Graphical abstract
Keywords: Laser, Livestock, Maturation, Oocyte, Sperm
Abstract
This article presents a brief review of the past and present literature pertinent to laser effects on sperm motility parameters, improvement of oocyte maturation and characterization of semen in livestock. The aim was, on one hand, to make the readers aware of such knowledge and on the other hand to trigger the interest of the animal reproduction scientific community in attempting some laser techniques that have not yet been fully exploited in the field of artificial insemination. With respect to the conventional methods, laser is a more sensitive and less costly technology that can be used for improving artificial insemination and embryo production system. Since 1980s, laser treatment came on the biological samples scene; its applications have continuously been developed thereafter. Exploitation of laser light by various researchers for improving the reproductive efficiency of sperm cells and the maturation rate in different livestock is demonstrated herein. Laser irradiation, in principal, can increase the production of adenosine triphosphate (ATP) and consequently increases the energy provided to the cell. Since sperm motility and oocyte maturation depend on the energy consumption, an increase in the energy supply to the cells will be of great importance. In addition, the authors also discuss the use of laser spectrochemical analytical techniques, such as laser induced breakdown spectroscopy (LIBS) and laser induced fluorescence (LIF), in characterization of semen samples.
Introduction
Over the past two decades, the assisted reproductive technologies (ARTs), namely artificial insemination (AI), in vitro maturation (IVM), in vitro fertilizations (IVF), intracytoplasmic sperm injection (ICSI), and somatic cell nuclear transfer (SCNT), for different species have been evolved effectively [1–6].
Artificial insemination offers many advantages to commercial livestock production and is routinely used in several domestic animals such as cattle, sheep and horses [7]. The technique represents a very important and a promising issue to increase animal production. Improvement of sperm motility is expected to have direct qualitative and quantitative impacts on AI of livestock. As it is well known, the role of a spermatozoon is to deliver the male’s genetic material to the oocyte during fertilization; consequently motility is considered as one of the most important parameters. For motility, spermatozoa require development of a tail (flagellum). Mammalian sperm is characterized by the fusion of mitochondria in a mitochondrial sheath located around the apical portion of the tail. In different species [8] a direct relationship between motility and mitochondrial activity was shown.
Evaluation of oocytes quality is of high importance, since improvement of maturation rate represents the corner stone of the above mentioned technologies. Many researchers conducted experiments and suggested procedures for improving oocytes maturity using different types of media such as a basal medium (M199) [9], and DMEM/F12-based media [10] and/or different incubation times [11,12]. The success of in vitro produced embryo depends on the quality and competence of gametes involved in oocyte maturation, fertilization, and early embryonic development.
During the last two decades of the 20th century, the effects of laser on biological tissues have been studied widely [13–17]. It has been clearly demonstrated that low level laser irradiation, also known as photobiomodulation, has pronounced biological effects. Laser irradiation of fibroblasts [18], and other biological structures, such as the neuromuscular junction [19] are some examples of such biological application of lasers. In addition of studying the cells’ response to laser light irradiation, recent studies dealt with laser effects on spermatozoa [20–23], and oocytes [24,25]. The positive effects of low level laser irradiation include the increase of cellular metabolism and improvement of structural characteristics, as has been confirmed in the literature [13,26,27].
Many studies have focused on utilization of laser effect on sperm motility parameters. Notwithstanding, there have been very few studies concerning the effects of laser on oocyte maturation. Over the last couple of years there has been an exponential growth in the areas of utilization of lasers (pulsed or continuous wave (CW)) which is reflected by an increasing number of publications, and thus its utility as an assisting technology has been proved. Below, a summary is presented of literature pertinent to the effect of laser irradiation on improving AI, oocyte maturation, and embryo production system in domestic animals.
