Abstract
Background
Rodent lungs undergo full histologic recovery within one week following unilateral lung contusion (LC). However, when LC is followed by hemorrhagic shock (HS), healing is impaired. We hypothesize that the intravenous administration of mesenchymal stem cells (MSC) to animals undergoing combined LC followed by HS (LCHS) will improve wound healing.
Methods
Male Sprague-Dawley rats (n=5-6/group) were subjected to LCHS with or without the injection of a single iv dose of 5 × 106 MSCs following return of shed blood after HS. Rats were sacrificed seven days following injury. Flow cytometry was used to determine the T regulatory (Treg) cell population in peripheral blood (PB). Lung histology was graded using a well-established lung injury score (LIS). Components of the LIS include average inflammatory cells/high power field (hpf) over 30 fields, interstitial edema, pulmonary edema, and alveolar integrity with total scores ranging from 0-11. Data analyzed by ANOVA followed by Tukey's multiple comparison test, expressed as mean ± SD. p<0.05 considered significant.
Results
Seven days following isolated LC animals demonstrate lung healing with a LIS unchanged from naive. The addition of HS results in a persistently elevated LIS score, whereas addition of MSC to LCHS decreased the LIS score back to naïve levels. The change in LIS was driven by a significant decrease in edema scores. In rats undergoing LC alone, 10.5 ± 3.3% of CD4+ cells were Tregs. The addition of HS caused no significant change in Treg population (9.3±0.7%), whereas LCHS+MSC significantly increased the population to 18.2±6.8% in PB (p<0.05 vs LCHS).
Conclusion
Impaired wound healing following trauma and hemorrhagic shock is improved by a single dose of MSCs given immediately after injury. This enhanced healing is associated with an increase in the T regulatory cell population and a significant decrease in lung edema score as compared to animals undergoing LCHS. Further study into the role of Tregs in MSC-mediated wound healing is warranted.
Keywords: Cell-based therapy, Lung Injury, Immunomodulation, MSCs
INTRODUCTION
Traumatic injury induces a systemic inflammatory response necessary to initiate wound healing and return of tissue integrity1. However, when response is potentiated by hemorrhagic shock, there is an excessive upregulation of inflammatory mediators and migration of neutrophils into the tissues. These changes contribute to end-organ damage and dysfunction following severe injury2. Impairment of wound healing has long been recognized as a consequence of hemorrhagic shock3-5 and strategies to improve healing could significantly reduce trauma-associated morbidity.
Cell based therapy using mesenchymal stem cells (MSCs) has great potential following traumatic injury. These multipotent cells have both paracrine and immunomodulatory properties and have shown promise in treatment of multiple disease states including myocardial infarction, traumatic brain injury, and graft versus host disease6-8. One putative mechanism by which MSCs have been shown to act is by expanding the circulating population of T regulatory cells (Treg)9. Tregs are an immunosuppressive subset of T cells important for self-tolerance and maintaining immune homeostasis10.
Our lab has previously shown MSCs enhance wound healing following unilateral lung contusion11, but has never examined their role in a more severe injury model involving the second hit of hemorrhagic shock (HS). We hypothesize that the treatment of animals undergoing combined lung contusion (LC) and HS with MSCs given at the time of resuscitation can reverse HS-induced impairment of lung healing. Furthermore we hypothesize that this improvement in healing will be associated with an expansion of the systemic Treg population.
METHODS
Experimental Groups
Rats (n=5-6/group) were randomly assigned to undergo unilateral lung contusion (LC), LC followed by hemorrhagic shock (LCHS), or LCHS followed by the administration of MSCs (LCHS +MSC). Seven days following injury, rats were sacrificed; lungs were examined for histologic evidence of healing and blood was collected to assess the Treg population by flow cytometry.
Lung Contusion Model
Rats received intraperitonal sodium pentobarbital (50mg/kg) (B&B Pharmacy, Bellflower, CA) for anesthesia prior to undergoing unilateral lung contusion (LC). A 12mm metal plate was secured to the right axilla and LC was induced using the blast wave of a percussive nail gun (Craftsman 968514 Stapler, Sears Brands, Chicago IL).
