Abstract
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) from a geno-identical matched sibling (MSD) is one of the most successful therapies in patients with non-malignant hematological disorders. This study included 273 patients with severe aplastic anemia (SAA), 152 patients with B-Thalassemia major (BTM), 31 patients with Fanconi’s anemia (FA), 20 patients with congenital immunodeficiency diseases (ID), and 13 patients with inherited metabolic disorders (IMD) allografted from a MSD. In SAA, the 8-year overall survival (OS) of the whole group patients was 74%. OS was significantly better in patients conditioned with fludarabine and cyclophosphamide (Flu/Cy) than in those who received cyclophosphamide and antithymocyte globulin (Cy/ATG) (p = 0.021). Acute graft-versus-host disease (aGVHD) grade II–IV occurred in 15% while chronic GVHD (cGVHD) occurred in 28%. In BTM, the 12-year disease-free survival (DFS) of the whole group of BTM patients was 72.4%. DFS was 74% for peripheral blood stem cell (PBSC) group compared to 64% in the BM stem cell group. The incidence of graft rejection was significantly lower in patients who received PBSC than in those who received BM (9% vs 25%) (p = 0.036). AGVHD grade II–IV and cGVHD occurred in 15% and 12% of the whole group of BTM patients respectively. In FA, the 5-year OS was 64.5%. Graft rejection occurred in 10% of patients. Grade II–IV aGVHD occurred in 16% while cGVHD occurred in 4%. In ID, the 5-year OS was 62%. Graft rejection occurred in two (10%) patients. Three patients (15%) developed grade II–IV aGVHD, 2 of them progressed to secondary cGVHD. In IMD, OS was 46% at 5 years. Graft rejection occurred in 8% of patients. AGVHD grade II–IV occurred in 15% while cGVHD occurred in 14%. In conclusion, Allo-HSCT provides a higher DFS rate over conventional therapies for patients with non-malignant hematological disorders with prolonged survival.
Keywords: Hematopoietic stem cell transplantation, B-thalassemia major, Fanconi’s anemia, Immunodeficiency diseases, Inherited metabolic disorders
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a potentially curative modality for a variety of nonmalignant disorders involving bone marrow (BM) failure and thalassemia [1]. It has been successfully used as a replacement therapy for patients with severe aplastic anemia (SAA), B-thalassemia major (BTM), Fanconi anemia (FA), immunodeficiency diseases (ID) and inherited metabolic disorders (IMD) [1,2].
SAA is characterized by profoundly hypocellular marrow with marked reduction in 2 or 3 peripheral blood parameters [3]. Allo-HSCT is a convincing treatment modality for children and young adults with SAA and best results are achieved from a matched family donor [4]. The expected 5 years OS for patients <20 years old receiving HLA identical sibling HSCT as upfront treatment is 88% and for patients aged 21–50 years 72% [5]. On the other hand, BTM is an inherited disease requiring lifelong red blood cell transfusion to treat the anemia caused by ineffective intramedullary erythropoiesis and enhanced red blood cell destruction in the peripheral circulation [6]. Prognosis is highly dependent on compliance and socio-economic status. Mortality is high due to therapy related complications especially liver fibrosis and heart disease. The best curative modality is replacement of defective marrow by allo-HSCT [7]. FA, an autosomal recessive disorder belonging to the group of chromosomal instability syndrome is characterized clinically by congenital malformations, hypersensitivity to alkylating agents, progressive marrow failure, and predisposition to acute myeloid leukemia [8]. Allo-HSCT has also been established as the only treatment modality that can restore normal hematopoiesis in these patients [1]. ID is a group of inherited disorders characterized by severe impairment of innate and adaptive immune systems, which leads to early death from infectious complications. Replacement of the defective lineage by allo-HSCT from healthy allogeneic donors remains the curative approach for most patients [9]. IMDs and osteopetrosis are a diverse group of diseases arising from genetic defects or osteoclast disorders. Onset in infancy or early childhood is typically accompanied by rapid deterioration and associated with early death. Timely diagnosis and immediate referral to a specialist with discussion of the patient by a multidisciplinary team including a transplant physician are essential steps in management of these disorders [10].
While improved supportive care has extended the life span of patients affected by these diseases, definitive cure is only achieved by allo HSCT. The careful selection of pre-transplant conditioning can significantly reduce early transplant related mortality (TRM), increase the probability of durable engraftment and leads to better survival especially when linked to an HLA identical donor.
The aim of this study was to illustrate and report the outcome of allo-HSCT in different non-malignant hematologic conditions treated at our Institute.
Patients and methods
Patient population
Between May 1997 and April 2012, a total of 489 patients with non-malignant hematological diseases were allografted at Nasser Institute for Research and Treatment, Ministry of Health, Cairo, Egypt, using different conditioning regimens. Allo-HSCT protocols were approved by the institutional review board. Written informed consent was obtained from patients or their parents.
Allo-HSCT
Intermediate-resolution DNA typing by polymerase chain reaction sequence specific oligonucleotides probe (PCR–SSO) for human leukocyte antigen (HLA) class I (HLA-A, -B, -C) and class-II (HLA-DRB1, -DQB1) was performed [11]. ALL donors were siblings or other family members and at least 6/6 HLA matched. PBSC Donors were injected subcutaneously with granulocyte-colony stimulating factor (G-CSF, 10 μg/kg daily for 5 days) and mobilized PBSC was collected the day of last injection. One to 2 apheresis procedures were planned by means of COBE Spectra continuous cell separator (Gambro, Lakewood, CO, USA) using Spin–Nebraska protocol [12]. For BM donors, aspirations were performed under general anesthesia from posterior ileum region. Enumeration of total WCC, MNC and CD34 +ve cells was done by flow cytometry (Coulter EPICS, Coulter electronics, Hialeah, FL, USA) using anti CD34 monoclonal antibody HPCA2 (BD, San Jose, CA, USA). The aim was to collect at least 5 × 108 mononuclear cells (MNC) and/or 3 × 106 viable CD34+ cells/kg recipient’s body weight. The products of PBSC apheresis and BM harvest were infused to patients on the same day of collection (day 0).