Laser and improvement of sperm parameters
Sperm consists of a head containing condensed DNA, followed by a short neck (midpiece) containing mitochondria and a thin tail (flagellum) responsible for motility [11,25]. The spermatozoon motility depends on energy supply. Both of energy metabolism in mitochondria and the motility system of the cells are involved in the activation of the sperm flagellum. ATP on the other hand can be produced by mammalian spermatozoa via both of aerobic and anaerobic glycolysis [28-30]. Previously published works showed the potential of low-power laser irradiation of spermatozoa in increasing their motility and raising the ATP amount in cells. The first paper published on this topic was that of Goldstein [31]. Thereafter, it has been clearly evidenced that He–Ne laser irradiation (at wavelength 632.8 nm) of active living human sperm improved their motility and speed [32–35]. During the years 1996 and 1997 other works have been published dealing with coherent light (laser) and incoherent light (visible) motility stimulation of bull, ram, mouse and human spermatozoa [36–41]. The hyperactivated motility of human sperm irradiated with visible light (400–800 nm) has been increased significantly while the concentration of intercellular Ca2+ accompanied with a reduction in the hyperactivated motility increased very fast when voltage-dependent Ca2+ channel was blocked [41]. Biochemical and topological analysis of bovine sperm cells showed change in sperm metabolism due to low-level laser treatment; however it has been mentioned that more studies are necessary to establish an optimal dose to increase the fertility potential of these cells [42]. Wenbin et al. [43] found that laser irradiation increased sperm fructose fermentation, respiration, the amount of phosphorus (32P) uptake and the Ca2+ absorption, thus there was an increase of motility and survival time of spermatozoa. According to Zan-Bar et al. [40], the effects of light are mediated through reactive oxygen species (ROS). Indeed, although high ROS level can lead to cell death (by ATP depletion and lipid peroxidation), at low level, ROS can play a major role in activation of many cellular processes. In the case of spermatozoa, ROS, including superoxide anion, H2O2, and reactive nitrogen species as nitric oxide (NO) can cause sperm hypercapacitation and the acrosome reaction [44–46]. The energy delivery time is, in fact, an essential parameter in laser irradiation, since there should be enough time for the cell to obtain more metabolites from the medium needed for reactions where laser irradiation is involved in. This is similar to the mechanisms of photodynamic therapy (PDT), where the presence of oxygen is needed for the production of an oxygen singlet (1O2) [47,48]. However, the laser light photon energy E (E = hc/λ where h is Planck’s constant, c is the speed of light and λ is the wavelength) is a decisive parameter for the time needed to obtain the required effect. Table 1 lists parameters of lasers mostly used in animal reproduction applications.
Table 1.
Types of lasers used in animal reproduction researches and relevant parameters.
| Laser | Operation mode | Wavelength (nm) (color) | Photon energy (eV) | Application | Species and reference |
|---|---|---|---|---|---|
| DPSS | CW | 405 (violet) | 3.07 | Molecular analysis (LIF) | Buffalo [68] |
| Nd:YAG second harmonic | CW and pulsed | 532 (green) | 2.34 | Irradiation | Buffalo [20] |
| He–Ne | CW | 632.8 (red) | 1.96 | Irradiation (activation and/or sterilization) | Bovine [21,23,62], chicken [39], Turkey [53,54], rabbit [55] |
| Diode laser | CW | 655 (red) | 1.90 | Irradiation | Dog [49,50] |
| Nd:YAG | Pulsed | 1064 (IR) | 1.17 | Elemental analysis (LIBS) | Buffalo [68] |
DPSS: Diode pumped solid state laser.
CW: Continuous wave.
IR: Infrared.
In the year 2005, Corral-Baqués et al. [49] demonstrated that irradiating dog sperm with a 655 nm diode laser light at 4.00, 6.00, and 10.00 J/cm2 improves its motility features and seems to maintain its functional characteristics up to 45 min after irradiation. In 2009 the same research group extended their investigations on the effects of low-level laser irradiation on dog spermatozoa and its dependence on the laser output power [50]. The results showed that irradiation with different output powers had different effects on semen parameters including motility, average velocity, linear coefficient and beat cross frequency. Yazdi et al. [51] reported on the effect of 830 nm diode laser irradiation on human sperm motility. Significant increase in the irradiated sperm motility has been obtained after exposure to 4.00 and 6.00 J/cm2 for 60 and 45 min respectively.
Zan-Bar et al. [40] reported the effect of red laser light (660 nm) on ram and tilapia sperm. They found that the use of such red light irradiation led to a slight increase in motility and fertility in ram spermatozoa compared with higher values for the two parameters in tilapia sperm. In an interesting study, Abdel-Salam et al. [20] reported that irradiation of buffalo semen with green laser light (532 nm) at 0.31 J/cm2 and 0.38 J/cm2 doses improved semen quality such as motility, progressive, VCL (curvilinear velocity), VSL (progressive velocity), VAP (average path velocity), and ALH (mean amplitude of lateral head displacement) for short exposure times from 4 to 5 min. They interpreted the obtained improvement in semen quality as being due to the shorter wavelength used where the photon energy is higher than in case of using red laser with longer wavelength and low photon energy.
Laser and semen storage
It is well known that semen of domestic animals cannot be stored for longer time without a loss of fertilizing capability even if oxygenated and stored with appropriate diluents at reduced temperature [52]. Recent article [53] reported that He Ne laser irradiation has differential action on biostimulation of turkey, chicken and pheasant spermatozoa. This work is considered the first elucidate of the possibility for restoration of motility of cryopreserved avian spermatozoa by biostimulation provided via He Ne laser irradiation. Previously, the quality of stored turkey semen was found to be improved significantly following He–Ne laser irradiation with energy doses ranging from 3.24 J/cm2 to 5.40 J/cm2 and in particular at fluences close to 4.00 J/cm2 laser prevented in vitro liquid storage-dependent damage [54]. It was found in another study [55] that laser irradiation increased the sperm motility parameter, viability, and cell energy charge in rabbit semen. The authors concluded that laser irradiation might be a viable technique for enhancing the quality of semen in long-term storage. Previous studies by Passarella et al. [13] demonstrated that irradiation with He–Ne laser of energy density 5 J/cm2 led to an increase in proton electrochemical potential and ATP synthesis in isolated mitochondria. The increase of ATP synthesis has been interpreted by the authors as being due to laser induced protomotive force. Sperm-fertilizing capacity can be affected by changing sperm motility due to all factors enhancing or hindering ATP production or availability. Iaffaldano et al. [55] found that He–Ne laser irradiation improved rabbit sperm preservation during liquid storage modulating sperm qualitative functions that may be related to biostimulation of rabbit spermatic cells and increased cytochrome c oxidize activity.
