Graphical abstract
Keywords: Physical fatigue, Muscle activation, Proprioception, Cognition
Abstract
Fatigue is a common worrying complaint among people performing physical activities on the basis of training or rehabilitation. An enormous amount of research articles have been published on the topic of fatigue and its effect on physical and physiological functions. The goal of this review was to focus on the effect of fatigue on muscle activity, proprioception, and cognitive functions and to summarize the results to understand the influence of fatigue on these functions. Attaining this goal provides evidence and guidance when dealing with patients and/or healthy individuals in performing maximal or submaximal exercises.
Introduction
Fatigue can be instigated by various mechanisms, ranging from accumulation of metabolites within muscle fibers to generation of an inadequate motor command in the motor cortex [1]. The effect of fatigue on other domains as physical or cognitive performance was not fully understood and it is still under investigation. The purpose of this review was to search the literature pertaining to the association between fatigue and muscle activity, proprioception, and cognition to help the health professionals in their planning of a training program and/or attempting to measure the performance in patient subjects
Fatigue is a common feature of many physical, neurological, and psychiatric disorders. Despite being commonly identified as a sign or a symptom of a disease or side effect of a treatment, fatigue has been considered as a subjective experience. Great efforts have been made to conceptualize or define it in a clear way to be at variance from normal experiences such as tiredness or sleepiness [2].
‘‘Fatigue’’ is a term used to describe a decrease in physical performance associated with an increase in the real/perceived difficulty of a task or exercise [3]. From another aspect, fatigue is defined as the inability of the muscles to maintain the required level of strength during exercises [4]. Alternatively, it can be defined as an exercise induced reduction in muscle’s capability to generate force. The term muscle fatigue was used to denote a transient decreases in the muscle capacity to perform physical activity [5]. Performing a motor task for long periods induces motor fatigue, which is generally accepted as a decline in a person’s ability to exert force [6]. Fatigue is reflected in the EMG signal as an increase of its amplitude and a decrease of its spectral characteristic frequencies [7].
Fatigue occurs due to the impairment of one or several physiological processes, which enable the contractile proteins to generate force. This effect was known as task dependency and was considered to be one of the principles that have been emerged in this era, so far [8–10]. According to this principle, there is no single cause of muscle fatigue [11]. The process of fatigue is gradual and includes important physiological changes, which occur before and during mechanical failure [12]. Boyas et al. [13] have introduced several principles to characterize the phenomena of muscle fatigue that occur in response to physical activity, namely “exercise induced fatigue”. These principles stress on the fact that there is no single mechanism to induce fatigue, but it is a complex mechanisms that may include organic central nervous system (CNS) abnormalities (central fatigue), peripheral nervous system dysfunction, or skeletal muscle disease [13]. The central fatigue designates a decrease in voluntary activation of the muscle (i.e. a decrease in the number and discharge rates of the motor units (MUs) recruited at the start of muscle force generation), whereas, peripheral fatigue indicates a decrease in the contractile strength of the muscle fibers and changes in the mechanisms underlying the transmission of muscle action potentials. These phenomena occur at the nerve endings and neuromuscular junction (NMJ) and are usually associated with peripheral fatigue [14]. However, data on this phenomenon are scarce and have only been gathered in animal experiments. Notably, intracortical inhibition could also be involved in the drop of muscle performance under fatiguing conditions. McNeil et al. [15] suggested increases in the intracortical inhibition as fatigue progressed, during 2-min maximum voluntary contraction (MVC) of the elbow flexors. Lastly, motoneurons (mainly those in fast-twitch MUs) are inhibited by Renshaw cells, which are stimulated by the same motoneurons and by the descending peripheral influence [16]. Using the indirect Hoffmann reflex method (a muscle response induced by excitation of group Ia afferents during electrical stimulation), several studies have suggested that intracortical inhibition increases during maximal efforts [17], however, it falls during submaximal contractions at 20% of the MVC, when central fatigue occurs [18,19].
Fatigue and muscle activity
In this section, the effects of fatigue on muscle activities using various methods, such as electromyography assessment, chemical biomarkers, and others are discussed [12,14].
Neuromuscular fatigue is a complex phenomenon involving physiological processes occurring in structures, from the motor cortex to muscle contractile proteins [13].
The motor unit denotes the basic functional element of the CNS and muscle that produces movement. It comprises a motor neuron in the ventral horn of the spinal cord, its axon, and the muscle fibers innervated by this axon [20]. The CNS controls muscle force by modifying the activity of motor units in the muscle.
It is well known that skeletal muscle is highly organized at the microscopic level, as can be seen from the incredible number and diversity of electronic micrographs and schematics of muscle sarcomeres. Skeletal muscle is based on myosin heavy chain (MHC) isoforms. The major types of muscle fiber are type I, IIa, IIx, and IIb. Type I is the slowest; type IIa is intermediate, and IIx/b is the fastest. Fast type II muscle fibers (also known as non-fatigue resistant) generally have a lower oxidative capacity than slow type I fibers (known as slow twitch or fatigue resistant) [21].
Most of the studies performed to investigate the effect of fatigue on the muscle activity patterns reported changes in force generation amplitude; motor unit potential; or the synaptic discharge and motor neuronal output.
