Abstract
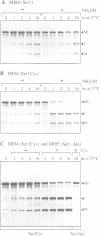
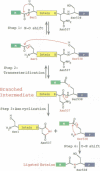
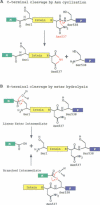
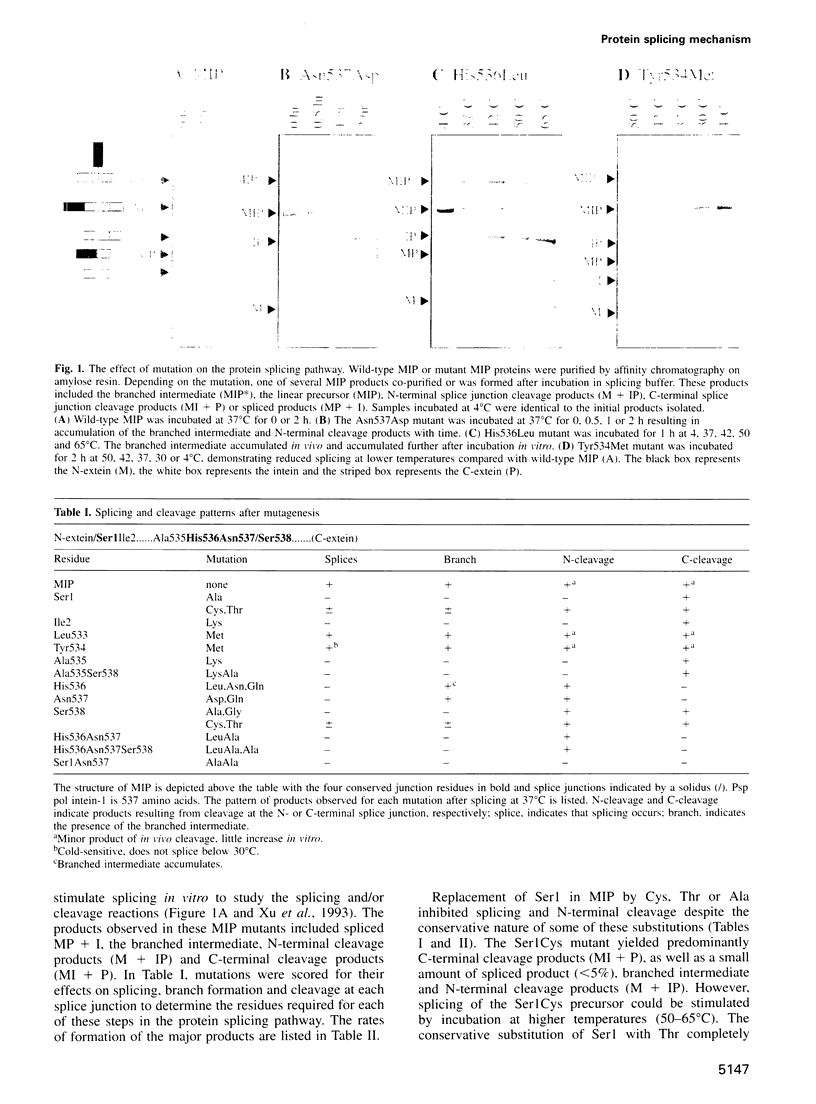
Protein splicing results in the expression of two mature proteins from a single gene. After synthesis of a precursor protein, an internal segment (the intein) is excised and the external domains are joined together. A self-catalyzed mechanism for this cleavage-ligation reaction is presented, based on mutagenesis data and analysis of splicing intermediates. Mutations were used to block various steps in the protein splicing pathway, allowing each isolated step to be studied independently. A linear ester intermediate was identified and functional roles for the four conserved splice junction residues were determined. Understanding the mechanism of protein splicing provides a basis for protein engineering studies. For example, inteins can be constructed which fail to splice, but instead cleave the peptide bond at a chosen splice junction.