Laser and bacterial contamination
The extent of microbial contamination is an important parameter to consider in quality control of semen that is used for artificial insemination or direct mating. Semen is an ideal medium for establishment and growth of many microorganisms including bacteria and fungi. Other sources of contamination include inflammatory foci in the genital tract, the skin of animals, semen collection, the processing equipment, animal handlers and laboratory personnel during manipulation. Bartlett [56] considered that low number of bacteria in frozen semen, capable of being transferred during coitus, should be accepted as “normal” because the endometrium of healthy animals could be highly resistant to microbial infection. Parez and Thibier [57] reported that there was no evidence of a relationship between the presence of potentially pathogenic bacteria and the fertilizing ability of semen. To minimize these adverse effects, antibiotics are included in the composition of the ram semen extenders to prevent bacterial growth [58,59]. Addition of variable alternatives antibiotics in extenders to control microbial contamination in semen has been weakened by the increasing appearance of antibiotic resistant strains and a growing list of opportunistic and potential pathogens in semen [60]. Novel methods for controlling the contaminants such as the use of antibodies (monoclonal and genetically-engineered antibodies), anti-idiotype vaccines, visible wavelengths radiation, and exploitation of natural inhibitors in the semen should be looked at as variable alternatives or supplements to antibiotic treatment [61]. Hussein et al. [62] reported that in Friesian bulls semen medium, reduced bacterial growth and improved semen quality were observed 2, 4, and 8 min after irradiation by light emitting diode (LED) (680 nm, 10 mW) and diode laser (DL) (660 nm, 100 mW). It has been found that such red wavelengths improved semen quality in some of the exposed samples. This coincided with the previous work [63], which showed the importance of red wavelength for the increase of semen viability.
Laser and oocyte maturation
In all assisted reproductive technologies, namely IVM, ICSI, and SCNT, the evaluation of the oocytes quality is of high importance; however the improvement of the maturation rate represents the corner stone of these technologies. In fact there are very few studies concerning the effects of laser on oocyte maturation. Soares et al. [24] used He–Ne Laser (632 nm) to irradiate bovine oocytes. These authors found that laser is capable of modulating events in granulosa cells that may lead to changes in oocyte. Also, Moreno-Millan and Ocaña-Quero [64] used low laser power to treat immature oocyte, and found that He–Ne laser with doses of 0.40 J/cm2 and 2.00 J/cm2 affected the maturation process negatively and caused nuclear damage. Researches indicate that the mechanisms involved in laser interaction with biological cells are due to photons absorption by cellular photoreceptors that trigger chemical reactions such as glycolysis and oxidative phosphorylation. This could accelerate RNA transcription and DNA replication. In addition, it is widely known that laser light accelerates mitosis and affects different metabolic processes through changes induced in the mitochondrial membrane [65]. As mentioned by Karu [66] the absorption of different light wavelengths by cytochromes from the internal mitochondrial membrane increases ATP synthesis. Oocyte suffers from numerous transformations during its maturation to be ready for fertilization and following embryo development. These transformations are mainly induced by granulosa cells, which facilitate communication to oocyte through the gap junctions [34]. Previously published results, using He–Ne laser for irradiation, revealed that the mitochondrial membrane potential has been increased [26,17]. It is most probable that the same mechanism takes place also in case of using other laser wavelengths. Shorter wavelength laser light is especially a good candidate to induce effects similar to those obtained by He–Ne laser irradiation at shorter irradiation time and lower energy doses. This is mainly due to the fact that the shorter is the laser light wavelength the higher is its photon energy. For example, the He–Ne laser photon energy is 1.87 eV while the second harmonic of Nd:YAG laser 532 nm (green) and the diode pumped solid state laser, DPSS, 405 nm (violet) have photon energies of 2.25 eV, 2.92 eV respectively. Consequently the effect of shorter wavelength laser irradiation will be higher on the mitochondria [24]. This is in agreement with what has been reported by Fujiwara et al. [34] who interpreted the induced chemical energy as being due to the absorption of laser energy by proteins of mitochondrial respiratory chain.
