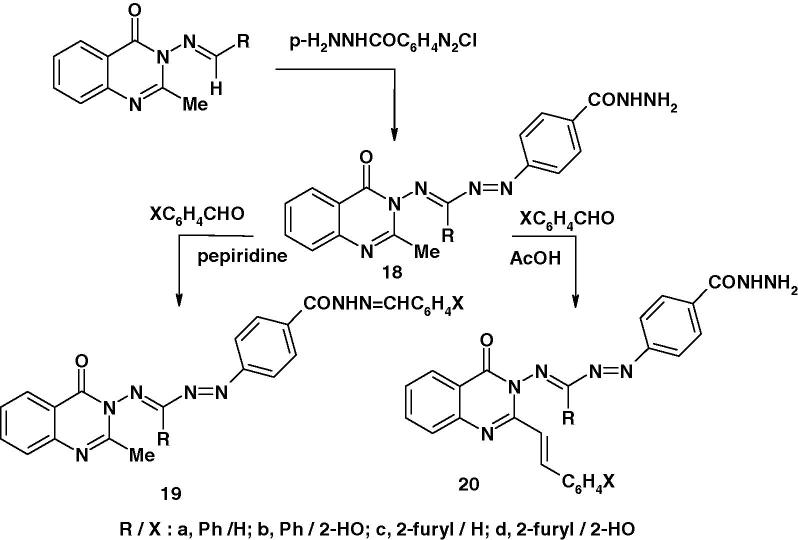
The substituted quinazolonoformazans 18–20 were also synthesized as depicted in Scheme 4 and were screened for their anti-inflammatory activity [68]. The results indicated that all compounds except 20a, possessed anti-inflammatory activity. The degree of protection provided by these compounds (100 mg/kg, po) against carrageenan-induced edema in rat paw ranged from 26% to 57%. Such protection was comparable to that of 51% observed with phenylbutazone (100 mg/kg, po) which was used as a standard reference drug. Regarding the structure–activity relationship, the results showed that compounds 19 possessed higher anti-inflammatory activity than that of 20 with the exception of 20d. The authors [68], in an attempt to gain some idea of their possible analgesic effectiveness, they screened also compounds 19a, b and 20b, d for their antiwrithmogenic activity. The results showed that three of such compounds namely 19a, b and 20b showed 10–50% protection against aconitine-induced writhing response in mice, whereas compound 20d exhibited 80% protection similar to that of acetylsalicylic acid.
Scheme 4.
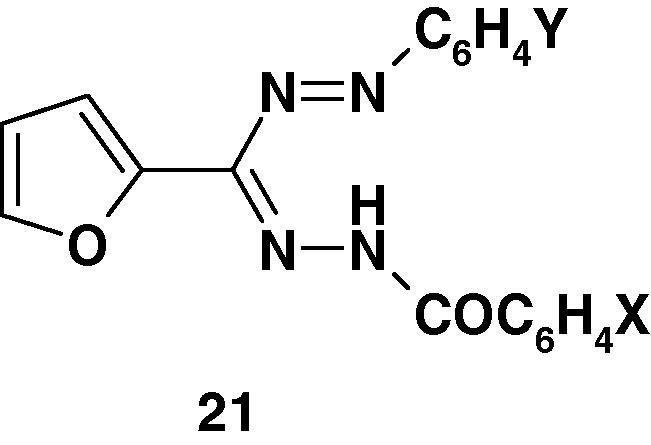
Furthermore, Kumar et al. [71] synthesized the formazans 21 (Chart 13) and tested them for their anti-inflammatory activity in albino rats. The results indicated that they exhibit only moderate activity.
Chart 13.
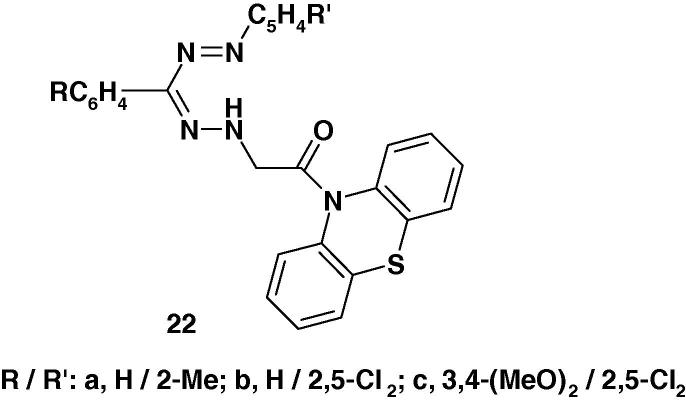
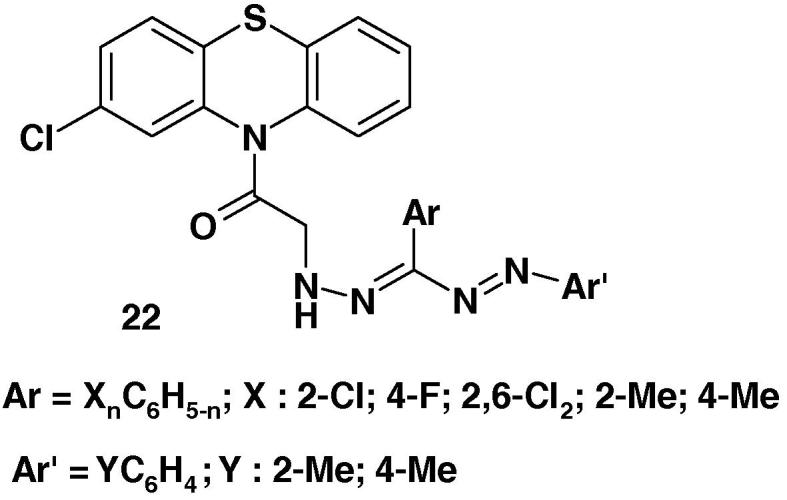
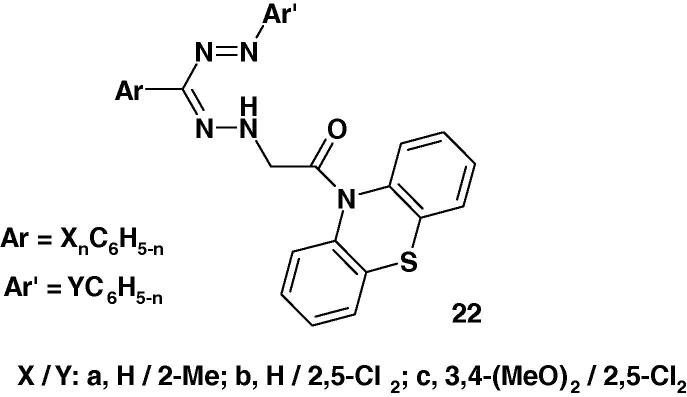
Formazans 22 (Chart 14) were synthesized by Kumar et al. [72] and their anti-inflammatory activity was screened against carrageenan-induced edema in albino rats. On the basis of the results of such screening, the authors concluded that such formazans exhibit promising anti-inflammatory activity.
Chart 14.
Very recently, Kumara Prasad et al. [59] reported the results of screening of compounds 9A and 9B (Chart 4b) for their anti-inflammatory activity by carrageenan induced rat paw edema inhibition method. The reported results indicate that compounds 9Ac and 9Bc are the most active among the tested compounds. The other compounds were found less active.
Chart 4b.
Anticancer and anti-HIV activities
Some formazans were tested for their anticancer and anti-HIV activities [73], [74], [75]. The results showed that most of the tested formazans were inactive.
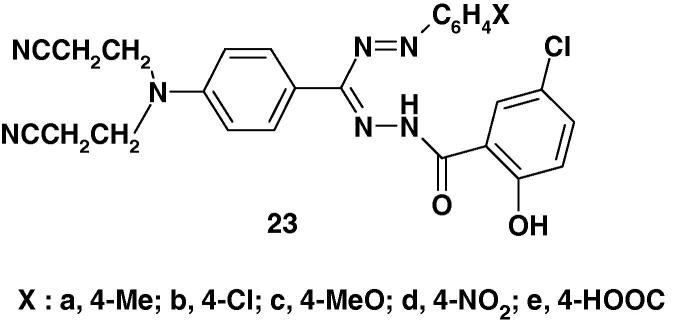
Bhardwaj and Jolly [76] synthesized the formazan derivatives 23 (Chart 15) and evaluated them for anticancer and ant-HIV activities. The results revealed that the studied compounds did not show any significant anticancer or anti-HIV activity [76].
Chart 15.
