Graphical abstract
Keywords: HCV, Direct antiviral agents (DAA), Protease inhibitors, Polymerase inhibitors
Abstract
The pegylated interferon regimen has long been the lone effective management of chronic hepatitis C with modest response. The first appearance of protease inhibitors included boceprevir and telaprevir. However, their efficacy was limited to genotype 1. Recently, direct antiviral agents opened the gate for a real effective management of HCV, certainly after FDA approval of some compounds that further paved the way for the appearance of enormous potent direct antiviral agents that may achieve successful eradication of HCV.
Introduction
The first generation of protease inhibitors included boceprevir and telaprevir. Combined with pegylated interferon and ribavirin, they improved the overall rates of sustained virological response (SVR). This included treatment naïve as well as experienced patients [1–10]. However, this efficacy was limited to patients with hepatitis C virus genotype 1 infection, was associated with numerous side effects and needed a frequent dosing schedule [11].
With recent advances, many direct antiviral agents (DAA) developed and led to a more promising future for HCV infected patients. Excellent advantages were related to their high potency, pangenotypic coverage and intermediate to high barrier to resistance [12]. They paved the way to the possible application of oral interferon-free regimens. In addition, these regimens can be taken once daily and may result in global HCV eradication in near future [13].
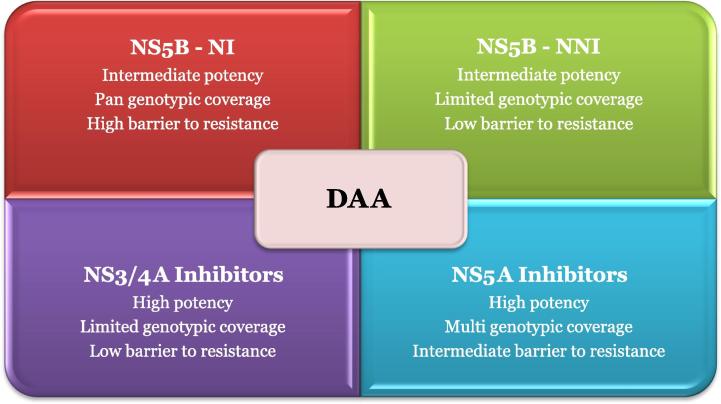
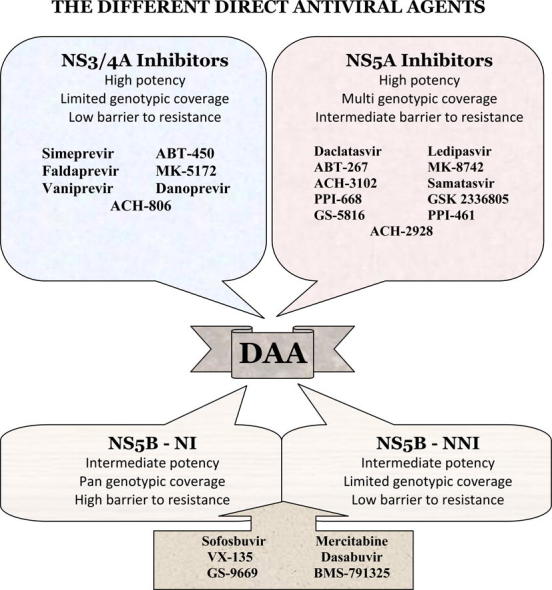
Three major classes of DAA dominated the scenario at different stages of development and clinical practice: NS3/4A protease inhibitors, NS5B polymerase inhibitors and NS5A direct inhibitors (Figs. 1 and 2). NS5B polymerase inhibitors are further categorized into two distinct groups: the nucleoside polymerase inhibitors (NIs) and the nonnucleoside polymerase inhibitors (NNIs). NIs interact with the catalytic site for NS5B affecting viral RNA synthesis. They are characterized by their high barrier of resistance [14,15]. NNIs bind to different allosteric sites on the NS5B protein and prevent effective viral RNA synthesis. Their efficacies differ according to HCV genotypes and subtypes. They have a lower barrier of resistance [16].
Fig. 1.
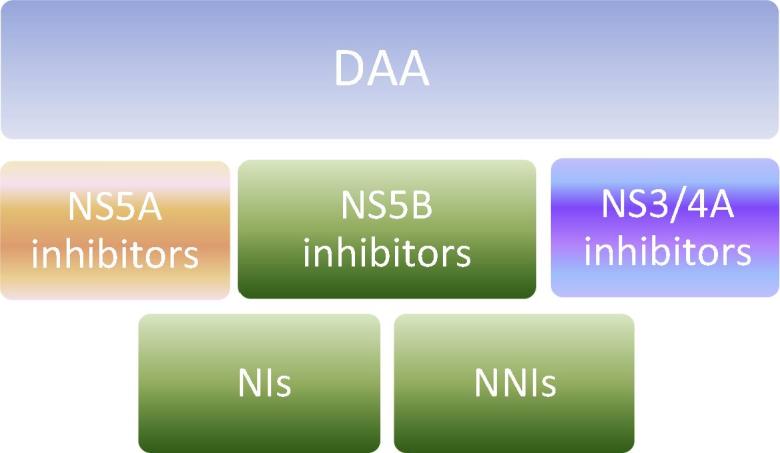
Different classes of direct antiviral agents (DAA).
Fig. 2.
Differences between the different DAA classes.
NS5A inhibitors showed promising results among the different DAA drug studies due to their multigenotypic efficacy, high potency but low to intermediate barrier to resistance. Such DAA are used in combination with interferon and ribavirin to prevent virologic breakthrough and subsequent resistance. Similarly, they synergize with other DAA to suppress the development of resistant virus strains [17–19].
The most important direct antiviral agents
Sofosbuvir
Sofosbuvir is a prodrug of 20-deoxy-20-fluoro-20-C-methyluridine monophosphate. It is a specific nucleotide analog inhibitor of the HCV NS5B polymerase acting as a false substrate for the RdRp and ending at chain termination after incorporation into the newly produced RNA chain [20]. It is characterized by its broad antiviral activity against all HCV genotypes [21]. Sofosbuvir is a 400 mg once-daily capsule, which can be taken with or without food. It is mainly excreted by the kidneys at a rate of 76% with a median half life ranging from 0.48 to 0.75 h [22]. Metabolism of sofosbuvir and the other related drugs of the same family have no relation with the CYP3A4 pathway. So, they have a low potential for drug-drug interaction [23,24].
S282T mutation is determined as the common mutation occurring in replicon studies with sofosbuvir [25]. This mutation has reduced replicative capacity as compared to the wild type containing replicons [26,27]. In a study using sofosbuvir containing regimen, patients who failed to achieve SVR showed undetectable S282T mutation, either at baseline or later [28].
Sofosbuvir has been studied in different combinations (Table 1). The value of adding sofosbuvir to the combination of Peg-IFNα in genotype 1 patients was first addressed in two phase II trials (PROTON and ATOMIC) [29,30]. The first study (PROTON) tested the addition of 400 mg sofosbuvir versus placebo to the combined IFN/RBV regimen for 12 weeks followed by an additional 12 or 36 weeks of Peg-IFNα and RBV. The 91% SVR12 in sofosbuvir arm compared to the 40% figure in placebo group was striking. The other trial (ATOMIC) evaluated the role of maintenance therapy after the initial 12 week response. SVR rated between 90% and 94%. This occurred regardless of the use of maintenance therapy. This based the 12 week triple therapy regimen for phase III NEUTRINO study [31]. In this study, 327 naïve patients with different genotypes (1, 4, 5 and 6) were treated for 12 weeks in an open-label single-arm design with sofosbuvir 400 mg and RBV 1000–1200 mg daily in addition to weekly Peg-IFNα 180 μg. The historic SVR12 of 60% was used for comparison. According to the treated HCV genotype, the results were 89% in HCV type 1, 96% in HCV type 4 and 100% in seven patients with HCV types 5 and 6. The overall response was 90%.