Laser characterization of semen
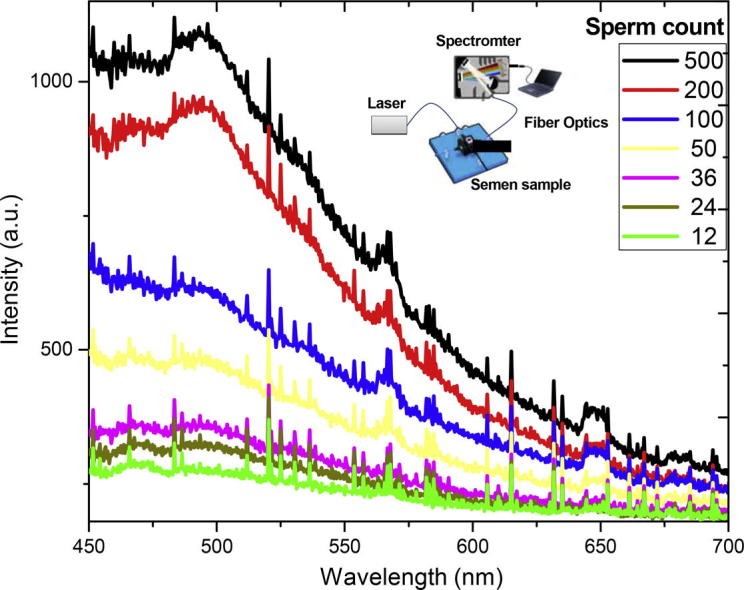
Smuk et al. [67] studied random laser action in bovine semen when excited by a Q-switched frequency doubled Nd:YAG laser, without any analytical investigations. Abdel-Salam and Harith [68] used laser spectrochemical analytical techniques such as laser induced breakdown spectroscopy (LIBS) and laser induced fluorescence (LIF) for characterization of semen samples. Via LIBS those authors obtained information about the elemental seasonal variations in the seminal plasma. Ca, Mg, Zn and Fe were found to be higher in winter than in summer in buffaloes’ seminal plasma. They also found that elements’ concentrations in seminal plasma have direct relation to the sperm parameters and consequently LIBS can be used for indirect assessment of semen parameters. LIF that is normally used for detection of selective species and studying structure of molecules has been exploited by the same authors to estimate sperm count in buffalo semen samples [68]. The sperm count could be correlated to the intensity of the fluorescence emission and provided the basis for instrumentation for in situ rapid determination of sperm counts with no need for conventional microscopic or time consuming imaging techniques in the laboratory.
Conclusions
As mentioned above, the aim of this brief review was to demonstrate what have been published in the topic of improvement of sperm parameters and maturation rate of domestic animals oocytes via laser irradiation. Such improvement can contribute effectively in enhancement of in vitro embryo production. The publications in this field are still very limited, and investigated mainly the irradiation effect of red laser (λ = 632.8 nm) to improve the system of sperm parameters and in vitro embryo production [20,24,33,49,68]. However, negative effects on sperm parameters and on the maturation process of the oocyte have been obtained using such red laser for irradiation [50,64].
We do expect that the use of the shorter wavelengths, e.g. λ = 532 nm and λ = 405 nm is promising and more reasonable than longer laser wavelength for biostimulative purposes because they will be better absorbed by the cellular chromophores. However, studies indicated that the cellular photoreceptors are capable of absorbing photons that may trigger chemical reactions at certain wavelengths [24–26,56]. The laser fluence (J/cm2) is the key point of the impacts on the biological cells. However, and as mentioned by Passarella and Karu [69] the overall mechanism of light interaction with the biological samples needs more research work to be fully understood. In view of the photosensitivity of numerous molecules, it is possible to exploit photobiomodulation in photomedicine and biotechnology [69]. In the above review it has been shown that lasers show up in the field of livestock reproduction as an easy, time saving, less costly and effective technique in addition of having the possibility of its use in situ, namely in cattle farms and veterinary clinics.
Conflict of interest
The authors have declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Biographies

Zienab Abdel-Salam (B.Sc., Animal Production, Faculty of Agriculture, Cairo University 1999; MSc, Application of Laser in Biotechnology, NILES, Cairo University; PhD, Laser Application in Biotechnology 2009, NILES, Cairo University). She is currently a lecturer of laser applications in biology at NILES. Her current research interests include biological applications of lasers especially in the field of animal production.

Mohamed A. Harith (B.Sc., Physics, Cairo University 1968; PhD, Physics, TU Dresden, Germany 1976). Former dean of the National Institute of Laser Enhanced Science (NILES), Cairo University. He is currently professor of laser physics at NILES and head of the Applied Laser Spectroscopy (ALS) group. His current research interests include industrial, archaeological, and biological applications of laser induced breakdown spectroscopy (LIBS) and laser-induced fluorescence (LIF).