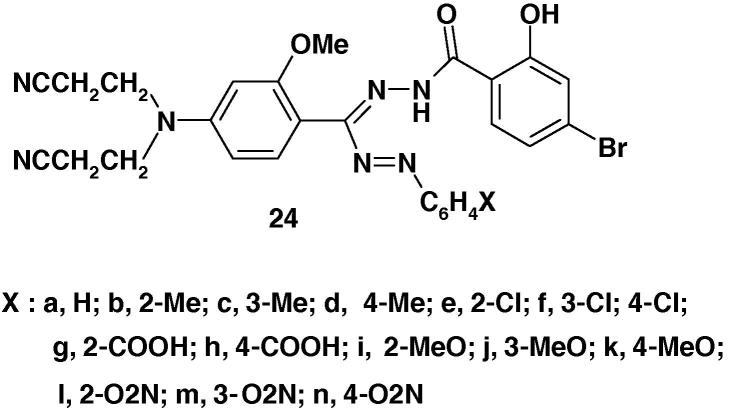
In another report, Bhardwaj [77] prepared also a similar series of formazans 24 (Chart 16) and the results of testing them for anticancer activity. The results revealed that none of the compounds was found active at the dose level tested. Also, the compounds did not show significant anti-HIV activity.
Chart 16.
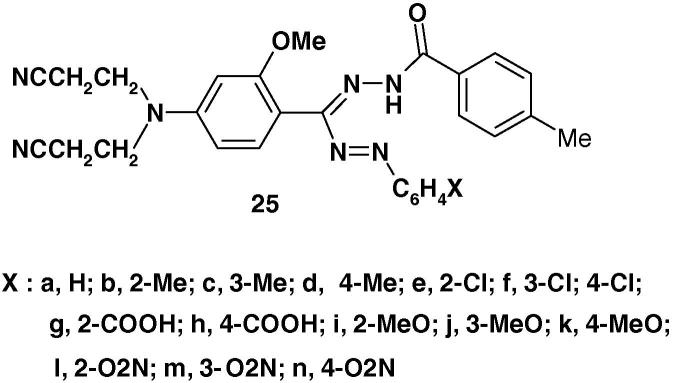
Also, the formazans 25 (Chart 17) were prepared and tested for anticancer and anti-HIV activities [78]. However, none of such compounds was found active at the dose level tested.
Chart 17.
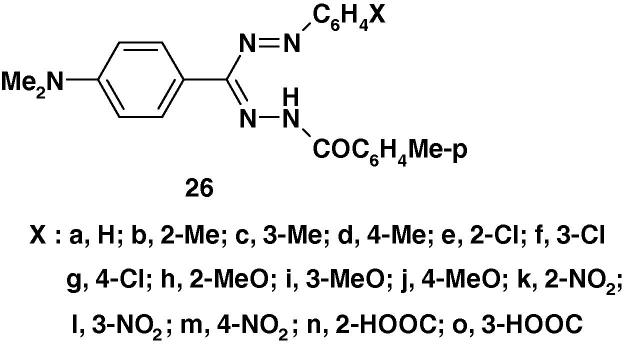
Furthermore, the formazans 26a–o (Chart 18) were prepared and screened for their anti-cancer and anti-HIV activities [79]. The results of such a study revealed that the compounds 26b, d, e, f, i, j, k, l, o did not show any significant anticancer activity or anti-HIV activity.
Chart 18.
Antimicrobial activities (Antibacterial and antifungal activities)
Several formazan derivatives have shown a broad spectrum of activity against pathogens [49], [69], [73], [80], [81]. Raval et al. [82] synthesized the formazans 27a–m (Chart 19a) and screened them for their antibacterial activity against representative Gram-positive organisms viz. Bacillus subtilis (MTCC 121), Micrococcus luteus (MTCC 106), Bacillus sphaericus (MTCC 11) Staphylococcus aureus (MTCC 96) and Gram-negative organisms viz. Chromobacterium violaceum (MTCC 2656), Klebsiella aerogenes (MTCC 39), Pseudomonas aeruginosa (NTCC 791), Escherichia coli (MTCC 443), Klebsiella pneumoniae (MTCC 109), Salmonella paratyphi A(MTCC 735) using standard antibacterial agents like penicillin and streptomycin under identical conditions for comparison [82]. The results showed that the tested compounds 27a, d, h, k possessed the highest degree of inhibition against all tested Gram + ve organisms, while compounds 27c and 27h showed highest degree of inhibition against all tested Gram –ve organisms. Compounds 27c, d, h, k showed highest degree of inhibition against Escherichia coli, Klebsiella pneumoniae, Salmonella paratyphi A, while compound 27k showed highest inhibition against Chromobacterium violaceum, Klebsiella aerogenes, Pseudomonas aeruginosa, Escherichia coli respectively [82].
Chart 19a.
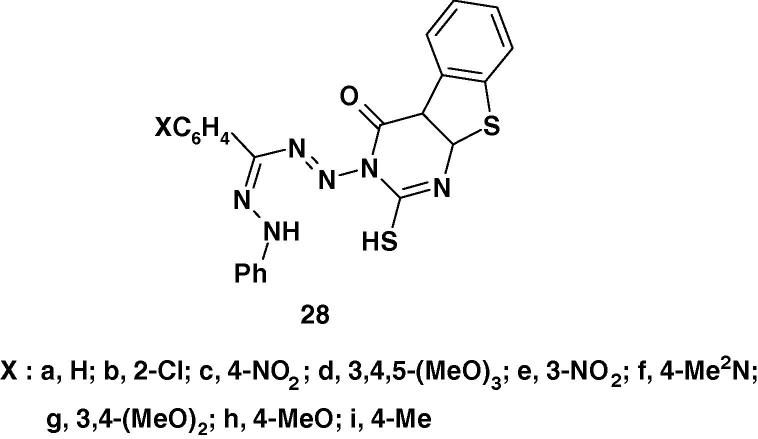
Lakshmi et al. [83] prepared the formazans 28a–i (Chart 20) and evaluated their antimicrobial activity by the cup–plate agar diffusion method. All the synthesized compounds were tested in vitro for their antibacterial activity against various microbes such as Bacillus subtilis NCIM 2063, Escherichia coli NCIM 2118, Pseudomonas aeruginosa NCIM 2036 and antifungal activity against Candida albicans NCIM 3102. Ciprofloxacin (10 μg/disk) and Fluconazole (2 μg/disk) were used as standard. The results showed that compounds 28a, c, g, h, i were active against Bacillus subtilis and 28b, d, e, h, i showed significant activity against Escherichia coli. Compounds 28a, c, d, e, g, h, i showed activity against Pseudomonas aeruginosa. The compounds 28a, b, c, f, g, h, i were found active against Candida albicans. The other compounds were resistant to the selected microbial strains [83].
Chart 20.
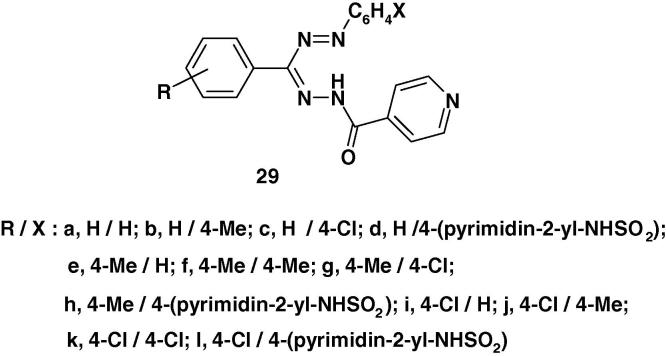
In another report Revanasiddappa and Subrahmanyam [84] synthesized the formazans 29a–l (Chart 21) by coupling the corresponding hydrazones with the appropriate diazonium salts and assayed them in vitro for antibacterial activity against S. aureus, P. aeruginosa, E. coli and B. subtilis and antifungal activity against C. albicans and A. ringer by the cup–plate method using DMF as solvent. The activity was compared with the known antibiotics namely Streptomycin and Griseofulvin under the same conditions. The results of the antibacterial studies indicated that the compounds 29b, e, g, i possess good activity against both the Gram positive and gram negative pathogenic organisms [84]. The rest of the compounds showed moderate activity against all the four organisms. In the antifungal activity, the compounds 29b and 29h showed highest activity against both the fungal organisms. The other compounds showed moderate activity [84].
Chart 21.
Recently, Mariappan et al. [58] screened the formazan derivatives 7a–d and 8 (Chart 3c) for antibacterial activity. All the compounds were reported to show remarkable antibacterial activity against Gram positive organism S. aureus except 7d. From the SAR point of view, it is interesting to observe that the formazan derived from hetero aniline 8 displayed profound antibacterial activities. The order of activity of 8 > 7c > 7a > 7b. Also, all the compounds showed remarkable antibacterial against Gram negative bacteria Vibrio cholerae. However, the antibacterial activity of the studied compounds was lower than that of streptomycin at 250 μg/ml [58].
Chart 3c.