Hemorrhagic Shock Model
As previously described24, The LCHS and LCHS/CS groups underwent cannulation of the right internal jugular vein with polyethylene (PE-50; Becton Dickinson and Co., Sparks, MD) and right femoral artery with Silastic (Dow Corning Corp., Midland, MI) tubing immediately following LC. All tubing was flushed with 10 units/ml of heparinized saline (Hospira Inc., Lakefront, IL) in order to prevent clotting. The femoral artery catheter was connected to a continuous blood pressure monitoring device for measurement of mean arterial pressure (MAP) and heart rate. Blood was withdrawn and rats were maintained at a MAP of 30-35mmHg for 45 minutes. Temperature was maintained at approximately 37°C with the use of an electric heating pad placed under the surgical platform. Following the completion of the shock period, shed blood was re-infused at a rate of 1mL/min.
Animals
Male Sprague-Dawley rats ranging from 250-350g (Charles River, Wilmington, MA), were maintained according to the recommendations of the Guide for the Care and Use of Laboratory Animals. Use was approved by the Rutgers New Jersey Medical School Amimal Care and Use Committee. Rats were housed in a 25° C barrier-sustained animal facility with 12-hour light/dark cycles and free access to water and chow (Teklad 22/5 Rodent Diet W-8640; Harlen, Madison WI).
Mesenchymal Stem Cell Culture
Sprague-Dawley rat mesenchymal stem cells (MSCs) (Cyagen Biosciences, Santa Clara, CA) were cultured and expanded. Briefly, after thawing, cells were transferred into 15mL OriCell MSC Growth Medium. Cells were then centrifuged at 250 × g for 5 minutes, supernatant removed, and cells resuspended and seeded into T25 flasks containing growth medium. Cells were incubated with 5% humidified CO2 at 37°C. Medium was changed the day following incubation then every three days. Once cells reached 80-90% confluence they were dissociated with Trypsin-EDTA and re-seeded. Cell lines were maintained until harvesting for injection. On the day of injection, MSCs were aliquoted into preparations of 5 × 106 cells in one mL Iscove's Modified Dulbecco's Medium (IMDM) and incubated at 37°C until injection.
Mesenchymal Stem Cell Injection
As above, animals were anesthetized with intraperitoneal sodium pentobarbital (50mg/kg) and underwent LCHS. Within ten minutes of completion of resuscitation, injection of 5 × 106 cells in one mL IMDM was given into the jugular vein over five minutes.
Lung Histology and Lung Injury Score
Lungs were placed in 10% buffered formalin following sacrifice. Slide preparation consisted of dehydrating samples and embedding them in paraffin blocks. Four-micrometer thick sections were stained with hematoxylin and eoisin (H&E). Degree of injury was scored according to a quantitative lung injury score (LIS) using standard light microscopy. LIS ranged between 0 and 11, with 11 representing the most severe injury12 (Table 1). 30 random high power fields/sample were examined for grading. Slides were coded and read by one of two interpreters blinded to their origin.
TABLE 1.
Lung Injury Score
| Subgroup | Points |
|---|---|
| Interstitial Edema | |
| None | 0 |
| Minimal | 1 |
| Moderate | 2 |
| Severe | 3 |
|
Pulmonary Edema | |
| <5% | 0 |
| 5-25% | 1 |
| >25% | 2 |
|
Alveolar Integrity | |
| Normal | 0 |
| Abnormal moderate | 1 |
| Abnormal severe | 2 |
|
Inflammatory Cells/HPF | |
| <5 | 0 |
| 6-10 | 1 |
| 11-15 | 2 |
| 16-20 | 3 |
| >20 | 4 |
Flow Cytometry for % T-regulatory Cells
At the time of sacrifice, whole blood was collected by direct cardiac puncture using a heparinized syringe. Blood was analyzed for the presence of Treg by flow cytometry with the use of the T-regulatory Cell Staining Kit from eBioscience, Inc (San Diego, CA). Briefly, 100μL aliquots (one million cells) of blood in 5mL polystyrene tubes were stained with 10μL of each stain: BD Pharmingen™ mouse anti-rat CD4 antibody conjugated with fluorescein isothiocyanate (FITC) and BD Pharmingen™ rat anti-mouse CD25 antibody conjugated to phycoerythrin (PE) (BD Biosciences, Franklin Lakes, NJ). Samples were incubated in the dark for 30 minutes. After being centrifuged at 300 × g for 5 minutes, cells were washed with stain buffer for a total of two washes. Cells were then resuspended in 1mL FoxP3 fixation/permeabilization working solution, pulse vortexed, and incubated for two hours in the dark. Without washing, 2mL fixation/permeabilization working solution was added to each tube prior to centrifugation and washing. Cells were resuspended in 100μL 1X permeabilization buffer and stained with 10μL eBioscience mouse anti-rat FoxP3 conjugated to APC. Samples were then incubated in the dark for 30 minutes. A final two washes with permeabilization buffer were performed prior to resuspension in stain buffer. Cells were analyzed using BD FACSCalibur flow cytometer (BD) equipped with CellQuest software (BD) with an event count of 300,000 for each run.
