Abstract
Diagnosing dizziness can be challenging, and the consequences of missing dangerous causes such as stroke can be substantial. Most physicians use a diagnostic paradigm developed over 40 years ago that focuses on the type of dizziness (e.g., vertigo vs. non-vertigo), but this approach is flawed. In this article we propose a new paradigm based on symptom timing, triggers, and targeted bedside eye examinations (‘TiTrATE’). Using timing and triggers, patients with recent-onset dizziness will fall into one of four major ‘syndrome’ categories (triggered episodic, spontaneous episodic, post-exposure acute, and spontaneous acute), each with its own differential diagnosis and set of targeted examination techniques that help clinicians make a specific diagnosis. Following an evidence-based approach such as this could help reduce the frequency of misdiagnosis of serious causes of dizziness.
Introduction
Dizziness accounts for 3.3–4.4% of emergency department (ED) visits.1–3 This translates into over 4.3 million ED patients with dizziness or vertigo annually in the US4 and probably 50–100 million worldwide.
‘Dizziness’ means different things to different people. Patients may describe feeling dizzy, lightheaded, faint, giddy, spacey, off-balance, rocking, swaying, or spinning. Expert international consensus definitions for vestibular5 and related symptoms6 are shown in Box 1. While historically much has been made of the distinction between the terms ‘dizziness’ and ‘vertigo,’ current evidence (described in chapter 3) suggests the distinction is of limited clinical utility. We will not make a distinction between these terms in this manuscript unless specifically noted.
Box 1. International consensus definitions for major vestibular symptoms.
Dizziness is the sensation of disturbed or impaired spatial orientation without a false or distorted sense of motion. This includes sensations sometimes referred to as giddiness, lightheadedness, or non-specific dizziness, but does not include vertigo.
Presyncope (also near syncope or faintness) is the sensation of impending loss of consciousness. This sensation may or may not be followed by syncope. When patients report “lightheadedness,” it should be classified as presyncope, dizziness, or both.
Syncope (also faint) is transient loss of consciousness due to transient global cerebral hypoperfusion characterized by rapid onset, short duration, and spontaneous complete recovery. Syncope usually leads to loss of postural control and falling.
Vertigo is the sensation of self-motion (of head/body) when no self-motion is occurring or the sensation of distorted self-motion during an otherwise normal head movement.
Unsteadiness is the feeling of being unstable while seated, standing, or walking without a particular directional preference. This sensation has previously been called disequilibrium or imbalance.
The differential diagnosis of dizziness is broad with no single cause accounting for more than 5–10% of cases.1 We will focus on the most common and most serious causes of new-onset dizziness in adults. More than 15% of patients presenting with dizziness to an ED will have dangerous causes.1 Sometimes, a serious etiology is obvious based on the presentation (e.g., dizziness with fever, cough and hypoxia due to pneumonia). Other times, dangerous conditions can present with isolated dizziness that mimics benign problems.7–11 Misdiagnosis in this latter group is not uncommon, even when patients are evaluated by neurologists.12–16 An important clinical goal is to distinguish serious from benign causes using the fewest resources possible. On average, however, diagnosing dizziness consumes disproportionate resources through extensive testing and hospital admission.1, 4 Indiscriminate application of CT, CTA, and MRI has low yield and low value in this patient population,17–21 yet brain imaging for dizziness continues to increase steadily over time.4 Use of brain imaging varies 1.5-fold across hospitals without differences in the detection of neurologic causes.22 Annual spending on patients with dizziness in US EDs is now $4 billion,4 with another $5 billion spent on those admitted.23
Previously, the evidence base for diagnosing patients with dizziness was limited.24 A proliferation of recent research, however, has supplied clinicians with high-quality data to guide bedside diagnosis and management, particularly with regard to identifying cerebrovascular causes. In this article we propose a new diagnostic paradigm based on symptom ‘timing and triggers,’ derived from recent advances in evidence-based, targeted bedside exams for specific dizziness subpopulations. We focus primarily on new, acute dizziness presentations, and limit discussions about treatment except where specifically relevant to initial ED management.
New Diagnostic Approach
Accumulating evidence over the past decade suggests using a different approach based on the timing and triggers for dizziness symptoms, rather than type.25, 26 Timing refers to the onset, duration, and evolution of the dizziness. Triggers refer to actions, movements or situations that provoke the onset of dizziness in patients who have intermittent symptoms.
A ‘timing and triggers’ history in dizziness results in six possible ‘syndromes’ (Table 1). This conceptual approach has been endorsed by an international committee of specialists tasked with formulating vestibular research definitions;27 (also see chapter 1). Each syndrome suggests a specific differential diagnosis and targeted bedside exam, described further in the sections below. The ‘Triage—TITRATE—Test’ method (Figure 1) results in a new diagnostic algorithm (Figure 2). We will focus on the four acute syndromes and not discuss the two chronic syndromes here. Some patients with a chief symptom of dizziness will have prominent associated features suggesting a likely diagnosis (Table 2). Our emphasis will be on those with ‘isolated’ dizziness or vertigo. ‘Isolated’ here excludes major medical or general neurologic symptoms, but includes headache and the typical otologic (e.g., hearing loss, tinnitus, ear fullness), autonomic (e.g., nausea/vomiting), or balance (e.g., gait unsteadiness/ataxia) accompaniments normally encountered in patients with acute vestibular symptoms.
Table 1.
Timing-and-trigger-based ‘vestibular* syndromes’
| Timing | Triggers† Present | No Triggers |
|---|---|---|
| New, episodic | Triggered episodic vestibular syndrome (t-EVS) (e.g., positional vertigo from BPPV) | Spontaneous episodic vestibular syndrome (s-EVS) (e.g., cardiac arrhythmia) |
| New, continuous | Post-exposure acute vestibular syndrome (t-AVS) (e.g., post gentamicin) | Spontaneous acute vestibular syndrome (s-AVS) (e.g., posterior fossa stroke) |
| Chronic, persistent | Context-specific chronic vestibular syndrome (t-CVS) (e.g., uncompensated unilateral vestibular loss, present only with head movement) | Spontaneous chronic vestibular syndrome (s-CVS) (e.g., chronic, persistent dizziness associated with cerebellar degeneration) |
Note that the use of the word ‘vestibular’ here connotes vestibular symptoms (dizziness or vertigo or imbalance or lightheadedness, etc.), rather than underlying vestibular causes (e.g., benign paroxysmal positional vertigo, vestibular neuritis).
‘Triggers’ here for non-spontaneous forms refer to obligate triggers (EVS), exposures (AVS), and contexts (CVS) that sharply distinguish these forms from their spontaneous counterparts. Spontaneous causes, as defined here, sometimes have underlying predispositions or precipitants, but these are not ‘only-and-always.’
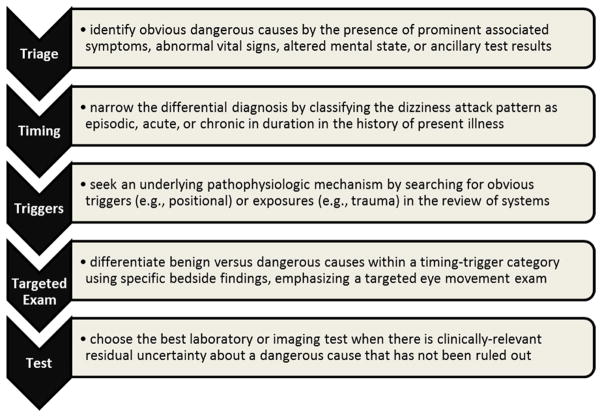
Figure 1. The “Triage – TITRATE – Test” approach to diagnosing dizziness and vertigo.
The ‘TI.TR.A.T.E.’ acronym stands for TIming, TRiggers, And Targeted Exams.
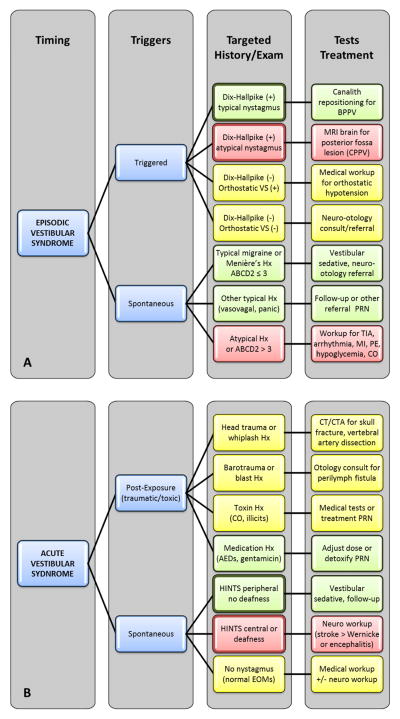
FIGURE 2. TITRATE algorithm for differential diagnosis and workup of dizziness and vertigo.
The TITRATE algorithm divides patients into four key categories: triggered and spontaneous forms of episodic (Panel A) and acute (Panel B) vestibular syndromes. Each syndrome determines a targeted bedside exam, differential diagnosis, and tests, regardless of symptom type (vertigo, presyncope, unsteadiness, non-specific dizziness). Some steps may occur after the ED visit, as part of follow-up or during inpatient hospital admission. Box color in the ‘Targeted’ and ‘Tests’ columns denotes risk of a dangerous disorder (red – high; yellow – intermediate; green – low). Bold outlines denote evidencebased, targeted eye exams that discriminate between benign and dangerous causes (see Tables 3, 4).
Table 2.