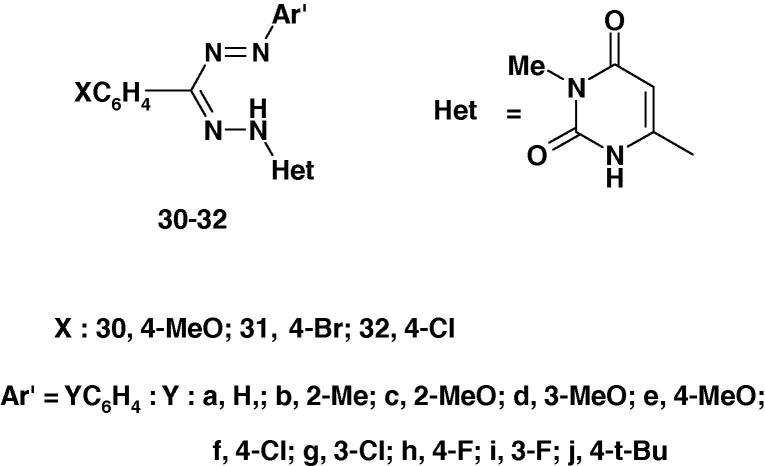
Three other series of uracil formazans 30–32 (Chart 22) were synthesized by coupling of the corresponding hydrazone derivatives with diazonium salts of various substituted aromatic amines and were screened for their antimicrobial activities against E. coli and S. Aureus and antifungal activity against S. Cerevisiae and C. albicans at a concentration of 60 μg/mL in DMF by cup–plate method [75]. Standard anti-bacterial and anti-fungal drug, gentamycin and miconazole, respectively were also used under similar conditions for comparison. The results revealed that compounds 30c and 31g are good antibacterial agents against E. coli whereas compounds 31b, 32a and 32b exhibit better activity against S. aureus.
Chart 22.
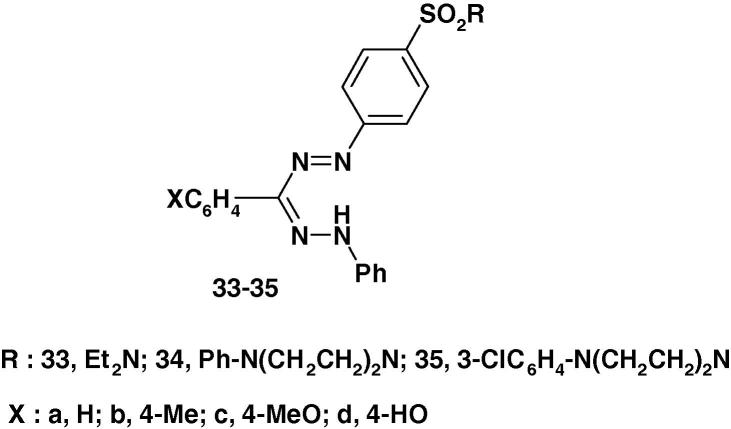
The formazans 33–35 (Chart 23) have been prepared by Vashi and Sheth [85] and were screened for antimicrobial activity against Gram positive (B. subtilis and S. aureus) and Gram negative (E. coli and P. aeruginosa) organisms using DMF as solvent at 50 mg/mL concentration at 37 °C. The activity was compared with the standard drugs viz, ampicillin, tetracycline, gentamycin and chloramphenicol. The results of such screening revealed that compounds 34a–d, 35a–c, 34a–d and 35b–d exhibited good activity against the tested Gram negative and Gram positive bacteria, whereas compounds 33a–d showed moderate to mild activity against all four bacteria as compared to standard drugs.
Chart 23.
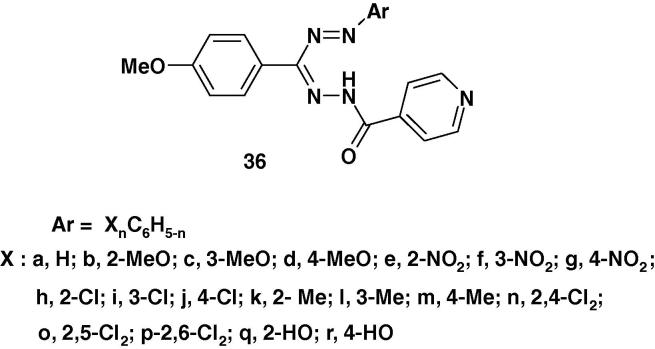
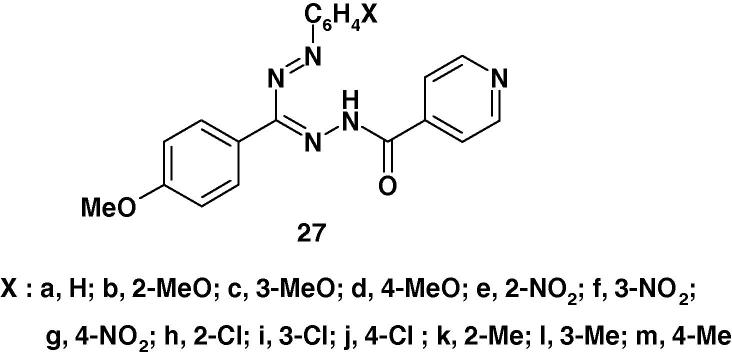
Raval et al. [82] reported the synthesis of a series of 3-(4-methoxyphenyl)-1-isonicotinoyl-5-(substituted phenyl)formazans (36a–r) (Chart 24) and their antimyco-bacterial activity in vitro against M. tuberculosis H37Rv using the BACTEC 460 radiometric system. The results showed that 3-(4-methoxyphenyl)-5-(2,4-dichlorophenyl)-1-isonicotinoylformazan (36n) and 3-(4-methoxyphenyl)-5-(2,6-dichlorophenyl)-1-isonicotinoyl-formazan (36p) produced highest efficacy and exhibited >90% inhibition at a concentration of 0.0179 μM and 0.0149 μM whereas 3-(4-methoxyphenyl)-5-(2-methoxyphenyl)-1-isonicotinoylformazan (36b), 3-(4-methoxyphenyl)-5-(4-methoxyphenyl)-1-isonicotinoylformazan (36d) and 3-(4-methoxyphenyl)-5-(2,5-dichlorophenyl)-1-isonicotinoylformazan (36o) showed moderate inhibitory activity with 0.0277 μM, 0.0203 μM, 0.0292 μM respectively. This finding was taken as evidence that the 2,4-dichloro and 2,6-dichloro groups substitution derivatives displayed relatively higher inhibitory activity in general. However, the electron rich groups such as, 2,4-dichloro, 2,5-dichloro, 2,6-dichloro, 2-methoxy, 3-methoxy and 4-methoxy substituted analogs produced significant increase in inhibitory activity against M. tuberculosis H37Rv [82]. On the other hand, analogs with methyl group substitution (36k–m) and phenyl substitution (36a) were reported to show relatively low inhibitory activity against M. tuberculosis H37Rv. Instead (OH) group, (CH3) group and (NO2) group substitution at phenyl ring in formazan analogs worsens the anti-mycobacterial activity in comparison with (Cl) group analogs.
Chart 24.
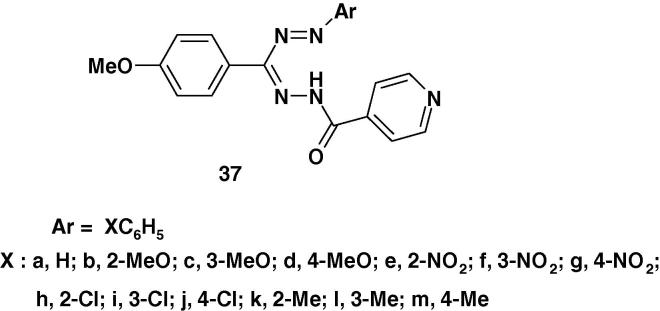
In another report, Raval et al. [61] indicated that the results of screening the antimicrobial activities of formazans 37a–m (Chart 19b) revealed that compounds 37a, d, h and 37k exhibited the highest degree of inhibition against all tested Gram +ve organisms, while compounds 37c, h showed highest degree of inhibition against all tested Gram −ve organisms compared to standard antibiotic drugs. Compounds 37c, d, h and 37k showed highest degree of inhibition against Escherichia coli, Klebsiella pneumoniae, Salmonella paratyphi A, while compound 37k showed highest inhibition against Chromobacterium violaceum, Klebsiella aerogenes, Pseudomonas aeruginosa, Escherichia coli, respectively [61].
Chart 19b.
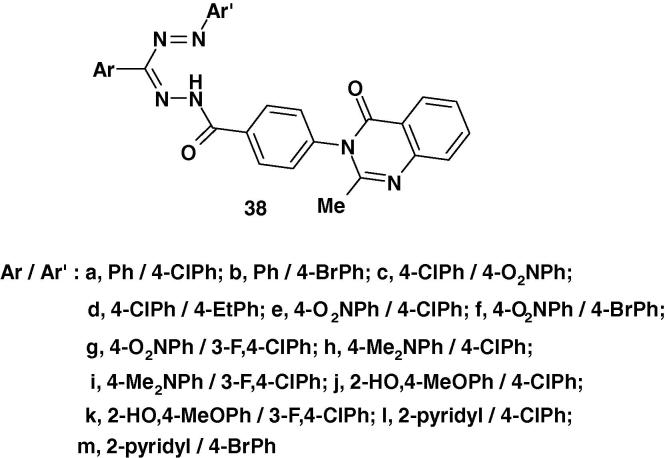
Nadendla et al. [60] synthesized a series of quinazolinone formazans 38a–m (Chart 25) and screened them for antibacterial activity against six bacterial strains namely Bacillus subtilis, Bacillus cereus, Escherichia coli, Staphylococcus aureus, Staphylococcus epidermidis, Pseudomonas aeruginosa and antifungal activity against four fungal strains: Candida albicans, Candida glabrata, Aspergillus niger and Saccharomyces cerevisiae by paper diffusion method. Of the compounds studied only compounds 38f, g, h and 38k showed moderate to good inhibition.