Statistical Analysis
Data presented as mean ± SD. Statistical analysis using GraphPad Prism (Version 4.0, San Diego, CA) consisted of one-way analysis of variance (ANOVA) followed by Tukey-Kramer's multiple comparison post-test. *p <0.05 vs. LC or **p <0.05 vs LCHS considered significant.
RESULTS
Mesenchymal Stem Cell Decrease Total Lung Injury Score
Seven days following unilateral LC, the lung injury has completely healed, with a mean lung injury score (LIS) of 0.8 ± 0.4. Those rats that underwent LCHS had a LIS of 3.7 ± 0.81 (p<0.05 vs LC), indicating that the addition of HS delays the normal healing pattern. When rats received MSCs following LCHS, LIS returned to 1.6 ± 0.6 at one week (p<0.05 vs LCHS), which is not statistically different from LC alone (Table 2).
Table 2.
LIS Total and Subgroup Score Seven Days Following Injury
| Group | Inflammatory cells/hpf | Interstitial Edema | Pulmonary Edema | Alveolar Integrity | Total LIS |
|---|---|---|---|---|---|
| LC | 0 ± 0 | 0.75 ± 0.5 | 0 ± 0 | 0 ± 0 | 0.8 ± 0.4 |
| LCHS | 0.4 ± 0.5* | 2.0 ± 0* | 1.0 ± 0.7* | 0.4 ± 0.5 | 3.7 ± 0.81* |
| LCHS+MSC | 1.0 ± 0** | 0.4 ± 0.5** | 0 ± 0** | 0.2 ± 0.4 | 1.6 ± 0.6** |
LC= lung contusion, LCHS= lung contusion hemorrhagic shock, MSC= mesenchymal stem cells. Data presented as mean score ± standard deviation; n= 5-6/group
p<0.05 vs LC
p<0.05 vs LCHS
Mesenchymal Stem Cells Affect Lung Subgroup Scores
Pulmonary and Interstitial Edema Scores
Lung injury subgroup scores at seven days for pulmonary and interstitial edema are 0 ± 0 and 0.75 ± 0.5 respectively. In those animals undergoing LCHS, there is still a moderate amount of interstitial edema, with a subgroup score of 2.0 ± 0 (p<0.05 vs LC). Pulmonary edema remains as well, affecting 5-25% of the lung parenchyma, with LIS subgroup score of 1.0 ± 0.7 (p<0.05 vs LC). MSC administration reversed the pulmonary and interstitial edema seen in rats undergoing LCHS with subgroup scores of 0.4 ± 0.5 and 0 ± 0 respectively (p<0.05 vs LCHS) (Supplementary Digital Content 1).
Alveolar Integrity Score
The alveolar integrity subscore is 0 ± 0 in rats undergoing LC alone seven days after injury. There is no significant change in either rats undergoing LCHS or LCHS + MSCs (subgroup scores of 0.4 ± 0.5 and 0.2 ± 0.4 respectively; p>0.05) (Table 2).
Number of Inflammatory Cells
In those rats undergoing LC alone, the subgroup score for inflammatory cells/high power field (hpf) is 0 ± 0. While this score is elevated in rats undergoing LCHS (0.4 ± 0.5, p<0.05 vs LC), it is even higher in those animals receiving MSCs (1.0 ± 0, p<0.05 vs LCHS) one week following injury (Table 2).