Prominent associated symptoms, signs, or laboratory results that may be available at the initial ‘Triage’ step to inform diagnosis in dizziness/vertigo
| Symptom or finding | Diagnoses that are suggested by the finding |
|---|---|
| Altered mental status | Wernicke’s encephalopathy; stroke; encephalitis; seizure; intoxication with alcohol, illicit drugs, carbon monoxide; hypertensive encephalopathy |
| Transient loss of consciousness | Arrhythmia; acute coronary syndrome; aortic dissection; pulmonary embolism; vasovagal syncope; hypovolemia; stroke; subarachnoid hemorrhage; seizure |
| Headache | Stroke; craniocervical vascular dissection; meningitis; carbon monoxide exposure; vestibular migraine; high or low intracranial pressure; subarachnoid hemorrhage |
| Neck pain | Craniocervical vascular dissection (esp. vertebral artery) |
| Chest/back pain | Acute coronary syndrome; aortic dissection |
| Abdominal/back pain | Ruptured ectopic pregnancy; aortic dissection |
| Dyspnea | Pulmonary embolism; pneumonia; anemia |
| Palpitations | Arrhythmia; vasovagal syncope; panic disorder |
| Bleeding or fluid losses | Hypovolemia; anemia |
| New/recent medication use | Medication side effects or toxicity (e.g., gentamicin) |
| Fever or chills | Systemic infection; encephalitis; mastoiditis; meningitis |
| Abnormal glucose | Symptomatic hypoglycemia, diabetic ketoacidosis |
Four Vestibular Syndromes
In the sections that follow, we describe the four key vestibular syndromes in ED patients presenting recent intermittent or continuous dizziness: triggered episodic, spontaneous episodic, post-exposure acute, and spontaneous acute. The word ‘vestibular’ here refers to vestibular symptoms (dizziness, vertigo, unsteadiness, or lightheadedness), not underlying vestibular causes. For ‘triggered episodic’ and ‘spontaneous acute’ syndromes, the focus is targeted bedside examination, emphasizing eye movements (Table 3, Table 4). For ‘spontaneous episodic’ and ‘post-exposure acute’ syndromes, the focus is targeted history-taking (Table 4). Although full details for individual diseases are presented in other chapters, we summarize here key aspects related to early differential diagnostic considerations.
Table 3.
Nystagmus characteristics in key peripheral and central vestibular disorders
| Vestibular Condition | Test Maneuver | Nystagmus Duration | Trajectory/Direction | Variation in Direction |
|---|---|---|---|---|
| Triggered Episodic Vestibular Syndrome* (episodic nystagmus triggered by specific positional maneuvers) | ||||
| posterior canal BPPV | head hanging with 45° turn to each side (Dix-Hallpike) | 5–30 seconds † | upbeat-torsional ‡ | direction reversal on arising |
| horizontal canal BPPV | supine roll to either side (Pagnini–McClure) | 30–90 seconds † | horizontal | spontaneous reversal during test |
| central paroxysmal positional vertigo | any (usually head hanging) | 5–60+ seconds † (sometimes persistent if position is held) | any (usually downbeat or horizontal) | any (often direction-fixed) |
| Spontaneous Acute Vestibular Syndrome* (spontaneous nystagmus that may be exacerbated non-specifically by various head maneuvers) | ||||
| vestibular neuritis or labyrinthitis | gaze testing § | persistent | dominantly horizontal | direction-fixed (acutely) |
| stroke | gaze testing § | persistent | any (usually dominantly horizontal, occasionally vertical or torsional) | direction-fixed or direction-changing with gaze position |
Abbreviations: BPPV – benign paroxysmal positional vertigo
Key – green – very likely peripheral nystagmus; red – very likely central nystagmus; black – indeterminate nystagmus (other eye movement features may be diagnostic)
Only two syndromes (t-EVS, s-AVS) are shown in this table because the other two syndromes (s-EVS, t-AVS) lack characteristic, diagnostic patterns of nystagmus.
BPPV nystagmus usually begins after a delay (latency) of a few seconds, peaks in intensity rapidly, then decays monophasically as long as the head is held stationary. In the horizontal canal variant, the nystagmus may be biphasic, with a spontaneous direction reversal after the initial nystagmus, even if the head is held motionless. Central paroxysmal positional vertigo may begin immediately or after a delay, may decay or persist, and may or may not change direction during testing.
Torsion with the 12 o’clock pole (top) of the eye beating towards down-facing (tested) ear, sometimes referred to as ‘geotropic’ (i.e., towards the ground).
In the acute vestibular syndrome, gaze testing is useful but positional tests are not. With peripheral lesions, nystagmus should increase in intensity when the patient’s gaze is directed towards the fast phase of the nystagmus, and should not reverse. With central lesions, this same pattern may occur, but more than one third of the time, the nystagmus will reverse direction when the patient’s gaze is directed away from the fast phase of the nystagmus (i.e., is ‘direction-changing’ with gaze position).
Table 4.
‘Safe to go’ features for the most common, benign vestibular* causes of isolated dizziness and vertigo
| Syndrome† | Targeted Exam | Benign Disorder | Dangerous Mimic | ‘Safe-to-Go’ Features |
|---|---|---|---|---|
| t-EVS | orthostatic vitals; positional tests for nystagmus | BPPV | posterior fossa mass |
|
| s-EVS | head, neck, & cranial nerve history; ear, hearing history | vestibular migraine or Meniere’s disease | TIA |
|
| s-AVS | HINTS; ear, hearing exam | vestibular neuritis | stroke ‡ |
|
Abbreviations: a.k.a. – also known as; BPPV – benign paroxysmal positional vertigo; HINTS – head impulse, nystagmus type, skew; s-AVS – spontaneous acute vestibular syndrome; s-EVS – spontaneous episodic vestibular syndrome; t-EVS – triggered episodic vestibular syndrome; TIA – transient ischemic attack
We highlight vestibular disorders in this Table because ED physicians have a high degree of comfort diagnosing other benign causes of isolated t-EVS (e.g., orthostatic hypotension) and isolated s-EVS (e.g., vasovagal syncope). Dangerous non-vestibular, non-neurologic causes (principally for s-EVS) are rarely isolated (see Table 2).
Only three syndromes (t-EVS, s-EVS, s-AVS) are shown in this table because the other syndrome (t-AVS) is typically diagnosed based largely on exposure history.
Findings on the HINTS exam (head impulse vestibular reflexes; primary and lateral gaze testing for nystagmus; cover test for vertical eye alignment) that suggest stroke are given the acronym ‘I.N.F.A.R.C.T.’ 96 – Impulse Normal; Fast-Phase Alternating; Refixation on Cover Test. Thus if an s-AVS patient has any one of these three eye signs (bilaterally normal head impulses; direction-changing, gaze-evoked nystagmus; or vertical skew deviation), stroke is likely.
Episodic Vestibular Syndrome
The episodic vestibular syndrome (EVS) involves intermittent dizziness lasting seconds, minutes, or hours. Episode duration is more important than total illness duration. Most such patients have multiple, discrete episodes spaced out over time. Relapsing and remitting symptoms lasting weeks at a time, such as sometimes seen in multiple sclerosis, should not be considered in this category. EVS is divided into triggered and spontaneous forms, each discussed below.
Triggered EVS (t-EVS)
Approach
Episodes of the t-EVS are precipitated by some specific obligate action or event. The most common triggers are head motion or change in body position (e.g., arising from a seated or lying position, tipping the head back in the shower to wash one’s hair, or rolling over in bed). Uncommon triggers include loud sounds or Valsalva maneuvers, among others.5 Attacks usually last seconds to minutes, depending on the underlying etiology. Because some vestibular forms are provoked repetitively and frequently, or patients’ nausea can linger between spells, some patients may overstate episode duration. This can usually be sorted out by careful history taking.
It bears emphasis that clinicians must distinguish triggers (head or body motion provokes new symptoms not present at baseline) from exacerbating features (head or body motion worsens pre-existing baseline dizziness). Head movement typically exacerbates any dizziness of vestibular cause (benign or dangerous, central or peripheral, acute or chronic). The concept that worsening of dizziness with head motion equates with a peripheral cause is a common misconception.28
The goal of physical examination in t-EVS is to reproduce the patient’s dizziness in order to witness the corresponding pathophysiology (e.g., falling blood pressure on arising or abnormal eye movements with Dix-Hallpike testing). A caveat for postural symptoms is that orthostatic dizziness and orthostatic hypotension are not always related.29, 30 Orthostatic hypotension may be incidental and misleading, especially in older patients taking anti-hypertensive medications.31 Conversely, dizziness on arising without systemic orthostatic hypotension may indicate hemodynamic transient ischemic attack (TIA) from hypoperfusion distal to a cranial vascular stenosis32 or, alternatively, intracranial hypotension.33 Neurological evaluation is probably indicated for patients with reproducible and sustained orthostatic dizziness but no demonstrable hypotension or BPPV.