Chimerism analysis
To assess engraftment, degree of chimerism in patients was monitored at regular intervals by Fluorescent In-situ Hybridization (FISH) XY chromosome analysis in case of sex mismatch and by PCR for variable number tandem repeats (VNTR) analysis at D+28 and D+56 post-transplant using loci Apo B, YNZ, DIS 80, 33.1, 33.4, 33.6 and H Ras [13].
Conditioning regimen
181 SAA patients received Flu/Cy regimen consisting of cyclophosphamide 50 mg/kg/day (days −5 to −2) and fludarabine 40 mg/m2/day (days −3 to −1). Another 92 SAA patients received Cy/ATG regimen consisting of cyclophosphamide 50 mg/kg/day (days −5 to −2) and antithymocyte globulin (ATG) 10 mg/kg/day (days −5 to −3). BTM patients were classified into Peasaro class I, II and III according to hepatomegaly (⩾2 cm below costal margin), irregular chelation and portal fibrosis on liver biopsy [14]. All BTM patients, whatever their risk class, received Bu/Cy/ATG regimen consisting of busulfan 5 mg/kg/day P.O. for 4 days for patients ⩽8 years or 4 mg/kg/day P.O. for 4 days for patients >8 years and cyclophosphamide 30 mg/kg/day for 4 days in addition to ATG at a total dose of 110 mg/kg divided into 10 doses (5 pre- and 5 post-transplant). FA patients received Flu/Cy/ATG regimen consisting of low-dose cyclophosphamide 5 mg/kg/day for 4 days and fludarabine 25 mg/m2 for 5 days; in addition ATG was administered pre-transplant (5 mg/kg for 4 days) to promote engraftment and post-transplant (2.5 mg/kg days +1, +3, +6 and +11) for additional GVHD prophylaxis. ID patients received Flu/Cy regimen consisting of cyclophosphamide 50 mg/kg/day (days −5 to −2) and fludarabine 40 mg/m2/day (days −3 to −1). Patients with IMD received Bu/Cy regimen consisting of busulfan 5 mg/kg/day P.O. for 4 days in patients <8 years or 4 mg/kg/day P.O. for 4 days for patients >8 years and cyclophosphamide 30 mg/kg/day for 4 days to all patients.
Graft rejection
Primary graft rejection was defined as failure to establish hematopoietic reconstitution of donor-origin after allografting, while secondary graft failure was defined as absolute neutrophil count (ANC) <0.5 × 109/L after initial neutrophil recovery.
GVHD prophylaxis
All patients received cyclosporine A (CSA) at a dose of 3 mg/kg/day IV from day −1 until oral intake was possible then shifted to oral dose 5 mg/kg/day divided on two daily doses and maintained till day 180 then gradually tapered off. Whole-blood CsA concentration was monitored weekly using the fluorescence polarization immunoassay technique, and the dosage was adjusted in order to maintain a trough goal of 150–250 ng/dL. Methotrexate (MTX) was given at a dose of 15 mg/m2 IV on day +1, then 10 mg/m2 on days +3, +6, and +11. For BTM patients MTX was replaced by methylprednisone 2 mg/kg (MP) starting from day −7 till day +4 then tapered gradually over two weeks. For FA patients, MTX was replaced by ATG (as mentioned before).
Supportive care
All patients received antibacterial prophylaxis (by levofloxacine), anti-fungal prophylaxis (by fluconazole), anti-herpes prophylaxis (by acyclovir), and anti-pneumocystis jiroveci prophylaxis (by trimethoprim/sulfamethoxazole) starting from two days before conditioning regimen till the end of immunosuppression. Febrile neutropenia was treated with piperacillin/tazobactam and amikacin. In case of persistent fever, piperacillin/tazobactam was switched to imepenem (or meropenem) with or without the addition of amphotericin-B. Packed red blood cells and platelet transfusions were given to maintain Hb level ⩾8 g/dl and platelet count ⩾10 × 109/L respectively. All blood products were irradiated and filtered for leukocyte depletion.
Hematopoietic recovery
Post-transplant neutrophil and platelet engraftment were defined by three successive days with absolute neutrophilic count (ANC) ⩾0.5 × 109/L and platelet count ⩾20 × 109/L (without transfusion).
Graft rejection
Primary graft rejection was defined as failure to establish hematopoietic reconstitution of donor-origin after allografting, while secondary graft failure was defined as absolute neutrophil count (ANC) <0.5 × 109/L after initial neutrophil recovery.
GVHD assessment and treatment
AGVHD was graded according to “the 1994 Consensus Conference on Acute GVHD Grading” [15]. Classification of cGVHD into mild, moderate, and severe subtypes was performed using the “National Institutes of Health Consensus Development Project Criteria” [16]. Patients were evaluated for cGVHD if they survived for at least 100 days after HSCT. Corticosteroids comprised the first-line therapy for aGVHD (grade II–IV) and extensive cGVHD.
Outcome definitions
Transplant related mortality (TRM) was defined as mortality from any cause directly related to conditioning regimen or due to graft rejection. Overall survival (OS) was calculated from time of transplant till death from any cause. Disease free survival (DFS) was calculated from time from obtaining a clinically documented complete remission till the time of first evidence of relapsed disease.