Full text
PDF


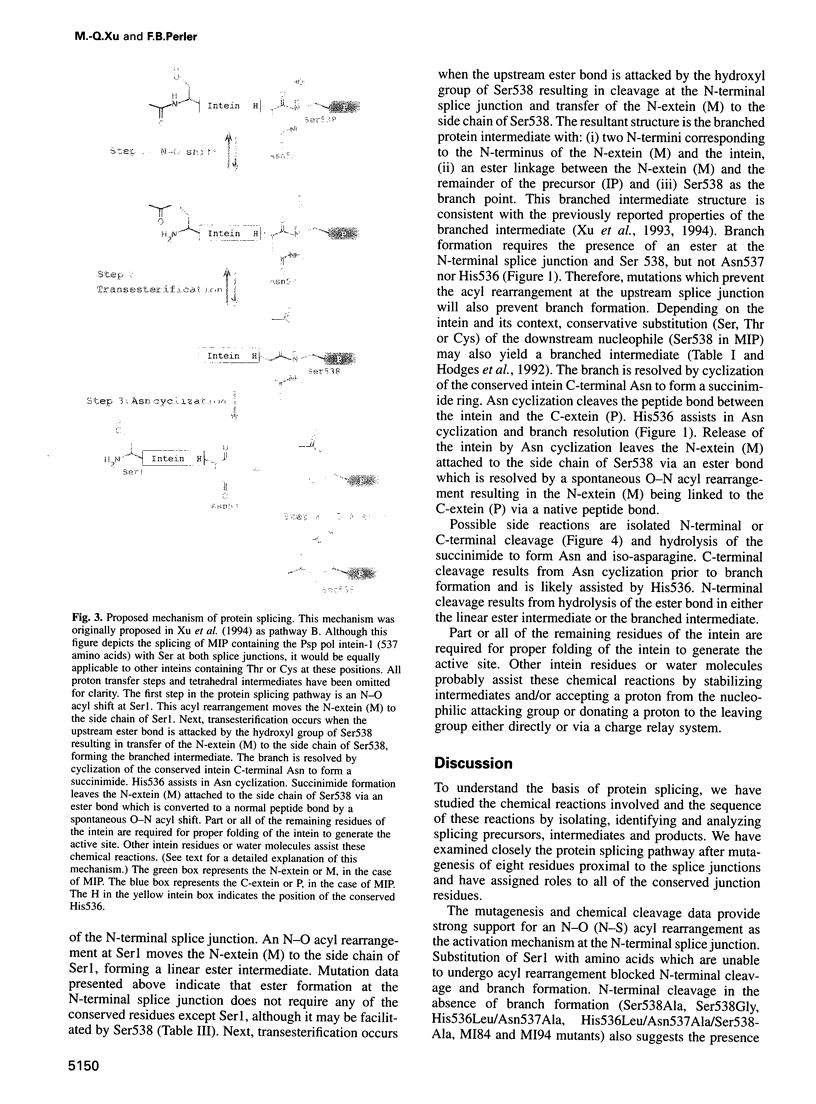
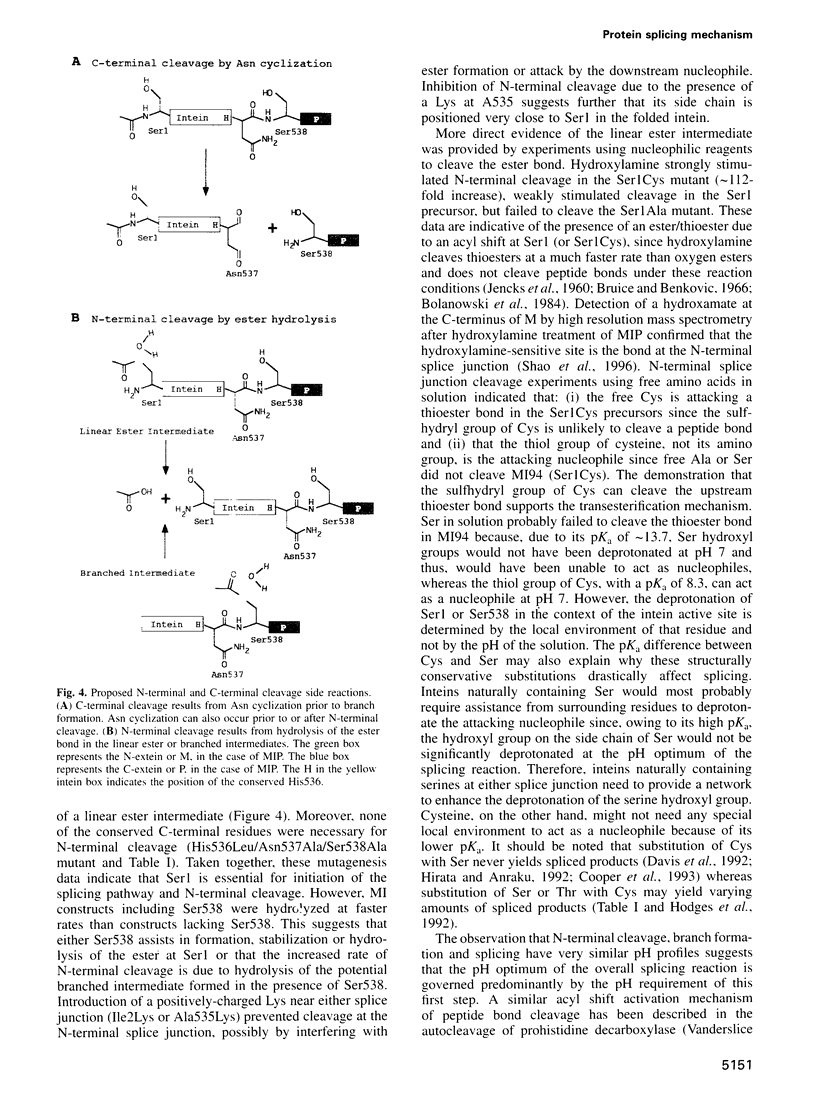


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolanowski M. A., Earles B. J., Lennarz W. J. Fatty acylation of proteins during development of sea urchin embryos. J Biol Chem. 1984 Apr 25;259(8):4934–4940. [PubMed] [Google Scholar]
- Clarke N. D. A proposed mechanism for the self-splicing of proteins. Proc Natl Acad Sci U S A. 1994 Nov 8;91(23):11084–11088. doi: 10.1073/pnas.91.23.11084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A. A., Chen Y. J., Lindorfer M. A., Stevens T. H. Protein splicing of the yeast TFP1 intervening protein sequence: a model for self-excision. EMBO J. 1993 Jun;12(6):2575–2583. doi: 10.1002/j.1460-2075.1993.tb05913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis E. O., Jenner P. J., Brooks P. C., Colston M. J., Sedgwick S. G. Protein splicing in the maturation of M. tuberculosis recA protein: a mechanism for tolerating a novel class of intervening sequence. Cell. 1992 Oct 16;71(2):201–210. doi: 10.1016/0092-8674(92)90349-h. [DOI] [PubMed] [Google Scholar]
- Davis E. O., Sedgwick S. G., Colston M. J. Novel structure of the recA locus of Mycobacterium tuberculosis implies processing of the gene product. J Bacteriol. 1991 Sep;173(18):5653–5662. doi: 10.1128/jb.173.18.5653-5662.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis E. O., Thangaraj H. S., Brooks P. C., Colston M. J. Evidence of selection for protein introns in the recAs of pathogenic mycobacteria. EMBO J. 1994 Feb 1;13(3):699–703. doi: 10.1002/j.1460-2075.1994.tb06309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson P. E., Muir T. W., Clark-Lewis I., Kent S. B. Synthesis of proteins by native chemical ligation. Science. 1994 Nov 4;266(5186):776–779. doi: 10.1126/science.7973629. [DOI] [PubMed] [Google Scholar]
- Gimble F. S., Thorner J. Homing of a DNA endonuclease gene by meiotic gene conversion in Saccharomyces cerevisiae. Nature. 1992 May 28;357(6376):301–306. doi: 10.1038/357301a0. [DOI] [PubMed] [Google Scholar]