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Joohyeong L., Yongjin L., Bola P., Fazle E., Yubyeol J., Sang-Hwan H. Developmental competence of IVM pig oocytes after SCNT in relation to the shrinkage pattern induced by hyperosmotic treatment. Theriogenology. 2014;81:974–981. doi: 10.1016/j.theriogenology.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 2.Gaoping Z., Kaifeng W., Liang C., Lixia Z., Yiyi L., Xiuwen T. In vitro maturation and artificial activation of donkey oocytes. Theriogenology. 2011;76:700–704. doi: 10.1016/j.theriogenology.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 3.Wang Q., Sun Q.Y. Evaluation of oocyte quality: morphological, cellular and molecular predictors. Reprod Fertil Develop. 2007;19:1–12. doi: 10.1071/rd06103. [DOI] [PubMed] [Google Scholar]
- 4.Hinrichs K., Schmidt A.L. Meiotic competence in horse oocytes: interactions among chromatin configuration, follicle size, cumulus morphology, and season. Biol Reprod. 2000;62:1402–1408. doi: 10.1095/biolreprod62.5.1402. [DOI] [PubMed] [Google Scholar]
- 5.Nagashima H., Grupen C.G., Ashman R.J., Nottle M.B. Developmental competence of in vivo and in vitro matured porcine oocytes after subzonal sperm injection. Mol Reprod Dev. 1996;45:359–363. doi: 10.1002/(SICI)1098-2795(199611)45:3<359::AID-MRD13>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 6.Li G.P., Tan J.H., Sun Q.Y., Meng Q.G., Yue K.Z., Sun X.S. Cloned piglets born after nuclear transplantation of embryonic blastomeres into porcine oocytes matured in vivo. Cloning. 2000;2:45–52. doi: 10.1089/15204550050145120. [DOI] [PubMed] [Google Scholar]
- 7.Vishwanath R. Artificial insemination: the state of the art. Theriogenology. 2000;59:571–584. doi: 10.1016/s0093-691x(02)01241-4. [DOI] [PubMed] [Google Scholar]
- 8.Eddy E.M., O’Brien A.D. The spermatozoon. In: Knobil E., Neill J.D., editors. The physiology of reproduction. Raven Press; New York: 1988. pp. 27–77. [Google Scholar]
- 9.Hinrich K., Choi Y.H., Love L.B., Varner D.D., Love C.C., Walckenaer B.E. Chromatin configuration within the germinal vesicle of horse oocytes: changes post mortem and relationship to meiotic and developmental competence. Biol Reprod. 2005;72:1142–1150. doi: 10.1095/biolreprod.104.036012. [DOI] [PubMed] [Google Scholar]
- 10.Galli C., Colleoni S., Duchi R., Lagutina I., Lazzari G. Developmental competence of equine oocytes and embryos obtained by in vitro procedures ranging from in vitro maturation and ICSI to embryo culture, cryopreservation and somatic cell nuclear transfer. Anim Reprod Sci. 2007;98:39–55. doi: 10.1016/j.anireprosci.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 11.Nandi S., Ravindranatha B.M., Gupta P.S.P., Sarma P.V. Timing of sequential changes in cumulus cells and first polar body extrusion during in vitro maturation of buffalo oocyte. Theriogenology. 2007;57:1151–1159. doi: 10.1016/s0093-691x(01)00709-9. [DOI] [PubMed] [Google Scholar]
- 12.Lin Y.H., Hwang J.L., Seow K.M., Huang L.W., Hsieh B.C., Chen H.J. Effect of incubation with different concentrations and durations of FSH for in-vitro maturation of murine oocytes. Reprod Biomed Online. 2011;23:3–19. doi: 10.1016/j.rbmo.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 13.Passarella S., Casamassima E., Molinari S., Pastone D. Increase of proton electrochemical potential and ATP synthesis in rat liver mitochondria irradiated in vitro by He–Ne laser. FEBS Lett. 1984;175:95–99. doi: 10.1016/0014-5793(84)80577-3. [DOI] [PubMed] [Google Scholar]
- 14.Levi A.C., Petrino R., Siccardi E. Laser irradiation on chicken embryos. Boll Sco Ital Biol Sper. 1987;3:233–236. [PubMed] [Google Scholar]
- 15.Karu T.I. Molecular mechanism of therapeutic effect of low intensity laser irradiation. Laser Life Sci. 1988;2:53–74. [Google Scholar]
- 16.Karu T.I. Photobiology of low power laser effects. Health Phys. 1988;56:691–704. doi: 10.1097/00004032-198905000-00015. [DOI] [PubMed] [Google Scholar]
- 17.Karu T.I., Ryabykh T.P., Fedoseyeva R., Puchkova G.E. Helium neon laser induced respiratory burst of phagocytic cells. Lasers Surg Med. 1989;9:585–588. doi: 10.1002/lsm.1900090608. [DOI] [PubMed] [Google Scholar]