Statistical analysis
All analyses were performed using the statistical package for social sciences (SPSS) software version 20. Comparison between groups was performed using independent samples t-tests for quantitative variables and p-values <0.05 were considered statistically significant. Survival analysis was done using Kaplan Meier test and survivals of different groups were compared by Log-rank (Mantel-Cox) test. [17].
Results
A total of 489 patients with non-malignant hematological disorders were included in this study; 344 males and 135 females (M/F ratio 2.5:1), including 273 SAA patients; 152 BTM patients; 31 FA patients, 20 patients with congenital ID [11 patients with severe combined immunodeficiency disease (SCID), 4 patients with leukocyte adhesion deficiency (LAD), 2 patients with Chediak–Higashi syndrome, 1 patient with common variable immunodeficiency (CVID), 1 patient with Griscelli syndrome, 1 patient with T-cell defect and 1 patient with Wiskott–Aldrich syndrome (WAS)] and 11 patients with IMD [7 patients with Niemann–Pick disease, 3 patients with adrenoleukodystrophy (ALD), 1 patient with mucopolysaccharidosis, in addition to 2 patients with osteopetrosis]. Table 1 represents major transplantation characteristics of these disease categories.
Table 1.
Major transplantation characteristics of all disease categories.
| No | Age | CD34+ stem cell dose (× 106/kg) | Time to neutrophil engraftment (days) | Time to platelet engraftment (days) | Graft rejection | AGVHD (grade II–IV) | CGVHDa | TRM | |
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD (Range) | Mean ± SD (Range) | Mean ± SD (Range) | Mean ± SD (Range) | No (%) | No (%) | No (%) | No (%) | ||
| SAA | 273 | 19.7 ± 0.54 (1.5–51) | 8.9 ± 0.32 (3.1–24.4) | 13.9 ± 0.36 (10–26) | 14.1 ± 0.61 (8–83) | 3/273 (1%) | 42/273 (15%) | 70/248 (28%) | 60/273 (22%) |
| BTM | 152 | 5.7 ± 0.35 (1.1–23) | 12.6 ± 0.66 (2–49) | 21.4 ± 0.64 (8–69) | 32.8 ± 1.7 (7–134) | 17/152 (11.2%) | 23/152 (15%) | 16/133 (12%) | 28/152 (18%) |
| PB: 9% | |||||||||
| BM: 25% | |||||||||
| (p = 0.036)b | |||||||||
| FA | 31 | 11.7 ± 0.8 (6–26) | 11.6 ± 1.7 (2–56) | 11.1 ± 1.2 (9–26) | 12.3 ± 1.9 (9–45) | 3/31 (10%) | 5/31 (16%) | 1/24 (4%) | 10/31 (32%) |
| ID | 20 | 2.4 ± 0.55 (1–10) | 21.1 ± 3.0 (4.3–45) | 15.4 ± 1.1 (9–22) | 16.4 ± 2.3 (8–40) | 2/20 (10%) | 3/20 (15%) | 2/14 (14%) | 7/20 (35%) |
| IMD | 13 | 3 ± 0.66 (1–7) | 13.4 ± 1.7 (5.8–28.6) | 17.8 ± 2.2 (11–32) | 17.3 ± 1.6 (12–23) | 1/13 (8%) | 2/13 (15%) | 1/7 (14%) | 7/13 (54%) |
Patients were evaluated for cGVHD if they survived for at least 100 days after HSCT.
P value was calculated by independent samples t-tests.
SAA (no = 273)
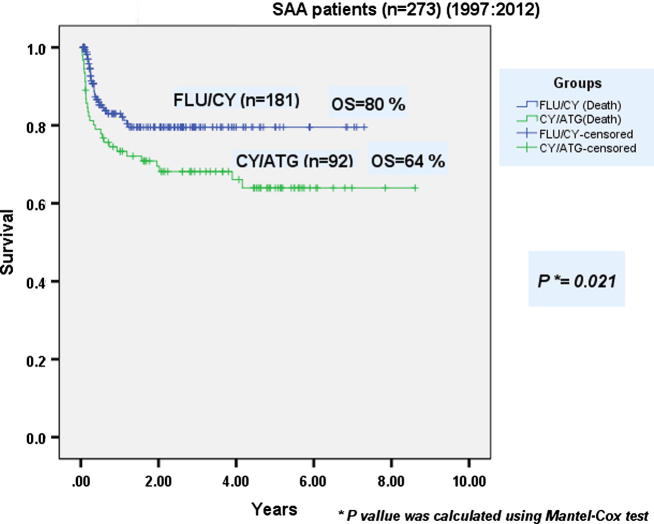
212 males and 61 females were allografted. Mean age at transplantation was 19.7 years (1.5–51). Patients received a mean CD34+ cell dose of 8.9 × 106/kg BW (3.1–24.4). Mean times to neutrophil and platelet engraftment were 13.9 days (10–26) and 14.1 days (8–83) respectively. Graft rejection occurred in 1% of patients. AGVHD grade II–IV occurred in 15% while cGVHD occurred in 28% of patients. The incidence of TRM in the whole group of SAA patients was 22%. Both OS and DFS of the whole group of SAA patients were 74% at 8 years. Major transplant characteristics of Flu/Cy group (no = 181) compared with Cy/ATG group (no = 92) are presented in Table 2. No statistically significant differences between both conditioning groups were observed in terms of mean time to neutrophil engraftment and incidence of extensive cGVHD (p-values = 0.136 and 0.651 respectively). Mean time to platelet engraftment was significantly longer in the Cy/ATG group when compared to Flu/Cy group (p = 0.016). The incidence of TRM in Flu/Cy group was 17%, significantly lower than that of Cy/ATG group (33%) (p = 0.002). After a median follow-up period of 8 years, OS was statistically significantly better in Flu/Cy group than that in the Cy/ATG group of patients (80% vs 64% respectively) (p = 0.021) (Fig. 1).
