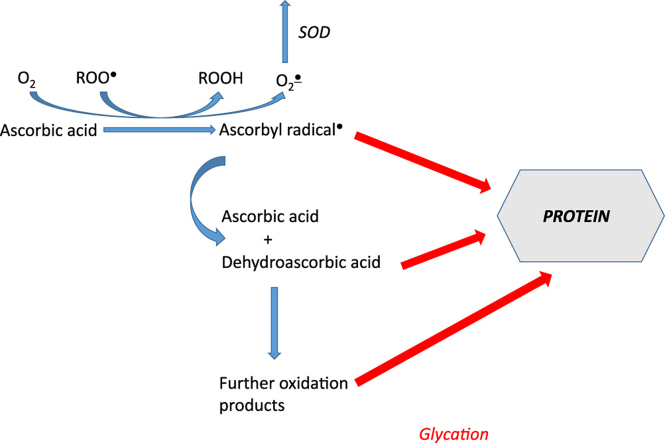
Abstract
Ascorbic acid (AA) has been reported to be both pro-and antiglycating agent. In vitro, mainly proglycating effects of AA have been observed. We studied the glycation of bovine serum albumin (BSA) induced by AA in vitro. BSA glycation was accompanied by oxidative modifications, in agreement with the idea of glycoxidation. Glycation was inhibited by antioxidants including polyphenols and accelerated by 2,2′-azobis-2-methyl-propanimidamide and superoxide dismutase. Nitroxides, known to oxidize AA, did not inhibit BSA glycation. A good correlation was observed between the steady-state level of the ascorbyl radical in BSA samples incubated with AA and additives and the extent of glycation. On this basis we propose that ascorbyl radical, in addition to further products of AA oxidation, may initiate protein glycation.
Keywords: Antioxidant, Ascorbic acid, Bovine serum albumin, Ascorbyl free radical, Glycation
Graphical abstract
Highlights
-
•
Ascorbic acid (AA) induced glycation of bovine serum albumin (BSA) in vitro.
-
•
Antioxidants, including polyphenols, inhibited glycation.
-
•
Nitroxides, known to oxidize AA, did not protect from glycation.
-
•
BSA glycation was accelerated by AAPH and superoxide dismutase.
-
•
Good correlation was found between the level of ascorbyl radical and extent of glycation.
-
•
We postulate that ascorbyl radical is able to induce protein glycation.
Introduction
One of unavoidable posttranslational protein modifications is the non-enzymatic glycosylation (glycation). Protein glycation is initiated by a nucleophilic addition reaction between the free amino group of a protein, lipid or nucleic acid and the carbonyl group of a reducing saccharide or aldehyde. This reaction forms a reversible Schiff base, which rearranges over a period of days to generate Amadori products. The Amadori products undergo dehydration and rearrangements followed by other reactions such as cyclization, oxidation, and dehydration to form more stable Advanced Glycation End Products (AGEs) [1,2].
Many substances affect protein glycation, some promoting and some inhibiting this process. One of such compounds is ascorbic acid (AA), a key exogenous non-enzymatic antioxidant present in plasma and within cells. In the general population the assumed optimal plasma AA concentration of 50 µM, as proposed by a consensus conference, can be achieved by the intake of 100 mg of vitamin C per day, which is the new recommendation of the Austrian, German, and Swiss Nutrition Societies [3]. AA has been reported to be both an anti- and proglycating agent. Numerous studies have demonstrated mainly pro-glycating activity of AA in in vitro systems and mainly anti-glycating activity of this compound in vivo [4–11].
A simple explanation of these divergent results implies that AA oxidation products i.e. dehydroascorbic acid (DHA) and compounds formed by its decomposition, and not AA itself are the glycating agents in AA-containing systems. Unlike AA, DHA is unstable both in the absence and in the presence of oxygen, and is a reactive electrophile, which generates further reactive degradation products over time in solution. These electrophilic products react with nucleophiles on proteins, specifically lysinyl and arginyl residues, resulting in structurally deleterious, non-enzymatic modifications of proteins. Increases in DHA degradation have been involved in the etiology of a variety of diseases, including age-related cataract [9,12], diabetes [13] and Alzheimer's disease [14]. These diseases have all been associated with increases in ROS production and protein glycation.