Mesenchymal Stem Cells Expand the T Regulatory Cell Population
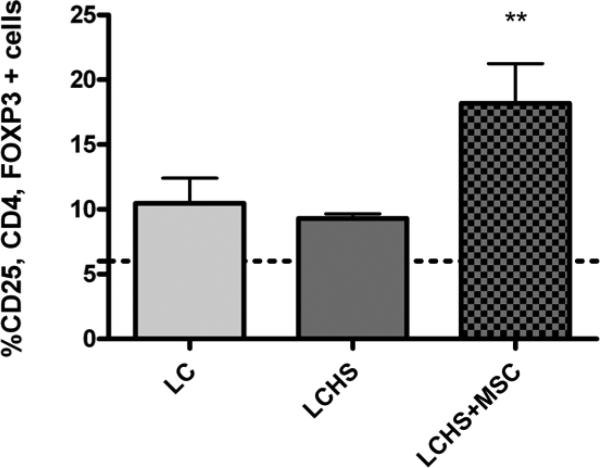
At day seven following injury, the percent of circulating T regulatory cells found in the peripheral blood of SD rats undergoing LC alone was 10.5 ± 3.3%, not significantly different from naïve rats (6.0 ± 1.5, p>0.05). The addition of HS resulted in no significant change in peripheral Tregs. (Figure 1). Rats receiving MSCs following LCHS had a significantly increased proportion of Tregs in their peripheral blood at 18.2 ± 6.8% (p<0.05 vs LCHS) (Figure 1).
Figure 1. T-Regulatory Cell Population Seven Days Following Injury.
Dotted line represents naïve. LC= lung contusion, LCHS= lung contusion hemorrhagic shock, MSC= mesenchymal stem cells. Data presented as mean score ± standard deviation; n= 5-6/group; *p<0.05 vs LCHS.
DISCUSSION
Following unilateral LC, lungs display complete histologic healing within seven days11, however, wound healing is delayed when LC is followed by a period of hemorrhagic shock. Hannoush et al previously demonstrated that MSCs hasten healing following isolated LC, with histologic recovery evident two days earlier, or five days post-injury11. Our data presented here demonstrates a single iv dose of MSCs given immediately following resuscitation reverses HS induced wound healing dysfunction. Furthermore, this improvement in wound healing is associated with an expansion of the peripheral Treg population.
MSCs have been studied as a potential cell based therapy in multiple clinical scenarios including fulminant hepatic failure, myocardial infarction, and acute renal failure6,13-14. Studies on the effect of MSCs on lung healing have predominately looked at acute injury induced by barotrauma, chemical injury, or remote injury in the setting of sepsis and shock15-17. The current study is one of the first times MSCs have been studied in the context of direct traumatic injury followed by shock. While it was initially believed that the mechanism of action of MSCs was secondary to their capacity to differentiate and engraft into tissues, it appears that their immunomodulatory properties are much more important18. Xu et al showed MSCs decreased pulmonary edema in mice undergoing endotoxin-induced ALI as well as decreasing the number of neutrophils within the alveolar airspace16. This decrease in edema and inflammatory cells was associated with decreased levels of pro-inflammatory cytokines including IFN- γ and IL-1β. In the current study we demonstrate impaired wound healing with the addition of hemorrhagic shock following unilateral lung contusion that is associated with persistently elevated edema scores in the injured lung and an increase in inflammatory cells as compared to LC alone. This is consistent with our findings in that our data indicate that the administration of MSCs results in significant decreases in both pulmonary and interstitial edema.