The central and peripheral mechanisms of fatigue have typically been examined during isolated muscle contractions; involving maximal [i.e., maximal voluntary contractions (MVCs)] or submaximal (i.e., submaximal voluntary contractions) torques. In a sustained MVC, the torque produced is the highest at the beginning of the contraction and progressively falls throughout the remainder of the contraction. Motor unit recruitment and firing rates are greatest at the beginning of MVC [22], subsequently de-recruitment occurs, and firing rates decline [23]. During the application of fatigue tests involving submaximal voluntary contractions, subjects are typically required to perform a contraction at a specific submaximal torque until they are no longer able to voluntarily produce the required torque. The number of motor units recruited at the beginning of a submaximal contraction depends on the strength of the contraction; however, it increases over time as the force developed by the initially recruited motor units declines [24]. Hoffman et al. studied the corticospinal responsiveness during a sustained submaximal contraction of lower limb muscles [12]. They reported that inducing a motor evoked potentials (MEPs) and cervicomedullary motor evoked potentials (CMEPs) in the triceps surae muscle during sustained planter flexion at 30% of the MVC, would increase the amplitude of the MEPs over the course of the fatiguing contractions, which indicate increases in corticospinal responsiveness during sustained submaximal exercise [12]. There was a difference between the growth of the two responses, MEPs and CMEPs, during the fatiguing contraction compared with non fatigue control responses. Both of these responses showed central fatigue during the sustained 30% MVC of triceps surae, which is typical in most sustained submaximal voluntary contraction protocols [25–28]. Torque fluctuation was measured throughout the sustained contraction to indicate that the central processes of torque production were also affected. It is thought that increased excitatory drive to the motor neuron pool leads to oscillations in the stretch-reflex arc and bursts of motor unit firing, which increased the fluctuations in torque production at ∼8–10 Hz [25,26,19]. Hoffman et al. [12] support the evidence that MEP and CMEP amplitudes would increase during sustained submaximal voluntary contractions, it is speculated that differences in spinal responsiveness between submaximal voluntary contractions and MVCs could be attributed to the motor unit recruitment and firing rate. In a sustained submaximal voluntary contraction, the number of motor units recruited at the initiation of contraction is dependent on its force [29], which increases over time as additional motor units were recruited to compensate for a reduction in the force-generating capacity of the originally active units. An increase in this increment (superimposed twitch) signifies the central fatigue and means that central processes proximal to the site of motor axon stimulation are contributing to a loss of force. Some central fatigue can be attributed to supraspinal mechanisms [30,31]. Testing of motor neuron excitability during fatiguing contractions shows that the slower firing rates are not due solely to a decrease in excitatory input. During a sustained maximal effort, the decrease in CMEP, measured in the electromyogram (EMG) of the active muscle, suggests that the motoneurons become less responsive to synaptic input [32–34]. Repetitive activation may decrease the responsiveness of motoneurons to synaptic input. The process known as late adaptation can be demonstrated when motoneurons are given a sustained input [19,35–37]. Initially the motoneurons fire repetitively, but with time, some motoneurons slow their firing rate and others stop [37,38]. The increase in excitatory input to the motoneurons pool is evidenced by increased surface EMG, which indicates that other motor units have been recruited or are firing more these changes in inputs to motoneurons also occur during fatiguing exercise. To estimate the extent to which the EMG–force relation can be changed during fatiguing contractions, Dideriksen et al. [38] developed a computational model based on an earlier model of motor unit recruitment and rate coding. The adjustments in motor unit activity during the fatiguing contractions were implemented with a compartment-model approach as functions of the metabolite concentration within each muscle fiber and in the extracellular space [38]. The simulated concentrations were related to decrease in conduction velocity of muscle fiber action potentials, increase of inhibitory afferent feedback, decline in twitch-force amplitude, and progressive inability of the CNS to produce an output that matched the target force. To determine the adjustments, which are responsible for the depression of EMG amplitude when a low-force isometric contraction is sustained for as long as possible a computational mode was used. The mode simulates the adjustments in motor unit activity that were required to sustain isometric contractions at target forces of 20%, 40%, and 60% of MVC force for as long as possible [39]. The depression of EMG amplitude at task failure of long-duration contractions was mainly caused by a decrease in muscle activation i.e. number of muscle fiber action potentials. This depression may be attributed to a decrease in net synaptic input to motor neurons, with less of an impact of the changes in the shapes of motor unit action potentials and no contribution of amplitude cancelation [40]. Significantly, EMG amplitude during the simulated fatiguing contractions was related to the number of muscle fiber action potentials (muscle activation), but not consistently to the number of motor unit action potentials (neural drive to the muscle).
Fatigue and proprioception
Proprioception accounts for the most misused term within the sensorimotor system. It has been incorrectly used synonymously and interchangeably with kinesthesia, joint position sense, somatosensation, balance, and reflexive joint stability. In Sherrington’s [41] original description of the proprioceptive system, proprioception was used to reference the afferent information arising from proprioceptors located in the proprioceptive field. The proprioceptive field was specifically defined as that area of the body screened from the environment by the surface cells, which contain receptors specially adapted for the changes occurring inside the organism independent of the interoceptive field (alimentary canal and visceral organs) [42]. Denny-Browen et al. declared that proprioception has been used for the regulation of total posture (postural equilibrium) and segmental posture (joint stability), as well as initiating several conscious peripheral sensations (‘‘muscle senses’’). Four submodalities of ‘‘muscle sense’’ have been described: (1) posture, (2) passive movement, (3) active movement, and (4) resistance to movement [42,43]. These submodalities type of sensations correspond to the contemporary terms joint position sense (posture of segment), kinesthesia (active and passive), and the sense of resistance or heaviness. Thus, proprioception correctly describes afferent information arising from internal peripheral areas of the body that contribute to postural control, joint stability, and several conscious sensations [44]. Depending upon the exact circumstances of a situation or task, sources contributing to conscious sensations of proprioception i.e. joint position sense, could potentially include the deeper receptors i.e., joint and muscle mechanoreceptors, mechanoreceptors conveying proprioceptive information are often labeled as proprioceptors [41–45]. However, in addition to mechanoreceptors located in Sherrington’s proprioceptive field being referred to as proprioceptors, the term has also been used for the mechanoreceptors located at the surface of the body, and portions of the vestibular apparatus responsible for conveying information regarding the orientation of the head with respect to gravity. The mechanoreceptors responsible for proprioceptive information are primarily found in muscle, tendon, ligament, and capsule [46–49] with the mechanoreceptors located in the deep skin and fascial layers traditionally associated with tactile sensations being theorized as supplementary sources [48–52]. Mechanoreceptors are specialized sensory receptors responsible for quantitatively transducing the mechanical events occurring in their host tissues into neural signals [49]. Although the process generally occurs in a similar manner across the various mechanoreceptors, each morphologic type possesses some degree of specificity for the sensory modality to which it responds (light touch versus tissue lengthening), as well as the range of stimuli within a sensory modality [53].