Although glycation of long-lived proteins is the most important from the physiological point of view, the effect of glycation on shorter-living proteins like plasma albumin is also of interest as their properties and functions may be affected by this process. Many in vitro glycation experiments have been done on human serum albumin and bovine serum albumin (BSA) which has high (76%) sequence homology to human serum albumin. The glycation of albumin induces several structural and functional modifications, including alterations in ligand binding [15,16].
We have found previously that AA enhanced BSA glycation induced by sugars, especially glucose, and induced glycation itself [17]. The aim of the present study was the characterization of the AA-induced glycation of BSA and of the effects of various compounds on this process.
Materials and methods
Materials
All basic reagents were from Sigma-Aldrich Company (Poznań, Poland) unless indicated otherwise. Genistein, 4-hydroxycinnamic acid, naringin, quercetin and genkwanin were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Sample preparation
BSA (purity of 96%) was dissolved in 0.1 M sodium phosphate buffer, pH 7.4, at a concentration of 0.09 mM. AA was used at a concentration of 1 mM. The samples without or with selected additives were incubated in closed vials at a temperature of 37 °C for 6 days with 1 mM sodium azide as a preservative. The following water-soluble additives were used: 0.05, 0.1, 0.2, 0.5 and 1 mM nitroxides: 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO), 4-hydroxy-TEMPO and 4-carboxy-TEMPO; oxidants: 0.1 mM H2O2, 5 mM 2,2′-azobis-2-methyl-propanimidamide hydrochloride (AAPH) and 1 mM FeCl3; antioxidants (1 mM): captopril, tiron and trolox; metal chelators: ethylenediminetetraacetic acid (EDTA), ethylene glycol tetraacetic acid (EGTA), diethylenetriaminepentaacetic acid (DTPA) and nitrilotriacetic acid (NTA); organic acids: 1-cyano-4-hydroxycinnamic acid, 4-hydroxycinnamic acid; polyphenols: caffeic acid, ellagic acid, ferulic acid, gallic acid and propyl gallate. Genistein, genkwanin, naringin, quercetin and rutin were dissolved in dimethylsulfoxide (DMSO) so the level of glycoxidation found in samples containing AA and DMSO was used as a reference in these cases. The above concentrations of selected anti-glycating compounds had been applied in our earlier in vitro experiments [17–20].
In studies of the effects of the effects of addition of superoxide dismutase (SOD) and catalase (CAT) on AA-induced BSA glycation, 10 µg/ml SOD and 10 µg/ml CAT were used.
AGE measurements
AGEs are a heterogeneous group of compounds with a characteristic fluorescence. AGEs were estimated by assessing the formation of AGE-derived fluorescence, termed glycophore, using the spectrofluorimetric method according to Henle et al. [21] and Münch et al. [22] at the excitation and emission wavelengths of 325 and 440 nm, respectively. Two hundred microliters of aliquots were applied to a 96-well plate. In order to check the validity of results based on the assay of fluorimetric indices of BSA oxidative damage, the AGEs content was also evaluated by enzyme-linked immunosorbent assay (ELISA) Kit for Advanced Glycation End Product (USCN Life Science Inc., Product no. CEB353Ge), according to the instruction of the manufacturer. Increase in the AGE content in samples incubated with AA only (in the absence of any potential protectors) with respect to a sample incubated with AA only or AA with DMSO (for compounds introduced from DMSO solutions) was assumed as 100%; the increase in samples containing selected anti-glycating protector was expressed as % of this value.
Content of dityrosine, kynurenine, and N′-formylkynurenine
The levels of dityrosine, kynurenine, N′-formylkynurenine and, to a lesser extent, kynurenine fluorescence are markers of protein oxidation [23]. The content of dityrosine, kynurenine, N′-formylkynurenine and kynurenine was estimated on the basis of their characteristic fluorescence at the wavelengths of 325/440 nm, 330/415 nm, 325/434 nm and 365/480 nm, respectively [24]. Two hundred microliters of aliquots were applied to a 96-well plate.