Chart 25.
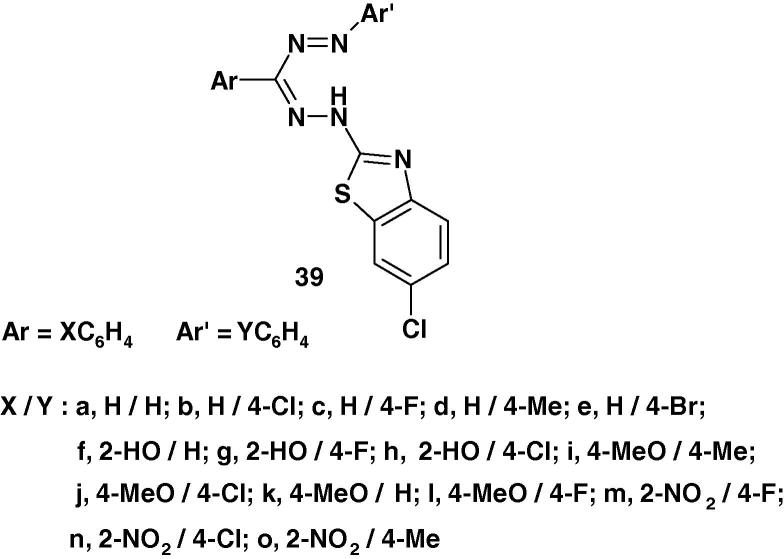
Various (6-chloro-benzothiazol-2-yl)formazans 39 (Chart 26) were synthesized by Basavaraja et al. [86] and evaluated for their antimicrobial activity against E. coli, Pseudomonas, Staphylococcus aureus, Bacillus subtilis and antifungal activity against Aspergillus. Flavus and Candida albicans using DMF as solvent at 50 and 100 μg/ml concentration by using cup–plate method. The activities were compared with known reference drugs like procaine penicillin, Streptomycin and Griseofulvin [86]. The results showed that compounds 39l–n are active against S. aerus whereas compounds 39h, j are active against B. subtilis. In addition, while the formazans 39j, n, m exhibited activities against E. coli and Pseudo Mona comparable to that of the standards used. Furthermore, compounds 39c, h, l and 39d, n possessed activities close to that of Griseofulvin against Candida albicans and Aspergillus Flavus, respectively [86].
Chart 26.
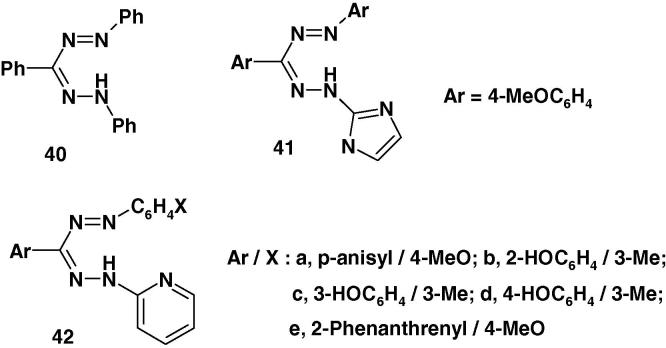
Recently, Uraz et al. [80] reported the synthesis of the formazans 40–42 (Chart 27) with various substituents on 1,3,5-sites and their antimicrobial activity against some selected microorganism namely, Staphylococcus aureus, S. epidermidis, S. saprophyticus, Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae. Moreover, the antifungal effects of these formazans were tested on the Candida kefir, C. glabrata, C. tropicalis, Cryptococcus neofarmans, and Saccharomyces cerevisiae. The results of that study indicated that the studied formazans were very active against Candida kefir, C. tropicalis, Cryptococcus neofarmans and Saccharomyces cerevisiae [80].
Chart 27.
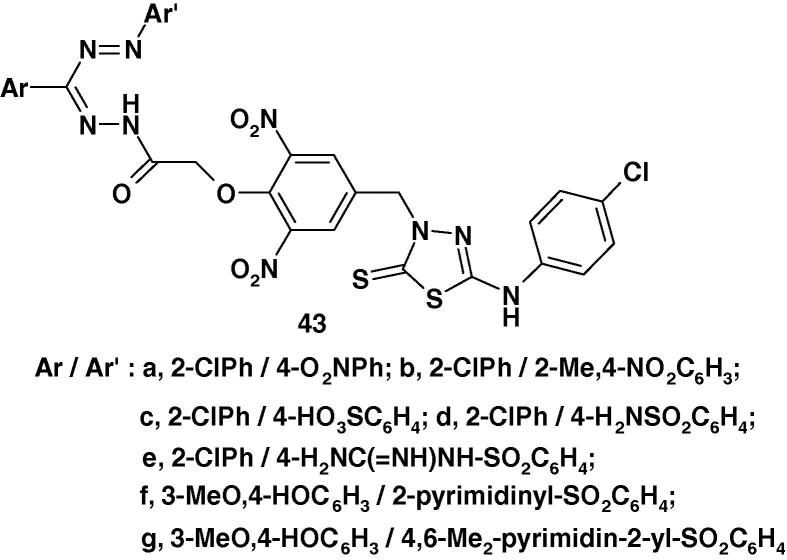
Furthermore, Sah et al. [87] reported the synthesis of the formazans 43a–g (Chart 28) containing 1,3,4-thiadiazole ring residue and their in vitro antibacterial activity against Escherichia coli and Salmonella typhi and antifungal activity against Aspergillus sp. and Candida albicans. The results revealed that all of the studied compounds showed moderate activities.
Chart 28.
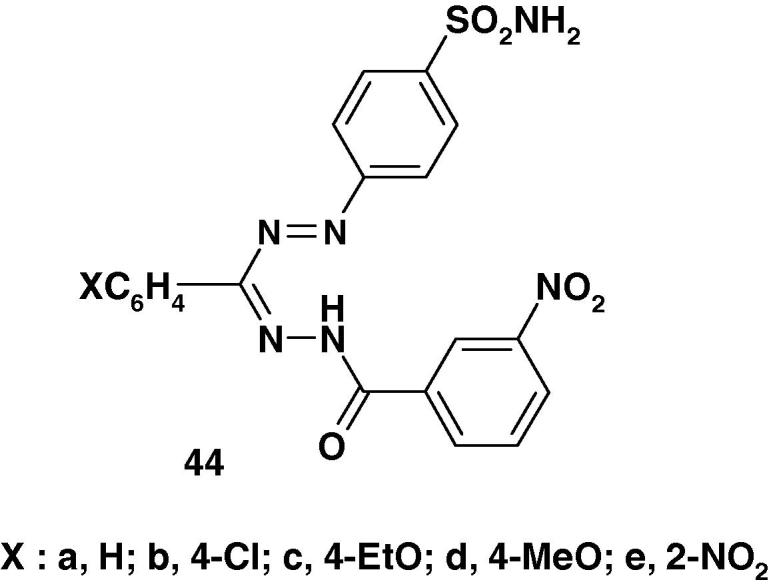
Other formazans 44a–e (Chart 29) were synthesized by Desai et al. [88] and were assayed for their in vitro antibacterial activity against E. coli, P. fluorescence, B. mega, B. subtilis, and antifungal activity against Aspergillus awamori using DMF as solvent at 50 μg concentration by cup–plate method. The activity was compared with the known antibiotic namely ampicillin, chloramphenicol, norfloxacin, and griseofulvin at the same concentration. The results showed that compounds 44b–d exhibit antibacterial activity similar to that of ampicillin and compound 44e showed comparable antifungal activity as that of griseofulvin.
Chart 29.
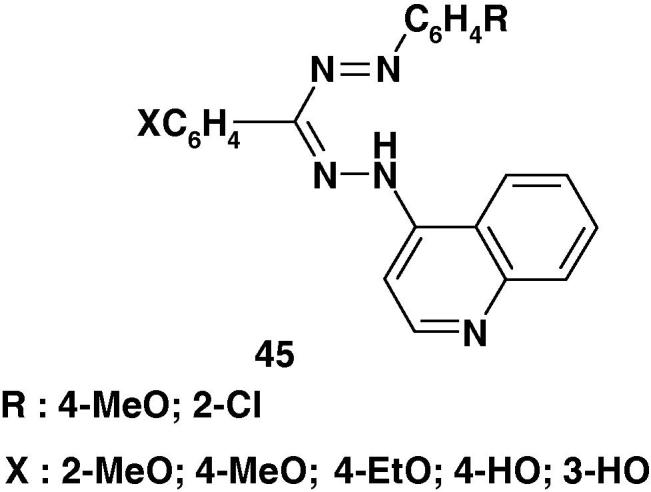
Also, Tandel et al. [89] reported the synthesis of formazans 45 (Chart 30) and indicated that they exhibited potent activity against E. coli, B. Subtilis, S. aureus, and S. paratyphi B bacteria.