Accurate sensory inputs regarding both internal and external conditions of the body are of major importance to effective motor control. Optimization of the performance of daily living and physical activities necessitates adequate postural control. Many studies reported changes in postural control during quiet standing after the performance of a fatiguing exercise. Assuming that joint proprioception plays an important role in maintaining the functional stability of the joint [54,55], deterioration in proprioception as a result of physical or mental fatigue may be a risk of ligamentous injury [56,57]. Muscle fatigue has been shown to adversely alter joint proprioception [58,59] and impair neuromuscular control in the lower extremities. Although many authors [56–60] have studied the changes that occur in proprioception after fatigue, they have not established what components in the proprioceptional pathway do not function sufficiently after fatigue. Therefore, researchers do not know whether muscle receptors, joint receptors, the central nervous system, or other components are mainly responsible for decrease in proprioceptive sense. In an attempt to determine which component in the neuromuscular control pathway may change after fatigue a study was conducted to evaluate the effects of local and general fatigue loads on knee joint proprioception [61]. Miura et al. hypothesized that the difference between local fatigue and general fatigue affects the changes in knee proprioception after exercise. The study was done on knee joint as it is more sensitive to fatigue loading regarding reproduction of joint angle than kinesthesia. Therefore, joint position sense was used to evaluate knee proprioception in this study [61]. It was noted that only the general fatigue load had a statistically significant effect on knee proprioception. Skinner et al. [57] also found a decrease of knee proprioception, with a 15% decrease of knee flexion and extension work output after general fatigue load.
The results of Miura et al. [61] were different from those of Skinner et al. [57] in that muscle weakness of the knee could not be seen. Proprioceptional decline without muscle weakness of knee after general load suggests a change in the proprioceptional pathway without influence from muscle mechanoreceptors the decline in joint sense of position after general load may be caused by deficiency of central processing of proprioceptive signals, that is, caused by central fatigue processes. Central fatigue may diminish precision of motor control; interrupt voluntary muscle-stabilizing activity to resist imparted joint forces [62].
The proprioceptive impairment due to muscle fatigue could be caused by changes in the discharge patterns of muscle afferents due to metabolite build up leading to potential altered muscle spindles information [63], altered central processing of proprioception via group III and IV afferents [64] and effects on the efferent pathways [65]. However, the relative contribution of fatigue-related changes in mechanical properties and proprioception for postural stability remains to be clarified. Studies [65–68] on the effect of muscle fatigue and postural stability have repeatedly suggested that proprioception could be the primary mechanism explaining changes in postural sway observed after fatigue. In their study to compare the extent to which fatigue of ankle extensor (plantarflexor) and flexor (dorsiflexor) muscles versus fatigue of hip extensor and flexor muscles affects postural sway in unipedal stance, Vuillerme et al., reported that ankle and hip fatigue increased sway variability and sway velocity in young healthy adults during a unipedal stance in the fatigued plane, anteroposterior (AP), whereas sway velocity in the non-fatigued plane, mediolateral (ML) increased only after hip fatigue, suggesting a greater decline in postural control with fatigue for this muscle group, agreeing with several others [67–70] show that fatiguing proximal muscles (hip and/or knee) have a greater effect on postural control than distal (ankle) muscles.
Fatigue and cognition
Cognitive function impairment is a growing public health problem and the relationship between physical activity and cognitive function is peculiar and controversial. It is known that physical activity has great benefit for the health and help reducing the risk of many cardiovascular and pulmonary disorders. However, until recently the relationship between acute physical exercise and cognitive function was not that clear as the literature on the topic seemed to provide somewhat contradictory findings. While one of the studies indicated that short periods of physical exercise improved cognitive functioning in adults [71], others either did not find any benefits [72] or even reported deterioration of cognitive function [73]. It has now been more clearly demonstrated that the effect of physical exercise on cognitive performance depends both on the intensity and the duration of the exercise [74,75]. Much of the evidence found in the literature suggests that the relationship between acute physical activity and cognitive performance has an inverted U shape. Some reported that physical exercise of moderate intensity and duration appears to ameliorate brain dysfunction. In fact, several studies found that immediately after an exercise session of sub-maximal intensity (i.e., heart rate of about 110–130 beats per minute) and a duration of 20–40 min, there is an improvement in sensori-motor and cognitive performance [76,77]. While others reveled that prolonged but sub-maximal physical exercise leading to dehydration is associated with a reduction in cognitive performance. For example, a two-hour run on a treadmill at 65% of maximal oxygen uptake (VO2max) results in a significant disruption of short-term memory, psycho-motor abilities, and visual discrimination [73].
One of the essential component of daily activities is maintaining a stable, upright stance, even though this is an automated process, numerous studies using the dual-task paradigm have shown that tasks like standing or walking require some attentional resources [78]. An increase in attentional demands can be concluded from a reduction in the performance of a secondary task (usually a cognitive task) while the performance on the primary (postural) task remains the same. It is known that the attentional demands needed for control postural sway increase with the difficulty of the task [79,80], with aging [81,82] and with the presence of pathology [83,84], particularly when proprioceptive information is reduced due to environmental constraints [85,86]. This is not surprising since ankle proprioception is one of the primary regulatory mechanisms for stabilization of the body [87,88]. In their study that was conducted to assess the effect of fatigue on postural sway and attentional demand Bisson et al. [89] asked the participants to focus on standing as still as possible during all conditions (primary task), according to such dual-task (DT) instructions (attentional task), it was expected that no difference would be observed in sway area and sway variability between the single task and dual-task, which was confirmed. In contrast, a significant increase in AP and ML sway velocity during the dual-task condition was noted. When the difficulty of a task increases, more activity of the supporting musculature may be needed to remain in a stable posture. Because participants did not sway more, the increase in sway velocity during the dual-task condition suggests an increase in corrective actions [89].