Content of amyloid cross-β structure
Formation of amyloid cross-β structure was measured using thioflavin T [25,26]. Briefly, 5 μl of 640 µM thioflavin T in 0.1 M sodium phosphate buffer, pH 7.4, were added to 95 μl of a sample. The fluorescence intensity was measured at excitation/emission wavelengths of 435/485 nm after 1-h incubation at room temperature. Fluorescence measurements were made in an Infinite 200 PRO multimode reader (Tecan Group Ltd., Männedorf, Switzerland).
Electron paramagnetic resonance (EPR) spectroscopy
ESR spectra of samples at “time 0” (ca 30 min after formulation of the incubation media) and after 6 days were analysed using a Bruker FT-EPR spectrometer ELEXSYS E580 (Teaching Center of Microelectronics and Nanotechnology, University of Rzeszów). 50 µl of each sample was introduced into a capillary tube for EPR measurement (non-heparinized microhematocrit tubes ~75 μL; 1.55×75 mm; Medlab Products, Raszyn, Poland). Sample capillaries were placed into a quartz EPR sample tube and centered in a standard rectangular microwave cavity under critical coupling conditions. The spectrometer operated at X-band (9.850537 GHz). The following settings were used: central field, 3507.00 G; modulation amplitude, 0.3 G; modulation frequency, 100 kHz; microwave power, 94.64 mW; power attenuation 2.0 dB; scan range, 50 G; conversion time, 25 ms; sweep time, 25.6 s. Two scans were typically accumulated to intensify the signals observed. The spectra were recorded and analysed using Xepr software. The signal was integrated twice to determine its area, and thus the concentration of the radical.
Statistical analysis