Table 2.
Major transplant characteristics SAA patients in Flu/Cy group compared with Cy/ATG group.
| FLU/Cy (n = 181) | Cy/ATG (n = 92) | p-valueb | |||
|---|---|---|---|---|---|
| CD34 +ve cells/kg BW (× 106) | Mean | 8.9 ± 0.35 | Mean | 8.5 ± 0.73 | 0.570 |
| Range | 2.9–42 | Range | 2.5–37 | ||
| Time to neutrophil engraftment (days) | Mean | 13.7 ± 0.42 | Mean | 14.9 ± 0.66 | 0.136 |
| Range | 7–42 | Range | 8–43 | ||
| Time to platelet engraftment (days) | Mean | 13.2 ± 0.72 | Mean | 16.6 ± 1.1 | 0.016 |
| Range | 9–83 | Range | 8–55 | ||
| Extensive cGVHDa: no (%) | 36/172 (21%) | 14/76 (18%) | 0.651 | ||
| TRM: no (%) | 30/181 (17%) | 30/92 (33%) | 0.002 | ||
Patients were evaluated for cGVHD if they survived for at least 100 days after HSCT.
p values were calculated by independent samples t-tests.
Fig. 1.
Overall survival of severe aplastic anemia patients after allogeneic hematopoietic stem cell transplantation: Flu/Cy group compared with Cy/ATG group.
BTM (no = 152)
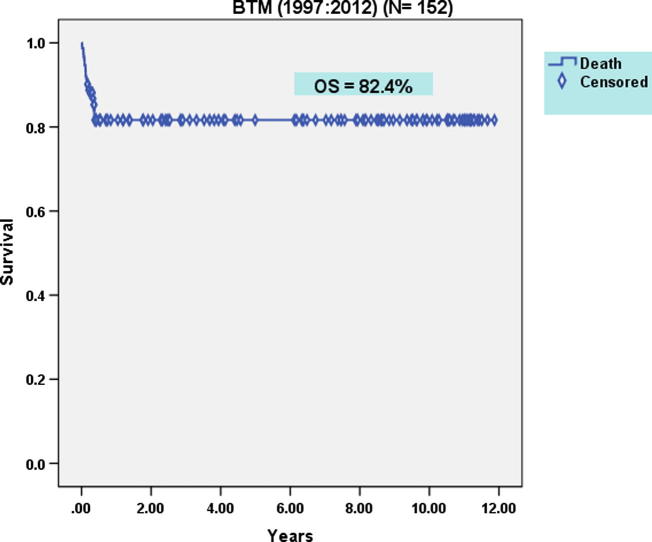
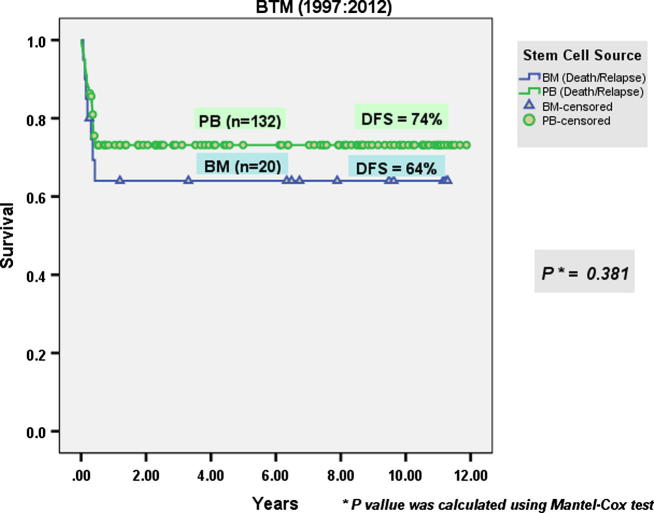
92 males and 50 females were allografted. Mean age at transplantation was 5.7 years (1.1–23). Twenty-six patients (17%) had Pesaro class I, 103 (68%) had class II and 23 (15%) had class III at the time of transplantation. 132 patients received PBSC while 20 patients received BM grafts. PBSC patients received a mean CD34+ cell dose of 8.9 × 106/kg BW (2–49). Mean times to neutrophil and platelet engraftment were 21.4 days (8–69) and 32.8 days (7–134) respectively. The incidence of graft rejection was significantly lower in patients who received PBSC than in those who received BM grafts (9% vs 25%) (p = 0.036). AGVHD grade II–IV occurred in 15% while cGVHD occurred in 12% of the whole group of patients. The incidence TRM of the whole group of BTM patients was 18%. After a median follow-up period of 12 years, the OS of the whole group of BTM patients was 82.4% (Fig. 2). DFS of the whole group of BTM patients was 72.4% [74% in the PBSC transplantation group compared to 64% in the BM stem cell transplantation group (p = 0.381)] (Fig. 3). This finding may be attributed to the higher incidence of graft rejection in BM group compared to PBSC group.
Fig. 2.
Overall survival of the whole group of B-thalassemia patients after allogeneic hematopoietic stem cell transplantation.
Fig. 3.
Disease-free survival of B-thalassemia in PBSC transplantation group compared with BM stem cell transplantation group.