Table 1.
Different sofosbuvir studies.
| Name of study | Design | Genotype | SVR (%) |
|---|---|---|---|
| PROTON | Sof, IFN/RBV | 1 | 91 |
| ATOMIC | Sof, IFN/RBV | 1,4,6 | 90–94 |
| NEUTRINO | Sof, IFN/RBV | 1,4,5,6 | 89–100 |
| ELECTRON | Sof, RBV | 1 | 88 |
| Sof, RBV, Ledipasvir | 100 | ||
| Sof, RBV, GS-9669 | 92 | ||
| QUANTUM | Sof, RBV | 1 | 56 |
| FISSION | Sof, RBV | 2,3 | 67 |
| FUSION | Sof, RBV | 2,3 | 56–73 |
| POSITRON | Sof, RBV | 2,3 | 61–93 |
| LONESTAR | Sof, RBV, Ledipasvir | 1 | 95–100 |
Sofosbuvir was the first drug to test the concept of “interferon free” regimens. Many phase II studies were conducted to evaluate the efficacy of various sofosbuvir combination regimens [32,33]. The sofosbuvir based interferon free regimens were either: sofosbuvir plus RBV, sofosbuvir plus another DAA or sofosbuvir plus another DAA and RBV for different treatment durations (8–24 weeks). Studies that tested for sofosbuvir in genotype 1 included QUANTUM and ELECTRON studies. Fifty naïve patients were treated with sofosbuvir and ribavirin for 12 weeks. SVR12 rates were 56% and 88% respectively [34,35]. No obvious improvement in SVR was noted when treatment duration extended up to 24 weeks in a subgroup of patients in the QUANTUM and NIH SPARE studies [34–36]. Results of FUSION trial that used the same duration (24 weeks) in genotypes 2 and 3 led to significant improvement of SVR from 56% to 73% [37].
Impressive results are shown in studies that used sofosbuvir combined with other DAA. Most of studies proved that ribavirin use will be of no value [32,36]. Many regimens with different DAA were used with either a non-nucleoside polymerase inhibitor or a NS5A inhibitor with no significant differences in response. The addition of one of the nucleotide analogs (GS-0938) to sofosbuvir for 12 weeks resulted in SVR12 in 88% while SVR4 after 12 weeks of a combination of sofosbuvir and daclatasvir (NS5A inhibitor) or ledipasvir (GS-5885) was as high as 98% and 100% [38,39]. Shortening of treatment duration in LONESTAR trial that used sofosbuvir and ledipasvir for 8 weeks resulted in 95% SVR8 [38].
Treatment experienced patients were the target group in many sofosbuvir trials. In ELECTRON study, 12-week sofosbuvir plus RBV were tested in 10 genotype 1 patients with previous treatment failure and led to high relapse rates [32]. It differed when another DAA was used with sofosbuvir for 12 weeks in this difficult to treat group of patients. The SVR results in these DAA combination studies ranged 90–100%. Many DAA were used in combination with sofosbuvir as NS5A inhibitor ledipasvir or daclatasvir, the protease inhibitor simeprevir or the non-nucleoside polymerase inhibitor GS-9669 [33,38,39].
ELECTRON study assessed the use of sofosbuvir plus ribavirin in genotypes 2 and 3 for 12 weeks with/without Peg-IFNα [32]. The nonsignificant value of added interferon paved the way for further interferon free trials.
For genotypes 2 and 3, interferon free regimens were assessed in phase III trials (FISSION, FUSION and POSITRON) where combined sofosbuvir and RBV were given for 12 weeks. The efficacy of such combination was tested in the FISSION study in 256 naïve patients with SVR reached about 67% [31]. The 16 week instead of 12 week duration was tested in the FUSION study that recruited previous treatment failure patients. The overall SVR rates were 50% in the 12 week and 73% in the 16 week arms [40]. The POSITRON trial studied the effectiveness of 12 weeks of combination of sofosbuvir and RBV in patients with HCV type 2 or 3 who are ineligible for Peg-IFNα-based therapies due to contraindications, unacceptable previous adverse events or unwillingness to receive Peg-IFNα therapy. The overall SVR rates were 61–93% according to RBV dose [37].
Daclatasvir
Daclatasvir, produced by Bristol-Myers Squibb, is the first-in-class NS5A inhibitor that demonstrated a satisfactory multiphase rapid HCV RNA decline without significant adverse events. It is highly effective against genotypes 1–4 [41,42]. Several clinical trials assessed the efficacy of daclatasvir with different compounds.
Using daclatasvir with interferon and ribavirin, Suzuki et al. managed HCV patients who were treatment naïve, prior null or partial responders. Two Different concentrations of daclatasvir were used (10 and 60 mg) versus a placebo group. SVR24 reached 66.7–90% in naïve group versus 62.5% in placebo group and much less satisfactory results in prior null responders (22–33.3%) [43]. Better results were achieved by Izumi and colleagues. Naïve group had SVR24 89–100% while null responders group 50–78% [44].
Other trials looked for daclatasvir combinations with asunaprevir. A phase III trial was accomplished in 24 Japanese centers to manage genotype 1b HCV patients who were either interferon ineligible/intolerant or nonresponders. SVR24 was 87.4% in interferon ineligible versus 80.5% in null responder patients. Cirrhotic patients achieved 90.9% SVR24. Side effects included nasopharyngitis, elevated liver enzymes, headache, diarrhea and pyrexia [45]. Another study found 90.5% SVR in null responders while 63.6% in ineligible/intolerant patients [46]. Moreover, further results were published by Lok et al. who used different combinations of daclatasvir with asunaprevir (Dual), ribavirin (Triple) or interferon and ribavirin (Quad) in genotype 1 null responder patients. Dual twice daily asunaprevir and the quad therapy were effective (SVR 78% and 95% respectively). Neither dual (once daily asunaprevir) nor triple therapy was sufficiently effective for such patients [47]. Ongoing studies added BMS791325 to daclatasvir and asunaprevir. SVR results were recorded as 94–100% according to treatment period (12 or 24 weeks) [48].
Excellent results were further published by Sulkowski et al. [49]. Daclatasvir was combined with sofosbuvir for treatment naïve and treatment experienced patients infected with HCV genotypes 1, 2 and 3. The best results were achieved with genotype 1 patients (98% SVR12 for both naïve and prior null responder patients). As for genotypes 2 and 3, SVR was achieved in 92% and 89% of patients respectively. Response rates were similar whether patients were taking ribavirin or not. In another phase II study, SVR12 was recorded in all genotype 1 naïve and treatment experienced patients (100%) while patients with genotype 2 or 3 succeeded to have 91% SVR [50].
Simeprevir
Simeprevir is a new direct acting antiviral drug and a second generation NS3/4A serine protease inhibitor. It was introduced by Janssen and Medivir for the oral treatment of patients with genotype 1 and/or genotype 4 chronic HCV infections [51]. In vitro, it is active against all six genotypes with lesser efficacy against genotype 3a [52,53]. Simeprevir proved to have several advantages such as its high efficacy, short treatment duration (12–24 weeks), better safety profile than first generation drugs and the decreased pill burden being taken once daily [54].
The PILLAR study is a phase IIb trial that was performed to test the efficacy of combined simeprevir, interferon and ribavirin in HCV G1 infected patients who were naïve and noncirrhotic. They were compared to another group taking interferon and ribavirin alone. SVR rates in the combined group reached 74.7–86.1% compared to 64.9% in the second group. Patients who met the RGT criteria had the best SVR rates (91%). Reversible hyperbilirubinemia was recorded as a side effect of simeprevir. Discontinuation rates were 8–16% in the simeprevir group versus 15% in the control group [55].
The ASPIRE trial is another phase II trial that was designed for treatment experienced HCV G1 patients. Triple therapy (simeprevir, interferon and ribavirin) led to 85% SVR as compared to 37% only in control group (interferon and ribavirin). More detailed, partial responders had a SVR 75% versus 9% while prior nonresponders recorded 51% versus 19% SVR [56].