All the experiments were done at least in triplicate. Data were shown in the form of mean values and standard deviations. The statistical analysis of the data was performed using STATISTICA software package (version 10, StatSoft Inc. 2010, Tulsa, OK, USA, www.statsoft.com). Differences between means were analysed using Student's t–test for independent samples and were considered significant or highly significant at ▲p-values <0.05, #p-values <0.01 and *p-values< 0.001.
Results and discussion
Ascorbic acid is present in blood plasma at relatively low concentrations (about 50 µM) [3,27] but can be accumulated in some cells expressing SVCT1 or SVCT2 transporter up to millimolar concentrations [28,29]. We chose an intermediate concentration of 1 mM for routine studies of the AA-induced glycation in vitro.
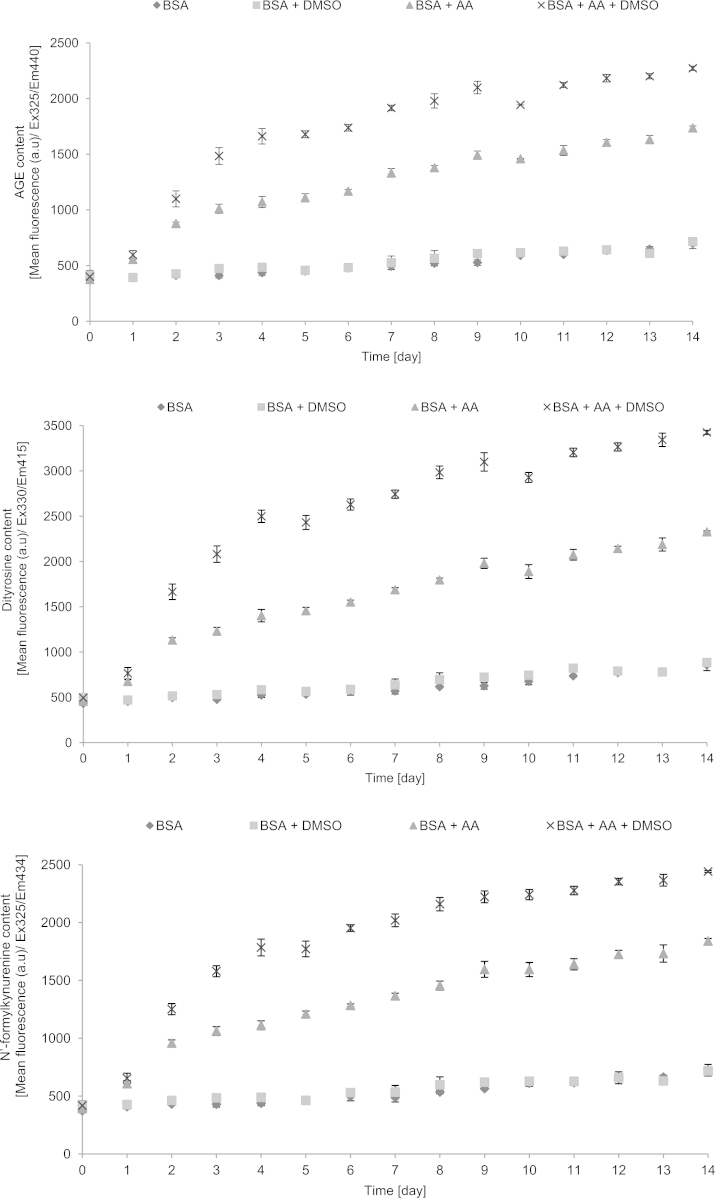
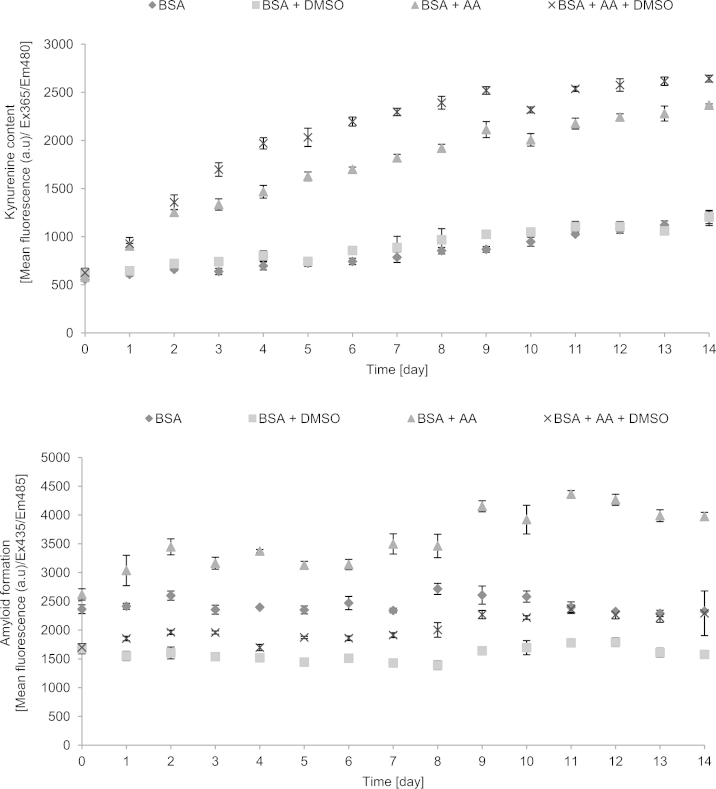
Studies of the kinetics of AA-induced AGE formation showed a hyperbolic-type course of the reaction, reaching saturation after several days. The increase in the level of AGEs was parallelled by increases in the levels of dityrosine, N′-formylkynurenine and kynurenine, indicating that protein glycation was accompanied by oxidative changes, in agreement with the idea of glycoxidation. Interestingly, DMSO enhanced the rate of AA-induced glycoxidation. The measurements of the formation of amyloid cross-β structure were less reproducible showing a high day-to-day variation. DMSO inhibited rather than enhanced amyloid cross-β structure formation (Fig. 1). On the basis of kinetic data, the incubation time of 6 days was chosen for the routine studies of the effects of additives on the initial stage BSA glycoxidation.
Fig. 1.
Kinetics of glycoxidation of BSA by ascorbic acid as measured by fluorimetric parameters of protein modification.
Glycoxidation of BSA by AA was enhanced by SOD (unlike glycation induced by glucose, fructose or ribose) [17] but not affected by CAT (Table 1).