FA (no = 31)
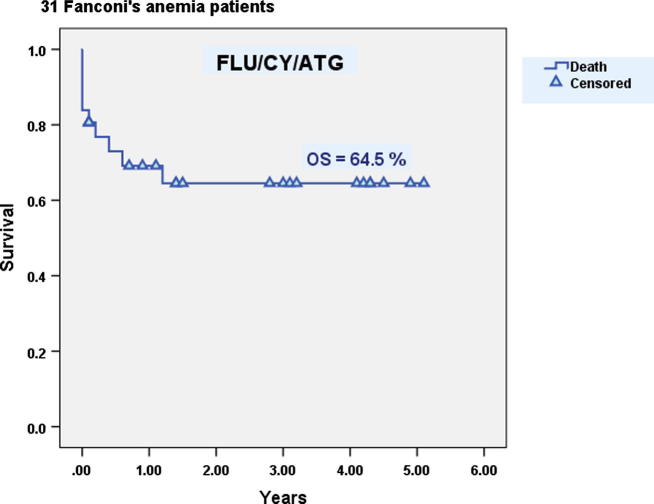
17 males and 14 females with FA were allografted. Mean age at transplantation was 11.7 years (6–26). Patients received a mean CD34+ cell dose of 11.6 × 106/kg BW (2–56). Mean times to neutrophil and platelet engraftment were 11.1 days (9–26) and 12.3 days (9–45) respectively. Graft rejection occurred in 10% of patients. AGVHD grade II–IV occurred in 16% while cGVHD occurred in 4%. The incidence TRM was 32%. At five years, the DFS was 52% while the OS was 64.5% (Fig. 4).
Fig. 4.
Overall survival of Fanconi’s anemia patients after allogeneic hematopoietic stem cell transplantation.
ID (no = 20)
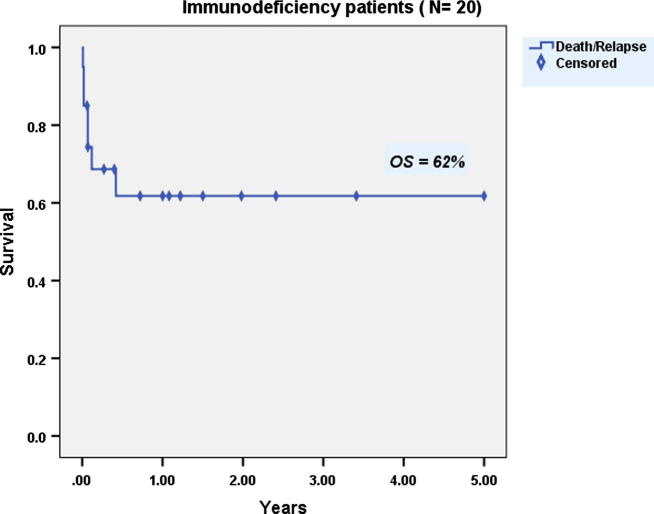
13 males and 7 females with ID were allografted. Mean age at transplantation was 2.4 years (1–10). Patients received a mean CD34+ cell dose of 21.1 × 106/kg BW (4.3–45). Mean times to neutrophil and platelet engraftment were 15.4 days (9–22) and 16.4 days (8–40) respectively. Graft rejection occurred in two (10%) patients. Three patients (15%) developed grade II–IV aGVHD, 2 of them progressed to secondary cGVHD. The incidence TRM was 35%. At 5-years, both DFS and OS were 62% (Fig. 5).
Fig. 5.
Overall survival of patients with immunodeficiency diseases after allogeneic hematopoietic stem cell transplantation.
IMD (n = 13)
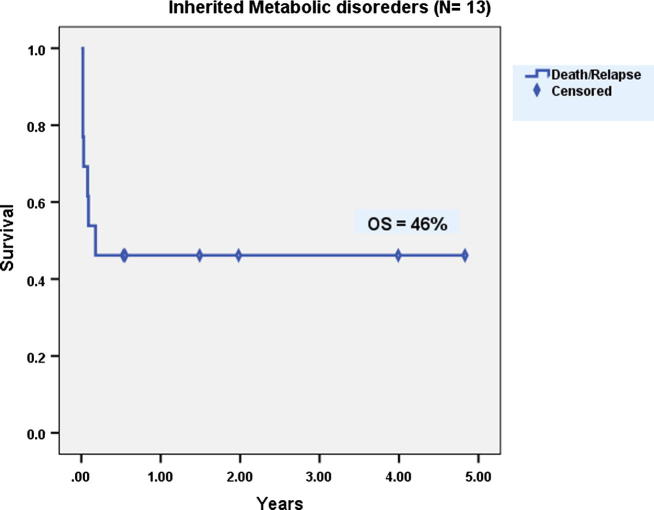
10 males and 3 females with IMD were allografted. Mean age at transplantation was 3 years (1–7). Patients received a mean CD34+ cell dose of 13.4 × 106/kg BW (6–29). Mean times to neutrophil and platelet engraftment were 17.8 days (11–32) and 17.3 days (12–23) respectively. Primary graft failure occurred in one patient only (7.7%). AGVHD grade II–IV occurred in 15% while cGVHD occurred in 14%. At 5-years, both DFS and OS were 46% (Fig. 6).
Fig. 6.
Overall survival of patients with inherited metabolic disorders after allogeneic hematopoietic stem cell transplantation.