A phase III trial for treatment experienced HCV genotype 1 patients, named PROMISE study, was performed with randomization of patients to simeprevir, interferon and ribavirin versus placebo, interferon and ribavirin. Results were 79% SVR12 versus 39% in placebo group. QUEST-1 and QUEST-2 studies also randomized to same regimen. Higher SVR12 rates were recorded in simeprevir group (80–81%) compared to the placebo group (50%) [57–60].
In parallel, important randomized clinical trials were performed in Japan. In the DRAGON study, 92 naïve patients infected with genotype 1 HCV were enrolled for simeprevir (12–24 weeks) with pegylated interferon and ribavirin (24–48 weeks) versus lone pegylated interferon and ribavirin for 48 weeks. Statistically significant difference was observed between simeprevir group (SVR 77–92% with relapse rate 8–17%) and the other group (SVR 46% with relapse rate 36%) [61]. CONCERTO-1 study for treatment naïve G1 HCV patients found SVR 88.6% versus 61.7%. CONCERTO-2 (for nonresponders) and CONCERTO-3 (for relapsers) concluded SVR12 rates as 35.8–52.8% for prior nonresponders and 95.9% for prior relapsers [62,63]. Most recently, CONCERTO-4 study for both treatment naïve and experienced patients with chronic HCV genotype 1 infection was published. The highest SVR12 was achieved by the treatment naïve patients (91.7%) and prior nonrelapsers (100%) while the prior nonresponders scored an SVR rate 38.5% only [64].
Ledipasvir
Ledipasvir is another NS5A inhibitor that showed promising results in different trials that evaluated its combination to sofosbuvir. It provided high potency against HCV genotypes 1a, 1b, 4a and 6a while it was less efficacious against genotypes 2a and 3a [65]. It cannot be used alone due to quick development of resistance [66].
The LONESTAR clinical trial evaluated the use of sofosbuvir and ledipasvir with and without ribavirin for HCV G1 patients, either treatment naïve or experienced. SVR was really successful (98%) [67]. ELECTRON trial is a similar trial that announced 100% SVR12 for both treatment naïve and prior nonresponder G1 patients [68]. These results are further proved in another large clinical trial using fixed doses for untreated G1 patients. Ledipasvir combined with sofosbuvir led to 99% SVR of total 865 patients (16% had liver cirrhosis) [69]. Concerning the previously treated patients (n = 440), SVR ranged 94–99% according to duration of therapy (12–24 weeks) and whether taken with or without ribavirin [70]. Reducing the duration of therapy to 8 weeks led to 93–94% SVR compared to 95% if the regimen was taken for 12 weeks [71].
Abt-450/ritonavir, ombitasvir and dasabuvir
ABT-450, new product of Abbvie Company (North Chicago, USA), is a potent inhibitor of NS3/4A protease. It has been studied with several drugs aiming to provide a possible interferon free/ribavirin free “all oral” drug regimen in a shorter duration of therapy [72]. Ombitasvir (ABT-267) is a pangenotypic inhibitor of NS5A with excellent potency, metabolic stability and pharmacokinetics [73].
Different studies provided optimistic results for a future of HCV eradication. The AVIATOR study used the combination of ABT-450/r, ombitasvir (ABT-267), dasabuvir (ABT-333) plus ribavirin for 8, 12 or 24 weeks in noncirrhotic G1 HCV patients (either naïve or previous treatment failure). Treatment naïve patients had a successful SVR (97.5%) as well as treatment experienced patients (93.3%) [74].
In another clinical trial performed by Zeuzem et al., they managed HCV G1 infected patients with ABT-450/r-ombitasvir and dasabuvir with ribavirin. 286 of 297 patients (96.3%) achieved SVR12. Enrolled patients were treatment relapsers, partial responders or null responders [75]. Feld et al. used the same drug regimen for treatment naïve patients and scored 96.2% SVR [76]. Moreover, Poordad and colleagues focused their clinical trial on cirrhotic patients, used the same regimen and finally concluded a successful SVR12 rate in such difficult to treat patients (91.8%) [77].
In a trial to exclude ribavirin, Ferenci et al. used the same drug regimen excluding ribavirin and found that the rates of virologic failure were higher in the ribavirin free group (7.8% versus 2%) [78]. Finally, Andreone et al. concluded a 97–100% SVR with or without ribavirin in treatment experienced patients with HCV G1 infection with well tolerability as evidenced by the low rates of discontinuation and the mild adverse events [79].
Faldaprevir
Faldaprevir is a new NS3/4A protease inhibitor that has an acceptable tolerability and safety at all dose levels. Several studies used it with different drug combinations. SILEN-C1 (for naïve G1 HCV patients) and SILEN-C2 (for prior nonresponders) were enrolled on 429 and 290 patients respectively. Faldaprevir was used in combination with pegylated interferon and ribavirin. SVR rates in naïve, prior partial responders and null responders were 72–84%, 32–50% and 21–35% respectively. SILEN-C3 is a phase II trial for 160 naïve G1 infected patients. Faldaprevir was taken for 12–24 weeks with 24–48 weeks of pegylated interferon and ribavirin. SVR rates were 67% (if 12 weeks) and 74% (if 24 weeks) [80–82].
Zeuzem et al. used a different drug combination. In a phase Ib trial (SOUND-C1), faldaprevir was taken with deleobuvir and ribavirin. Studied patients were treatment naïve G1 infected persons. Two out of 32 managed patients suffered from virological breakthrough that was successfully treated with interferon containing therapy. SVR24 was 73% (deleobuvir 400 mg) and 94% (deleobuvir 600 mg) [83]. In another phase IIb trial by Zeuzem et al., SVR12 was 52–69% according to the drug regimen using 120 mg once daily faldaprevir with deleobuvir 600 mg (2–3 times daily) and ribavirin (16, 28 or 40 weeks) [84].
MK-5172, MK-8742 and vaniprevir
MK-5172 is an NS3/4A protease inhibitor with a high potency and barrier to resistance. It is taken once daily. MK-5172 is active against multiple genotypes associated with resistance to first generation protease inhibitors [85,86]. It has been tested in combination with pegylated interferon and ribavirin to manage patients infected with genotype 1 HCV. Achieved SVR24 ranged between 86% and 93% according to the used dose (100, 200, 400 or 800 mg). The combination was generally well tolerated [87]. Vaniprevir (MK-7009) is another NS3/4A protease inhibitor that reached phase III clinical trials. Vaniprevir monotherapy showed potent antiviral activity in genotype 1 HCV patients. It is generally well tolerated without serious adverse events [88]. Despite the efficacy of both MK-5172 and vaniprevir, emergence of resistance reduced their effectiveness against viral replication [89]. MK-8742 is an NS5A inhibitor, currently in phase IIb clinical trials as an all oral, interferon free regimen for management of HCV [90]. Being combined with MK-5172, they led to a breakthrough therapy for HCV therapy. This study named C-WORTHY study treated genotype 1 (1a and 1b) and declared 89–100% SVR12 for patients using this combination with or without ribavirin [91]. Still more progression is ongoing to provide further MK compounds with retained high potency and pangenotypic activity [92].