- Gu H. H., Xu J., Gallagher M., Dean G. E. Peptide splicing in the vacuolar ATPase subunit A from Candida tropicalis. J Biol Chem. 1993 Apr 5;268(10):7372–7381. [PubMed] [Google Scholar]
- Hirata R., Ohsumk Y., Nakano A., Kawasaki H., Suzuki K., Anraku Y. Molecular structure of a gene, VMA1, encoding the catalytic subunit of H(+)-translocating adenosine triphosphatase from vacuolar membranes of Saccharomyces cerevisiae. J Biol Chem. 1990 Apr 25;265(12):6726–6733. [PubMed] [Google Scholar]
- Hodges R. A., Perler F. B., Noren C. J., Jack W. E. Protein splicing removes intervening sequences in an archaea DNA polymerase. Nucleic Acids Res. 1992 Dec 11;20(23):6153–6157. doi: 10.1093/nar/20.23.6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENCKS W. P., CORDES S., CARRIUOLO J. The free energy of thiol ester hydrolysis. J Biol Chem. 1960 Dec;235:3608–3614. [PubMed] [Google Scholar]
- Kane P. M., Yamashiro C. T., Wolczyk D. F., Neff N., Goebl M., Stevens T. H. Protein splicing converts the yeast TFP1 gene product to the 69-kD subunit of the vacuolar H(+)-adenosine triphosphatase. Science. 1990 Nov 2;250(4981):651–657. doi: 10.1126/science.2146742. [DOI] [PubMed] [Google Scholar]
- Lambowitz A. M., Belfort M. Introns as mobile genetic elements. Annu Rev Biochem. 1993;62:587–622. doi: 10.1146/annurev.bi.62.070193.003103. [DOI] [PubMed] [Google Scholar]
- Perler F. B., Comb D. G., Jack W. E., Moran L. S., Qiang B., Kucera R. B., Benner J., Slatko B. E., Nwankwo D. O., Hempstead S. K. Intervening sequences in an Archaea DNA polymerase gene. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5577–5581. doi: 10.1073/pnas.89.12.5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perler F. B., Davis E. O., Dean G. E., Gimble F. S., Jack W. E., Neff N., Noren C. J., Thorner J., Belfort M. Protein splicing elements: inteins and exteins--a definition of terms and recommended nomenclature. Nucleic Acids Res. 1994 Apr 11;22(7):1125–1127. doi: 10.1093/nar/22.7.