Prototype t-EVS causes are BPPV and orthostatic hypotension. Dangerous causes include neurologic mimics known as ‘central paroxysmal, positional vertigo’ (CPPV) (e.g., posterior fossa mass lesions34) and serious causes of orthostatic hypotension,35 such as internal bleeding. All are associated with episodic positional symptoms, but can be readily distinguished from one another using targeted bedside history and exam. Orthostatic hypotension causes symptoms only on arising, whereas BPPV causes symptoms both on arising and on lying back, or when rolling in bed.36 BPPV and CPPV can be distinguished based on characteristic eye exam differences on standard positional tests for nystagmus including the Dix-Hallpike test (Table 3).37
Diseases
BPPV is the most common vestibular disorder in the general population, with a lifetime prevalence of 2.4% and increasing incidence with age.36 In the ED, it is probably the second most common cause, accounting for nearly 10% of ED dizzy presentations.16 It results from mobile crystalline debris trapped in one or more semicircular canals (“canaliths”) within the vestibular labyrinth. Symptoms and signs vary based on the canal(s) involved and whether the crystals are free-floating or trapped.38 Classical symptoms are repetitive brief, triggered episodes of rotational vertigo lasting more than a few seconds but less than one minute, although non-vertiginous symptoms of dizziness or even presyncope are frequent.39
The diagnosis is confirmed by reproducing symptoms and signs using canal-specific positional testing maneuvers and identifying a canal-specific nystagmus (Table 3).38 Since the offending canal(s) are generally not known in advance, multiple diagnostic maneuvers are typically performed. Proven bedside treatments to displace the offending crystals (canalith repositioning maneuvers) are also canal-specific.37, 40 As mentioned above, BPPV mimics include orthostatic hypotension and CPPV. Patients with atypical nystagmus forms (e.g., downbeat or horizontal) on Dix-Hallpike testing usually have CPPV, and some cases are due to posterior fossa tumors or strokes.37 CPPV includes common, benign causes such as intoxication with alcohol or sedative drugs, but such patients are more apt to complain of continuous, persistent dizziness exacerbated (rather than triggered) by position change and are usually readily diagnosed based on context and other signs of intoxication.
Orthostatic hypotension is common, accounting for 24% of acute syncopal spells.41 Classical symptoms are brief lightheadedness or a feeling of near syncope on arising, but vertigo is common42 and underappreciated.28 Orthostatic hypotension is caused by numerous conditions that produce hypovolemia, cardiac dysfunction, or reduced vasomotor tone. The most common causes are medications and hypovolemia.41
The primary dangerous concern is internal bleeding. Strong bedside predictors of moderate hypovolemia from blood loss are postural dizziness so severe as to prevent standing or a postural pulse increment >30 beats per minute, but the sensitivity of these findings is only 22%.43 Furthermore, the benign postural orthostatic tachycardia syndrome (POTS) produces similar clinical findings.6 Heart rate is not a consistent predictor of serious disease; absence of tachycardia or even relative bradycardia can occur in catastrophic conditions such as ruptured ectopic pregnancy. Coexistent chest, back, abdominal, or pelvic pain should suggest intrathoracic or intra-abdominal emergencies. Dangerous diseases presenting severe orthostatic hypotension but sometimes lacking overt clues include myocardial infarction, occult sepsis, adrenal insufficiency, and diabetic ketoacidosis.35
Spontaneous EVS (s-EVS)
Approach
Episode duration for s-EVS varies, ranging from seconds to a few days, but the majority of spells last minutes to hours.44 Patients are often asymptomatic at the time of ED presentation. Since episodes cannot usually be provoked at the bedside (as they can with the t-EVS), evaluation relies almost entirely on history-taking. The frequency of spells varies from multiple times a day to monthly, depending on the cause. Although precipitants may exist (e.g., red wine prior to vestibular migraine), many spells occur without apparent provocation. This differs from BPPV and other diseases with obligate, immediate triggers. Diagnosis may be clear-cut in typical cases. Unfortunately, classical features such as frank loss of consciousness in vasovagal syncope,45 headache in vestibular migraine,46 and fear in panic attacks47 are absent in 25–35% of cases. Atypical case presentations probably contribute to diagnostic confusion in patients with such transient neurological attacks.48
Prototype s-EVS causes include common benign, recurrent disorders such as vestibular migraine, vasovagal syncope, and panic attacks. Although Meniere’s disease is often mentioned as a common cause of s-EVS, its estimated population prevalence (0·1%49) is much lower than that of the three other episodic disorders mentioned. Principal dangerous causes are cerebrovascular (vertebrobasilar TIA, subarachnoid hemorrhage), cardiorespiratory (cardiac arrhythmia, unstable angina, pulmonary embolus), and endocrine (hypoglycemia). Temporary or intermittent carbon monoxide exposure is a rare serious cause.50
Diseases
Patients with Meniere’s disease classically present with episodic vertigo accompanied by unilateral tinnitus and aural fullness, often with reversible sensorineural hearing loss.51 Only one in four initially present with the complete symptom triad,52 and non-vertiginous dizziness is common.53 Patients with suspected Meniere’s disease should generally be referred to an otolaryngologist, but care must be taken to avoid missing TIA mimics with audio-vestibular symptoms.54
Vestibular migraine (previously called migrainous vertigo, migraine-associated vertigo, or migraine-associated dizziness) is a newly-described form of migraine. It is related to basilar-type migraine,55 but episodes lack a second defining brainstem symptom such as diplopia, quadriparesis, or paresthesias.56 The two migraine types may exist along a continuum.57 With a population prevalence of roughly 1%,58 vestibular migraine is a common cause of s-EVS. A definite diagnosis of vestibular migraine requires ≥5 attacks with vestibular symptoms, a history of migraine headaches, and migraine-like symptoms with at least half the attacks.59 Episode duration ranges from seconds to days.56 Nystagmus, if present, may be peripheral, central, or mixed type.56 Headache is often absent.46 When headache does occur, it may begin before, during, or after the dizziness and may differ from the patient’s other typical migraine headaches.56 Nausea, vomiting, photophobia, phonophobia, and visual auras may occur. There are no pathognomonic signs or biomarkers, so diagnosis is currently based on clinical history and exclusion of alternative causes.59 An episode similar to prior spells with long illness duration, migraine features, no red-flags, and low vascular risk is sufficient for diagnosis without testing (Table 4).
Reflex syncope (also called neurocardiogenic or neurally-mediated syncope) usually has prodromal symptoms, typically lasting 3–30 minutes.60 Dizziness, the most common prodrome, occurs in 70–75%,61–63 and may be of any type, including vertigo.61 Although rarely seen in clinical practice, central forms of nystagmus may be identified during provocative testing, suggesting a TIA-like mechanism producing central vertigo.64 In reflex syncope, episodes of near syncope (no loss of consciousness) substantially outnumber spells with syncope,63 so many patients likely present with isolated dizziness. The diagnosis is readily suspected if classic contextual precipitants (e.g., pain/fear for vasovagal syncope, micturition/defecation for situational syncope) are present,65 but these are absent in atypical forms, including those due to carotid sinus hypersensitivity.6 Diagnosis is based on clinical history, excluding dangerous mimics (especially cardiac arrhythmia), and, if clinically necessary, can be confirmed by formal head-up tilt table testing.6
Panic attacks, with or without hyperventilation, are often accompanied by episodic dizziness. Dizziness begins rapidly, peaks within 10 minutes and, by definition, is accompanied by at least three other symptoms.66 There may be a situational precipitant (e.g., claustrophobia), but spells often occur spontaneously. Fear of dying or “going crazy” are classical symptoms but are absent in 30% of cases.47 Ictal panic attacks from temporal lobe epilepsy generally last only seconds, and altered mental status is frequent.67 Hypoglycemia, cardiac arrhythmias, pheochromocytoma, and basilar TIA can all mimic panic attacks presenting with dizziness; each can produce a multi-symptom complex with neurologic and autonomic features.
The most common dangerous diagnoses for s-EVS are TIA and cardiac arrhythmias. In 1975, a National Institutes of Health consensus report on TIA recommended that isolated dizziness or vertigo not be considered a TIA,68 a pronouncement that has been widely accepted. Recent data, however, contradict this classic teaching. Multiple studies show that dizziness and vertigo, even when isolated, are the most common premonitory vertebrobasilar TIA symptoms and are more frequent in the days to weeks preceding posterior circulation stroke.69–71
TIAs can present with isolated episodes of dizziness weeks to months prior to a completed infarction.72, 73 Dizziness is the most common presenting symptom of vertebral artery dissection,74 which affects younger patients, mimics migraine, and is easily misdiagnosed.13 Dizziness and vertigo are the most common symptoms in basilar artery occlusion and are sometimes early and isolated.75, 76 Because roughly 5% of TIA patients suffer a stroke within 48 hours,77 and rapid treatment reduces stroke risk by up to 80%,78, 79 prompt diagnosis is critical. Patients with posterior circulation TIA have an even higher stroke risk than those with anterior circulation spells.80, 81 The presence of three or more vascular risk factors or an ABCD2 score ≥4 are predictors of TIA in patients with s-EVS,82, 83 although high-risk vascular lesions may predict stroke risk more accurately than risk-factor-based scoring.84
Cardiac arrhythmias should be considered in any patient with s-EVS, particularly when syncope occurs, or when exertion is a precipitant, even if the lead symptom is ‘true’ spinning vertigo.10, 42 Although some clinical features during the attack may increase or decrease the odds of a dangerous cardiac cause,65 additional testing (e.g., cardiac loop recording) is often required to confirm the final diagnosis.6
Acute Vestibular Syndrome
The acute vestibular syndrome (AVS) involves acute, persistent dizziness lasting days to weeks, sometimes with lingering sequelae thereafter. Temporal evolution at onset and in the first week is more important than total illness duration. Most such patients have a monophasic course with an early peak in symptom severity, rapid improvement in symptoms over the first week, and gradual recovery over weeks to months. Unusual cases resolve in less than 48–72 hours. AVS is divided into postexposure and spontaneous forms, each discussed below.