Other compounds
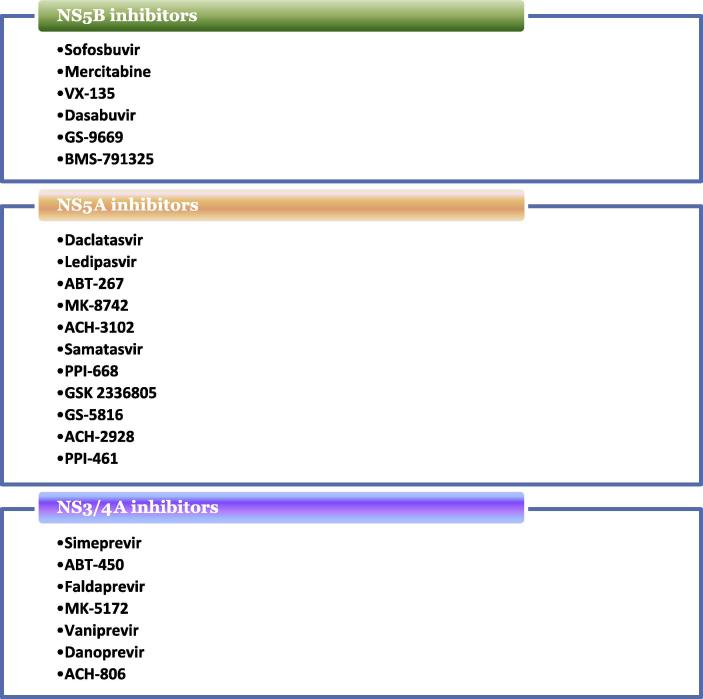
Apart from the previously discussed major compounds, there are numerous drugs and molecules at various stages of production and different phases of trials (Fig. 3). Phase II clinical trials include NS3/4A inhibitors such as danoprevir [93–95] and NS5A inhibitors such as ACH-3102 [96], samatasvir [97], PPI-668 [98], GSK 2336805 [99] and GS-5816 [100,101]. Similarly, phase I clinical trials include NS3/4A inhibitors such as ACH-806 [102] and NS5A inhibitors such as ACH-2928 [103] and PPI-461 [104].
Fig. 3.
The different direct antiviral agent drugs.
Conclusions
The last few years witnessed the development and appearance of many drugs that led to potent changes in the management of HCV. Still the future will evidence the development of much more compounds that will provide 100% efficacy within very short periods of therapy and the dream of HCV eradication seems to be possible in the near future.
Conflict of interest
The authors have declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Biographies

Tamer Elbaz an Assistant Professor of Hepatology and Gastroenterology working at Endemic Medicine and Hepatogastroenterology Department, Faculty of Medicine, Cairo University. His studies focused on the management of viral hepatitis and its sequelae as hepatocellular carcinoma. He is a consultant member of Liver Transplantation Unit, Manial Specialized Hospital, Cairo University. He is an active participant of the multidisciplinary hepatocellular carcinoma clinic at Kasr Al Ainy Hospital. In addition, he is a member of several projects enrolled in Egypt for management of HCV using the recently discovered direct antiviral agents.

Mohamed El-Kassas a consultant of Hepatology and Tropical Medicine at National Hepatology and Tropical Medicine Research Institute. He received MD degree in Tropical Medicine from Cairo University. He has some international publications especially in viral hepatitis and hepatocellular carcinoma. He is included in a number of collaborative research work projects with American, French and Japanese universities. He is an assistant executive secretary of Egyptian National Committee for Control of Viral Hepatitis and consultant of Hepatology in some private and authority hospitals. Finally, he is member of European and American associations for Study of Liver.

Gamal Esmat a distinguished Professor at Endemic Medicine and Hepatogastroenterology Department, Cairo University. He is Vice President of Cairo University for Graduate Studies and Research. He published a vast number of scientific papers in top journals and was mainly concerned with hepatitis, hepatocellular carcinoma and liver transplantation. Since 2001, he is a director of Clinical Research Unit, Hepatitis C Project, International Health Division, University of Maryland Baltimore. He is a member of Numerous Scientific Societies and Organizations and per se was the president of IASL (International Association for the Study of the Liver) during the period 2005–2008 and is recently the WHO consultant for management of HBV.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Zeuzem S., Hezode C., Ferenci P., Dusheiko G.M., Alves K., Bengtsson L. Telaprevir in combination with peginterferon alfa-2a with or without ribavirin in the treatment of chronic hepatitis C. final results of the PROVE 2 study. Hepatology. 2008;48(1):418–419. [Google Scholar]
- 2.Hézode C., Forestier N., Dusheiko G., Ferenci P., Pol S., Goeser T. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N Engl J Med. 2009;360(18):1839–1850. doi: 10.1056/NEJMoa0807650. [DOI] [PubMed] [Google Scholar]
- 3.McHutchison J.G., Everson G.T., Gordon S.C., Jacobson I.M., Sulkowski M., Kauffman R. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med. 2009;360(18):1827–1838. doi: 10.1056/NEJMoa0806104. [DOI] [PubMed] [Google Scholar]
- 4.McHutchison J.G., Manns M.P., Muir A.J., Terrault N.A., Jacobson I.M., Afdhal N.H. Telaprevir for previously treated chronic HCV infection. N Engl J Med. 2010;362(14):1292–1303. doi: 10.1056/NEJMoa0908014. [DOI] [PubMed] [Google Scholar]
- 5.Kwo P.Y., Lawitz E.J., McCone J., Schiff E.R., Vierling J.M., Pound D. SPRINT-1 investigators. Efficacy of boceprevir, an NS3 protease inhibitor, in combination with peginterferon alfa-2b and ribavirin in treatment-naïve patients with genotype 1 hepatitis C infection (SPRINT-1): an open-label, randomised, multicentre phase 2 trial. Lancet. 2010;376:705–716. doi: 10.1016/S0140-6736(10)60934-8. [DOI] [PubMed] [Google Scholar]
- 6.Sherman K.E., Flamm S.L., Afdhal N.H., Nelson D.R., Sulkowski M.S., Everson G.T. Telaprevir in combination with peginterferon alfa2a and ribavirin for 24 or 48 weeks in treatment-naïve genotype 1 HCV patients who achieved an extended rapid viral response: final results of phase 3 ILLUMINATE study. AASLD 2010: 61st annual meeting for the American association for the study of liver disease; October 29–November 2, 2010. Boston, MAHepatology. 2010;52(4):401A–402A. [Google Scholar]
- 7.Poordad F., McCone J., Bacon B.R., Bruno S., Manns M.P., Sulkowski M.S. SPRINT-2 investigators. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364(13):1195–1206. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bacon B.R., Gordon S.C., Lawitz E., Marcellin P., Vierling J.M., Zeuzem S. HCV RESPOND-2 Investigators. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364(13):1207–1217. doi: 10.1056/NEJMoa1009482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobson I.M., McHutchison J.G., Dusheiko G., Di Bisceglie A.M., Reddy K.R., Bzowej N.H. ADVANCE study team. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364(25):2405–2416. doi: 10.1056/NEJMoa1012912. [DOI] [PubMed] [Google Scholar]
- 10.Zeuzem S., Andreone P., Pol S., Lawitz E., Diago M., Roberts S. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364(25):2417–2428. doi: 10.1056/NEJMoa1013086. [DOI] [PubMed] [Google Scholar]
- 11.Chae H.B., Park S.M., Youn S.J. Direct-acting antivirals for the treatment of chronic hepatitis C: open issues and future perspectives. Sci World J. 2013:704912. doi: 10.1155/2013/704912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaetano J.N. Benefit-risk assessment of new and emerging treatments for hepatitis C: focus on simeprevir and sofosbuvir. Drug Healthc Patient Saf. 2014;6:37–45. doi: 10.2147/DHPS.S43304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kohler J.J., Nettles J.H., Amblard F., Hurwitz S.J., Bassit L., Stanton R.A. Approaches to hepatitis C treatment and cure using NS5A inhibitors. Infect Drug Resist. 2014;7:41–56. doi: 10.2147/IDR.S36247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lesburg C.A., Cable M.B., Ferrari E., Hong Z., Mannarino A.F., Weber P.C. Crystal structure of the RNA-dependent RNA polymerase from hepatitis C virus reveals a fully encircled active site. Nat Struct Biol. 1999;6(10):937–943. doi: 10.1038/13305. [DOI] [PubMed] [Google Scholar]