Tracey et al. [90] compared cognitive functions after physical exercise to vo2max and resting state. They suggested that cognitive impairments on verbal memory composite scores occur after a maximal exercise test and measured by Immediate Post-Concussion Assessment and Cognitive Testing (ImPACT) test [90]. Examining the individual ImPACT test modules that compile the verbal memory composite score revealed a significant deterioration on both the immediate and delayed recall tasks after exercise intervention. These findings support those who showed deterioration on verbal memory tasks after a bout of exercise. Cian et al. [91] attributed the deterioration in performance to dehydration, whereas Frey et al. [92] suggested a decrease in performance on memory tasks resulted from changes in cortical activity in the brain and hypoxia brought about by exercise.
Perspective
In this review we have outlined the complex mechanisms of fatigue; how it occurs and what are the major sequels of fatigue. It was noted that fatigue is not a result of one mechanism only but due to multiple factors. Fatigue process should be considered as two way relationship when muscle fatigue occurs muscle activity declines which hinder the proprioception function and vice versa; if the proprioception is affected muscle does not function properly as was reported by Voight et al., that muscle fatigue adversely affects joint proprioception and impairs neuromuscular control [58,59]. It is generally accepted that the greatest contribution to position sense and kinesthesia is from muscle receptors, primarily muscle spindles and Golgi tendon organs. Since fatigue process would presumably affect muscle tissue more than joint tissue, then diminished position sense may conceptually be thought of as secondary to loss of muscle receptor input [62].
In addition, the contribution of cognitive function to the process of motor performance and the effect of fatigue on this process should be considered. Many research studies had been done regarding the relationship between physical fatigue and cognitive impairment, most of these studies looked at the effect of fatigue on cognitive functions and few examined the effect of cognitive dysfunction on the physical performance. Executive cognitive functions considered as a key factor in locomotor control and its deficits are associated with increased risk of falling. Various dual task (DT) studies have affirmed that difficulty in assigning attention to each task simultaneously may contribute significantly to increased motor dysfunction. The altered prioritization between the two tasks could be the main cause of Poor DT performance in either the motor or cognitive task [93]. So it has now been more clearly demonstrated what effect physical exercise has on cognitive performance but the effect of cognitive impairment on the physical performance has not been clarified, so this should be further investigated.
So when planning a training or rehabilitation program for a patient or for a healthy individual, a great consideration should be taken. There are multiple factors that contribute to the initiation and persistence of fatigue. These factors may not be only damaging to the muscles and/or joints but also could result in mental fatigue.
Conclusions
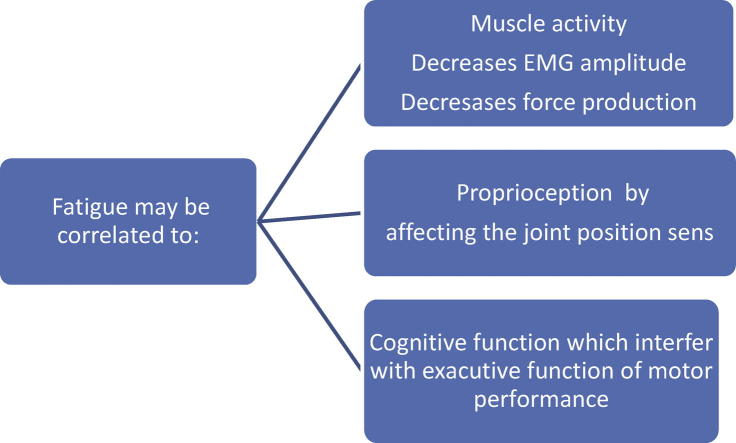
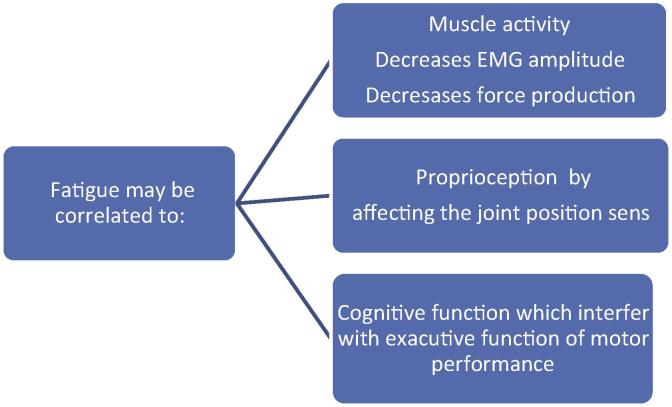
In this review an outline on the effect of fatigue on different functions, muscle activity, Proprioception and cognitive functions was presented in order to understand the underlying mechanisms that lead to the deterioration of these functions (Fig. 1). In summary, muscle fatigue causes decrease in muscle activation pattern, which in turn affects the joint sense of position leading to disturbed balance and an increase in the risks of falls. Furthermore, fatigue appears to have an effect on cognitive functions, regardless of the controversy found by research, it can be safely said that a relation does exist between the intensity and duration of physical activity and the cognitive function.
Fig. 1.
Exploratory framework of correlation of fatigue with muscle activity, proprioception, and cognitive function.
Conflict of interest
The authors have declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Biographies

Hoda Mohammed Abdelfattah received her master degree in Pediatric Physical Therapy Department, Cairo University, Egypt in 2010. Her research focused on the relationship between fatigue and muscle activity, proprioception, and cognition.

Faten H. Abdelazeim, graduated from Faculty of Physical Therapy, Cairo University. Her areas of interest are; neuromuscular, cognitive training and evidence based Medicine. She is a member of many Society Associations and Committees in Supreme Council University, Egypt.

Shorouk Elshennawy, PT, MSc, Ph.D Received her MSc (2005) and PhD (2009) degree from Faculty of Physical Therapy-Cairo University. She teaches courses relating to pediatric physical therapy. She worked as pediatric rehabilitator for 10 years. Her research focuses on pediatrics rehabilitation. Specific areas of study include motor control and cognition. Currently, she is studying biostatistics diploma and a member of editorial office of Journal of Advanced Research, the Official Journal of Cairo University.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Enoka R.M., Duchateau J. Muscle fatigue: what, why and how it influences muscle function. J Physiol. 2008;586(Pt 1):11–23. doi: 10.1113/jphysiol.2007.139477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dittnera A.J., Wesselyb S.C., Browna R.G. The assessment of fatigue, a practical guide for clinicians and researchers. J Psychosom Res. 2004;56:157–170. doi: 10.1016/S0022-3999(03)00371-4. [DOI] [PubMed] [Google Scholar]
- 3.MacInstosh B, Gardiner P, McComas A. Skeletal muscle: form and function: Human kinetics. 2nd ed. Champaign, IL, USA; 2005.