While MSCs have been shown to interact with most cells of the immune system, of particular interest is their interaction with Tregs. Following traumatic injury, the initial pro-inflammatory response is mediated by activation of T helper 1 (Th1) lymphocytes, whereas the late-occurring anti-inflammatory response is driven by T helper 2 (Th2) cells19. T regulatory cells (Treg) interact with cells of both the innate and adaptive immune system and work to restore Th1/Th2 balance, shifting the immune response toward an anti-inflammatory, or Th2 phenotype20. Zhang et al recently examined the changes in Treg population as well as Th1/Th2 balance in rats undergoing hemorrhagic shock and found an early decrease in Tregs and shift toward Th121. When looked at later after injury, the Treg population has been shown to increase. Cook et al investigated the Treg population following LC alone and showed an increase in Treg 5 days after injury. This was further increased by treatment with MSCs9. There is mounting evidence that this Treg response is necessary for healing. When Cook looked at the effect of MSCs in unilateral LC and blocked Tregs, MSCs no longer had a therapeutic effect on healing22. In addition, Kavanagh et al showed a protective effect of MSCs by expansion of the Treg population in allergic airway inflammation that was no longer seen when the Treg population was depleted23. Our data similarly indicate that a MSC-mediated expansion of the peripheral T regulatory cell population may be responsible for the improvement in wound healing. In those animals receiving MSCs, edema scores were significantly lower than those in animals undergoing LCHS. While the inflammatory cell score is higher as compared to both LC alone and LCHS it may be that these cells, while physically present within the lung parenchyma, have been inhibited either directly by the MSCs or by the immunosuppressive Tregs and are functionally inactivated.
In summary, a single dose of MSCs given immediately following resuscitation improves wound healing following trauma and hemorrhagic shock. This enhanced healing is associated with significant decreases in both pulmonary and interstitial edema as well as an increase in the peripheral Treg population. While the inflammatory cell component of the LIS remains elevated, this may be secondary to either functional inactivation of inflammatory cells within the lung by MSCs or Tregs, or an increase in the number of Tregs within the lung itself. Further study is needed to elucidate the mechanisms by which MSCs enhance healing and how their apparent benefit in pre-clinical animal models can be translated to the care of human trauma patients. This further characterization of the immunomodulatory pathways of MSCs will enhance our understanding of the inflammatory pathway and immune interactions following severe injury, potentially opening up new avenues for therapy. As MSCs themselves do not incite an immune response and treatment with MSCs does not require concomitant immunosuppression, they have the potential to become a readily available cellular therapy to limit the secondary effects of trauma in selected patients.
Supplementary Material
Acknowledgments
This research was supported by the National Institutes of Health grant T32 GM069330.
Footnotes
This study was presented at the 28th annual meeting of the Eastern Association for the Surgery of Trauma, January 13–17, 2015, in Lake Buena Vista, Florida.
Authorship Statement: The role of each author is as described below. Amy Gore was involved in experimental design, data acquisition, analysis and interpretation of data, and manuscript preparation. Letitia Bible and Kimberly Song in data acquisition and analysis. David Livingston, Alicia Mohr, and Ziad Sifri in design, data analysis and interpretation, and critical revision.
The authors have nothing else to disclose nor any conflicts to report.
REFERENCES
- 1.Thornton FJ, Schaffer MR, Barbul A. Wound healing in sepsis and trauma. Shock. 1997;8(6):391–401. [PubMed] [Google Scholar]
- 2.Hierholzer CH, Billiar TR. Molecular mechanisms in the early phase of hemorrhagic shock. Langenbeck's Arch Surg. 2001;386:302–308. doi: 10.1007/s004230100242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGinn FP. Effects of haemorrhage upon surgical operations. Br J Surg. 1976;63(10):742–746. doi: 10.1002/bjs.1800631005. [DOI] [PubMed] [Google Scholar]
- 4.Guiney DJ, Morris PJ, Donaldson GA. Wound dehiscence. A continuing problem in abdominal surgery. Arch Surg. 1966;92:47–51. doi: 10.1001/archsurg.1966.01320190049011. [DOI] [PubMed] [Google Scholar]