- 15.Ali S., Leveque V., Le Pogam S., Ma H., Philipp F., Inocencio N. Selected replicon variants with low-level in vitro resistance to the hepatitis C virus NS5B polymerase inhibitor PSI-6130 lack cross-resistance with R1479. Antimicrob Agents Ch. 2008;52(12):4356–4369. doi: 10.1128/AAC.00444-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Powdrill M.H., Bernatchez J.A., Götte M. Inhibitors of the hepa titis C virus RNA-dependent RNA polymerase NS5B. Viruses. 2010;2(10):2169–2195. doi: 10.3390/v2102169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao M. Antiviral activity and resistance of HCV NS5A replication complex inhibitors. Curr Opin Virol. 2013;3(5):514–520. doi: 10.1016/j.coviro.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 18.Zeuzem S., Buggish P., Agarwal K., Manns M., Marcellin P., Foster G.R. Dual, triple, and quadruple combination treatment with a protease inhibitor (GS 9256) and a polymerase inhibitor (GS-9190) alone and in combination with ribavirin (RBV) or PegIFN/RBV for up to 28 days in treatment naïve, genotype 1 HCV subjects. Hepatology. 2010;52(S1) LB-1. [Google Scholar]
- 19.Gane E.J., Roberts S.K., Stedman C.A., Angus P.W., Ritchie B., Elston R. Oral combination therapy with a nucleoside polymerase inhibitor (RG7128) and danoprevir for chronic hepatitis C genotype 1 infection (INFORM-1): a randomised, double-blind, placebo-controlled, dose-escalation trial. Lancet. 2010;376:1467–1475. doi: 10.1016/S0140-6736(10)61384-0. [DOI] [PubMed] [Google Scholar]
- 20.Sofia M.J., Bao D., Chang W., Du J., Nagarathnam D., Rachakonda S. Discovery of a beta-d-2′-deoxy-2′-alpha-fluoro-20-beta-Cmethyluridine nucleotide prodrug (PSI-7977) for the treatment of hepatitis C virus. J Med Chem. 2010;53:7202–7218. doi: 10.1021/jm100863x. [DOI] [PubMed] [Google Scholar]
- 21.Hassanein T., Lawitz E., Crespo I., Davis M., DeMicco M.P., Nelson D.R. Once daily sofosbuvir (GS-7977) plus PEG/RBV: high early response rates are maintained during post-treatment follow-up in treatment-naive patients with HCV genotype 1, 4, and 6 infection in the ATOMIC study. Hepatology. 2012;56:307A. [Google Scholar]
- 22.Rodriguez-Torres M., Lawitz E., Kowdley K.V., Nelson D.R., Dejesus E., McHutchison J.G. Sofosbuvir (GS-7977) plus peginterferon/ribavirin in treatment-naïve patients with HCV genotype 1: a randomized, 28-day, dose-ranging trial. J Hepatol. 2013;58:663–668. doi: 10.1016/j.jhep.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 23.Mathias A., Cornpropst M., Clemons D., Denning J., Symonds W.T. No clinically significant pharmacokinetic drug–drug interactions between sofosbuvir (GS-7977) and the immunosuppressants, cyclosporine a or tacrolimus in healthy volunteers. Hepatology. 2012;56:1063A–1064A. [Google Scholar]
- 24.Kirby B, Mathias A, Rossi S, Moyer C, Shen G, Kearney BP. No clinically significant pharmacokinetic interactions between sofosbuvir (GS-7977) and HIV antiretrovirals Atripla, rilpivirine, darunavir/ritonavir, or raltegravir in healthy volunteers. In: 63rd annual meeting of the American association for the study of liver diseases (AASLD), Abstract 1877. Boston (MA); November 9–13, 2012.
- 25.Lam A.M., Espiritu C., Bansal S., Micolochick Steuer H.M., Niu C., Zennou V. Genotype and subtype profiling of PSI-7977 as a nucleotide inhibitor of hepatitis C virus. Antimicrob Agents Ch. 2012;56(6):3359–3368. doi: 10.1128/AAC.00054-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuntzen T., Timm J., Berical A., Lennon N., Berlin A.M., Young S.K. Naturally occurring dominant resistance mutations to hepatitis C virus protease and polymerase inhibitors in treatment-naïve patients. Hepatology. 2008;48(6):1769–1778. doi: 10.1002/hep.22549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu S, Rajyaguru S, Chiu S, Hebner C, Evguenia S, Svarovskaia ES, et al. Sofosbuvir selects the NS5B S282T mutation in vitro in genotype 1–6 replicons and is not cross-resistant to resistant associated variants selected by other classes of antiviral inhibitors. In: 64rd annual meeting of the American association for the study of liver diseases. Washington (DC); November 1–5 2013.
- 28.Evguenia S, Svarovskaia ES, Hadas S, Dvory HS, Hebner C, Doehle B, et al. No resistance detected in four phase 3 clinical studies in HCV genotype 1–6 of sofosbuvir + ribavirin with or without peginterferon. In: 64rd annual meeting of the American association for the study of liver diseases. Washington (DC); November 1–5 2013.
- 29.Lawitz E., Lalezari J.P., Hassanein T., Kowdley K.V., Poordad F.F., Sheikh A.M. Sofosbuvir in combination with peginterferon alfa-2a and ribavirin for non-cirrhotic, treatment-naive patients with genotypes 1, 2, and 3 hepatitis C infection: a randomised, double-blind, phase 2 trial. Lancet Infect Dis. 2013;13:401–408. doi: 10.1016/S1473-3099(13)70033-1. [DOI] [PubMed] [Google Scholar]
- 30.Kowdley K.V., Lawitz E., Crespo I., Hassanein T., Davis M.N., DeMicco M. Sofosbuvir with pegylated interferon alfa-2a and ribavirin for treatment-naive patients with hepatitis C genotype-1 infection (ATOMIC): an open-label, randomised, multicentre phase 2 trial. Lancet. 2013;381:2100–2107. doi: 10.1016/S0140-6736(13)60247-0. [DOI] [PubMed] [Google Scholar]
- 31.Lawitz E., Mangia A., Wyles D., Rodriguez-Torres M., Hassanein T., Gordon S.C. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878–1887. doi: 10.1056/NEJMoa1214853. [DOI] [PubMed] [Google Scholar]
- 32.Gane E.J., Stedman C.A., Hyland R.H., Ding X., Pang P.S., Symonds W.T. All-oral sofosbuvir-based 12-week regimens for the treatment of chronic HCV infection: the ELECTRON study. J Hepatol. 2013;58:S6. [Google Scholar]
- 33.Lawitz E, Ghalib R, Rodriguez-Torres M, et al. SVR4 results of a once daily regimen of simeprevir (TMC435) plus sofosbuvir (GSs-7977) with or without ribavirin (RBV) in HCV GT 1 null responders. Digestive Disease Week, Orlando, Abstract Sa2073; May 18–21 2013.
- 34.Lalezari J.P., Nelson D.R., Hyland R.H., Lin M., Rossi S.J., Symonds W.T. Once daily sofosbuvir plus ribavirin for 12 and 24 weeks in treatment-naïve patients with HCV infection: the QUANTUM study. J Hepatol. 2013;58:S236. [Google Scholar]
- 35.Gane E.J., Stedman C.A., Hyland R.H., Ding X., Svarovskaia E., Symonds W.T. Nucleotide polymerase inhibitor sofosbuvir plus ribavirin for hepatitis C. N Engl J Med. 2013;368:34–44. doi: 10.1056/NEJMoa1208953. [DOI] [PubMed] [Google Scholar]
- 36.Osinusi A, Meissner EG, Bon D, Lee YJ, Proschan M, Herrmann E, et al. High efficacy of sofosbuvir in combination with weight based ribavirin for 24 weeks in difficult to treat HCV infected genotype-1 patients. In: 20th conference on retroviruses and opportunistic infections, Abstract 157B. Atlanta (GA); March 3–6, 2013.