Table 1.
Effect of additives on fluorimetric parameters of glycoxidative modifications of BSA. Mean+SD.
| Additive | AGE | Dityrosine | N′-formylkynurenine | Kynurenine | Thioflavin T |
|---|---|---|---|---|---|
| BSA | 7.19 ±4.11* | 4.99±2.74* | 7.18±4.12* | 9.26 ±4.79* | 12.06±5.47* |
| BSA+ascorbic acid, no additive | 100 | 100 | 100 | 100 | 100 |
| DMSO | 157.86±11.33* | 185.23±10.62* | 166.92±13.12* | 140.60±9.14Δ | 46.93±14.48Δ |
| Antioxidants | |||||
| Captopril 1 mM | 80.04±6.15Δ | 84.22±5.78Δ | 86.37± 6.35# | 105.51±7.62 | 77.06±11.53# |
| Tiron 1 mM | 47.00±7.15* | 48.45±10.40Δ | 40.03±5.67* | 100.56±3.23 | 136.80±24.19 |
| Trolox 1 mM | 46.23 ±0.38* | 35.35±0.52* | 44.07±1.48* | 110.14±1.41* | 35.59±26.85# |
| Metal chelators | |||||
| EDTA 1 mM | 126.99±6.04Δ | 135.29±7.76Δ | 130.02±7.38Δ | 134.79±7.81Δ | 186.99±15.27* |
| EGTA 1 mM | 101.81±2.65 | 102.72±5.81 | 105.25±8.94 | 95.68±6.53 | 73.25±6.04Δ |
| DTPA 1 mM | 145.12±8.08* | 156.67±5.78* | 149.57±6.02* | 148.85±16.85Δ | 31.93±39.78# |
| NTA 1mM | 110.41±12.98 | 119.76±12.84 | 113.27±10.90 | 111.70±11.36 | 176.30±31.27# |
| Organic acids | |||||
| 1-Cyano-4-hydroxycinnamic acid 1 mM | 4.24±0.20* | 3.07±0.16* | 3.32±0.47* | 9.71±0.81* | 64.01±20.36# |
| 4-Hydroxycinnamic acid 1 mM | 107.35±6.65 | 109.01±6.04 | 111.26±7.49 | 110.73±7.30 | 55.30±18.55# |
| Polyphenols | |||||
| Caffeic acid 1 mM | <0* | <0* | <0* | <0* | 261.65 ± 20.65* |
| Ellagic acid 1 mM | 52.71±0.81* | 28.62±0.45* | 46.94±0.62* | 89.63±2.07* | 519.37±29.54* |
| Ferulic acid 1 mM | <0* | 15.43±4.28 | <0* | 166.44±3.25 | 45.16±12.69Δ |
| Gallic acid 1 mM | 191.78 ±3.17* | 105.68±3.46# | 188.74 ±4.13* | 284.02±8.56 | 176.77 ±16.98Δ |
| Genistein 1 mM | 8.74 ±0.42* | 6.43± 0.35* | 7.85 ± 0.45* | 16.90±0.40* | 359.27±25.08* |
| Genkwanin 1 mM | 25.89±5.12* | 18.95 ±3.25* | 25.41 ±5.48* | 23.91 ±9.77* | <0* |
| Naringin 1 mM | 20.18 ± 0.72* | 13.12 ± 0.35* | 17.47 ± 0.43* | 99.14 ± 1.28 | 319.05 ± 25.32* |
| Propyl gallate 1 mM | <0* | <0* | <0* | 70.17 ±2.08* | 233.83±32.31Δ |
| Quercetin 1 mM | 21.21±0.10* | 11.21±0.14* | 19.82±0.10* | 79.60±1.90* | 403.74 ±28.77* |