- 4.Edwards R.H. Human muscle function and fatigue. Ciba Found Symp. 1981;82:1–18. doi: 10.1002/9780470715420.ch1. [DOI] [PubMed] [Google Scholar]
- 5.Friedman J.H., Brown R.G., Comella C., Garber C.E., Krupp L.B., Lou J.S. Fatigue in Parkinson’s disease: a review. Movement Disord. 2007;22:297–308. doi: 10.1002/mds.21240. [DOI] [PubMed] [Google Scholar]
- 6.Lorist M.M., Kernell D., Meijman T.F., Zijdewind I. Motor fatigue and cognitive task performance in humans. J Physiol. 2002;545:313–319. doi: 10.1113/jphysiol.2002.027938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kallenberg L.A.C., Schulte E., Disselhorst-Klug C., Hermens H.J. Myoelectric manifestations of fatigue at low contraction levels in subjects with and without chronic pain. J Electromyogr Kinesiol. 2007;17:264–274. doi: 10.1016/j.jelekin.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Asmussen E. Muscle fatigue. Med Sci Sports. 1979;11:313–321. [PubMed] [Google Scholar]
- 9.Enoka R.M., Stuart D.G. Neurobiology of muscle fatigue. J Appl Physiol. 1992;72:1631–1648. doi: 10.1152/jappl.1992.72.5.1631. [DOI] [PubMed] [Google Scholar]
- 10.Bigland-Ritchie B., Rice C.L., Garland S.J., Walsh M.L. Task-dependent factors in fatigue of human voluntary contractions. In: Gandevia S.C., Enoka R.M., Stuart D.G., Thomas C.K., editors. Fatigue: neural & muscular mechanisms. Plenum Press; New York: 1995. pp. 361–380. [Google Scholar]
- 11.Cairns S.P., Knicker A.J., Thompson M.W., Sjøgaard G. Evaluation of models used to study neuromuscular fatigue. Exerc Sport Sci Rev. 2005;33:9–16. [PubMed] [Google Scholar]
- 12.Hoffman B.W., OyaT, Carroll T.J., Cresswell A.G. Increases in corticospinal responsiveness during a sustained submaximal plantar flexion. J Appl Physiol. 2009;107:112–120. doi: 10.1152/japplphysiol.91541.2008. [DOI] [PubMed] [Google Scholar]
- 13.Boyas S., Guével A. Neuromuscular fatigue in healthy muscle: underlying factors and adaptation mechanisms. Ann Phys Rehabil Med. 2011;54:88–108. doi: 10.1016/j.rehab.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Gandevia S.C. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- 15.McNeil C.J., Martin P.G., Gandevia S.C., Taylor J.L. The response to paired motor cortical stimuli is abolished at a spinal level during human muscle fatigue. J Physiol. 2009;587:5601–5612. doi: 10.1113/jphysiol.2009.180968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hultborn H., Lipski J., Mackel R., Wigstrom H. Distribution of recurrent inhibition within a motor nucleus. I. Contribution from slow and fast motor units to the excitation of Renshaw cells. Acta Physiol Scand. 1988;134:347–361. doi: 10.1111/j.1748-1716.1988.tb08503.x. [DOI] [PubMed] [Google Scholar]
- 17.Kukulka C.G., Moore M.A., Russell A.G. Changes in human alpha motoneurons excitability during sustained maximum isometric contractions. Neurosci Lett. 1986;68:327–333. doi: 10.1016/0304-3940(86)90511-2. [DOI] [PubMed] [Google Scholar]
- 18.Khaslavskaia S., Ladouceur M., Sinkjaer T. Increase in tibialis anterior motor cortex excitability following repetitive electrical stimulation of the common peroneal nerve. Exp Brain Res. 2002;145:309–315. doi: 10.1007/s00221-002-1094-9. [DOI] [PubMed] [Google Scholar]
- 19.Loscher W.N., Cresswell A.G., Thorstensson A. Recurrent inhibition of soleus alpha-motoneurons during a sustained submaximal plantar flexion. Electroencephalogr Clin Neurophysiol. 1996;101:334–338. doi: 10.1016/0924-980x(96)95670-2. [DOI] [PubMed] [Google Scholar]
- 20.Duchateau J., Semmler J.G., Enoka R.M. Training adaptations in the behavior of human motor units. J Appl Physiol. 2006;101:1766–1775. doi: 10.1152/japplphysiol.00543.2006. [DOI] [PubMed] [Google Scholar]
- 21.Lieber RL. Functional and clinical significance of skeletal muscle architecture, PhD; 2000. [DOI] [PubMed]
- 22.Baldwin K.M., Klinkerfuss G.H., Terjung R.L., Mole P.A., Holloszy J.O. Respiratory capacity of white, red, and intermediate muscle: adaptative response to exercise. Am J Physiol. 1972;1972(222):373–378. doi: 10.1152/ajplegacy.1972.222.2.373. [DOI] [PubMed] [Google Scholar]
- 23.Enoka R.M. Morphological features and activation patterns of motor units. J Clin Neurophysiol. 1995;12:538–559. doi: 10.1097/00004691-199511000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Löscher W.N., Cresswell A.G., Thorstensson A. Excitatory drive to the-motoneuron pool during a fatiguing submaximal contraction in man. J Physiol. 1996;491:271–280. doi: 10.1113/jphysiol.1996.sp021214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cresswell A.G., Löscher W.N. Significance of peripheral afferent input to the motoneuron pool for enhancement of tremor during an isometric fatiguing contraction. Eur J Appl Physiol. 2000;82:129–136. doi: 10.1007/s004210050662. [DOI] [PubMed] [Google Scholar]