- 18.Hawkins D., Abrahamse H. Effect of multiple exposures of low-level laser therapy on the cellular responses of wounded human skin fibroblasts. Photomed Laser Surg. 2006;24:705–714. doi: 10.1089/pho.2006.24.705. [DOI] [PubMed] [Google Scholar]
- 19.Nicolau R.A., Martinez M.S., Rigau J., Tomas J. Effect of low power 655 nm diode laser irradiation on the neuromuscular junctions of the mouse diaphragm. Lasers Surg Med. 2004;34:277–284. doi: 10.1002/lsm.20006. [DOI] [PubMed] [Google Scholar]
- 20.Abdel-Salam Z., Dessouki S.H.M., Abdel-Salam S.A.M., Ibrahim M.A.M., Harith M.A. Green laser irradiation effects on buffalo semen. Theriogenology. 2011;75:988–994. doi: 10.1016/j.theriogenology.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Ocaña-Quero J.M., Gomez Villamandos R.J., Moreno Millan M., Santisteban Valenzuela J.M. Helium–neon (He–Ne) laser irradiation increases the incidence of unreduced bovine oocytes during the first meiotic division in vitro. Laser Med Sci. 1998;13(13):260–264. doi: 10.1007/s101030050005. [DOI] [PubMed] [Google Scholar]
- 22.Ocaña-Quero J.M., Gomez Villamandos R.J., Moreno Millan M., Santisteban Valenzuela J.M. Biological effects of helium–neon (He–Ne) laser irradiation on acrosome reaction in bull sperm cells. J Photochem Photobiol B. 1997;40:294–298. doi: 10.1016/s1011-1344(97)00072-9. [DOI] [PubMed] [Google Scholar]
- 23.Ocaña-Quero J.M., Gomez Villamandos R.J., Moreno Millan M., Santisteban Valenzuela J.M. The effect of helium neon laser on in vitro maturation and fertilization of immature bovine oocytes. Laser Med Sci. 1995;10:113–119. [Google Scholar]
- 24.Soares C.A., Annes K., Dreyer T.R., Magrini T., Sonoda M.T., Martinho H. Photobiological effect of low-level laser irradiation in bovine embryo production system. J Biomed Opt. 2014;19:035006–035009. doi: 10.1117/1.JBO.19.3.035006. [DOI] [PubMed] [Google Scholar]
- 25.Karu T.I. Laser in infertility treatment: irradiation of oocytes and spermatozoa. Photomed Laser Surg. 2012;30:239–241. doi: 10.1089/pho.2012.9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karu T.I. Mitochondrial mechanisms of photobiomodulation in context of new data about multiple roles of ATP. Photomed Laser Surg. 2010;28:159–160. doi: 10.1089/pho.2010.2789. [DOI] [PubMed] [Google Scholar]
- 27.Al Ghamdi K.M., Kumar A., Moussa N.A. Low-level laser therapy: a useful technique for enhancing the proliferation of various cultured cells. Lasers Med Sci. 2012;27:237–249. doi: 10.1007/s10103-011-0885-2. [DOI] [PubMed] [Google Scholar]
- 28.Albarracin J.L., Mogas T., Paloma M.J., Peña A., Rigua T., Rodriguez-Gil J.E. In vitro capacitation and acrosome reaction of dog spermatozoa can be feasibly attained in a defined medium without glucose. Reprod Dom Anim. 2004;39:129–135. doi: 10.1111/j.1439-0531.2004.00485.x. [DOI] [PubMed] [Google Scholar]
- 29.Peterson R.N., Freund M. ATP synthesis and oxidative metabolism in human spermatozoa. Biol Reprod. 1970;3:47–54. doi: 10.1093/biolreprod/3.1.47. [DOI] [PubMed] [Google Scholar]
- 30.Mukai C., Okino M. Glycolysis plays a major role for adenosine triphosphate supplementation in mouse sperm flagellar movement. Biol Reprod. 2004;71:540–547. doi: 10.1095/biolreprod.103.026054. [DOI] [PubMed] [Google Scholar]
- 31.Goldstein S.F. Irradiation of sperm tails by laser microbeam. J Exp Biol. 1969;51:431–441. doi: 10.1242/jeb.51.2.431. [DOI] [PubMed] [Google Scholar]
- 32.Lenzi A., Claroni F., Gandini L., Lombardo F., Barbieri C., Lino A. Laser radiation and motility patterns of human sperm. Syst Biol Reprod Med. 1989;23:229–234. doi: 10.3109/01485018908986845. [DOI] [PubMed] [Google Scholar]
- 33.Singer R., Sagiv M., Barnett M., Levinsky H., Segenreich F., Fuchs Y. Low energy narrow band non-coherent infrared illumination of human semen and isolated sperm. Andrologia. 1991;23:181–184. doi: 10.1111/j.1439-0272.1991.tb02532.x. [DOI] [PubMed] [Google Scholar]
- 34.Fujiwara A., Tazawa E., Yasumasu I. Activating effects of light irradiation at various wavelength on the respiration in sperm of the echiuroid, Urechis unicinctus, in the presence of carbon monoxide. J Biochem. 1991;109:486–491. doi: 10.1093/oxfordjournals.jbchem.a123408. [DOI] [PubMed] [Google Scholar]