| Rutin 1 mM | 2.61 ±0.22* | 1.48±0.13* | 1.76±0.25* | 3.08±0.16* | 7.56 ±3.80* |
| Oxidants | |||||
| AAPH 5 mM | 367.67±10.24* | 349.04±9.40* | 374.63±12.31* | 525.06±18.21* | 689.92±13.59* |
| FeCl3 1 mM | 57.77±1.54* | 64.12±1.33* | 64.00±2.87* | 94.02±1.66Δ | 180.82±12.86* |
| H2O2 0.1 mM | 101.86 ±7.17 | 99.78±7.24 | 98.15±6.87 | 100.19±5.19 | 124.15±14.87# |
| Nitroxides | |||||
| TEMPO 0.05 mM | 116.18±3.51Δ | 120.31±4.68Δ | 118.30±3.77Δ | 104.61±4.75 | 134.19±14.23Δ |
| TEMPO 0.1 mM | 111.52±5.12# | 114.20 ±3.14Δ | 110.01±5.24# | 101.19 ±4.68 | 82.81±20.48 |
| TEMPO 0.2 mM | 110.97 ± 4.49# | 113.17 ± 3.93Δ | 111.73 ± 2.01* | 101.61 ± 3.58 | 144.07 ± 18.13Δ |
| TEMPO 0.5 mM | 102.98±5.56 | 104.50±4.26 | 105.19 ±4.85 | 91.31 ±5.18# | 139.83±15.44Δ |
| TEMPO 1 mM | 97.27 ±4.54 | 98.39±4.17 | 100.15 ± 5.22 | 85.87±5.6# | 138.61 ±19.08Δ |
| 4-Hydroxy-TEMPO 0.05 mM | 128.54 ± 6.06Δ | 128.18 ± 3.52* | 127.75 ± 4.21* | 118.00 ± 6.64Δ | 114.30 ± 11.47 |
| 4-Hydroxy-TEMPO 0.1 m mM | 122.91 ±6.78Δ | 123.93±5.67Δ | 123.68 ± 4.63* | 105.89 ±5.65 | 75.75 ±15.24 |
| 4-Hydroxy-TEMPO 0.2 mM | 119.29 ± 5.46Δ | 123.14 ± 4.11* | 120.58 ± 4.68Δ | 108.89 ± 5.24# | 124.05 ±14.73Δ |
| 4-Hydroxy-TEMPO 0.5 mM | 117.19 ± 7.77# | 118.36 ±5.43Δ | 117.86 ±6.76# | 102.44 ±4.19 | 176.33 ± 13.67* |
| 4-Hydroxy-TEMPO 1 mM | 113.32 ±12.39 | 112.37 ±9.83 | 110.50±6.87 | 100.24±9.18 | 145.35±19.59Δ |
| 4-Carboxy-TEMPO 0.05 mM | 93.79 ±4.53 | 90.36 ±2.67Δ | 91.83 ±2.7 | 100.19±3.44 | 380.08 ±30.19* |
| 4-Carboxy-TEMPO 0.1 m mM | 108.40±3.61# | 104.56±1.99# | 106.09 ±2.4# | 114.03±1.8* | 328.22 ±42.91* |
| 4-Carboxy-TEMPO 0.2 mM | 120.19±5.37Δ | 118.63±5.74Δ | 119.83 ±3.88* | 119.74±5.15Δ | 336.72 ±38.29* |
| 4-Carboxy-TEMPO 0.5 mM | 120.76±2.18* | 120.06±2.18* | 119.80±3.28* | 115.26±3.93Δ | 133.4 ±23.03 |
| 4-Carboxy-TEMPO 1 mM | 120.26±2.69* | 119.39 ±2.07* | 118.43 ±2.38* | 111.50±1.74* | 122.41±21.2 |
Compounds written in italics were dissolved in DMSO and compared with samples incubated with AA and DMSO.
p<0.001 (with respect to BSA incubated with AA alone).
p<0.01 (with respect to BSA incubated with AA alone).
p<0.05 (with respect to BSA incubated with AA alone).