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrokovski S. Conserved sequence features of inteins (protein introns) and their use in identifying new inteins and related proteins. Protein Sci. 1994 Dec;3(12):2340–2350. doi: 10.1002/pro.5560031218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter J. A., von Kessler D. P., Ekker S. C., Young K. E., Lee J. J., Moses K., Beachy P. A. The product of hedgehog autoproteolytic cleavage active in local and long-range signalling. Nature. 1995 Mar 23;374(6520):363–366. doi: 10.1038/374363a0. [DOI] [PubMed] [Google Scholar]
- Shao Y., Xu M. Q., Paulus H. Protein splicing: characterization of the aminosuccinimide residue at the carboxyl terminus of the excised intervening sequence. Biochemistry. 1995 Aug 29;34(34):10844–10850. doi: 10.1021/bi00034a017. [DOI] [PubMed] [Google Scholar]
- Shao Y., Xu M. Q., Paulus H. Protein splicing: evidence for an N-O acyl rearrangement as the initial step in the splicing process. Biochemistry. 1996 Mar 26;35(12):3810–3815. doi: 10.1021/bi952592h. [DOI] [PubMed] [Google Scholar]
- Vanderslice P., Copeland W. C., Robertus J. D. Site-directed alteration of serine 82 causes nonproductive chain cleavage in prohistidine decarboxylase. J Biol Chem. 1988 Aug 5;263(22):10583–10586. [PubMed] [Google Scholar]
- Wallace C. J. The curious case of protein splicing: mechanistic insights suggested by protein semisynthesis. Protein Sci. 1993 May;2(5):697–705. doi: 10.1002/pro.5560020501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M. Q., Comb D. G., Paulus H., Noren C. J., Shao Y., Perler F. B. Protein splicing: an analysis of the branched intermediate and its resolution by succinimide formation. EMBO J. 1994 Dec 1;13(23):5517–5522. doi: 10.1002/j.1460-2075.1994.tb06888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M. Q., Southworth M. W., Mersha F. B., Hornstra L. J., Perler F. B. In vitro protein splicing of purified precursor and the identification of a branched intermediate. Cell. 1993 Dec 31;75(7):1371–1377. doi: 10.1016/0092-8674(93)90623-x. [DOI] [PubMed] [Google Scholar]
- van Poelje P. D., Snell E. E. Cloning, sequencing, expression, and site-directed mutagenesis of the gene from Clostridium perfringens encoding pyruvoyl-dependent histidine decarboxylase. Biochemistry. 1990 Jan 9;29(1):132–139. doi: 10.1021/bi00453a016. [DOI] [PubMed] [Google Scholar]