Discussion
Allo-HSCT is considered a valuable therapeutic option for a variety of non-malignant hematopoietic disorders. Recently, there is an increasing interest in the use of G-CSF mobilized PBSCs (G-PBSCs) as a source of stem cells for allo-HSCT. G-PBSCs contain an increased number of CD34+ hematopoietic progenitor cells and an approximately 10-fold increased numbers of T-cells compared with marrow [18]. With the infusion of a larger number of donor cells, G-PBSCs could potentially decrease the risk of graft rejection in patients with SAA but also may increases the incidence of severe cGVHD. Cyclophosphamide and ATG (Cy/ATG) can be considered a standard protocol for conditioning patients with SAA, with excellent outcomes reported by several groups including successful engraftment rates exceeding 95%, low rates (<30%) of both grade II–IV aGVHD and cGVHD; and an excellent long-term OS (at 15-year) exceeding 85% [19]. But due to the high cost of ATG in our country, the use of another, cheaper yet, still potent and effective immunosuppressor like fludarabine was considered. Most of our SAA patients received a Flu/Cy conditioning without ATG and showed a statistically significant better OS than Cy/ATG group (80% vs 64% respectively) (p = 0.021). Fludarabine based regimen was sufficiently immunosuppressive for engraftment. Moreover, comparable rates of graft rejection were encountered due to selection of patients earlier after diagnosis for transplantation before allosensitization by frequent blood transfusions and by the use of an HLA matched related family donor. Champlin et al. in their randomized trial involving 134 SAA patients did not show any significant benefit from addition of ATG in conditioning regimen to standard cyclophosphamide (200 mg/kg), but the study was underpowered [20]. However, recent large registry based analysis showed that addition of ATG resulted in lower incidence of GVHD and improved OS (84% vs 74%) [21]. Acute GVHD grade II–IV occurred in 15% of our patients while chronic GVHD occurred in 28%. These results are comparable to others although we used PBSC as a source of stem cells. Results among 94 patients conditioned with Cy/ATG and treated with CSA and MTX after transplant showed overall cumulative incidence of grade II–IV acute GVHD of 29% and chronic GVHD of 32% [2].
B-thalassemia is the most common hereditary hemolytic anemia in Egypt, with a carrier state varying between 6% and 10% [22]. Considering the chronicity of the disease, the huge psychological, social and financial burden on both the patient and his family, as well as the different complications associated with the conventional therapy available, it becomes clear that we were interested in initiating a transplant program in Egypt for this entity in an effort to offer a radical cure or at least a stable chimeric state that makes it possible to avoid regular blood transfusion and chelation. It is obvious that the strategy for thalassemia in the developing world should be primarily preventive. However, there is no doubt that in a country like Egypt, the economic advantage of SCT for thalassemia is compelling. The major reason for transplant failure is graft rejection due to previous polytransfusions. As most of our patients are referred relatively late to the transplant center owing to logistic and administrative problems, we had to look for an innovative approach for such candidates. In an attempt to overcome the problem of graft rejection, we have added antithymocyte globulin to the conditioning regimen (using Bu/Cy/ATG) to increase immunosuppression of recipients, and we also shifted our program from allogeneic BMT to allogeneic PBSC to take advantage of the higher T-cell content of PBSC grafts, which in turn is known to have a graft-letting effect [23]. With these changes, the OS and DFS rates for BTM patients post-allogeneic SCT at our center were 82.4% and 72.4% respectively, at a median follow-up of 12 years. DFS for PBSC transplantation group (no = 132) was 74% compared to 64% in the BM stem cell transplantation group (no = 20) (p = 0.381). Results on almost 1400 transplants performed for BTM after the year 2000 in 128 centers in 23 countries showed that 5 year OS and DFS were 89% and 79%, respectively [24].
All our FA patients were allografted using Flu/Cy/ATG as conditioning regimen from a matched sibling donor. Twenty patients (64.5%) had sustained engraftment and were transfusion-independent at median follow-up of 13.5 months. One patient (3.2%) had primary graft failure and two patients (6.4%) developed secondary graft failure. Five patients (16%) developed aGVHD and 1 patient (4%) developed cGVHD. The 5 years DFS and OS were 52% and 64.5% respectively. Pasquini et al. on behalf of CIBMTR compared the early outcome of HSCT using non-irradiation conditioning regimens to the outcome of regimens with irradiation for FA patients transplanted with HLA identical sibling donors. Hematopoietic recovery, acute and chronic GVHD and mortality were similar after the two regimens [25]. The 5-year probability of OS was 78% after irradiation and 81% after non-irradiation regimen. We showed here an OS of 64.5% using Flu/Cy/ATG. Many reports showed successful HSCT using non-radiotherapy based conditioning regimens in patients with FA [26–29]. In light of these data, the most frequently used conditioning regimen for HLA-identical sibling HSCT for FA is currently low dose cyclophosphamide, fludarabine and ATG.
IDs are often accompanied by life threatening infections. The OS for allo-HSCT in ID is now probably in excess of 90% [30]. Recognition of the specific molecular defect may alter the approach to HSCT for IDs. Patients with adenosine deaminase (ADA) deficient SCID will develop adequate immunological reconstitution following an unconditioned infusion from HLA-identical sibling stem cells, whereas those with other forms of SCID such as recombinant activating gene (RAG) deficiency will require chemotherapy conditioning to achieve stem cell engraftment and immunological reconstitution. The major difference with non-SCID patients e.g. Leukocyte adhesion deficiency (LAD) in comparison with SCID patients is the usual requirement for a conditioning regimen to achieve engraftment [31]. Careful selection of pre-transplant conditioning can significantly reduce early transplant related mortality in ID and increase the probability of durable engraftment. We used a Flu/Cy conditioning regimen in all our ID patients. Mean age at transplantation was 2.4 years, which contributed to better outcome. Most of the patients were infused with mega doses of CD34 (mean = 21.1 × 106/kg), which resulted in an incidence of graft rejection of only 10%. Acute GVHD occurred in 15%, while cGVHD developed in 14% of patients. At 5 years, OS of our ID patients was 62%. According to the European series of 475 ID patients collected between years 1968 and 1999, survival rates for all ID phenotypes combined ranged from 80% with matched related donors (MRD) to 50% with haplo-identical donors, with transplant from unrelated donors at approximately 70% [32,33]. CIBMTR data from 1990 to 2004 (748 ID patients) reveal very similar rates of survival according to donor source. More recent experience in 30 patients from Toronto, Canada, using matched unrelated donors (MUD) and myeloablative conditioning (Bu/Cy/ATG) has resulted in 76% OS [30].