- 37.Jacobson I.M., Gordon S.C., Kowdley K.V., Yoshida E.M., Rodriguez-Torres M., Sulkowski M.S. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013;368:1867–1877. doi: 10.1056/NEJMoa1214854. [DOI] [PubMed] [Google Scholar]
- 38.Lawitz E., Poordad F.F., Pang P.S., Hyland R.H., Ding X., Mo H. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naïve and previously treated patients with genotype 1 hepatitis C: the LONESTAR study. Hepatology. 2013;58:1092A. doi: 10.1016/S0140-6736(13)62121-2. [DOI] [PubMed] [Google Scholar]
- 39.Sulkowski MS, Gardiner DF, Rodriguez-Torres M, Reddy KR, Hassanein T, Jacobson I, et al. AI444040 study group. High rate of sustained virologic response with the all-oral combination of daclatasvir (NS5a inhibitor) plus sofosbuvir (nucleotide NS5b inhibitor) with or without ribavirin, in treatment naive patients chronically infected with HCV GT 1, 2 or 3. In: 63rd annual meeting of the American association for the study of liver diseases (AASLD), Abstract LB-2. Boston (MA); 2012 November 9–13.
- 40.Dahari H., Cotler S.J., Layden T.J., Perelson A.S. Understanding triphasic HCV decline during treatment in the era of IL28B polymorphisms and direct acting antiviral agents via mathematical modeling. J Hepatol. 2013;58(4):840–842. doi: 10.1016/j.jhep.2012.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guedj J., Dahari H., Rong L., Sansone N.D., Nettles R.E., Cotler S.J. Modeling shows that the NS5A inhibitor daclatasvir has two modes of action and yields a shorter estimate of the hepatitis C virus half-life. Proc Natl Acad Sci USA. 2013;110(10):3991–3996. doi: 10.1073/pnas.1203110110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nettles R.E., Gao M., Bifano M., Chung E., Persson A., Marbury T.C. Multiple ascending dose study of BMS-790052, a nonstructural protein 5A replication complex inhibi tor, in patients infected with hepatitis C virus genotype 1. Hepatology. 2011;54(6):1956–1965. doi: 10.1002/hep.24609. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki F., Toyota J., Ikeda K., Chayama K., Mochida S., Hayashi N. A randomized trial of daclatasvir with peginterferon alfa-2b and ribavirin for HCV genotype 1 infection. Antivir Ther. 2014;19:491–499. doi: 10.3851/IMP2730. [DOI] [PubMed] [Google Scholar]
- 44.Izumi N., Yokosuka O., Kawada N., Osaki Y., Yamamoto K., Sata M. Daclatasvir combined with peginterferon alfa-2a and ribavirin in Japanese patients infected with hepatitis C genotype 1. Antivir Ther. 2014;19:501–510. doi: 10.3851/IMP2731. [DOI] [PubMed] [Google Scholar]
- 45.Kumada H., Suzuki Y., Ikeda K., Toyota J., Karino Y., Chayama K. Daclatasvir plus asunaprevir for chronic HCV genotype 1b infection. Hepatology. 2014;59(6):2083–2091. doi: 10.1002/hep.27113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suzuki Y., Ikeda K., Suzuki F., Toyota J., Karino Y., Chayama K. Dual oral therapy with daclatasvir and asunaprevir for patients with HCV genotype 1b infection and limited treatment options. J Hepatol. 2013;58(4):655–662. doi: 10.1016/j.jhep.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 47.Lok A.S., Gardiner D.F., Hézode C., Lawitz E.J., Bourlière M., Everson G.T. Randomized trial of daclatasvir and asunaprevir with or without PegIFN/RBV for hepatitis C virus genotype 1 null responders. J Hepatol. 2014;60(3):490–499. doi: 10.1016/j.jhep.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 48.Everson G.T., Sims K.D., Rodriguez-Torres M., Hézode C., Lawitz E., Bourlière M. Efficacy of an interferon- and ribavirin-free regimen of daclatasvir, asunaprevir, and BMS-791325 in treatment-naive patients with HCV genotype 1 infection. Gastroenterology. 2014;146(2):420–429. doi: 10.1053/j.gastro.2013.10.057. [DOI] [PubMed] [Google Scholar]
- 49.Sulkowski M.S., Gardiner D.F., Rodriguez-Torres M., Reddy K.R., Hassanein T., Jacobson I. AI444040 study group. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370(3):211–221. doi: 10.1056/NEJMoa1306218. [DOI] [PubMed] [Google Scholar]
- 50.Pawlotsky J.M. NS5A inhibitors in the treatment of hepatitis C. J Hepatol. 2013;59(2):375–382. doi: 10.1016/j.jhep.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 51.Vaidya A., Perry C.M. Simeprevir: first global approval. Drugs. 2013;73(18):2093–2106. doi: 10.1007/s40265-013-0153-9. [DOI] [PubMed] [Google Scholar]
- 52.Gottwein J.M., Scheel T.K., Jensen T.B., Ghanem L., Bukh J. Differential efficacy of protease inhibitors against HCV genotypes 2a, 3a, 5a, and 6a NS3/4A protease recombinant viruses. Gastroenterology. 2011;141:1067–1079. doi: 10.1053/j.gastro.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 53.Tsantrizos Y.S. TMC-435, an NS3/4A protease inhibitor for the treatment of HCV infection. Curr Opin Investig Drugs. 2009;10(8):871–881. [PubMed] [Google Scholar]
- 54.Asselah T., Marcellin P. Second-wave IFN-based triple therapy for HCV genotype 1 infection: simeprevir, faldaprevir and sofosbuvir. Liver Int. 2014;34(1):60–68. doi: 10.1111/liv.12424. [DOI] [PubMed] [Google Scholar]
- 55.Fried M.W., Buti M., Dore G.J., Flisiak R., Ferenci P., Jacobson I. Once-daily simeprevir (TMC435) with pegylated interferon and ribavirin in treatment-naïve genotype 1 hepatitis C: the randomized PILLAR study. Hepatology. 2013;58(6):1918–1929. doi: 10.1002/hep.26641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lenz O, Fevery B, Vijgen L, Verbeeck J, Peeters M, Beumont-Mauviel M, et al. TMC 435 in patients infected with HCV genotype 1 who have failed previous pegylated interferon/ribavirin treatment: virologic analysis of the ASPIRE trial. In: Proceedings of the 47th annual meeting of the European association for the study of the liver, Abstract 9. Barcelona (Spain); April 2010.
- 57.Lawitz E, Forns X, Zeuzem S, Gane Ed, Bronowicki JP, Andreone P, et al. Simeprevir (TMC435) with peginterferon/ribavirin for treatment of chronic HCV genotype 1 infection in patients who relapsed after previous interferon-based therapy: results from PROMISE, a phase III trial. Digestive Disease Week 2013. Orlando (FL); May 18–21, 2013.