Post-Exposure AVS (t-AVS)
Approach
Sometimes AVS results directly from trauma or a toxic exposure (t-AVS). The exposure history is usually obvious. The most common causes are blunt head injury and drug intoxication, particularly with medications (e.g., anticonvulsants) or illicit substances affecting the brainstem, cerebellum, or peripheral vestibular apparatus.
Most patients experience a single, acute attack resolving gradually over days to weeks once the exposure has stopped. Depending on the nature of the trauma or toxin, other symptoms such as headache or altered mental status may predominate. Rotatory vertigo, spontaneous nystagmus (looking straight ahead), and head-motion intolerance may be absent or unimpressive if the pathologic effects are bilateral and relatively symmetric, as with most toxins.
Diseases
Blunt head trauma,85 blast injuries,86 whiplash,87 and barotrauma88 may cause direct vestibular nerve injury, labyrinthine concussion, or mechanical disruption of inner ear membranes, resulting in an AVS presentation. Care should be taken not to miss a basal skull fracture or traumatic vertebral artery dissection. Traumatic brain injury may cause the post-concussion syndrome. Patients typically present with a combination of dizziness, headaches, fatigue, and minor cognitive impairments, with dizziness the most common symptom in the first two weeks after injury.89
Anticonvulsant side effects or toxicity are a frequent cause of dizziness and vertigo in the ED, and may present with an acute clinical picture.90 Carbon monoxide intoxication is an uncommon but important cause to consider.91 Aminoglycoside toxicity is a well-known cause of acute bilateral vestibular failure.92, 93 Gentamicin produces profound, permanent loss of vestibular function with relatively spared hearing, and toxicity may occur after even a single antibiotic dose.93 Although this problem is often discovered during the course of an inpatient admission, patients may develop symptoms later and present to the ED. Patients usually present with predominantly gait unsteadiness and oscillopsia (bouncing vision) while walking.94
Spontaneous AVS (s-AVS)
Approach
Classical AVS is defined as the acute onset of persistent, continuous dizziness or vertigo in association with nausea or vomiting, gait instability, nystagmus, and head-motion intolerance that lasts days to weeks.95 Patients are usually symptomatic at the time of ED presentation and focused physical examination is usually diagnostic. Patients generally experience worsening of AVS symptoms with any head motion, including provocative tests (e.g., Dix-Hallpike). Contrary to conventional wisdom, these exacerbating features do not suggest an etiologic or anatomic diagnosis95 and must be distinguished from head movements that trigger dizziness.96 This common source of confusion probably contributes to misdiagnosis of a ‘peripheral’ problem or ‘positional’ vertigo when dizziness worsens with head movement or testing.28, 97 The difference is that a patient with s-AVS is dizzy at rest and feels worse with any head motion, whereas a patient with t-EVS is normal at rest and specific head motions induce transient dizziness. This means that positional tests such as Dix-Hallpike should not be applied to AVS patients but reserved for use in EVS.
The prototype s-AVS cause is vestibular neuritis (often incorrectly called labyrinthitis), an acute peripheral vestibulopathy without hearing loss. The primary dangerous mimic is ischemic stroke in the lateral brainstem, cerebellum, or inner ear.95 Cerebellar hemorrhages rarely mimic a peripheral vestibular process.98 Uncommon dangerous causes are thiamine deficiency11 and listeria encephalitis.99
Although it is often assumed that strokes usually exhibit neurological features,28 obvious focal signs are present in less than 20% of stroke patients with s-AVS.95 Patients are usually symptomatic at initial assessment and often have diagnostic eye signs. Strong evidence95 suggests that a physical exam clinical decision rule using three bedside eye exam findings (HINTS – head impulse test, nystagmus type, and skew deviation, Table 4) rules out stroke more accurately than early MRI.90, 100, 101 Importantly, the mere presence of nystagmus (found in both neuritis and stroke) is not as useful as the nystagmus attributes, which help differentiate the two (Table 3).
Eye movement tests have excellent performance characteristics in the hands of neuro-otologists, and similar findings have been replicated by multiple investigative teams around the world.102–107 Nevertheless, care should be taken before applying these tests in routine ED practice, since interpretation differs between experts and novices108 and limited instruction may not always be sufficient to yield optimal results.109 More extensive training with subspecialists directly observing trainees and providing immediate feedback may facilitate skill-building at tertiary care institutions with access to such expertise,110 but new technologies may offer more widely available help in the near future. Recent studies have found accurate diagnosis using a portable video-oculography device that measures key eye movements quantitatively.111, 112 Such devices could eventually make subspecialty-level expertise in eye movement assessment widely available for diagnosis or training, although artifacts and related issues with quantitative recordings still currently require expert interpretation.113
Neuroimaging studies are often insufficient to accurately diagnose s-AVS cases. CT, the most commonly applied test, is useful to detect (or rule out) brain hemorrhages, but is far less helpful for investigating suspected ischemic strokes. Retrospective studies suggest CT may have up to 42% sensitivity for ischemic stroke in dizziness.19, 114 In prospective studies, however, CT has even lower sensitivity (16%) for detecting early acute ischemic stroke,115 especially in the posterior fossa (7%).107 CT should therefore not be used to exclude ischemic stroke in s-AVS.116 Lack of understanding of CT’s limitations for assessment of dizziness may lead to CT overuse and misdiagnosis.13, 28 Less well known is that even MRI with diffusion-weighted imaging (DWI) misses 10–20% of strokes in s-AVS during the first 24–48 hours.95, 101 When smaller strokes (<1cm in diameter) present with s-AVS, early MRI sensitivity is only ~50%.117 Repeat delayed MRI-DWI (3–7 days after onset of symptoms) may be required to confirm a new infarct.90, 118 Routine MRI in all ED dizziness also has a low yield.21 Imaging only older patients with vascular risk factors is a common practice, but the countervailing concern is that young age predisposes to missed stroke.13, 119, 120 Stroke risk in patients presenting isolated s-AVS and no vascular risk factors is still roughly 10–20%, and one in four strokes occurs in a patient under age 50.95 Overreliance on youth, low vascular risk, normal neurologic exam, and normal CT likely explain the relatively high odds of missed stroke in isolated dizziness.12, 14, 121
Diseases
Vestibular neuritis is a benign, self-limited condition affecting the vestibular nerve. Some cases are linked to specific causes (e.g., multiple sclerosis122), but most are idiopathic and possibly related to herpes simplex infections.123 Although vestibular neuritis is usually a monophasic illness, 25% of cases have a single brief prodrome in the week prior to the attack124 and others have recurrences months or years later.125 MRI with or without contrast is normal and unnecessary.126 Diagnosis is based on nystagmus type and vestibular reflexes.127 Early treatment with oral or intravenous steroids is supported by some evidence, but remains controversial.128
When hearing loss accompanies vertigo in a ‘neuritis-like’ s-AVS presentation, the syndrome is known as viral labyrinthitis, although cochleo-vestibular neuritis might be more appropriate. This benign presentation must be differentiated from bacterial labyrinthitis, a dangerous disorder resulting from spread of middle ear or systemic infection that may lead to meningitis if left untreated.129 Even in the absence of systemic or local (otitis or mastoiditis) infection, however, this presentation should still be viewed suspiciously, since inner ear strokes typically present this way,54, 106, 130 and may often be the cause of s-AVS with hearing loss in the ED.101
The prevalence of stroke in ED dizziness is 3–5%,1, 2, 12, 16, 131, 132 and probably less for those with isolated dizziness.12 Among ED dizzy patients, those with AVS are a high-risk subgroup for stroke (~25% of s-AVS cases).95 Posterior circulation stroke typically presents with s-AVS, sometimes following a series of spontaneous episodes in the preceding weeks or months (i.e., TIAs, usually from posterior circulation stenosis, culminating in stroke).95 Nearly all of these strokes (96%) are ischemic.95, 98 Most are initially associated with minor neurologic deficits that recover well, absent recurrent stroke. Delays in prompt diagnosis and treatment, however, can result in disability or death.13, 95 Although most such patients are not thrombolysis candidates by current guidelines, they may benefit from early secondary prevention treatments and interventions to prevent posterior fossa stroke complications.95, 116
Bedside Approach Summary
For the usual ED patient with isolated dizziness or vertigo that is not obviously of traumatic or toxic cause, the goal for the syndrome-specific targeted exam will be to firmly diagnose the specific benign conditions described above. The majority of cases with initial diagnostic uncertainty are due to common cardiovascular (medication-induced orthostatic hypotension; vasovagal syncope), psychiatric (panic disorder), or vestibular disorders (BPPV, vestibular migraine, vestibular neuritis). These benign conditions can each be diagnosed confidently at the bedside using a syndrome-targeted history and exam. Patients whose presentations are atypical or whose targeted exam findings are suspicious for dangerous underlying causes should undergo appropriate lab tests, imaging, or consultation.
Bedside exams for benign vestibular disorders probably deserve special attention in emergency medicine education and in developing decision support tools.115, 129, 130 Confusion over the conduct of these exams may stem from the fact that a given clinical feature (e.g., upbeat-torsional nystagmus) predicts a benign condition in one syndrome (t-EVS, indicating typical posterior-canal BPPV) but a dangerous one in another (s-AVS, indicating a brainstem stroke).28 Thus, it is crucial to identify the timing-and-trigger syndrome before targeting the exam, something seldom done in current practice, and, unfortunately, often omitted in prominent textbooks133–135 and journal articles.136 Key criteria that define typical benign vestibular disorder cases and differentiate them from dangerous neurologic causes are shown in Tables 3, 4.