- 26.Sacco P., Thickbroom G.W., Thompson M.L., Mastaglia F.L. Changes in corticomotor excitation and inhibition during prolonged submaximal muscle contractions. Muscle Nerve. 1997;20:1158–1166. doi: 10.1002/(sici)1097-4598(199709)20:9<1158::aid-mus11>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 27.Søgaard K., Gandevia S.C., Todd G., Petersen N.T., Taylor J.L. The effect of sustained low-intensity contractions on supraspinal fatigue in human elbow flexor muscles. J Physiol. 2006;573:511–523. doi: 10.1113/jphysiol.2005.103598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gandevia S.C., Allen G.M., Butler J.E., Taylor J.L. Supraspinal factors in human muscle fatigue: evidence for suboptimal output from the motor cortex. J Physiol. 1996;490:529–536. doi: 10.1113/jphysiol.1996.sp021164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor J.L., Todd G., Gandevia S.C. Evidence for a supraspinal contribution to human muscle fatigue. Clin Exp Pharmacol Physiol. 2006;33:400–405. doi: 10.1111/j.1440-1681.2006.04363.x. [DOI] [PubMed] [Google Scholar]
- 30.Andersen B., Westlund B., Krarup C. Failure of activation of spinal motoneurons after muscle fatigue in healthy subjects studied by transcranial magnetic stimulation. J Physiol. 2003;551:345–356. doi: 10.1113/jphysiol.2003.043562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Butler J.E., Taylor J.L., Gandevia S.C. Responses of human motoneurons to corticospinal stimulation during maximal voluntary contractions and ischemia. J Neurosci. 2003;23:10224–10230. doi: 10.1523/JNEUROSCI.23-32-10224.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin P.G., Smith J.L., Butler J.E., Gandevia S.C., Taylor J.L. Fatigue sensitive afferents inhibit extensor but not flexor motoneurons in humans. J Neurosci. 2006;26:4796–4802. doi: 10.1523/JNEUROSCI.5487-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kernell D., Monster A.W. Time course and properties of late adaptation in spinal motoneurons of the cat. Exp Brain Res. 1982;46:191–196. doi: 10.1007/BF00237176. [DOI] [PubMed] [Google Scholar]
- 34.Sawczuk A., Powers R.K., Binder M.D. Contribution of outward currents to spike-frequency adaptation in hypoglossal motoneurons of the rat. J Neurophysiol. 1997;78:2246–2253. doi: 10.1152/jn.1997.78.5.2246. [DOI] [PubMed] [Google Scholar]
- 35.Spielmann J.M., Laouris Y., Nordstrom M.A., Robinson G.A., Reinking R.M., Stuart D.G. Adaptation of cat motoneurons to sustained and intermittent extracellular activation. J Physiol. 1993;464:75–120. doi: 10.1113/jphysiol.1993.sp019625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peters E.J., Fuglevand A.J. Cessation of human motor unit discharge during sustained maximal voluntary contraction. Neurosci Lett. 1999;274:66–70. doi: 10.1016/s0304-3940(99)00666-7. [DOI] [PubMed] [Google Scholar]
- 37.Fuglevand A.J., Winter D.A., Patla A.E. Models of recruitment and rate coding organization in motor-unit pools. J Neurophysiol. 1993;70:2470–2488. doi: 10.1152/jn.1993.70.6.2470. [DOI] [PubMed] [Google Scholar]
- 38.Dideriksen J.L., Farina D., Bækgaard M., Enoka R.M. An integrative model of motor unit activity during sustained submaximal contractions. J Appl Physiol. 2010;108:1550–1560. doi: 10.1152/japplphysiol.01017.2009. [DOI] [PubMed] [Google Scholar]
- 39.Fuglevand A.J., Zackowski K.M., Huey K.A., Enoka R.M. Impairment of neuromuscular propagation during human fatiguing contractions at submaximal forces. J Physiol. 1993;460:549–572. doi: 10.1113/jphysiol.1993.sp019486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dideriksen J.L., Enoka R.M., Farina D. Neuromuscular adjustments that constrain submaximal EMG amplitude at task failure of sustained isometric contractions. J Appl Physiol. 2011;111:485–494. doi: 10.1152/japplphysiol.00186.2011. [DOI] [PubMed] [Google Scholar]
- 41.Sherrington C.S. C Scribner’s Sons; New York, NY: 1906. The integrative action of the nervous system. [Google Scholar]
- 42.Denny-Brown D. Hamish Hamilton Medical Books; London, England: 1939. Selected writings of sir Charles Sherrington. [Google Scholar]
- 43.Matthews P.B. Where does Sherrington’s ‘‘muscular sense’’ originate? Muscles, joints, corollary discharges? Annu Rev Neurosci. 1982;5:189–218. doi: 10.1146/annurev.ne.05.030182.001201. [DOI] [PubMed] [Google Scholar]
- 44.Hasan Z., Stuart D.G. Animal solutions to problems of movement control: the role of proprioceptors. Annu Rev Neurosci. 1988;11:199–223. doi: 10.1146/annurev.ne.11.030188.001215. [DOI] [PubMed] [Google Scholar]
- 45.Enoka R.M. 2nd ed. Human Kinetics; Champaign, IL: 1994. Neuromechanical basis of kinesiology. [Google Scholar]
- 46.Ghez C. The control of movement. In: Kandel E.R., Schwartz J.H., Jessell T.M., editors. Principles of neural science. 3rd ed. Elsevier Science; New York, NY: 1991. pp. 533–547. [Google Scholar]
- 47.Johansson H., Sjolander P. The neurophysiology of joints. In: Wright V., Radin E.L., editors. Mechanics of joints: physiology, pathophysiology and treatment. Marcel Dekker Inc.; New York, NY: 1993. pp. 243–290. [Google Scholar]
- 48.Freeman M.A., Wyke B. Articular reflexes at the ankle joint: an electromyographic study of normal and abnormal influences of ankle joint mechanoreceptors upon reflex activity in the leg muscles. Br J Surg. 1967;54:990–1001. doi: 10.1002/bjs.1800541204. [DOI] [PubMed] [Google Scholar]
- 49.Grigg P. Peripheral neural mechanisms in proprioception. J Sport Rehabil. 1994;3:2–17. [Google Scholar]