Chart 30.
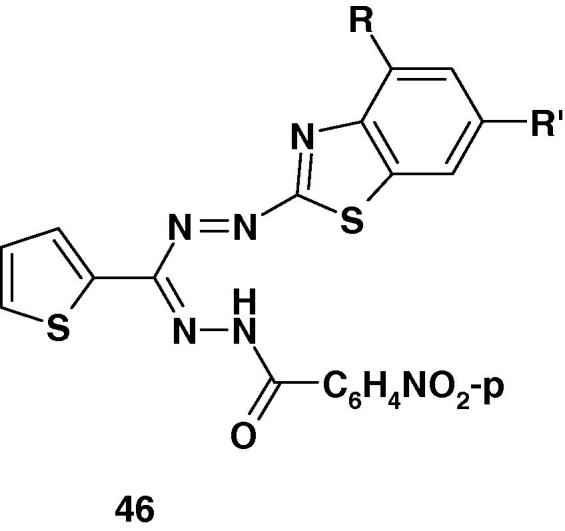
The formazans 46a–j (Chart 31) have been prepared by Desai et al. [90] and screened them for antifungal activity against Candida albicans, Candida krusei, and Candida parapsilosis and antibacterial activity against Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa and Bacillus subtilis.
Chart 31.
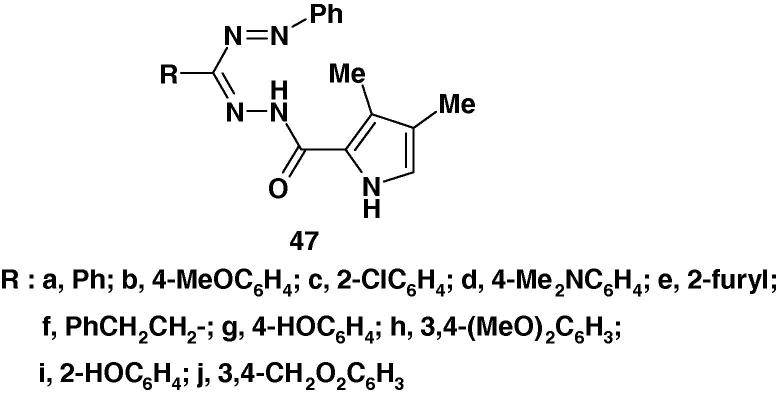
Very recently, the synthesis of the formazans 47a–j (Chart 32) and the results of screening of their antibacterial and antifungal activities were reported by Bhosale et al. [56]. The results indicated that all compounds 47a–j showed good activities against all strains of bacteria. For example, compound 47i showed excellent activities against the species Pseudomonas aeruginosa, Klebsiella pneumonia and Bacillus subtilis comparable with the standard antibacterial agent tetracycline. Compound 47g showed good activity among all the compounds studied against Salmonella typhi. In antifungal assay, while compounds 47a–i demonstrated moderate results against the fungi Aspergillus niger, compounds 47a–j displayed good antifungal activities against Aspergillus flavus.
Chart 32.
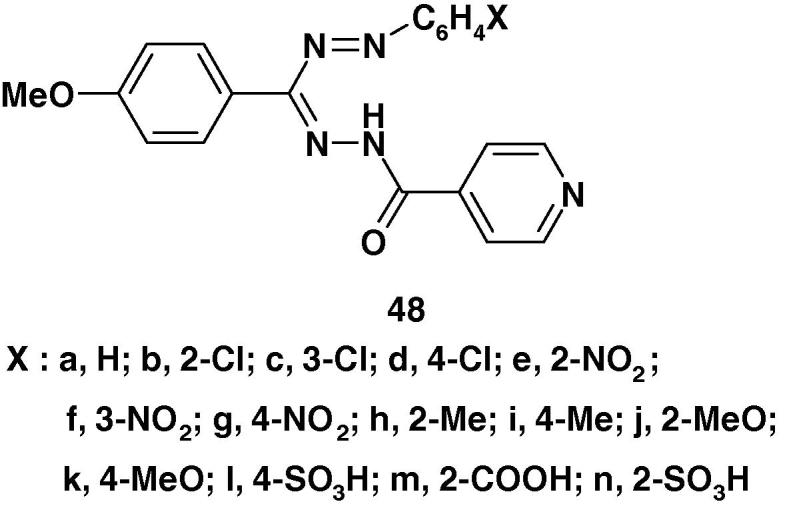
Also, Rajput [91] synthesized a series of 1-isonicotinoyl-3-(4-methoxyphenyl)-5-substituted phenyl)formazans 48a–n (Chart 19c) and screened them for their antimicrobial activity against Escherichia coli, Bacillus, Salmonella typhimurium and A niger using DMF as solvent and nutrient agar was employed as culture media. The results showed that the studied compounds were only active against E. coli.
Chart 19c.
Very recently, Kumara Prasad et al. [59] reported the results of assaying the formazan derivatives 9A(B) (Chart 4c) for antibacterial activity against Staphylococcus aureus and Escherichia coli and anti fungal activity against Aspergillus Niger in comparison with ofloxacin and fluconazole as standard. The reported results indicate that compounds with electron withdrawing substituents namely 9Ab and 9Ad are the most active among the tested compounds. The other compounds were found less active.
Chart 4c.
Analgesic activity
Nadendla and Babu [92] prepared a series of 1-substituted phenyl-3-(4-nitrophenyl)-4-[benzamido-(2-methyl-3-quinazolin)-4-one]formazans 10Aa–h (Chart 6b) and screened them for analgesic activities. The reported results revealed that only compounds 10d, e, f exhibit significant analgesic activity compared to the standard namely paracetamol.
Chart 6b.
Very recently, Kumara Prasad et al. [59] synthesized the two series of formazan derivatives 9A, B (Chart 4d) and screened them for their analgesic activity using Eddy’s Hot Plate Method. The reported results indicate that compounds 9Ac and 9Bc are the most active among the tested compounds. The other compounds were found less active.
Chart 4d.
Antiparkinsonian activity
The formazans 22a–c (Chart 33a) were prepared by coupling diazotized anilines with aldehyde N-(10-phenothiazinyl-acetyl)hydrazones. Compounds 22a–c were reported to exhibit prominent antiparkinsonian activity better than 1-dopa and bromocriptine in mice/rats (in vivo) with no toxicity effects with doses ⩽1000 mg/kg [93], [94].
Chart 33a.
Very recently, Mariappan et al. [58] reported that the formazan derivatives 7–8 (Chart 3d) showed significant anticonvulsant effect at 100 mg/kg po and the experimental data were statistically significant at P < 0.001 level.
Chart 3d.
Cardiovascular activity
Three 1,3-disubstituted phenyl-5[(2-acetyl-phenothiazin-10-yl)-2-oxo-ethyl]formazans 22 (Chart 33b) have been reported and were evaluated for their cardiovascular activity [95].
Chart 33b.
Anti-proliferative activity
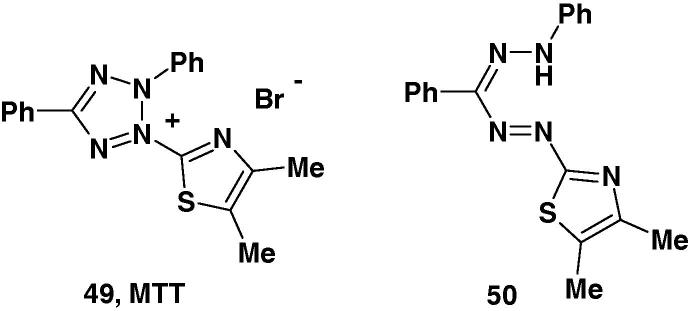
As prolactin and other lactogenic hormones are mitogenic for the rat T-cell lymphoma line Nb2 and glucocorticoids have antiproliferative effects on these cells, Alder et al. used the tetrazolium salt 49 (MTT) (Chart 34) for proliferation assay for the Nb2 cell line. Reduction of the yellow MTT to form the corresponding purple formazan 50 increases with cellular metabolic activity due to elevated NAD(P)H flux [96]. Resting cells such as thymocytes and splenocytes that are viable but metabolically quiet reduce very little MTT. In contrast, rapidly dividing cells exhibit high rates of MTT reduction.
Chart 34.