- 35.Vesich I.L. Some features of rehabilitating action of laser emission on native and cryopreserved human spermatozoa. Probl Cryobiol. 1994;4:33–35. [Google Scholar]
- 36.Lubart R., Friedmann H., Levinshal T., Lavi R., Breibart H. Effect of light on calcium transport in bull sperm cells. J Photochem Photobiol B. 1992;15:337–341. doi: 10.1016/1011-1344(92)85139-l. [DOI] [PubMed] [Google Scholar]
- 37.Lubart R., Friedmann H., Sinjukov H., Cohen N., Breibart H. Changes in Ca transport in mammalian sperm mitochondria and plasma membranes caused by 780 nm irradiation. Lasers Surg Med. 1997;21:493–499. doi: 10.1002/(sici)1096-9101(1997)21:5<493::aid-lsm12>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 38.Cohen N., Lubart R., Rubinstein S., Breitbart H. Light irradiation of mouse spermatozoa: stimulation of in vitro fertilization and calcium signals. J Photochem Photobiol B. 1998;68:407–413. [PubMed] [Google Scholar]
- 39.Breitbart H., Levinshal T., Cohen N., Friedmann H., Lubart R. Changes in calcium transport in mammalian sperm mitochondria and plasma membrane irradiated at 633 nm (He–Ne laser) J Photochem Photobiol B. 1996;34:117–121. doi: 10.1016/1011-1344(95)07281-0. [DOI] [PubMed] [Google Scholar]
- 40.Zan-Bar T., Bartoov B., Segal R., Yehuda R., Lavi R., Lubart R. Influence of visible light and UV radiation on motility and fertility of mammalian and fish sperm. Photomed Laser Surg. 2005;23:549–555. doi: 10.1089/pho.2005.23.549. [DOI] [PubMed] [Google Scholar]
- 41.Shahar S., Wiser A., Ickowicz D., Lubart R., Shulman A., Breitbart H. Light-mediated activation reveals a key role for protein kinase A and sarcoma protein kinase in the development of sperm hyper-activated motility. Hum Reprod. 2011;26:2274–2282. doi: 10.1093/humrep/der232. [DOI] [PubMed] [Google Scholar]
- 42.Dreyer TR, Siqueira AFP, Magrini TD, Fiorito ME, Assumpção OA, Nichi M, et al. Biochemical and topological analysis of bovine sperm cells induced by low power laser irradiation. In: Sroka R, Lilge L, editors. Medical laser applications and laser-tissue interactions. Proc. SPIE 2011. Paper 8092 0V. p. 8092.
- 43.Wenbin Y, Wenzhong L, Mengzhao L, Baotian Z, Laizeng AI, Tongya L. Effects of laser radiation on Saanen buck’s sperm energy metabolism. In: Proceedings of the sixth international conference on goats, Beijing, China, May 5–11; 1996.
- 44.Aitken R.J. Free radicals, lipid peroxidation and sperm function. Reprod Fertil Dev. 1995;7:659–668. doi: 10.1071/rd9950659. [DOI] [PubMed] [Google Scholar]
- 45.Aitken R.J., Paterson M., Fisher H., Buckingham D.W., VanDuim M. Redox regulation of tyrosine phosphorylation in human spermatozoa and its role in the control of human sperm function. J Cell Sci. 1995;108:2017–2025. doi: 10.1242/jcs.108.5.2017. [DOI] [PubMed] [Google Scholar]
- 46.Martínez-Pastor F., Aisen E., Fernández-Santos M.R., Esteso M.C., Maroto-Morales A., García-Álvarez O. Reactive oxygen species generators affect quality parameters and apoptosis markers differently in red deer spermatozoa. Reproduction. 2009;137:225–235. doi: 10.1530/REP-08-0357. [DOI] [PubMed] [Google Scholar]
- 47.Weishaupt K.R., Gomer C.J., Dougherty T.J. Identification of singlet oxygen as the cytotoxic agent in photo-inactivation of a marine tumor. Cancer Res. 1976;36:2326–2329. [PubMed] [Google Scholar]
- 48.Moan J., Sommer S. Oxygen dependence of the photosensitizing effect of hematoporphyrin derivative in NHIK cells. Cancer Res. 1985;45:1608–1610. [PubMed] [Google Scholar]
- 49.Corral-Baqués M.I., Rivera M.M., Rigau T., Rodriguez-Gil J.E., Rigau J. Effect of 655 nm diode laser on dog sperm motility. Lasers Med Sci. 2005;20:703–713. doi: 10.1007/s10103-005-0332-3. [DOI] [PubMed] [Google Scholar]
- 50.Corral-Baqués M.I., Rivera M.M., Rigau T., Rodriguez-Gil J.E., Rigau J. The effect of low-level laser irradiation on dog spermatozoa motility is dependent on laser output power. Lasers Med Sci. 2009;24:28–34. doi: 10.1007/s10103-008-0606-7. [DOI] [PubMed] [Google Scholar]
- 51.Yazdi S.R., Bakhshi S., Yanat A.F., Akhound H.R., Ansari A. Effect of 830 nm diode laser irradiation on human sperm motility. Int J Fertil Steril. 2010;4:31–32. [Google Scholar]