- 50.Warren S., Yezierski R.P., Capra N.F. The somatosensory system I: discriminative touch and position sense. In: Haines D.E., Ard M.D., editors. Fundamental neuroscience. Churchill Livingstone Inc.; New York, NY: 1997. pp. 220–235. [Google Scholar]
- 51.Macefield G., Gandevia S.C., Burke D. Perceptual responses to microstimulation of single afferents innervating joints, muscles and skin of the human hand. J Physiol. 1990;429:113–129. doi: 10.1113/jphysiol.1990.sp018247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Erickson R.P. Stimulus coding in topographic and nontopographic afferent modalities: on the significance of the activity of individual sensory neurons. Psychol Rev. 1968;75:447–465. doi: 10.1037/h0026752. [DOI] [PubMed] [Google Scholar]
- 53.Leonard C.T. Mosby-Year Book Inc.; St Louis, MO: 1998. The neuroscience of human movement. [Google Scholar]
- 54.Tsuda E., Okamura Y., Otsuka H. Direct evidence of the anterior cruciate ligament-hamstring reflex arc in humans. Am J Sports Med. 2001;29:83–87. doi: 10.1177/03635465010290011801. [DOI] [PubMed] [Google Scholar]
- 55.Swanik C.B., Harner C.D., Kinkiewicz J., Lephart S.M. Neurophysiology of the knee. 3rd ed. Churchill Livingstone; New York: 2001. pp. 175–189. (Surgery of the knee). [Google Scholar]
- 56.Lattanzio P.-J., Petrella R.J., Sproule J.R., Fowler P.J. Effects of fatigue on knee proprioception. Clin J Sports Med. 1997;7:22–27. doi: 10.1097/00042752-199701000-00005. [DOI] [PubMed] [Google Scholar]
- 57.Skinner H.B., Wyatt M.P., Hodgdon J.A. Effects of fatigue on joint position sense of the knee. J Orthop Res. 1986;4:112–118. doi: 10.1002/jor.1100040115. [DOI] [PubMed] [Google Scholar]
- 58.Voight M.L., Hardin J.A., Blackburn T.A., Tippett S., Canner G.C. The effects of muscle fatigue on and the relationship of arm dominance to shoulder proprioception. J Orthop Sports Phys Ther. 1996;23:348–352. doi: 10.2519/jospt.1996.23.6.348. [DOI] [PubMed] [Google Scholar]
- 59.Blasier R.B., James E.C., Laura J.H. Shoulder proprioception: effect of joint laxity, joint position, direction of motion, and muscle fatigue. Orthop Rev. 1993;23:45–50. [PubMed] [Google Scholar]
- 60.Shape M.H., Miles T.S. Position sense at the elbow after fatiguing contractions. Exp Brain Res. 1993;94:179–182. doi: 10.1007/BF00230480. [DOI] [PubMed] [Google Scholar]
- 61.Miura k, Ishibashi Y., Tsuda E., Okamura Y., Otsuka H., Toh S. The effect of local and general fatigue on knee proprioception. J Arthrosc Relat Surgery. 2004;20(4):414–418. doi: 10.1016/j.arthro.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 62.Hiemstra L.A., Lo I.K., Fowler P.J. Effect of fatigue on knee proprioception: implications for dynamic stabilization. J Orthop Sports Phys Ther. 2001;31:598–605. doi: 10.2519/jospt.2001.31.10.598. [DOI] [PubMed] [Google Scholar]
- 63.Forestier N., Teasdale N., Nougier V. Alteration of the position sense at the ankle induced by muscular fatigue in humans. Med Sci Sports Exerc. 2002;34:117–122. doi: 10.1097/00005768-200201000-00018. [DOI] [PubMed] [Google Scholar]
- 64.Taylor J.L., Butler J.E., Gandevia S.C. Changes in muscle afferents, motoneurons and motor drive during muscle fatigue. Eur J Appl Physiol. 2000;83:106–115. doi: 10.1007/s004210000269. [DOI] [PubMed] [Google Scholar]
- 65.Caron O. Effects of local fatigue of the lower limbs on postural control and postural stability in standing posture. Neurosci Lett. 2003;340:83–86. doi: 10.1016/s0304-3940(02)01455-6. [DOI] [PubMed] [Google Scholar]
- 66.Vuillerme N., Forestier N., Nougier V. Attentional demands and postural sway: the effect of the calf muscles fatigue. Med Sci Sports Exerc. 2002;34:1907–1912. doi: 10.1097/00005768-200212000-00008. [DOI] [PubMed] [Google Scholar]
- 67.Vuillerme N., Burdet C., Isableu B., Demetz S. The magnitude of the effect of calf muscles fatigue on postural control during bipedal quiet standing with vision depends on the eye-visual target distance. Gait Posture. 2006;24:169–172. doi: 10.1016/j.gaitpost.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 68.Gribble P.A., Hertel J. Effect of lower-extremity muscle fatigue on postural control. Arch Phys Med Rehabil. 2004;85:589–592. doi: 10.1016/j.apmr.2003.06.031. [DOI] [PubMed] [Google Scholar]
- 69.Bizid R., Margnes E., Francois Y., Jully J.L., Gonzalez G., Dupui P. Effects of knee and ankle muscle fatigue on postural control in the unipedal stance. Eur J Appl Physiol. 2009;106:375–380. doi: 10.1007/s00421-009-1029-2. [DOI] [PubMed] [Google Scholar]
- 70.Salavati M., Moghadam M., Ebrahimi I., Arab A.M. Changes in postural stability with fatigue of lower extremity frontal and sagittal plane movers. Gait Posture. 2007;26:214–218. doi: 10.1016/j.gaitpost.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 71.Hancock S., McNaughton L. Effects of fatigue on ability to process visual information by experienced orienters. Perceptual Motor Skills. 1986;62:491–498. doi: 10.2466/pms.1986.62.2.491. [DOI] [PubMed] [Google Scholar]