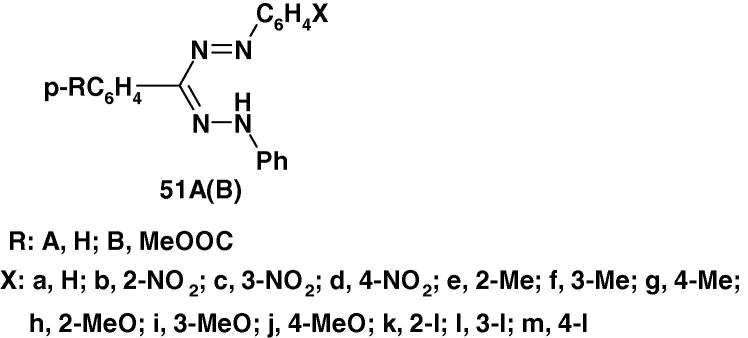
Recently, Yaglıoglu and Senöz [97] reported the results of antiproliferation activity of 1,3,5-triphenylformazan 51Aa and 1-substituted phenyl-3-(4-methoxycarbonyl)-phenyl-5-phenylformazans 51Ba–m (Chart 35) against HeLa an C6 cell line using BrdU cell proliferation ELISA assay. Cisplatin and 5-florouracil were used as standards. The activities of the studied compounds and standards were investigated on eight concentrations. The results revealed that compounds with 2-OCH3, 3-CH3 and 4-I substituents displayed maximum activity against HeLa while those having 2-NO2, 3-I and also 4-I groups were active against C6.
Chart 35.
Conclusions
This review presents an up-to-date survey of the various biological activities of formazans. The literature reports covered in this review indicate that formazans exhibit versatile biological activities. It is hoped that this review will capture the attention of other research groups to develop newer formazans that could be better agents in terms of efficacy and safety.
Conflict of Interest
The authors have declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Biographies

Ahmad S. Shawali is presently Emeritus Professor of Physical Organic Chemistry, Department of Chemistry, Faculty of Science, University of Cairo, Giza, Egypt. He graduated with B.Sc. from the University of Cairo in 1958. He received his M.Sc. and Ph.D. degrees in 1962 and 1966, respectively, from Lowell Technological Institute, presently the University of Lowell, Massachusetts, USA. He was awarded the degree of Doctor of Science (D.Sc.) from the university of cairo after recommendation from British committee from the Royal Chemical Society in 1995. Prof. Shawali has been the recipient of the state award and Egypt State Medal of Science and Arts in 1977. He holds several national and international certificates of merit for his distinguished services. He was appointed Vice-Dean for student affairs in 1989 and he was elected Dean of the Faculty of Science in 1991. He was visiting professor at the university of Texas at el Paso, Texas, USA from 1979 to 1980, University of Kuwait from 1973 to 1977 and King Abdulaziz University, Jeddah, Saudi Arabia from 1982 to 1988. He has published 231 scientific papers and 17 review articles all in international journals. At present there are more than 1950 citations of his work from 1970 till mid 2006 (i.e. about 50 citations/year or 8 citations/paper). He supervised till now 45 graduate M.Sc. and 15 Ph.D. theses. He was invited to present plenary lectures in 23 conferences. His research interests are in the fields of reaction mechanisms, applications of LFERs, Chemistry of hydrazonoic acid derivatives, 1,3-dipolar cycloadditions and 1,5-electrocyclizations.

Nevien A. Samy is presently Associate Professor of Dermatology, Department of Medical Applications of Laser, National Institute of Laser Enhanced Sciences, University of Cairo, Giza, Egypt. She graduated with B.Sc. from the University of Cairo in 1985 from faculty of Medicine. She received her M.Sc. and Ph.D. degrees in 1997 and 2003, respectively, from the University of Cairo. She has published 13 scientific papers and 2 review articles all in international journals. She supervised till now 3 M.Sc. and 20 Ph.D. theses. She was invited to present plenary lectures in 12 conferences. Her research interests are in the field of Laser applications in Dermatology.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Şenöz H. The chemistry of formazans and tetrazolium salts. Hacettepe J Biol Chem. 2012;40(3):293–301. [Google Scholar]
- 2.Buzykin B.I. Formazans in the synthesis of heterocycles. I. Synthesis of azoles (Review) Chem Heterocycl Compd. 2010;46(4):379–408. [Google Scholar]
- 3.Buzykin B.I. Formazans in the synthesis of heterocycles. I. Synthesis of azines (Review) Chem Heterocycl Compd. 2010;46(4):1043–1062. [Google Scholar]
- 4.Berry D.E., Hicks R.G., Gilroy J.E. The chemistry of formazan dyes synthesis and characterization of a stable verdazylradical and related boron-containing heterocycles. J Chem Educ. 2009;86:76–79. [Google Scholar]
- 5.De Rosa T.F. Elsevier; 2006. Advances in synthetic organic chemistry and methods reported in US Pat. p. 272. [Google Scholar]
- 6.Fryšová I., Růžičková V., Slouka J., Gucký T. α-Ketoacids for synthesis of heterocyclic compounds I. Synthesis and oxidative cyclization of some substituted benzimidazol-2-yl-formazans. Acta Univ Palacki Olomuc Fac Rerum Nat, Chem. 2005;44:55–61. [Google Scholar]
- 7.Fryšová I., Slouka J., Hlavka J. The synthesis of condensed imidazoles II. A simple synthesis of some 1,5-diaryl-3-[2-(naphtho[2,3-d]imidazol-2-yl)]formazans and its derivatives. Arkivoc. 2006;2:207–212. [Google Scholar]
- 8.Ibrahim Y.A., Abbas A.A., Elwahy A.H.M. New trends in the chemistry of condensed heteromacrocycles. J Heterocycl Chem. 2004;41:135–149. [Google Scholar]
- 9.El-Khadem H.S., Fatiadi A.J. Hydrazine derivatives of carba sugars and related compounds. Adv Carbohydr Chem Biochem. 2004;59:135–173. doi: 10.1016/S0065-2318(04)59004-2. [DOI] [PubMed] [Google Scholar]
- 10.Katritzky A.R., Belyakov S.A., Cheng D., Durst H.D. Synthesis of formazans under phase-transfer conditions. Synthesis. 1995;5:577–581. [Google Scholar]
- 11.Buzykin BI, Lipunova GN, Sysoeva LP, Rusinova LI. Khimiya formazanov (Chemistry of Formazans). Formazans [in Russian]. Moscow: Nauka; 1992.
- 12.Bednyaigna N.P., Postovskii IYa, Osipov O.A. Hetarylformazans. Russian Chem Rev. 1975;44(6):493–506. [Google Scholar]; Bednyaigna N.P., Postovskii IYa, Osipov O.A. Translated from Uspekhi Khimii. 1975;44:1052–1083. [Google Scholar]
- 13.Kitaev Yu P, Buzykin BI. Hydrazones [in Russian]. Moscow: Nauka, A; 1974.
- 14.Pütter R., Müller E., editors. vol. 10. Thieme; Stuttgart: 1965. (Methoden der organischen Chemie (Houben-Weyl)). p. 627–94. [Google Scholar]
- 15.Nineham A.W. The chemistry of formazans and tetrazolium salts. Chem Rev. 1955;55:355–483. [Google Scholar]
- 16.Reid W. Formazane und Tetrazoliumsalze, ihre Synthesen und ihre Bedeutung als Reduktion-sindikatoren und Vitalfarbstoffe. Angew Chem. 1951;64:391–415. [Google Scholar]
- 17.Shawali A.S., Sayed A.R. A facile entry for synthesis of 3-arylazo derivatives of [1,2,4]triazolo[4,3-a]benzimidazole and [1,2,4]triazolo[3,4-b]quinazolin-5- one. J Chem Res. 2005:285–288. [Google Scholar]
- 18.Shawali A.S., Sayed A.R. A convenient one-pot synthesis of 3-arylazo derivatives of azino[b][1,2,4,5]-tetrazines. J Chem Res. 2004:399–401. [Google Scholar]
- 19.Shawali A.S., Abdelkhalek A.A., Sayed A.R. Kinetics and mechanism of Dehydrochlorination of 3-Chloro-1,5-diarylformazans and Their Mass Spectra. J Chin Chem Soc. 2001;48:693–699. [Google Scholar]
- 20.Shawali A.S., Abd El-Galil M., Cynoacetarylamides I. Preparation and reactions of their arylazo derivatives with diazonium ion and Grignard reagents. Tetrahedron. 1971;27(18):4305–4316. [Google Scholar]
- 21.Abdelhamid A.O., Abbas I.M., Abdallah M.A., Fahmi A.A., Shawali A.S. Convenient synthesis of 3-arylazopyrazoles and 2-arylazo-1,3,4-Δ2-thiadiazo-line derivatives from 3-nitroformazans. J Heterocycl Chem. 1985;22(3):813–816. [Google Scholar]
- 22.Shawali A.S., Abdelhamid A.O. Synthesis of spiro-heteroycles via 1,3-dipolar cycloadditions of nitrilimines to exoheterocyclic enones. Site-, region-and stereo-selectivities overview. Curr Org Chem. 2012;16:2673–2689. [Google Scholar]