- 52.Thurston R.J. Storage of poultry semen above freezing for 24–48 hours. In: Bakst M.R., Wishart G.J., editors. Proceedings of the first international symposium on the artificial insemination of poultry. Poultry Science Association Inc.; Savoy, IL: 1995. pp. 107–122. [Google Scholar]
- 53.Iaffaldano N., Paventi G., Pizzuto R., Passarella S., Cerolini S., Zaniboni L. The post-thaw irradiation of avian spermatozoa with He–Ne laser differently affects chicken, pheasant and turkey sperm quality. Anim Reprod Sci. 2013;142:168–172. doi: 10.1016/j.anireprosci.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 54.Iaffaldano N., Meluzzi A., Manchisi A., Passarella S. Improvement of stored turkey semen quality as a result of He–Ne laser irradiation. Anim Reprod Sci. 2005;85:317–325. doi: 10.1016/j.anireprosci.2004.04.043. [DOI] [PubMed] [Google Scholar]
- 55.Iaffaldano N., Rosato M.P., Paventi G., Pizzuto R., Gambacorta M., Manchisi A. The irradiation of rabbit sperm cells with He–Ne laser prevents the in vitro liquid storage dependent damage. Anim Reprod Sci. 2010;119:123–129. doi: 10.1016/j.anireprosci.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 56.Bartlett D.E. Bull semen: specific microorganisms in disease control in semen and embryo. FAO Anim Health Prod. 1981;23:29–48. [Google Scholar]
- 57.Parez M., Thibier M. Contrôle de la fonction sexuelle chez le jeune taurillon. Elevage Inséminat. 1983;197:3–16. [Google Scholar]
- 58.Menchaca A., Pinczak A., Queirolo D. Storage of ram semen at 5 °C: effects of preservation period and timed artificial insemination on pregnancy rate in ewes. Anim Reprod. 2005;2:195–198. [Google Scholar]
- 59.Salamon S., Maxwell W.M.C. Storage of ram semen. Anim Reprod Sci. 2000;62:77–111. doi: 10.1016/s0378-4320(00)00155-x. [DOI] [PubMed] [Google Scholar]
- 60.Bielanski A. Disinfection procedures for controlling microorganism in the semen and embryos of humans and farm animals. Theriogenology. 2007;68:1–22. doi: 10.1016/j.theriogenology.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 61.Eglesome M.D., Bielanski A., Hare W.C.D., Ruhnke H.L. Studies on inactivation of pathogenic microorganisms in culture media and in bovine semen by photosensitive agents. Vet Microbiol. 1993;35:277–284. doi: 10.1016/0378-1135(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 62.Hussein Z.M., El-Tayeb T., El-Keraby F., Harith M.A. The effect of diode laser and light emitting diode on the bacterial contamination of semen medium for artificial insemination. Biologicals. 2008;36:303–307. doi: 10.1016/j.biologicals.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 63.Lubart R., Friedmann H., Levinshal T., Lavie R., Breitbart H. Effect of light on calcium transport in bull sperm cells. J Photochem Photobiol B. 1992;15:337–341. doi: 10.1016/1011-1344(92)85139-l. [DOI] [PubMed] [Google Scholar]
- 64.Moreno-Millán M., Ocaña-Quero J.M. Preliminary results of the evaluation of the use of clinical laser He–Ne radiation in the process of bovine ‘in vitro fertilization. Bulletin UASVM Vet Med. 2009;66:495. [Google Scholar]
- 65.Almeida-Lopes L., Rigau J., Amaro Zângaro R., Guidugli-Neto J., Jaeger M.M. Comparison of the low level laser therapy effects on cultured human gingival fibroblasts proliferation using different irradiance and same fluence. Lasers Surg Med. 2001;29:179–184. doi: 10.1002/lsm.1107. [DOI] [PubMed] [Google Scholar]
- 66.Karu T.I. Gordon and Breach; Amsterdam, The Netherlands: 1998. The science of low power laser therapy. [Google Scholar]
- 67.Smuk A., Lazaro E., Olson L.P., Lawandy N.M. Random laser action in bovine semen. Opt Commun. 2011;248:1257–1258. [Google Scholar]
- 68.Abdel-Salam Z., Harith M.A. Laser spectrochemical characterization of semen. Talanta. 2012;99:140–145. doi: 10.1016/j.talanta.2012.05.031. [DOI] [PubMed] [Google Scholar]
- 69.Passarella S., Karu T.I. Absorption of monochromatic and narrow band radiation in the visible and near IR by both mitochondrial and non-mitochondrial photoacceptors results in photobiomodulation. Photochem Photobiol B. 2014;140:344–358. doi: 10.1016/j.jphotobiol.2014.07.021. [DOI] [PubMed] [Google Scholar]