- 72.Cote J., Salmela J.H., Papthanasopoloulu K.P. Effects of progressive exercise on attentional focus. Percept Motor Skills. 1992;75:351–354. doi: 10.2466/pms.1992.75.2.351. [DOI] [PubMed] [Google Scholar]
- 73.Cian C., Barraud P.A., Melin B., Raphel C. Effects of fluid ingestion on cognitive function after heat stress or exercise-induced dehydration. Int J Psychophysiol. 2001;42:243–251. doi: 10.1016/s0167-8760(01)00142-8. [DOI] [PubMed] [Google Scholar]
- 74.Kamijo K., Nishihira Y., Higashiura T., Kuroiwa K. The interactive effect of exercise intensity and task difficulty on human cognitive processing. Int J Psychophysiol. 2007;65:114–121. doi: 10.1016/j.ijpsycho.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 75.Tomporowski P.D. Effects of acute bouts of exercise on cognition. Acta Psychol. 2003;112:297–324. doi: 10.1016/s0001-6918(02)00134-8. [DOI] [PubMed] [Google Scholar]
- 76.Clarkson-Smith L., Hartley A.A. Relationships between physical exercise and cognitive abilities in older adults. Psychol Aging. 1989;4:183–189. doi: 10.1037//0882-7974.4.2.183. [DOI] [PubMed] [Google Scholar]
- 77.Hogervorst E., Riedel W., Jeukendrup A., Jolles J. Cognitive performance after strenuous physical exercise. Percept Motor Skills. 1996;83:479–488. doi: 10.2466/pms.1996.83.2.479. [DOI] [PubMed] [Google Scholar]
- 78.Fraizer E.V., Mitra S. Methodological and interpretive issues in posture-cognition dual-tasking in upright stance. Gait Posture. 2008;27:271–279. doi: 10.1016/j.gaitpost.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 79.Lajoie Y., Teasdale N., Bard C., Fleury M. Attentional demands for static and dynamic equilibrium. Exp Brain Res. 1993;97:139–144. doi: 10.1007/BF00228824. [DOI] [PubMed] [Google Scholar]
- 80.Redfern M.S., Jennings J.R., Martin C., Furman J.M. Attention influences sensory integration for postural control in older adults. Gait Posture. 2001;14:211–216. doi: 10.1016/s0966-6362(01)00144-8. [DOI] [PubMed] [Google Scholar]
- 81.Shumway-Cook A., Woollacott M., Kerns K.A., Baldwin M. The effects of two types of cognitive tasks on postural stability in older adults with and without a history of falls. J Gerontol A Biol Sci Med Sci. 1997;52:M232–M240. doi: 10.1093/gerona/52a.4.m232. [DOI] [PubMed] [Google Scholar]
- 82.Lajoie Y., Teasdale N., Bard C., Fleury M. Upright standing and gait: are there changes in attentional requirements related to normal aging? Exp Aging Res. 1996;22:185–198. doi: 10.1080/03610739608254006. [DOI] [PubMed] [Google Scholar]
- 83.Brown L.A., Sleik R.J., Winder T.R. Attentional demands for static postural control after stroke. Arch Phys Med Rehabil. 2002;83:1732–1735. doi: 10.1053/apmr.2002.36400. [DOI] [PubMed] [Google Scholar]
- 84.Marchese R., Bove M., Abbruzzese G. Effect of cognitive and motor tasks on postural stability in Parkinson’s disease: a posturographic study. Mov Disord. 2003;18:652–658. doi: 10.1002/mds.10418. [DOI] [PubMed] [Google Scholar]
- 85.Woollacott M., Shumway-Cook A. Attention and the control of posture and gait: a review of an emerging area of research. Gait Posture. 2002;16:1–14. doi: 10.1016/s0966-6362(01)00156-4. [DOI] [PubMed] [Google Scholar]
- 86.Shumway-Cook A., Woollacott M. Attentional demands and postural control: the effect of sensory context. J Gerontol A Biol Sci Med Sci. 2000;55:M10–M16. doi: 10.1093/gerona/55.1.m10. [DOI] [PubMed] [Google Scholar]
- 87.Di Giulio I., Maganaris C.N., Baltzopoulos V., Loram I.D. The proprioceptive and agonist roles of gastrocnemius, soleus and tibialis anterior muscles in maintaining human upright posture. J Physiol. 2009;587:2399–2416. doi: 10.1113/jphysiol.2009.168690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wikstrom E.A., Tillman M.D., Chmielewski T.L., Cauraugh J.H., Borsa P.A. Dynamic postural stability deficits in subjects with self-reported ankle instability. Med Sci Sports Exerc. 2007;39:397–402. doi: 10.1249/mss.0b013e31802d3460. [DOI] [PubMed] [Google Scholar]
- 89.Bisson J., McEwena D., Lajoie Y., Bilodeau M. Effects of ankle and hip muscle fatigue on postural sway and attentional demands during unipedal stance. Gait Posture. 2011;33:83–87. doi: 10.1016/j.gaitpost.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 90.Tracey C., Leigh W., John P., Christopher W. Effects of a maximal exercise test on neurocognitive function. J Sports Med. 2007;41:370–374. doi: 10.1136/bjsm.2006.032334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cian C., Koulmann N., Barraud P.A., Raphel C., Jimenez C., Melin B. Influence of variations in body hydration on cognitive function: effects of hyperhydration, heat stress, and exercise-induced dehydration. J Psychophysiol. 2000;14:29–36. [Google Scholar]
- 92.Frey Y., Ferry A., VomHofe A., Rieu M. Effect of physical exhaustion on cognitive functioning. Percept Mot Skill. 1997;84:291–298. doi: 10.2466/pms.1997.84.1.291. [DOI] [PubMed] [Google Scholar]
- 93.Szturm T., Maharjan P., Marotta J., Shay B., Shrestha S., Sakhalkar V. The interacting effect of cognitive and motor task demands on performance of gait, balance and cognition in young adults. Gait Posture. 2013;38:596–602. doi: 10.1016/j.gaitpost.2013.02.004. [DOI] [PubMed] [Google Scholar]