- 23.Shawali A.S. Hydrazonoyl halides: a bubbling fountain of biologically active compounds. Curr Org Chem. 2010;14:784–815. [Google Scholar]
- 24.Shawali A.S., Samy N.A. Hydrazonoyl halides: their versatile biological activities. Open Bioact Compd J. 2009;2:8–16. [Google Scholar]
- 25.Shawali A.S., Farghaly T.A. Reactions of hydrazonoyl halides with heterocyclic thiones. Convenient methodology for heteroannulation, synthesis of Spiro-heterocycles and heterocyclic ring transformation. Arkivoc. 2008;1:18–64. [Google Scholar]
- 26.Shawali A.S., Sherif S.M. The chemistry of hydrazonates. Curr Org Chem. 2007;11(9):773–799. [Google Scholar]
- 27.Shawali A.S., Edrees M.M. Reactions of nitrilimines with heterocyclic amines and enamines. Convenient methodology for synthesis and annulations of heterocycles. Arkivoc. 2006;ix:292–365. [Google Scholar]
- 28.Shawali A.S., Mosselhi M.A.N. The chemistry of thiohydrazonates and their utility in organic synthesis. J Sulfur Chem. 2005;26(3):267–303. [Google Scholar]
- 29.Shawali A.S., Mosselhi M.A.N. Hydrazonoyl halides: useful building blocks for the synthesis of arylazoheterocycles. J Heterocycl Chem. 2003;40(5):725–746. [Google Scholar]
- 30.Shawali A.S., Elsheikh S.M. Annelated [1,2,4,5]tetrazines. J Heterocycl Chem. 2001;38(3):541–549. [Google Scholar]
- 31.Shawali A.S., Abdallah M.A. The chemistry of heterocyclic hydrazonoyl halides. Adv Heterocycl Chem. 1995;63(C):277–338. [Google Scholar]
- 32.Shawali A.S. Reactions of heterocyclic compounds with nitrilimines and their precursors. Chem Rev. 1993;93(8):2731–2777. [Google Scholar]
- 33.Shawali A.S. Reactions of hydrazidoyl halides with sulfur compounds. Heterocycles. 1983;20(11):2239–2285. [Google Scholar]
- 34.Shawali A.S., Parkanyi C. Hydrazidoyl halides in the synthesis of heterocycles. J Heterocycl Chem. 1980;17(5):833–854. [Google Scholar]
- 35.Shawali A.S. Synthesis and tautomerism of aryl- and hetaryl-azo derivatives of bi- and tri-heterocycles. J Adv Res. 2010;1:255–290. [Google Scholar]
- 36.Babu A.N., Nadendla R.R. Synthesis of some new quinazolinone formazans as anti-inflammatory and anthelmintic agents. J Pharm Res. 2011;4:983–985. [Google Scholar]
- 37.Halve A.K., Kankoriya A., Samadhiya S.K., Pant B.B., Gupta J.K. Synthesis and therapeutic evaluation of some formazans as potential antimicrobial agents. Heteroletters Org. 2011;1(3):221–226. [Google Scholar]
- 38.Marjadi S.I., Solanki J.H., Patel A.L. Synthesis and antimicrobial activity of some new formazan derivatives. Eur J Chem. 2009;63:844–848. [Google Scholar]
- 39.Singh N., Bhati S.K., Kumar A. Thiazolyl/oxazolyl formazanyl indoles as potent anti-inflammatory agents. Eur J Med Chem. 2008;43:2597–2609. doi: 10.1016/j.ejmech.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 40.Desai J.M., Shah V.H. Synthesis and antimicrobial profile of 5-imidazo-lones, sulphonamides, azomethines, 2-azetidinones and formazans derived from 2-amino-3-cyano-5-(5′-chloro-3′-methyl-1′-phenylpyrazol-4′-yl-vinyl)-7,7′-dimethyl-6,7-dihydrobenzothiophenes. Indian J Chem. 2003;42B(3):631–635. [Google Scholar]
- 41.Schiele C. The nature of tetrazolium salts and formazans. Angew Chem. 1966;5(7):681–682. [Google Scholar]
- 42.Plumb J.A., Milroy R., Kaye S.B. Effects of pH dependence of 3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide-formazan absorption on chemosensitivity determined by a novel tetrazolium-based assay. Cancer Res. 1989;49(16):4435–4440. [PubMed] [Google Scholar]
- 43.Mattson A.M., Jensen C.O. Triphenyltetra-zolium chloride as a dye for vital tissue. Science. 1947;106:294–295. doi: 10.1126/science.106.2752.294-a. [DOI] [PubMed] [Google Scholar]
- 44.Scudiero D.A., Shoemaker R.H., Paull K.D., Monks A., Tierney S., Nofziger T.H., et al. Evaluation of a soluble tetrazlium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988;48:4827–4833. [PubMed] [Google Scholar]
- 45.Wan H., Williams R.L., Doherty P.J., Williams D. The cytotoxicity evaluation of Kevlar and silicon carbide by MTT assay. J Mater Sci. 1994;5(6–7):441–445. [Google Scholar]
- 46.O’Toole S.A., Sheppard B.L., McGuinness E.P.J., Gleeson N.C., Yoneda M., Bonnar J. The MTS assay as an indicator of chemoselecti-vity/resistance in malignant gynaecological tumours. Cancer Detect Prev. 2003;27(1):47–54. doi: 10.1016/s0361-090x(02)00171-x. [DOI] [PubMed] [Google Scholar]
- 47.Aziz D.M. Assessment of bovine sperm viability by MTT reduction assay. Anim Reprod Sci. 2006;92:1–8. doi: 10.1016/j.anireprosci.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 48.Tezcan H., Özkan N. Substituent effects on the spectral properties of some 3-substituted formazans. Dyes Pigm. 2003;56:159–166. [Google Scholar]
- 49.Guerra R., Iacondini A., Abbondanzi F., Matteucci C., Bruzzi L. A new microbial assay for the toxicity detection of contaminated soils. Ann Chim. 2002;92(9):847–854. [PubMed] [Google Scholar]
- 50.Koren E., Kohen R., Gınsburg I. A cobalt-based tetrazolium salts reduction test to assay polyphenols. J Agric Food Chem. 2009;57:7644–7650. doi: 10.1021/jf9006449. [DOI] [PubMed] [Google Scholar]
- 51.Williams C., Espinosa O.A., Monenegro H., Cubilla L., Capson T.L., Ortega-Barria E., et al. Hydrosoluble formazan XTT: its application to natural products drug discovery for Leishmania. J Microbiol Methods. 2003;55(3):813–816. doi: 10.1016/j.mimet.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 52.Ishiyama M., Sasamoto K., Ohkura Y., Ueno K., Nishiyama K., Tanogucho I. Novel disulfonated tetrazolium salt that can be reduced to a water soluble formazan and its application to the assay of lactate dehyrogenase. Analyst. 1995;120(1):113–116. [Google Scholar]
- 53.Roslev P., King G.M. Application of a tetrazolium salt with water soluble formazan as an indicator of viability in respiring bacteria. Appl Environ Microbiol. 1993;59(9):2891–2896. doi: 10.1128/aem.59.9.2891-2896.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berlowska J., Kregiel D., Klimek L., Orzeszyna B., Ambroziak W. Novel yeast cell dehydrogenase activity assay in situ. Polish J Microbiol. 2006;55(2):127–131. [PubMed] [Google Scholar]
- 55.Sehl T., Simon R.C., Hailes H.C., Ward J.M., Shell U., Pohl M., et al. TTC-based screening assay for w-transaminases: a rapid method to detect reduction of 2-hydroxy ketones. J Biotechnol. 2012;159:188–194. doi: 10.1016/j.jbiotec.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 56.Bhosale J.D., Shirolkar A.R., Pete U.D., Zade C.M., Mahajan D.P., Hadole C.D., et al. Synthesis, characterization and biological activities of novel substituted formazans of 3,4-dimethyl-1H-pyrrole-2-carbohydrazide derivatives. J Pharm Res. 2013;7(7):582–587. [Google Scholar]
- 57.Srivastava A., Kumar A.V. Synthesis of newer formazan potential anticonvulsant agents. Indian J Pharma Sci. 2001;65:358–362. [Google Scholar]
- 58.Mariappan G., Korim R., Joshi N.M., Alam F., Hazarika R., Kumar D., et al. Synthesis and biological evaluation of formazan derivatives. J Adv Pharm Technol Res. 2010;1(4):396–400. doi: 10.4103/0110-5558.76438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kumara Prasad S.A., Subrahmanyam E.V.S., Shabaraya A.R. Design and biological screening of some novel formazan derivatives from Schiff bases of gallic acid. World J Pharm Res. 2014;3(2):2741–2752. [Google Scholar]
- 60.Nadendla R.R., Mukkanti K., Rao G.S., Babu A.N. Microwave synthesis of some new quinazolinone formazans for their antimicrobial and anthelmintic activities. Curr Trends Biotech Pharm. 2010;4:545–550. [Google Scholar]