Conclusions
The prevailing diagnostic paradigm for diagnosing ED patients with dizziness is based on dizziness symptom quality or ‘type.’ Recent research suggests that the logic underlying this traditional approach is flawed. A newer approach based on ‘timing and triggers’ of the dizziness likely offers a better diagnostic approach, especially in an unselected ED dizziness population. Using this approach allows targeted bedside examinations of proven value to be used effectively. Future research should seek to prospectively study the new approach to dizziness for its overall diagnostic accuracy, resource efficiency, and impact on health outcomes.
The prevailing diagnostic paradigm for diagnosing ED patients with dizziness is based on dizziness symptom quality or ‘type.’
Recent research suggests that the logic underlying this traditional approach is flawed.
A newer approach based on ‘timing and triggers’ of the dizziness likely offers a better diagnostic approach, especially in an unselected ED dizziness population.
Future research should seek to prospectively study the new approach to dizziness for its overall diagnostic accuracy, resource efficiency, and impact on health outcomes
Abbreviations
- AED
anti-epileptic drug
- BPPV
benign paroxysmal positional vertigo
- CO
carbon monoxide
- CPPV
central paroxysmal positional vertigo
- CT
computed tomography
- CTA
CT angiography
- EOMs
extra-ocular movements
- HINTS
head impulse, nystagmus type, skew
- Hx
history
- MI
myocardial infarction
- PE
pulmonary embolus
- PRN
pro re nata (as needed)
- VS
vital signs
- TIA
transient ischemic attack
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Newman-Toker DE, Hsieh YH, Camargo CA, Jr, et al. Spectrum of dizziness visits to US emergency departments: cross-sectional analysis from a nationally representative sample. Mayo Clin Proc. 2008;83(7):765–775. doi: 10.4065/83.7.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheung CS, Mak PS, Manley KV, et al. Predictors of important neurological causes of dizziness among patients presenting to the emergency department. Emerg Med J. 2010;27(7):517–521. doi: 10.1136/emj.2009.078014. [DOI] [PubMed] [Google Scholar]
- 3.Newman-Toker DE, Cannon LM, Stofferahn ME, et al. Imprecision in patient reports of dizziness symptom quality: a cross-sectional study conducted in an acute care setting. Mayo Clin Proc. 2007;82(11):1329–1340. doi: 10.4065/82.11.1329. [DOI] [PubMed] [Google Scholar]
- 4.Saber Tehrani AS, Coughlan D, Hsieh YH, et al. Rising annual costs of dizziness presentations to u.s. Emergency departments. Acad Emerg Med. 2013;20(7):689–696. doi: 10.1111/acem.12168. [DOI] [PubMed] [Google Scholar]
- 5.Bisdorff A, Von Brevern M, Lempert T, et al. Classification of vestibular symptoms: towards an international classification of vestibular disorders. J Vestib Res. 2009;19(1–2):1–13. doi: 10.3233/VES-2009-0343. [DOI] [PubMed] [Google Scholar]
- 6.Moya A, Sutton R, Ammirati F, et al. Guidelines for the diagnosis and management of syncope (version 2009) Eur Heart J. 2009;30(21):2631–2671. doi: 10.1093/eurheartj/ehp298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malouf R, Brust JC. Hypoglycemia: causes, neurological manifestations, and outcome. Ann Neurol. 1985;17(5):421–430. doi: 10.1002/ana.410170502. [DOI] [PubMed] [Google Scholar]
- 8.Lee H, Sohn SI, Cho YW, et al. Cerebellar infarction presenting isolated vertigo: frequency and vascular topographical patterns. Neurology. 2006;67(7):1178–1183. doi: 10.1212/01.wnl.0000238500.02302.b4. [DOI] [PubMed] [Google Scholar]
- 9.Demiryoguran NS, Karcioglu O, Topacoglu H, et al. Painless aortic dissection with bilateral carotid involvement presenting with vertigo as the chief complaint. Emerg Med J. 2006;23(2):e15. doi: 10.1136/emj.2005.027862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newman-Toker DE, Camargo CA., Jr ‘Cardiogenic vertigo’--true vertigo as the presenting manifestation of primary cardiac disease. Nat Clin Pract Neurol. 2006;2(3):167–172. doi: 10.1038/ncpneuro0125. quiz 173. [DOI] [PubMed] [Google Scholar]
- 11.Kattah JC, Dhanani SD, Pula JH, et al. Vestibular signs of thiamine deficiency during the early phase of suspected Wernicke’s encephalopathy. Neurol Clin Pract. 2013;3:460–468. doi: 10.1212/01.CPJ.0000435749.32868.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kerber KA, Brown DL, Lisabeth LD, et al. Stroke among patients with dizziness, vertigo, and imbalance in the emergency department: a population-based study. Stroke. 2006;37(10):2484–2487. doi: 10.1161/01.STR.0000240329.48263.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Savitz SI, Caplan LR, Edlow JA. Pitfalls in the diagnosis of cerebellar infarction. Acad Emerg Med. 2007;14(1):63–68. doi: 10.1197/j.aem.2006.06.060. [DOI] [PubMed] [Google Scholar]
- 14.Kim AS, Fullerton HJ, Johnston SC. Risk of vascular events in emergency department patients discharged home with diagnosis of dizziness or vertigo. Ann Emerg Med. 2011;57(1):34–41. doi: 10.1016/j.annemergmed.2010.06.559. [DOI] [PubMed] [Google Scholar]
- 15.Braun EM, Tomazic PV, Ropposch T, et al. Misdiagnosis of acute peripheral vestibulopathy in central nervous ischemic infarction. Otol Neurotol. 2011;32(9):1518–1521. doi: 10.1097/MAO.0b013e318238ff9a. [DOI] [PubMed] [Google Scholar]
- 16.Royl G, Ploner CJ, Leithner C. Dizziness in the emergency room: diagnoses and misdiagnoses. Eur Neurol. 2011;66(5):256–263. doi: 10.1159/000331046. [DOI] [PubMed] [Google Scholar]
- 17.Wasay M, Dubey N, Bakshi R. Dizziness and yield of emergency head CT scan: is it cost effective? Emerg Med J. 2005;22(4):312. doi: 10.1136/emj.2003.012765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kerber KA, Schweigler L, West BT, et al. Value of computed tomography scans in ED dizziness visits: analysis from a nationally representative sample. Am J Emerg Med. 2010;28(9):1030–1036. doi: 10.1016/j.ajem.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawhn-Heath C, Buckle C, Christoforidis G, et al. Utility of head CT in the evaluation of vertigo/dizziness in the emergency department. Emerg Radiol. 2013;20(1):45–49. doi: 10.1007/s10140-012-1071-y. [DOI] [PubMed] [Google Scholar]
- 20.Fakhran S, Alhilali L, Branstetter BFt. Yield of CT angiography and contrast-enhanced MR imaging in patients with dizziness. AJNR. 2013;34(5):1077–1081. doi: 10.3174/ajnr.A3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahsan SF, Syamal MN, Yaremchuk K, et al. The costs and utility of imaging in evaluating dizzy patients in the emergency room. Laryngoscope. 2013;123(9):2250–2253. doi: 10.1002/lary.23798. [DOI] [PubMed] [Google Scholar]
- 22.Kim AS, Sidney S, Klingman JG, et al. Practice variation in neuroimaging to evaluate dizziness in the ED. Am J Emerg Med. 2012;30(5):665–672. doi: 10.1016/j.ajem.2011.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newman-Toker DE, McDonald KM, Meltzer DO. How much diagnostic safety can we afford, and how should we decide? A health economics perspective. BMJ Qual Saf. 2013;22(Suppl 2):ii11–ii20. doi: 10.1136/bmjqs-2012-001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kerber KA, Fendrick AM. The evidence base for the evaluation and management of dizziness. J Eval Clin Pract. 2010;16(1):186–191. doi: 10.1111/j.1365-2753.2009.01133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newman-Toker DE. ProQuest Digital Dissertations. Baltimore, MD: The Johns Hopkins University; 2007. [Accessibility verified April 15, 2015]. Diagnosing Dizziness in the Emergency Department-Why “What do you mean by ‘dizzy’?” Should Not Be the First Question You Ask [Doctoral Dissertation, Clinical Investigation, Bloomberg School of Public Health] [database on Internet, http://www.proquest.com/]; publication number: AAT 3267879. Available at: http://gateway.proquest.com/openurl?url_ver=Z39.88-2004&res_dat=xri:pqdiss&rft_val_fmt=info:ofi/fmt:kev:mtx:dissertation&rft_dat=xri:pqdiss:3267879. [Google Scholar]
- 26.Edlow JA. Diagnosing dizziness: we are teaching the wrong paradigm! Acad Emerg Med. 2013;20(10):1064–1066. doi: 10.1111/acem.12234. [DOI] [PubMed] [Google Scholar]