Conclusions
The central role of allo-HSCT in non-malignant hematologic disorders is fully established. The decision to allo-HSCT is highly individualized. Age, clinical status, willingness to undergo treatment, donor availability, capability and compliance to adhere to the appropriate transfusion/chelation regimen, quality of life and resources must all be considered. Efforts aimed at early diagnosis favorably affect the outcome of transplantation. The use of PB as a source of stem cells did not affect incidence of cGVHD especially with the selection of a genotypically MSD and the most appropriate conditioning regimen.
Conflict of interest
The authors have declared no conflict of interest.
Acknowledgements
We acknowledge the work of the BMT lab at the National Cancer Institute, Cairo University and we thank them for their efforts and support.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Sevilla J., Fernandez-Plaza S., Diaz M.A., Madero L. Hematopoietic transplantation for bone marrow failure syndromes and thalassemia. Bone Marrow Transpl. 2005;35:S17–S21. doi: 10.1038/sj.bmt.1704838. [DOI] [PubMed] [Google Scholar]
- 2.Storb R., Blume K.G., O’Dennell M.R., Chauncey T., Forman S.J., Deeg H.J. Cyclophosphamide and antithymocyte globulin to condition patients with aplastic anemia for allogeneic marrow transplantation: the experience in four centers. Biol Blood Marrow Transpl. 2001;7:39–44. doi: 10.1053/bbmt.2001.v7.pm11215697. [DOI] [PubMed] [Google Scholar]
- 3.Camitta B.M., Thomas E.D., Nathan D.G., Santos G., Gordon-Smith E.C., Gale R.P. Severe aplastic anemia: a prospective study of the effect of early marrow transplantation on acute mortality. Blood. 1976;48:63–70. [PubMed] [Google Scholar]
- 4.Korthof E.T., Bekassy A.N., Hussein A.A. Management of acquired aplastic anemia in children. Bone Marrow Transpl. 2013;48:191–195. doi: 10.1038/bmt.2012.235. [DOI] [PubMed] [Google Scholar]
- 5.Armand P., Antin J.H. Allogeneic stem cell transplantation for aplastic anemia. Biol Blood Marrow Transpl. 2007;13:505–516. doi: 10.1016/j.bbmt.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Weatherall D.J., Clegg J.B. Thalassemia – a global public health problem. Nat Med. 1996;2:847–849. doi: 10.1038/nm0896-847. [DOI] [PubMed] [Google Scholar]
- 7.Lucarelli G., Andreani M., Angelucci E. The cure of thalassemia by bone marrow transplantation. Blood Rev. 2002;16:81–85. doi: 10.1054/blre.2002.0192. [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg P.S., Huang Y., Alter B.P. Individualized risks of adverse events in patients with Fanconi anemia. Blood. 2004;104(2):350–355. doi: 10.1182/blood-2004-01-0083. [DOI] [PubMed] [Google Scholar]
- 9.Dovorak C.C., Cowan M.J. Hematopoietic stem cell transplantation for primary immunodeficiency disease. Bone Marrow Transpl. 2008;41:119–126. doi: 10.1038/sj.bmt.1705890. [DOI] [PubMed] [Google Scholar]
- 10.Boelens J.J., Prasad V.K., Tolar J., Wynn R.F., Peters C. Current international perceptional perspectives on hematopoietic stem cell transplantation for inherited metabolic disorders. Pediatr Clin North Am. 2010;57:123–145. doi: 10.1016/j.pcl.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Saiki R.K., Walsh P.S., Levenson C.H., Erlich H.A. Genetic analysis of amplified DNA with immobilized sequence specific oligonucleotide probes. Proc Natl Acad Sci USA. 1989;86:6230–6234. doi: 10.1073/pnas.86.16.6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kessinger A., Armitage J.O., Landmark J.D., Smith D.M., Weisenburger D.D. Autologous peripheral hematopoietic stem cell transplantation restores hematopoietic function following marrow ablative therapy. Blood. 1998;71:723–727. [PubMed] [Google Scholar]
- 13.Kamel A., Mossallam G., Mahmoud H.K., Hamdy N., El-Haddad A., Fahmy O. Variable number tandem repeat polymorphism as a tool of chimerism detection in allogeneic stem cell transplantation. J Egypt Nat Cancer Inst. 2002;14(4):275–282. [Google Scholar]
- 14.Lucarelli G., Galimberti M., Polchi P., Angelucci E., Baronciani D., Giardini C. Bone marrow transplantation in patients with thalassemia. New Engl J Med. 1990;332:417–421. doi: 10.1056/NEJM199002153220701. [DOI] [PubMed] [Google Scholar]
- 15.Przepiorka D., Weisdorf D., Martin P., Klingemann H.G., Beatty P., Hows J. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transpl. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 16.Filipovich A.H., Weisdorf D., Pavletic S., Socie G., Wingard J.R., Lee S.J. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: 1. Diagnosis and staging working group report. Biol Blood Marrow Transpl. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 17.IBM SPSS Statistics for Windows. Version 20.0. Armonk (NY): IBM Corp; 2011.