- 61.Raval J.P., Patel P., Pradip S., Patel P.S. In vitro antitubercular activity of novel 3-(4-methoxyphenyl)-1-isonicotinoyl-5-(substituted phenyl) formazans. Int J Pharm Tech Res. 2009;1(4):1548–1553. [Google Scholar]
- 62.Misra V.S., Dhar S., Chowdhary B.L. Synthesis of some newer formazans and tetrazolium salts as antiviral agents. Pharmazie. 1978;33(12):790–792. [PubMed] [Google Scholar]
- 63.Misra V.S., Dhar S. Synthesis of some newer formazans and tetrazolium salts and their effect on Ranikhet disease virus and the vaccinia virus. Pharmazie. 1980;35(10):585–586. [PubMed] [Google Scholar]
- 64.Mukerjee D.D., Shukla S.K., Chowdhary B.L. Synthesis of some new formazans as potential antiviral agents. Arch Pharm (Weinheim) 1981;314:991–994. doi: 10.1002/ardp.19813141204. [DOI] [PubMed] [Google Scholar]
- 65.Tiwari M.M., Agarwal M., Saxena V.K., Bajpai S.K., Joshi M.M. Synthesis and antiviral activity of some new formazans. Indian J Pharm Sci. 1995;57(3):113–116. [Google Scholar]
- 66.Pandey V.K., Negi H.S. Synthesis of 1-(2′aryl-4′-oxo-3H-quinazolyl)-3- aryl-5-phenyl-formazans as potential anti-viral agents. Indian Drugs. 1990;36(1):37–40. [Google Scholar]
- 67.Kalsi R., Pande K., Bhalla T.N., Parmar S.S., Barthwal J.P. Novel formazans as potent anti-inflammatory and analgesic agents. Pharmacology. 1988;37:218–224. doi: 10.1159/000138469. [DOI] [PubMed] [Google Scholar]
- 68.Kaisi R., Pande K., Bhalla T.N., Barthwal J.P., Gupta G.P., Parmar S.S. Anti-inflammatory activity of quinazolinoformazans. J Pharm Sci. 1990;79(4):317–320. doi: 10.1002/jps.2600790409. [DOI] [PubMed] [Google Scholar]
- 69.Stefănescu E., Dorneanu M., Dănilă C., Aprotosoaie C., Grosu G., Pavelescu M. Hydrazones and formazans with possible biological activity. Rev Med Chir Soc Med Nat Iasi. 1997;101(3–4):178–182. [PubMed] [Google Scholar]
- 70.Babu A.N., Naderndia R.R. Synthesis and antimicrobial activity of 1-substituted phenyl-4-[(3,4,5-trimethoxy)-5-benzyl-4-aminopyrimidine formazans. Asian J Chem. 2011;23(1):278–280. [Google Scholar]
- 71.Kumar A., Verma M., Bhalla M., Saxena A.K., Shanker K. New heterocyclic compounds as anti-inflammatory agents. Indian J Heterocycl Chem. 1991;1(3):129–132. [Google Scholar]
- 72.Kumar A., Ram T., Tyagi R., Goel B., Bansal E., Srivastava V.K. Synthesis and anti-inflammatory activity of some potential cyclic phenothiazines. Bollettino Chim Farmac. 1998;137(5):152–156. [PubMed] [Google Scholar]
- 73.Baltrop J.A., Owen T.C., Cory A.H., et al. 5-(3-Carboxymethoxyphenyl)-2-(4,5-dimethylhiazo-lyl)-3-(4-sulfophenyl)tetrazolium, inner salt (MTS) and related analogs of 3-(4,5-dimethylthiazolyl)-2, 5-diphenyltetrazolium bromide (MTT) reducing to purple water-soluble formazans as cell-viability indicators. Bioorg Med Chem Lett. 1991;1:611–614. [Google Scholar]
- 74.Kidwai M., Negi N., Gupta S.D. Synthesis and antifertility activity of 1,5-diaryl-3-(3′-indolyl)formazans. Chem Pharm Bull (Tokyo) 1994;42(11):2363–2364. doi: 10.1248/cpb.42.2363. [DOI] [PubMed] [Google Scholar]
- 75.Samel A.B., Pai N.R. Synthesis and antimicrobial activity of some novel formazan derivatives. J Chem Pharm Res. 2010;2:60–67. [Google Scholar]
- 76.Bhardwaj S.D., Jolly V.S. Synthesis, anti-HIV and anticancer activities of some new formazans. Asian J Chem. 1997;9:48–51. [Google Scholar]
- 77.Bhardwaj S.D. Synthesis, anti-cancer and anti-HIV activities of some new formazans. Asian J Chem. 1998;10:39–42. [Google Scholar]
- 78.Bharadwaj S.D. Synthesis and biological activities of some new formazans, Part-I. Asian J Chem. 2002;14(2):767–770. [Google Scholar]
- 79.Bharadwaj S.D. Synthesis of some new formazans and their biological activity. Int J Chem Sci. 2003;1:272–277. [Google Scholar]
- 80.Uraz G., Yilmaz E., Tezcan H., Porsuk N., Imamoglu G., Kartli O.S. Synthesis and antimicrobial effects of 1,3,5-substituted phenylformazans. Asian J Chem. 2012;24(5):1924–1926. [Google Scholar]
- 81.Chavan S.B., Zangade S.B., VIbhute A.Y., Vibhute Y.B. Synthesis and evaluation of antimicrobial activity of some new Schiff bases and formazans. Res J Pharm Biol Chem Sci. 2012;3(1):262–269. [Google Scholar]
- 82.Raval J.P., Patel P.R., Patel N.H., Patel P.S., Bhatt V.D., Patel K.N. Synthesis, characeterization and in vitro antibacterial activity of novel 3-(4-methoxyphenyl)-1-isonicotinoyl-5-(substituted phenyl)-formazans. Intern J Chem Tech Res. 2009;1(3):610–615. [Google Scholar]
- 83.Lakshmi N., Haritha V., Sreeram V., Rajalakshmi D., Sindhura N., Visagaperumal D. Synthesis and their possible biological activities of few formazans of 3-amino-2-sulphanyl-2,3,4,5,6,7,8-hexahydrobenzothieno-[2,3-d]pyrimidin-4(1H)- one. Rasayan J Chem. 2009;2:71–74. [Google Scholar]
- 84.Revanasiddappa B.C., Subrahmanyam E.V.S. Synthesis and biological studies of some novel formazans. Oriental J Chem. 2010;26(1):243–246. [Google Scholar]
- 85.Vashi R.T., Sheth N.M. Synthesis and antimicrobial activity of novel formazans and their tetrazolium brmides. Asian J Chem. 2010;22:7827–7832. [Google Scholar]
- 86.Basavaraja K.M., Somasekhar B., Shivakumar B. Synthesis of 2-[(1-phenyl) (aryl) azo] methyleneimino-6-chloro/fluoro benzothiazoles and their antibacterial activity. Int J Pharm Tech Res. 2010;2(2):1139–1143. [Google Scholar]
- 87.Sah P., Bidawat P., Seth M., Gharu C.P. Synthesis of formazans from Mannich base of 5-(4-chlorophenyl amino)-2-mercapto-1,3,4-thiadiazole as antimicrobial agents. Arabian J Chem. 2010;10:23–29. [Google Scholar]
- 88.Desai R.M., Desai J.M., Shah V.H. Synthesis and antimicrobial profiles of 1,3,4-oxadiazoles, sulphonamides, 5-imidazolinones, azomethines, 4-thiazolidinones, 2-azetidinones, formazans and tetrazolium chlorides. Indian J Heterocycl Chem. 1999;8(4):329–334. [Google Scholar]
- 89.Tandel D.C., Desai C.M., Patel D., Naik B., Matjad S. Synthesis of quinolinyldiarylformazans as antitubercular/antibacterial agents. Oriental J Chem. 2001;17(3):529–530. [Google Scholar]
- 90.Desai K.G., Desai K.R. Microbial screening of novel synthesized formazans having amide linkages. J Heterocycl Chem. 2006;43(4):1083–1089. [Google Scholar]
- 91.Rajput S.S. Synthesis, characterisation and antimicrobial activity of 1-isonicotinoyl -3-(4-methoxy phenyl)-5-(substituted phenyl) formazanes. Int J Adv Pharm Biol Chem. 2013;2(2):376–379. [Google Scholar]
- 92.Nadendla R.R., Babu A.N. Synthesis and biological evaluation of some novel formazans. J Pharm Res. 2011;4:3–5. [Google Scholar]
- 93.Khanna R., Palit G., Srivastava V.K., Shanker K. Newer heterocycles of phenothiazine and their antiparkinsonian activity. Indian J Chem. 1990;29B:556–560. [Google Scholar]
- 94.Kumar P., Nath C., Shanker K. Newer dopamine quinazolones as anti-parkinsonian agents. Pharmazie. 1985;40(4):267–268. doi: 10.1002/chin.198531244. [DOI] [PubMed] [Google Scholar]
- 95.Kumar A., Verma R.S., Bhatia S.K., Gupta S., Jettly U.K., Bajpai S.K., et al. Synthesis and cardiovascular activity of new phenothiazines. Pakistan J Sci Ind Res. 1996;39:1–4. [Google Scholar]
- 96.Adler R.A., Naumann S.A., Mansouri A., Krieg R.J., Jr., Latta K., Sanders K.M. Anti-proliferative effects of deflazacort on NB2 cells as quantitated by formazan production. Life Sci. 1994;55(23):1823–1831. doi: 10.1016/0024-3205(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 97.Yaglıoglu A.S., Senöz H. The Antiproliferative effects of substituents in formazan derivatives aganist HeLa and C6 cell line. Hacettepe J Biol Chem. 2013;41(4):371–378. [Google Scholar]