- 5.Angele MK, Knoferl MW, Ayala A, Albina JE, Cioffi WG, Bland KI, Chaudry IH. Trauma-hemorrhage delays wound healing potentially by increasing pro-inflammatory cytokines at the wound site. Surgery. 1999;126(2):279–285. [PubMed] [Google Scholar]
- 6.Miyahara Y, Nagaya N, Kataoka M, Yanagawa B, Tanaka K, Hao H, Ishino K, Ishida H, Shimizu T, Kangawa K, et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006;12:459–465. doi: 10.1038/nm1391. [DOI] [PubMed] [Google Scholar]
- 7.Walker PA, Bedi SS, Shah SK, Jimenez F, Xue J, Hamilton JA, Smith P, Thomas CP, Mays RW, Pati S, et al. Intravenous multipotent adult progenitor cell therapy after traumatic brain injury: modulation of the resident microglia population. J Neruoinflam. 2012;9:228–240. doi: 10.1186/1742-2094-9-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371(9624):1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 9.Cook K, Sifri Z, Baranski G, Rameshwar P, Mohr A, Livingston D. The role of T regulatory cells in mesenchymal stromal cell (MSC)-mediated wound healing. Surgical Infections. 2012;13(1):S15. [Google Scholar]
- 10.Vignali DAA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8(7):523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hannoush EJ, Elhassan I, Sifri ZC, Mohr AM, Alzate WD, Livingston DH, et al. Role of bone marrow and mesenchymal stem cells in healing after traumatic injury. Surgery. 2013;153(1):44–51. doi: 10.1016/j.surg.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 12.Claridge JA, Enelow RI, Young JS. Hemorrhage and resuscitation induce inflammation and pulmonary dysfunction in mice. J Surg Res. 2000;92:206–213. doi: 10.1006/jsre.2000.5899. [DOI] [PubMed] [Google Scholar]
- 13.Parekkadan B, van Poll D, Suganuma K, Carter EA, Berthiaume F, Tilles AW, Yarmush ML. Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS One. 2007;2:e941. doi: 10.1371/journal.pone.0000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Togel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31–F42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 15.Curley GF, Hayes M, Ansari B, Shaw G, Ryan A, Barry F, O'Brien T, O'Toole D, Laffey JG. Mesenchymal stem cells enhance recovery and repair following ventilator-induced lung injury in the rat. Thorax. 2012;67:496–501. doi: 10.1136/thoraxjnl-2011-201059. [DOI] [PubMed] [Google Scholar]
- 16.Xu J, Woods CR, Mora AL, Joodi R, Brigham KL, Iyer S, Rojas M. Prevention of endotoxin-induced systemic response by bone marrow-derived mesenchymal stem cells in mice. Am J Physiol Lung Cell Mol Physiol. 2007;293:L131–L141. doi: 10.1152/ajplung.00431.2006. [DOI] [PubMed] [Google Scholar]
- 17.Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, Phinney DG. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A. 2003;100(14):8407–11. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matthay MA, Goolaerts A, Howard J, Lee JW. Mesenchymal stem cells for acute lung injury: preclinical evidence. Crit Care Med. 2010;38(10):S569–S573. doi: 10.1097/CCM.0b013e3181f1ff1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore FA, Moore EE. Evolving concepts in the pathogenesis of postinjury multiple organ failure. Surg Clin North Am. 1995;75:257–77. doi: 10.1016/s0039-6109(16)46587-4. [DOI] [PubMed] [Google Scholar]
- 20.Venet F, Chung CS, Monneret G, Huang X, Horner B, Garber M, Ayala A. Regulatory T cell populations in sepsis and trauma. J Leuk Biol. 2008;83(3):523–535. doi: 10.1189/jlb.0607371. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Q, Lu YQ, Jiang JK, Gu LH, Mou HZ. Early changes of CD4+CD25+Foxp3+ regulatory T cells and Th1/Th2, Tc1/Tc2 profiles in the peripheral blood of rats with controlled hemorrhagic shock and no fluid resuscitation. Chin Med J. 2012;125(12):2163–2167. [PubMed] [Google Scholar]
- 22.Cook K, Pasupuleti L, Alzate W, Mohr A, Livingston D, Sifri Z. Blocking T- regulatory Cell Response Impairs MSC-Mediated Wound Healing. Journal of Surgical Research. 2013;179(2):321. [Google Scholar]
- 23.Kavanagh H, Mahon BP. Allogeneic mesenchymal stem cells prevent allergic airway inflammation by inducing murine regulatory T cells. Allergy. 2011;66:523–531. doi: 10.1111/j.1398-9995.2010.02509.x. [DOI] [PubMed] [Google Scholar]
- 24.Pasupuleti LV, Cook KM, Sifri ZC, Kotamarti S, Calderon GM, Alzate WD, Livingston DH, Mohr AM. Does selective beta-1 blockade provide bone marrow protection after trauma/hemorrhagic shock? Surgery. 2012;152(3):322–30. doi: 10.1016/j.surg.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.