- 27.Newman-Toker DE, Staab JP, Bronstein A, et al. Proposed multi-layer structure for the International Classification of Vestibular Disorders [abstract]. Bárány Society XXVI International Congress; Reykjavik, Iceland. 2010. [Google Scholar]
- 28.Stanton VA, Hsieh YH, Camargo CA, Jr, et al. Overreliance on symptom quality in diagnosing dizziness: results of a multicenter survey of emergency physicians. Mayo Clin Proc. 2007;82(11):1319–1328. doi: 10.4065/82.11.1319. [DOI] [PubMed] [Google Scholar]
- 29.Radtke A, Lempert T, von Brevern M, et al. Prevalence and complications of orthostatic dizziness in the general population. Clin Auton Res. 2011;21(3):161–168. doi: 10.1007/s10286-010-0114-2. [DOI] [PubMed] [Google Scholar]
- 30.Wu JS, Yang YC, Lu FH, et al. Population-based study on the prevalence and correlates of orthostatic hypotension/hypertension and orthostatic dizziness. Hypertens Res. 2008;31(5):897–904. doi: 10.1291/hypres.31.897. [DOI] [PubMed] [Google Scholar]
- 31.Poon IO, Braun U. High prevalence of orthostatic hypotension and its correlation with potentially causative medications among elderly veterans. J Clin Pharm Ther. 2005;30(2):173–178. doi: 10.1111/j.1365-2710.2005.00629.x. [DOI] [PubMed] [Google Scholar]
- 32.Stark RJ, Wodak J. Primary orthostatic cerebral ischaemia. J Neurol Neurosurg Psychiatry. 1983;46(10):883–891. doi: 10.1136/jnnp.46.10.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blank SC, Shakir RA, Bindoff LA, et al. Spontaneous intracranial hypotension: clinical and magnetic resonance imaging characteristics. Clin Neurol Neurosurg. 1997;99(3):199–204. doi: 10.1016/s0303-8467(97)00015-2. [DOI] [PubMed] [Google Scholar]
- 34.Buttner U, Helmchen C, Brandt T. Diagnostic criteria for central versus peripheral positioning nystagmus and vertigo: a review. Acta Otolaryngol. 1999;119(1):1–5. doi: 10.1080/00016489950181855. [DOI] [PubMed] [Google Scholar]
- 35.Gilbert VE. Immediate orthostatic hypotension: diagnostic value in acutely ill patients. South Med J. 1993;86(9):1028–1032. [PubMed] [Google Scholar]
- 36.von Brevern M, Radtke A, Lezius F, et al. Epidemiology of benign paroxysmal positional vertigo: a population based study. J Neurol Neurosurg Psychiatry. 2007;78(7):710–715. doi: 10.1136/jnnp.2006.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhattacharyya N, Baugh RF, Orvidas L, et al. Clinical practice guideline: benign paroxysmal positional vertigo. Otolaryngol Head Neck Surg. 2008;139(5 Suppl 4):S47–S81. doi: 10.1016/j.otohns.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 38.von Brevern M, Bertholon P, Brandt T, et al. Benign paroxysmal positional vertigo: diagnostic criteria. J Vestib Res. 2015 doi: 10.3233/VES-150553. (in press) [DOI] [PubMed] [Google Scholar]
- 39.Lawson J, Johnson I, Bamiou DE, et al. Benign paroxysmal positional vertigo: clinical characteristics of dizzy patients referred to a Falls and Syncope Unit. QJM. 2005;98(5):357–364. doi: 10.1093/qjmed/hci057. [DOI] [PubMed] [Google Scholar]
- 40.Fife TD, Iverson DJ, Lempert T, et al. Practice parameter: therapies for benign paroxysmal positional vertigo (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2008;70(22):2067–2074. doi: 10.1212/01.wnl.0000313378.77444.ac. [DOI] [PubMed] [Google Scholar]
- 41.Sarasin FP, Louis-Simonet M, Carballo D, et al. Prevalence of orthostatic hypotension among patients presenting with syncope in the ED. Am J Emerg Med. 2002;20(6):497–501. doi: 10.1053/ajem.2002.34964. [DOI] [PubMed] [Google Scholar]
- 42.Newman-Toker DE, Dy FJ, Stanton VA, et al. How often is dizziness from primary cardiovascular disease true vertigo? A systematic review. J Gen Intern Med. 2008;23(12):2087–2094. doi: 10.1007/s11606-008-0801-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McGee S, Abernethy WB, 3rd, Simel DL. The rational clinical examination. Is this patient hypovolemic? JAMA. 1999;281(11):1022–1029. doi: 10.1001/jama.281.11.1022. [DOI] [PubMed] [Google Scholar]
- 44.Lempert T. Recurrent spontaneous attacks of dizziness. Continuum. 2012;18(5 Neuro-otology):1086–1101. doi: 10.1212/01.CON.0000421620.10783.ac. [DOI] [PubMed] [Google Scholar]
- 45.Mathias CJ, Deguchi K, Schatz I. Observations on recurrent syncope and presyncope in 641 patients. Lancet. 2001;357(9253):348–353. doi: 10.1016/S0140-6736(00)03642-4. [DOI] [PubMed] [Google Scholar]
- 46.Dieterich M, Brandt T. Episodic vertigo related to migraine (90 cases): vestibular migraine? J Neurol. 1999;246(10):883–892. doi: 10.1007/s004150050478. [DOI] [PubMed] [Google Scholar]
- 47.Chen J, Tsuchiya M, Kawakami N, et al. Non-fearful vs. fearful panic attacks: a general population study from the National Comorbidity Survey. J Affect Disord. 2009;112(1–3):273–278. doi: 10.1016/j.jad.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 48.Fonseca AC, Canhao P. Diagnostic difficulties in the classification of transient neurological attacks. Eur J Neurol. 2011;18:644–648. doi: 10.1111/j.1468-1331.2010.03241.x. [DOI] [PubMed] [Google Scholar]
- 49.Radtke A, von Brevern M, Feldmann M, et al. Screening for Meniere’s disease in the general population - the needle in the haystack. Acta Otolaryngol. 2008;128(3):272–276. doi: 10.1080/00016480701509933. [DOI] [PubMed] [Google Scholar]
- 50.Keles A, Demircan A, Kurtoglu G. Carbon monoxide poisoning: how many patients do we miss? Eur J Emerg Med. 2008;15(3):154–157. doi: 10.1097/MEJ.0b013e3282efd519. [DOI] [PubMed] [Google Scholar]
- 51.Sajjadi H, Paparella MM. Meniere’s disease. Lancet. 2008;372(9636):406–414. doi: 10.1016/S0140-6736(08)61161-7. [DOI] [PubMed] [Google Scholar]
- 52.Mancini F, Catalani M, Carru M, et al. History of Meniere’s disease and its clinical presentation. Otolaryngol Clin North Am. 2002;35(3):565–580. doi: 10.1016/s0030-6665(02)00017-8. [DOI] [PubMed] [Google Scholar]
- 53.Faag C, Bergenius J, Forsberg C, et al. Symptoms experienced by patients with peripheral vestibular disorders: evaluation of the Vertigo Symptom Scale for clinical application. Clin Otolaryngol. 2007;32(6):440–446. doi: 10.1111/j.1749-4486.2007.01552.x. [DOI] [PubMed] [Google Scholar]
- 54.Lee H. Audiovestibular loss in anterior inferior cerebellar artery territory infarction: a window to early detection? J Neurol Sci. 2012;313(1–2):153–159. doi: 10.1016/j.jns.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 55.Kirchmann M, Thomsen LL, Olesen J. Basilar-type migraine: clinical, epidemiologic, and genetic features. Neurology. 2006;66(6):880–886. doi: 10.1212/01.wnl.0000203647.48422.dd. [DOI] [PubMed] [Google Scholar]
- 56.Lempert T, Neuhauser H, Daroff RB. Vertigo as a symptom of migraine. Ann N Y Acad Sci. 2009;1164:242–251. doi: 10.1111/j.1749-6632.2009.03852.x. [DOI] [PubMed] [Google Scholar]
- 57.Wang CT, Lai MS, Young YH. Relationship between basilar-type migraine and migrainous vertigo. Headache. 2009;49(3):426–434. doi: 10.1111/j.1526-4610.2008.01283.x. [DOI] [PubMed] [Google Scholar]
- 58.Neuhauser HK, Radtke A, von Brevern M, et al. Migrainous vertigo: prevalence and impact on quality of life. Neurology. 2006;67(6):1028–1033. doi: 10.1212/01.wnl.0000237539.09942.06. [DOI] [PubMed] [Google Scholar]
- 59.Lempert T, Olesen J, Furman J, et al. Vestibular migraine: Diagnostic criteria. J Vestib Res. 2012;22(4):167–172. doi: 10.3233/VES-2012-0453. [DOI] [PubMed] [Google Scholar]
- 60.Sheldon RS, Amuah JE, Connolly SJ, et al. Design and use of a quantitative scale for measuring presyncope. J Cardiovasc Electrophysiol. 2009;20(8):888–893. doi: 10.1111/j.1540-8167.2009.01466.x. [DOI] [PubMed] [Google Scholar]
- 61.Sloane PD, Linzer M, Pontinen M, et al. Clinical significance of a dizziness history in medical patients with syncope. Arch Intern Med. 1991;151(8):1625–1628. [PubMed] [Google Scholar]
- 62.Calkins H, Shyr Y, Frumin H, et al. The value of the clinical history in the differentiation of syncope due to ventricular tachycardia, atrioventricular block, and neurocardiogenic syncope. Am J Med. 1995;98(4):365–373. doi: 10.1016/S0002-9343(99)80315-5. [DOI] [PubMed] [Google Scholar]
- 63.Romme JJ, van Dijk N, Boer KR, et al. Influence of age and gender on the occurrence and presentation of reflex syncope. Clin Auton Res. 2008;18(3):127–133. doi: 10.1007/s10286-008-0465-0. [DOI] [PubMed] [Google Scholar]