- 18.Korbling M., Anderlini P. Peripheral blood stem cell versus bone marrow allotransplantation: does the source of hematopoietic stem cells matter? Blood. 2001;98(10):2900–2908. doi: 10.1182/blood.v98.10.2900. [DOI] [PubMed] [Google Scholar]
- 19.Storb R., Etzioni R., Anasetti C., Appelbaum F.R., Buckner C.D., Bensinger W. Cyclophosphamide combined with antithymocyte globulin in preparation for allogeneic marrow transplants in patients with aplastic anemia. Blood. 1994;84:941–949. [PubMed] [Google Scholar]
- 20.Champlin R.E., Perez W.S., Passweg J.R., Klein J.P., Camitta B.M., Gluckman E. Bone marrow transplants for aplastic anemia: a randomized controlled study of conditioning regimens. Blood. 2007;109:4582–4585. doi: 10.1182/blood-2006-10-052308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bacigalupo A., Socie G., Schrezenmeier H., Tichelli A., Locasciulli A., Fuehrer M. Matched sibling transplants for aplastic anemia: survival advantage for marrow vs peripheral blood transplants in all age groups. Blood. 2010;116(21) doi: 10.3324/haematol.2011.054841. Abstract 523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Beshlawy A., Ragab L., Hussein I., El-Tagui M. Genotypes, phenotypes in beta-thalassemia and sickle cell anemia in Egypt. JAC. 1995;6:147. [Google Scholar]
- 23.Mahmoud HK, El-Haddad AA, Samra MA, El-Emary MA, Kamel AM, Beelen DW, et al. Allogeneic peripheral stem transplantation in thalassemia. Lectures to the fourth Fresenius Satellite Symposium, Current trends for the treatment of hemoglobinopathies with focus on Thalassemia, on the occasion of the 30th EBMT, March 2004, Barcelona, Spain.
- 24.Angelucci E, Baronciani D. HSCT for children and adolescents: hemoglobinopathies. In: Apperley J, Carreras E, Gluckman E, et al., editors. The EBMT handbook. 6th ed. Haematopoietic stem cell transplantation. Italy: Forum Service Editore; 2012. p. 584–96.
- 25.Pasquini R., Carreras J., Pasquini M.C., Camitta B.M., Fasth A.L., Hale G.A. HLA matched sibling hematopoietic stem cell transplantation for Fanconi anemia: comparison of irradiation and non-irradiation containing regimens. Biol Blood Marrow Transpl. 2008;14:1141–1147. doi: 10.1016/j.bbmt.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Motwani J., Lawson S.E., Darbyshire P.J. Successful HSCT using nonradiotherapy-based conditioning regimens and alternative donors in patients with Fanconi anemia – experience in a single UK center. Bone Marrow Transpl. 2005;36:405–410. doi: 10.1038/sj.bmt.1705071. [DOI] [PubMed] [Google Scholar]
- 27.George B., Mathews V., Shaji R.V., Srivastava V., Srivastava A., Chandy M. Fludarabine-based conditioning for allogeneic stem cell transplantation for multiply transfused patients with Fanconi’s anemia. Bone Marrow Transpl. 2005;35:341–343. doi: 10.1038/sj.bmt.1704785. [DOI] [PubMed] [Google Scholar]
- 28.Maschan A.A., Trakhtman P.E., Balashov D.N., Shipicina I.P., Skorobogatova E.V., Skvortsova Y.V. Fludarabine, low-dose busulfan and antithymocyte globulin as conditioning for Fanconi anemia patients receiving bone marrow transplantation from HLA-compatible related donors. Bone Marrow Transpl. 2004;34:305–307. doi: 10.1038/sj.bmt.1704580. [DOI] [PubMed] [Google Scholar]
- 29.Ayas M., Al-Jefri A., Al-Mahr M., Rifai S., Al-Seraihi A., Tbakhi A. Stem cell transplantation for patients with Fanconi anemia with low-dose cyclophosphamide and antithymocyte globulins without the use of radiation therapy. Bone Marrow Transpl. 2005;35:463–466. doi: 10.1038/sj.bmt.1704787. [DOI] [PubMed] [Google Scholar]
- 30.Grunebaum E., Mazzolari E., Porta F., Dallera D., Atkinson A., Reid B. Bone marrow transplantation for severe combined immune deficiency. JAMA. 2006;295:508–518. doi: 10.1001/jama.295.5.508. [DOI] [PubMed] [Google Scholar]
- 31.Gennery A.R., Slatter M.A., Grandin L., Taupin P., Cant A.J., Veys P. Transplantation of hematopoietic stem cells and long-term survival for primary immunodeficiencies in Europe: entering a new century, do we do better? J Allergy Clin Immunol. 2010;126:602–610. doi: 10.1016/j.jaci.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 32.Antoine C., Muller S., Cant A., Cavazzana-Calvo M., Veys P., Vossen J. Long-term survival and transplantation of hemopoietic stem cells for immunodeficiencies: report of the European Experience 1968–99. Lancet. 2003;361:553–560. doi: 10.1016/s0140-6736(03)12513-5. [DOI] [PubMed] [Google Scholar]
- 33.Gennery A.R., Slatter M.A., Grandin L., Taupin P., Cant A.J., Veys P. Transplantation of hematopoietic stem cells and long term survival for primary immunodeficiencies in Europe: entering a new century, do we do better? J Allergy Clin Immunol. 2010;126:602–610. doi: 10.1016/j.jaci.2010.06.015. [DOI] [PubMed] [Google Scholar]