- 58.Jacobson I., Dore G.J., Foster G.R., Fried M.W., Radu M., Rafalskiy V. Simeprevir (TMC435) with peginterferon/ribavirin for chronic HCV genotype 1 infection in treatment-naïve patients: results from QUEST-1, a phase III trial. The international liver congress™ 2013, 48th annual meeting of the European association for the study of the liver. Amsterdam (The Netherlands); April 24–28, 2013J Hepatol. 2013;58(1):S574. [Google Scholar]
- 59.Poordad F.P., Manns M.P., Marcellin P., Arujo E.S., Buti M., Horsmans Y. Simeprevir (TMC435) with peginterferon/ribavirin for treatment of chronic HCV genotype-1 infection in treatment-naıve patients: results from QUEST-2, a phase III trial. Gastroenterology. 2013;144(5):S151. [Google Scholar]
- 60.Jacobson I.M., Dore G.J., Foster G.R., Fried M., Manns M., Marcellin P. Simeprevir (TMC435) with peginterferon/ribavirin for treatment of chronic HCV genotype 1 infec tion in treatment-naïve patients: efficacy in difficult-to-treat patient sub-populations in the QUEST 1 and 2 phase III trials. AASLD 2013: 64th annual meeting of the American association for the study of liver diseases: the liver meeting 2013. Washington (DC); November 1–5, 2013Hepatology. 2013;58(S1):756A–757A. [Google Scholar]
- 61.Hayashi N., Seto C., Kato M., Komada Y., Goto S. Once-daily simeprevir (TMC435) with peginterferon/ribavirin for treatment-naïve hepatitis C genotype 1-infected patients in Japan: the DRAGON study. J Gastroenterol. 2014;49(1):138–147. doi: 10.1007/s00535-013-0875-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hayashi N., Izumi N., Kumada H., Okanoue T., Tsubouchi H., Yatsuhashi H. Simeprevir with peginterferon/ribavirin for treatment-naïve hepatitis C genotype 1 patients in Japan: CONCERTO-1, a phase III trial. J Hepatol. 2014;61(2):219–227. doi: 10.1016/j.jhep.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 63.Izumi N., Hayashi N., Kumada H., Okanoue T., Tsubouchi H., Yatsuhashi H. Once-daily simeprevir with peginterferon and ribavirin for treatment-experienced HCV genotype 1-infected patients in Japan: the CONCERTO-2 and CONCERTO-3 studies. J Gastroenterol. 2014;49(5):941–953. doi: 10.1007/s00535-014-0949-8. [DOI] [PubMed] [Google Scholar]
- 64.Kumada H., Hayashi N., Izumi N., Okanoue T., Tsubouchi H., Yatsuhashi H. Simeprevir (TMC435) once daily with peginterferon α-2b and ribavirin in patients with genotype-1 hepatitis-C virus infection: the CONCERTO-4 study. Hepatol Res. 2014 doi: 10.1111/hepr.12375. [DOI] [PubMed] [Google Scholar]
- 65.Wong K.A., Worth A., Martin R., Svarovskaia E., Brainard D.M., Lawitz E. Characterization of hepatitis C virus resistance from a multiple dose clinical trial of the novel NS5A inhibitor GS-5885. Antimicrob Agents Ch. 2013;57(12):6333–6340. doi: 10.1128/AAC.02193-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lawitz E.J., Gruener D., Hill J.M., Marbury T., Moorehead L., Mathias A. A phase 1, randomized, placebo-controlled, 3-day, dose-ranging study of GS-5885, an NS5A inhibitor, in patients with genotype 1 hepatitis C. J Hepatol. 2012;57(1):24–31. doi: 10.1016/j.jhep.2011.12.029. [DOI] [PubMed] [Google Scholar]
- 67.Lawitz E., Poordad F.F., Pang P.S., Hyland R.H., Ding X., Mo H. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): an open-label, randomised, phase 2 trial. Lancet. 2014;383(9916):515–523. doi: 10.1016/S0140-6736(13)62121-2. [DOI] [PubMed] [Google Scholar]
- 68.Gane E., Stedman C.A., Hyland R., Ding X., Svarovskaia G.M., Subramanian G.M. Efficacy of nucleotide polymerase inhibitor sofosbuvir plus the NS5A inhibitor ledipasvir or the NS5B non-nucleoside inhibitor GS-9669 against HCV genotype 1 infection. Gastroenterology. 2014;146(3):736–743. doi: 10.1053/j.gastro.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 69.Afdhal N., Zeuzem S., Kwo P., Chojkier M., Gitlin N., Puoti M. ION-1 investigators. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370(20):1889–1898. doi: 10.1056/NEJMoa1402454. [DOI] [PubMed] [Google Scholar]
- 70.Afdhal N., Reddy K.R., Nelson D.R., Lawitz E., Gordon S.C., Schiff E. ION-2 investigators. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370(16):1483–1493. doi: 10.1056/NEJMoa1316366. [DOI] [PubMed] [Google Scholar]
- 71.Kowdley K.V., Gordon S.C., Reddy K.R., Rossaro L., Bernstein D.E., Lawitz E. ION-3 investigators. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370(20):1879–1888. doi: 10.1056/NEJMoa1402355. [DOI] [PubMed] [Google Scholar]
- 72.Carrion A.F., Gutierrez J., Martin P. New antiviral agents for the treatment of hepatitis C: ABT-450. Expert Opin Pharmacother. 2014;15(5):711–716. doi: 10.1517/14656566.2014.889116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.DeGoey D.A., Randolph J.T., Liu D., Pratt J., Hutchins C., Donner P. Discovery of ABT-267, a pan-genotypic inhibitor of HCV NS5A. J Med Chem. 2014;57(5):2047–2057. doi: 10.1021/jm401398x. [DOI] [PubMed] [Google Scholar]
- 74.Kowdley K.V., Lawitz E., Poordad F., Cohen D.E., Nelson D., Zeuzem S. A 12-week interferon-free treatment regimen with ABT-450/r, ABT-267, ABT-333 and ribavirin achieves SVR rates (observed data) of 99% in treatment-naïve patients and 93% in prior null responders with HCV genotype 1 infection. Hepatology. 2012;56:LB1. [Google Scholar]
- 75.Zeuzem S., Jacobson I.M., Baykal T., Marinho R.T., Poordad F., Bourlière M. Retreatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370(17):1604–1614. doi: 10.1056/NEJMoa1401561. [DOI] [PubMed] [Google Scholar]
- 76.Feld J.J., Kowdley K.V., Coakley E., Sigal S., Nelson D.R., Crawford D. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370(17):1594–1603. doi: 10.1056/NEJMoa1315722. [DOI] [PubMed] [Google Scholar]
- 77.Poordad F., Hezode C., Trinh R., Kowdley K.V., Zeuzem S., Agarwal K. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med. 2014;370(21):1973–1982. doi: 10.1056/NEJMoa1402869. [DOI] [PubMed] [Google Scholar]
- 78.Ferenci P., Bernstein D., Lalezari J., Cohen D., Luo Y., Cooper C. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370(21):1983–1992. doi: 10.1056/NEJMoa1402338. [DOI] [PubMed] [Google Scholar]
- 79.Andreone P., Colombo M.G., Enejosa J.V., Koksal I., Ferenci P., Maieron A. ABT-450, ritonavir, ombitasvir, and dasabuvir achieves 97% and 100% sustained virologic response with or without ribavirin in treatment-experienced patients with HCV genotype 1b infection. Gastroenterology. 2014;147(2):359–365.e1. doi: 10.1053/j.gastro.2014.04.045. [DOI] [PubMed] [Google Scholar]
- 80.Sulkowski M.S., Asselah T., Lalezari J., Ferenci P., Fainboim H., Leggett B. Faldaprevir combined with pegylated interferon alfa-2a and ribavirin in treatment-naïve patients with chronic genotype 1 HCV: SILEN-C1 trial. Hepatology. 2013;57(6):2143–2154. doi: 10.1002/hep.26276. [DOI] [PubMed] [Google Scholar]
- 81.Sulkowski M.S., Bourlière M., Bronowicki J.P., Asselah T., Pawlotsky J.M., Shafran S.D. Faldaprevir combined with peginterferon alfa-2a and ribavirin in chronic hepatitis C virus genotype-1 patients with prior nonresponse: SILEN-C2 trial. Hepatology. 2013;57(6):2155–2163. doi: 10.1002/hep.26386. [DOI] [PubMed] [Google Scholar]