- 64.Choi JH, Seo JD, Kim MJ, et al. Vertigo and nystagmus in orthostatic hypotension. Eur J Neurol. 2015;22(4):648–655. doi: 10.1111/ene.12622. [DOI] [PubMed] [Google Scholar]
- 65.van Dijk JG, Thijs RD, Benditt DG, et al. A guide to disorders causing transient loss of consciousness: focus on syncope. Nat Rev Neurol. 2009;5(8):438–448. doi: 10.1038/nrneurol.2009.99. [DOI] [PubMed] [Google Scholar]
- 66.Katon WJ. Clinical practice. Panic disorder. N Engl J Med. 2006;354(22):2360–2367. doi: 10.1056/NEJMcp052466. [DOI] [PubMed] [Google Scholar]
- 67.Kanner AM. Ictal panic and interictal panic attacks: diagnostic and therapeutic principles. Neurol Clin. 2011;29(1):163–175. ix. doi: 10.1016/j.ncl.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 68.A classification and outline of cerebrovascular diseases. II. Stroke. 1975;6(5):564–616. doi: 10.1161/01.str.6.5.564. [DOI] [PubMed] [Google Scholar]
- 69.Compter A, Kappelle LJ, Algra A, et al. Nonfocal symptoms are more frequent in patients with vertebral artery than carotid artery stenosis. Cerebrovasc Dis. 2013;35(4):378–384. doi: 10.1159/000348849. [DOI] [PubMed] [Google Scholar]
- 70.Hoshino T, Nagao T, Mizuno S, et al. Transient neurological attack before vertebrobasilar stroke. J Neurol Sci. 2013;325(1–2):39–42. doi: 10.1016/j.jns.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 71.Paul NL, Simoni M, Rothwell PM, et al. Transient isolated brainstem symptoms preceding posterior circulation stroke: a population-based study. Lancet Neurol. 2013;12(1):65–71. doi: 10.1016/S1474-4422(12)70299-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grad A, Baloh RW. Vertigo of vascular origin. Clinical and electronystagmographic features in 84 cases. Arch Neurol. 1989;46(3):281–284. doi: 10.1001/archneur.1989.00520390047014. [DOI] [PubMed] [Google Scholar]
- 73.Gomez CR, Cruz-Flores S, Malkoff MD, et al. Isolated vertigo as a manifestation of vertebrobasilar ischemia. Neurology. 1996;47(1):94–97. doi: 10.1212/wnl.47.1.94. [DOI] [PubMed] [Google Scholar]
- 74.Gottesman RF, Sharma P, Robinson KA, et al. Clinical characteristics of symptomatic vertebral artery dissection: a systematic review. Neurologist. 2012;18(5):245–254. doi: 10.1097/NRL.0b013e31826754e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fisher CM. Vertigo in cerebrovascular disease. Arch Otolaryngol. 1967;85(5):529–534. doi: 10.1001/archotol.1967.00760040531010. [DOI] [PubMed] [Google Scholar]
- 76.von Campe G, Regli F, Bogousslavsky J. Heralding manifestations of basilar artery occlusion with lethal or severe stroke. J Neurol Neurosurg Psychiatry. 2003;74(12):1621–1626. doi: 10.1136/jnnp.74.12.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shah KH, Kleckner K, Edlow JA. Short-term prognosis of stroke among patients diagnosed in the emergency department with a transient ischemic attack. Ann Emerg Med. 2008;51(3):316–323. doi: 10.1016/j.annemergmed.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 78.Lavallee PC, Meseguer E, Abboud H, et al. A transient ischaemic attack clinic with round-the-clock access (SOS-TIA): feasibility and effects. Lancet Neurol. 2007;6(11):953–960. doi: 10.1016/S1474-4422(07)70248-X. [DOI] [PubMed] [Google Scholar]
- 79.Rothwell PM, Giles MF, Chandratheva A, et al. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet. 2007;370(9596):1432–1442. doi: 10.1016/S0140-6736(07)61448-2. [DOI] [PubMed] [Google Scholar]
- 80.Flossmann E, Rothwell PM. Prognosis of vertebrobasilar transient ischaemic attack and minor stroke. Brain. 2003;126(Pt 9):1940–1954. doi: 10.1093/brain/awg197. [DOI] [PubMed] [Google Scholar]
- 81.Gulli G, Khan S, Markus HS. Vertebrobasilar stenosis predicts high early recurrent stroke risk in posterior circulation stroke and TIA. Stroke. 2009;40(8):2732–2737. doi: 10.1161/STROKEAHA.109.553859. [DOI] [PubMed] [Google Scholar]
- 82.Moubayed SP, Saliba I. Vertebrobasilar insufficiency presenting as isolated positional vertigo or dizziness: a double-blind retrospective cohort study. Laryngoscope. 2009;119(10):2071–2076. doi: 10.1002/lary.20597. [DOI] [PubMed] [Google Scholar]
- 83.Navi BB, Kamel H, Shah MP, et al. Application of the ABCD2 Score to Identify Cerebrovascular Causes of Dizziness in the Emergency Department. Stroke. 2012;43(6):1484–1489. doi: 10.1161/STROKEAHA.111.646414. [DOI] [PubMed] [Google Scholar]
- 84.Amarenco P, Labreuche J, Lavallee PC. Patients with transient ischemic attack with ABCD2 <4 can have similar 90-day stroke risk as patients with transient ischemic attack with ABCD2 >/=4. Stroke. 2012;43(3):863–865. doi: 10.1161/STROKEAHA.111.636506. [DOI] [PubMed] [Google Scholar]
- 85.Davies RA, Luxon LM. Dizziness following head injury: a neuro-otological study. J Neurol. 1995;242(4):222–230. doi: 10.1007/BF00919595. [DOI] [PubMed] [Google Scholar]
- 86.Hoffer ME, Balaban C, Gottshall K, et al. Blast exposure: vestibular consequences and associated characteristics. Otol Neurotol. 2010;31(2):232–236. doi: 10.1097/MAO.0b013e3181c993c3. [DOI] [PubMed] [Google Scholar]
- 87.Vibert D, Hausler R. Acute peripheral vestibular deficits after whiplash injuries. Ann Otol Rhinol Laryngol. 2003;112(3):246–251. doi: 10.1177/000348940311200310. [DOI] [PubMed] [Google Scholar]
- 88.Klingmann C, Praetorius M, Baumann I, et al. Barotrauma and decompression illness of the inner ear: 46 cases during treatment and follow-up. Otol Neurotol. 2007;28(4):447–454. doi: 10.1097/MAO.0b013e318030d356. [DOI] [PubMed] [Google Scholar]
- 89.Yang CC, Tu YK, Hua MS, et al. The association between the postconcussion symptoms and clinical outcomes for patients with mild traumatic brain injury. J Trauma. 2007;62(3):657–663. doi: 10.1097/01.ta.0000203577.68764.b8. [DOI] [PubMed] [Google Scholar]
- 90.Kattah JC, Talkad AV, Wang DZ, et al. HINTS to diagnose stroke in the acute vestibular syndrome: three-step bedside oculomotor examination more sensitive than early MRI diffusion-weighted imaging. Stroke. 2009;40(11):3504–3510. doi: 10.1161/STROKEAHA.109.551234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Trevino R. A 19-year-old woman with unexplained weakness and dizziness. J Emerg Nurs. 1997;23(5):499–500. doi: 10.1016/s0099-1767(97)90154-9. [DOI] [PubMed] [Google Scholar]
- 92.Ariano RE, Zelenitsky SA, Kassum DA. Aminoglycoside-induced vestibular injury: maintaining a sense of balance. Ann Pharmacother. 2008;42(9):1282–1289. doi: 10.1345/aph.1L001. [DOI] [PubMed] [Google Scholar]
- 93.Ahmed RM, Hannigan IP, MacDougall HG, et al. Gentamicin ototoxicity: a 23-year selected case series of 103 patients. Med J Austral. 2012;196(11):701–704. doi: 10.5694/mja11.10850. [DOI] [PubMed] [Google Scholar]
- 94.JC LIVING without a balancing mechanism. N Engl J Med. 1952;246(12):458–460. doi: 10.1056/NEJM195203202461207. [DOI] [PubMed] [Google Scholar]
- 95.Tarnutzer AA, Berkowitz AL, Robinson KA, et al. Does my dizzy patient have a stroke? A systematic review of bedside diagnosis in acute vestibular syndrome. CMAJ. 2011;183(9):E571–592. doi: 10.1503/cmaj.100174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Newman-Toker DE. Symptoms and Signs of Neuro-otologic Disorders. Continuum (Minneap Minn) 2012;18(5 Neuro-otology):1016–1040. doi: 10.1212/01.CON.0000421618.33654.8a. [DOI] [PubMed] [Google Scholar]
- 97.Newman-Toker DE, Stanton VA, Hsieh YH, et al. Frontline providers harbor misconceptions about the bedside evaluation of dizzy patients. Acta Otolaryngol. 2008;128(5):601–604. doi: 10.1080/00016480701596096. [DOI] [PubMed] [Google Scholar]
- 98.Kerber KA, Burke JF, Brown DL, et al. Does intracerebral haemorrhage mimic benign dizziness presentations? A population based study. Emerg Med J. 2011 doi: 10.1136/emj.2010.104844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Smiatacz T, Kowalik MM, Hlebowicz M. Prolonged dysphagia due to Listeria-rhombencephalitis with brainstem abscess and acute polyradiculoneuritis. J Infect. 2006;52(6):e165–e167. doi: 10.1016/j.jinf.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 100.Newman-Toker DE, Kattah JC, Alvernia JE, et al. Normal head impulse test differentiates acute cerebellar strokes from vestibular neuritis. Neurology. 2008;70(24 Pt 2):2378–2385. doi: 10.1212/01.wnl.0000314685.01433.0d. [DOI] [PubMed] [Google Scholar]
- 101.Newman-Toker DE, Kerber KA, Hsieh YH, et al. HINTS Outperforms ABCD2 to Screen for Stroke in Acute Continuous Vertigo and Dizziness. Acad Emerg Med. 2013;20(10):986–996. doi: 10.1111/acem.12223. [DOI] [PubMed] [Google Scholar]
- 102.Cnyrim CD, Newman-Toker D, Karch C, et al. Bedside differentiation of vestibular neuritis from central “vestibular pseudoneuritis”. J Neurol Neurosurg Psychiatry. 2008;79(4):458–460. doi: 10.1136/jnnp.2007.123596. [DOI] [PubMed] [Google Scholar]
- 103.Chen L, Lee W, Chambers BR, et al. Diagnostic accuracy of acute vestibular syndrome at the bedside in a stroke unit. J Neurol. 2010 doi: 10.1007/s00415-010-5853-4. [DOI] [PubMed] [Google Scholar]
- 104.Casani AP, Dallan I, Cerchiai N, et al. Cerebellar infarctions mimicking acute peripheral vertigo: how to avoid misdiagnosis? Otolaryngol Head Neck Surg. 2013;148(3):475–481. doi: 10.1177/0194599812472614. [DOI] [PubMed] [Google Scholar]
- 105.Kim MB, Boo SH, Ban JH. Nystagmus-based approach to vertebrobasilar stroke presenting as vertigo without initial neurologic signs. Eur Neurol. 2013;70(5–6):322–328. doi: 10.1159/000353285. [DOI] [PubMed] [Google Scholar]
- 106.Huh YE, Koo JW, Lee H, et al. Head-Shaking Aids in the Diagnosis of Acute Audiovestibular Loss due to Anterior Inferior Cerebellar Artery Infarction. Audiol Neurootol. 2013;18(2):114–124. doi: 10.1159/000345643. [DOI] [PubMed] [Google Scholar]
- 107.Ozono Y, Kitahara T, Fukushima M, et al. Differential diagnosis of vertigo and dizziness in the emergency department. Acta Otolaryngol. 2013 doi: 10.3109/00016489.2013.832377. [DOI] [PubMed] [Google Scholar]
- 108.Jorns-Haderli M, Straumann D, Palla A. Accuracy of the bedside head impulse test in detecting vestibular hypofunction. J Neurol Neurosurg Psychiatry. 2007;78(10):1113–1118. doi: 10.1136/jnnp.2006.109512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Royl G, Ploner CJ, Leithner C. Dizziness in the emergency room: diagnoses and misdiagnoses. Eur Neurol. 2011;66(5):256–263. doi: 10.1159/000331046. [DOI] [PubMed] [Google Scholar]
- 110.Vanni S, Nazerian P, Casati C, et al. Can emergency physicians accurately and reliably assess acute vertigo in the emergency department? Emerg Med Australas. 2015 Apr;27(2):126–31. doi: 10.1111/1742-6723.12372. [DOI] [PubMed] [Google Scholar]
- 111.Newman-Toker DE, Saber Tehrani AS, Mantokoudis G, et al. Quantitative video-oculography to help diagnose stroke in acute vertigo and dizziness: toward an ECG for the eyes. Stroke. 2013;44(4):1158–1161. doi: 10.1161/STROKEAHA.111.000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mantokoudis G, Tehrani AS, Wozniak A, et al. VOR gain by head impulse video-oculography differentiates acute vestibular neuritis from stroke. Otol Neurotol. 2015;36(3):457–465. doi: 10.1097/MAO.0000000000000638. [DOI] [PubMed] [Google Scholar]
- 113.Mantokoudis G, Saber Tehrani AS, Kattah JC, et al. Quantifying the vestibuloocular reflex with video-oculography: nature and frequency of artifacts. Audiol Neurootol. 2015;20(1):39–50. doi: 10.1159/000362780. [DOI] [PubMed] [Google Scholar]
- 114.Hwang DY, Silva GS, Furie KL, et al. Comparative sensitivity of computed tomography vs. magnetic resonance imaging for detecting acute posterior fossa infarct. J Emerg Med. 2012;42(5):559–565. doi: 10.1016/j.jemermed.2011.05.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chalela JA, Kidwell CS, Nentwich LM, et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet. 2007;369(9558):293–298. doi: 10.1016/S0140-6736(07)60151-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Edlow JA, Newman-Toker DE, Savitz SI. Diagnosis and initial management of cerebellar infarction. Lancet Neurol. 2008;7(10):951–964. doi: 10.1016/S1474-4422(08)70216-3. [DOI] [PubMed] [Google Scholar]
- 117.Saber Tehrani AS, Kattah JC, Mantokoudis G, et al. Small strokes causing severe vertigo: frequency of false-negative MRIs and nonlacunar mechanisms. Neurology. 2014;83(2):169–173. doi: 10.1212/WNL.0000000000000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Morita S, Suzuki M, Iizuka K. False-negative diffusion-weighted MRI in acute cerebellar stroke. Auris Nasus Larynx. 2011;38(5):577–582. doi: 10.1016/j.anl.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 119.Bhattacharya P, Nagaraja N, Rajamani K, et al. Early use of MRI improves diagnostic accuracy in young adults with stroke. J Neurol Sci. 2013;324(1–2):62–64. doi: 10.1016/j.jns.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 120.Newman-Toker DE, Moy E, Valente E, et al. Missed diagnosis of stroke in the emergency department: a cross-sectional analysis of a large population-based sample. Diagnosis. 2014;1(2):155–166. doi: 10.1515/dx-2013-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lee CC, Ho HC, Su YC, et al. Increased risk of vascular events in emergency room patients discharged home with diagnosis of dizziness or vertigo: a 3-year follow-up study. PLoS One. 2012;7(4):e35923. doi: 10.1371/journal.pone.0035923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pula JH, Newman-Toker DE, Kattah JC. Multiple sclerosis as a cause of the acute vestibular syndrome. J Neurol. 2013;260(6):1649–1654. doi: 10.1007/s00415-013-6850-1. [DOI] [PubMed] [Google Scholar]
- 123.Arbusow V, Theil D, Strupp M, et al. HSV-1 not only in human vestibular ganglia but also in the vestibular labyrinth. Audiol Neurootol. 2001;6(5):259–262. doi: 10.1159/000046131. [DOI] [PubMed] [Google Scholar]
- 124.Lee H, Kim BK, Park HJ, et al. Prodromal dizziness in vestibular neuritis: frequency and clinical implication. J Neurol Neurosurg Psychiatry. 2009;80(3):355–356. doi: 10.1136/jnnp.2008.155978. [DOI] [PubMed] [Google Scholar]
- 125.Bergenius J, Perols O. Vestibular neuritis: a follow-up study. Acta Otolaryngol. 1999;119(8):895–899. doi: 10.1080/00016489950180243. [DOI] [PubMed] [Google Scholar]
- 126.Strupp M, Jager L, Muller-Lisse U, et al. High resolution Gd-DTPA MR imaging of the inner ear in 60 patients with idiopathic vestibular neuritis: no evidence for contrast enhancement of the labyrinth or vestibular nerve. J Vestib Res. 1998;8(6):427–433. [PubMed] [Google Scholar]
- 127.Kim JS, Kim HJ. Inferior vestibular neuritis. J Neurol. 2012;259(8):1553–1560. doi: 10.1007/s00415-011-6375-4. [DOI] [PubMed] [Google Scholar]
- 128.Wegner I, van Benthem PP, Aarts MC, et al. Insufficient evidence for the effect of corticosteroid treatment on recovery of vestibular neuritis. Otolaryngol Head Neck Surg. 2012;147(5):826–831. doi: 10.1177/0194599812457557. [DOI] [PubMed] [Google Scholar]
- 129.Bergmann K. Fatal complications of otitis 60 years ago. HNO. 1995;43(8):478–481. [PubMed] [Google Scholar]
- 130.Kim JS, Cho KH, Lee H. Isolated labyrinthine infarction as a harbinger of anterior inferior cerebellar artery territory infarction with normal diffusion-weighted brain MRI. J Neurol Sci. 2009;278(1–2):82–84. doi: 10.1016/j.jns.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 131.Navi BB, Kamel H, Shah MP, et al. Rate and predictors of serious neurologic causes of dizziness in the emergency department. Mayo Clin Proc. 2012;87(11):1080–1088. doi: 10.1016/j.mayocp.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lee DH, Kim WY, Shim BS, et al. Characteristics of central lesions in patients with dizziness determined by diffusion MRI in the emergency department. Emerg Med J. 2013 doi: 10.1136/emermed-2013-202674. [DOI] [PubMed] [Google Scholar]
- 133.Raynor EM. Vertigo. In: Wolfson AB, Hendey GW, Ling LJ, et al., editors. Harwood-Nuss’ Clinical Practice of Emergency Medicine. 5. Philadelphia, PA: Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 134.Goldman B. Vertigo and Dizziness. In: Tintinalli JE, Stapczynski JS, Ma OJ, et al., editors. Emergency Medicine: a Comprehensive Study Guide. 7. New York: McGraw-Hill, Medical Pub. Division; 2011. [Google Scholar]
- 135.Chang AK, Olshaker JS. Rosen’s emergency medicine concepts and clinical practice. 8. Philadelphia: Saunders/Elsevier; 2014. [Google Scholar]
- 136.Ning M, Gonzalez RG. Case records of the Massachusetts General Hospital. Case 34-2013. A 69-year-old man with dizziness and vomiting. New Engl J Med. 2013;369(18):1736–1748. doi: 10.1056/NEJMcpc1302431. [DOI] [PubMed] [Google Scholar]