- 82.Dieterich D., Asselah T., Guyader D., Berg T., Schuchmann M., Mauss S. SILEN-C3, a phase 2 randomized trial with faldaprevir plus pegylated interferon α-2a and ribavirin in treatment-naive hepatitis C virus genotype 1-infected patients. Antimicrob Agents Ch. 2014;58(6):3429–3436. doi: 10.1128/AAC.02497-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zeuzem S., Asselah T., Angus P., Zarski J.P., Larrey D., Müllhaupt B. Faldaprevir (BI 201335), deleobuvir (BI 207127) and ribavirin oral therapy for treatment-naive HCV genotype 1: SOUND-C1 final results. Antivir Ther. 2013;18(8):1015–1019. doi: 10.3851/IMP2567. [DOI] [PubMed] [Google Scholar]
- 84.Zeuzem S., Soriano V., Asselah T., Bronowicki J.P., Lohse A.W., Müllhaupt B. Faldaprevir and deleobuvir for HCV genotype 1 infection. N Engl J Med. 2013;369(7):630–639. doi: 10.1056/NEJMoa1213557. [DOI] [PubMed] [Google Scholar]
- 85.Gentile I., Buonomo A.R., Borgia F., Zappulo E., Castaldo G., Borgia G. MK-5172: a second-generation protease inhibitor for the treatment of hepatitis C virus infection. Expert Opin Investig Drugs. 2014;23(5):719–728. doi: 10.1517/13543784.2014.902049. [DOI] [PubMed] [Google Scholar]
- 86.Summa V., Ludmerer S.W., McCauley J.A., Fandozzi C., Burlein C., Claudio G. MK-5172, a selective inhibitor of hepatitis C virus NS3/4a protease with broad activity across genotypes and resistant variants. Antimicrob Agents Ch. 2012;56(8):4161–4167. doi: 10.1128/AAC.00324-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Manns M.P., Vierling J.M., Bacon B.R., Bruno S., Shibolet O., Baruch Y. The combination of MK-5172, peginterferon, and ribavirin is effective in treatment-naive patients with hepatitis C virus genotype 1 infection without cirrhosis. Gastroenterology. 2014;147(2):366–376. doi: 10.1053/j.gastro.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 88.Lawitz E., Sulkowski M., Jacobson I., Kraft W.K., Maliakkal B., Al-Ibrahim M. Characterization of vaniprevir, a hepatitis C virus NS3/4A protease inhibitor, in patients with HCV genotype 1 infection: safety, antiviral activity, resistance, and pharmacokinetics. Antiviral Res. 2013;99(3):214–220. doi: 10.1016/j.antiviral.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 89.Xue W., Ban Y., Liu H., Yao X. Computational study on the drug resistance mechanism against HCV NS3/4A protease inhibitors vaniprevir and MK-5172 by the combination use of molecular dynamics simulation, residue interaction network, and substrate envelope analysis. J Chem Inf Model. 2014;54(2):621–633. doi: 10.1021/ci400060j. [DOI] [PubMed] [Google Scholar]
- 90.Coburn C.A., Meinke P.T., Chang W., Fandozzi C.M., Graham D.J., Hu B. Discovery of MK-8742: an HCV NS5A inhibitor with broad genotype activity. Chem Med Chem. 2013;8(12):1930–1940. doi: 10.1002/cmdc.201300343. [DOI] [PubMed] [Google Scholar]
- 91.Lawitz E, Vierling J, Murillo A, Kugelmas M, Gerstoft J, Winkle P, et al. High efficacy and safety of the all-oral combination regimen, MK-5172/MK-8742 ± RBV for 12 weeks in HCV genotype 1 infected patients: the C-WORTHY study. In: 64rd annual meeting of the American association for the study of liver diseases. Washington (DC); November 1–5 2013.
- 92.Shah U., Jayne C., Chackalamannil S., Velázquez F., Guo Z., Buevich A. Novel quinoline-based P2–P4 macrocyclic derivatives as pan-genotypic HCV NS3/4a protease inhibitors. ACS Med Chem Lett. 2014;5(3):264–269. doi: 10.1021/ml400466p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gane E.J., Pockros P.J., Zeuzem S., Marcellin P., Shikhman A., Bernaards C. Mericitabine and ritonavir-boosted danoprevir with or without ribavirin in treatment-naive HCV genotype 1 patients: INFORM-SVR study. Liver Int. 2015;35(1):79–89. doi: 10.1111/liv.12588. [DOI] [PubMed] [Google Scholar]
- 94.Everson G., Cooper C., Hézode C., Shiffman M.L., Yoshida E., Beltran-Jaramillo T. DAUPHINE: a randomized phase II study of danoprevir/ritonavir plus peginterferon alpha-2a/ribavirin in HCV genotypes 1 or 4. Liver Int. 2015;35(1):108–119. doi: 10.1111/liv.12471. [DOI] [PubMed] [Google Scholar]
- 95.Jiang Y., Andrews S.W., Condroski K.R., Buckman B., Serebryany V., Wenglowsky S. Discovery of danoprevir (ITMN-191/R7227), a highly selective and potent inhibitor of hepatitis C virus (HCV) NS3/4A protease. J Med Chem. 2014;57(5):1753–1769. doi: 10.1021/jm400164c. [DOI] [PubMed] [Google Scholar]
- 96.Marcellin P, Manns MP, Janczewska E, Muir AJ, Wu X, Trenkle JD, et al. 12 week response-guided treatment with the NS5A inhibitor, GS-5885, the NS3 protease inhibitor, GS-9451, plus pegylated interferon/ribavirin in treatment naive genotype 1 hepatitis C infected patients. In: Abstract presented at the 48th annual meeting of the European association for the study of the liver. Amsterdam (The Netherlands); April 24–28, 2013.
- 97.Vince B., Hill J.M., Lawitz E.J., O’Riordan W., Webster L.R., Gruener D.M. A randomized, double-blind, multiple-dose study of the pan-genotypic NS5A inhibitor samatasvir in patients infected with hepatitis C virus genotype 1, 2, 3 or 4. J Hepatol. 2014;60(5):920–927. doi: 10.1016/j.jhep.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 98.Lalezari J, Farrell G, Shah P, Lawitz E, Schwabe C, Walsh D, et al. PPI-668, a potent new pan-genotypic HCV NS5A inhibitor: phase 1 efficacy and safety. In: Presented at the 63rd annual meeting of the American association for the study of liver diseases. Boston (MA); November 9–13, 2012.
- 99.Spreen W, Wilfret D, Bechtel J, Adkison K, Lou Y, Willsie S, et al. GSK2336805 HCV NS5A inhibi tor demonstrates potent antiviral activity in chronic hepatitis C (CHC) genotype 1 infection: results from a first time in human (FTIH) single and repeat dose study. In: Presented at the 62nd annual meeting of the American association for the study of liver diseases. San Francisco (CA); November 6–9, 2011.
- 100.German P, Mathias A, Pang P, Yang C, Yang C, Han L, et al. Healthy volunteer first-in-human evaluation of GS-5816, a novel second generation broad-genotypic NS5A inhibitor with potential for once-daily dosing. In: Abstract presented at the 48th annual meeting of the European association for the study of the liver. Amsterdam (The Netherlands); April 24–28, 2013.
- 101.Cheng G, Tian Y, Yu M, Lee YJ, Gong R, Trejo-Martin A, et al. GS-5816, a second generation HCV NS5A inhibitor with potent antiviral activity, broad genotypic coverage and a high resistance barrier. In: Abstract presented at the 48th annual meeting of the European association for the study of the liver. Amsterdam (The Netherlands); April 24–28, 2013.
- 102.Yang W., Sun Y., Hou X., Zhao Y., Fabrycki J., Chen D. ACH-806, an NS4A antagonist, inhibits hepatitis C virus replication by altering the composition of viral replication complexes. Antimicrob Agents Ch. 2013;57(7):3168–3177. doi: 10.1128/AAC.02630-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vince B., Lawitz E., Searle S., Marbury T., Robison H., Robarge L. Novel NS5A inhibitor ACH-2928 phase I results in HCV GT-1 patients. J Hepatol. 2012;56(2):S480–S481. [Google Scholar]
- 104.Lalezari J, Agarwal K, Dusheiko G, Brown A, Weis N, Christensen P, et al. Dose-ranging trial of PPI-461, a potent new pan-genotypic HCV NS5A inhibitor, in patients with HCV genotype-1 infection. In: Abstract presented at the 62nd annual meeting of the American association for the study of liver diseases. San Francisco (CA); November 5–9, 2011.