We checked the effect of chosen additives, known to affect BSA glycoxidation by sugars and aldehydes [17–20], on BSA glycoxidation by AA. Metal chelators did not decrease the extent of BSA modifications or even augmented them. On the other hand, antioxidants, in particular polyphenols were efficient in preventing glycoxidation. Interestingly, 1-cyano-4-hydroxycinnamic acid, in contrast to 4-hydroxycinnamic acid, was an efficient inhibitor of AA-induced glycation like in the case of glycation induced by sugars [19] and reactive aldehydes [18]. As noted previously [19], the effects of additives on changes in the fluorescence of AGEs, dityrosine and N’-formylkunurenine were similar, while those on the kynurenine fluorescence were less concordant. Effects of additives, especially polyphenols, on BSA modifications measured with thioflavine T were also divergent in many cases from those due to formation of AGEs and amino acid modifications (Table 2).
Table 2.
Effect of SOD and catalase on the glycoxidation of BSA by ascorbic acid. The increase in the values of glycoxidation parameters induced by 1 mM AA alone assumed as 100%. Mean values+SD.
| AGE [%] | Dityrosine [%] | N′-formylkynurenine [%] | Kynurenine [%] | |
|---|---|---|---|---|
| SOD [10 µg/ml] | 137.3±16.2 | 142.1±7.4 | 139.7 ±7.3 | 145.2±5.7 |
| Catalase [10 µg/ml] | 99.9±8.4 | 97.7±8.9 | 95.6 ±10.2 | 97.9 ±11.7 |
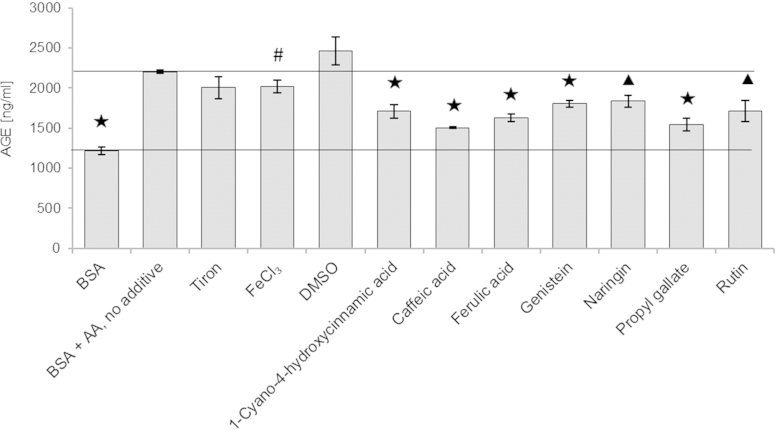
As fluorimetric measurements of glycation may be due to interference by the additives, we checked the extent of BSA glycation using an ELISA kit for chosen compounds. The results obtained are in general agreement with those obtained by fluorimetry, confirming, i.a., protection against glycation by FeCl3, 1-cyano-4-hydroxycinnamic acid, caffeic acid, ferulic acid, genistein, naringin, propyl gallate and rutin (Fig. 2 vs Table 2). A similar conclusion has been drawn in our previous studies of glycation induced by sugars [19].
Fig. 2.
Effect of selected additives on the extent of BSA glycation as estimated by ELISA.
We evaluated also the effects of oxidants on AA-induced glycoxidation. Among the oxidants employed, AAPH significantly enhanced glycoxidation but hydrogen peroxide was without effect and FeCl3 even decreased the extent of glycoxidation.
We have reported previously that nitroxides, especially tetramethylpiperidine derivatives, protect against glycation induced by glucose [19]. These compounds proved completely ineffective in protecting against AA-induced glycation (Table 2).
These results are generally in agreement with the view that products of AA oxidation and not AA itself induce protein glycoxidation. Enhancement of AA-induced glycoxidation by AAPH appears to be due to one-electron oxidation of AA by peroxyl radicals generated upon thermal decomposition of this compound [30]. The lack of effect of nitroxides, contrasting with their effects on glucose-induced glycoxidation, seems to be due to AA oxidation by these compounds [31]. Augmentation of glycoxidation by DMSO may be ascribed to oxidation of AA by DMSO; such an effect has been reported previously [32]. We did not observe aby increase in the extent of glycation induced by hydrogen peroxide. It has been reported that hydrogen peroxide, even at much lower concentrations than that used in the present study, enhanced human serum albumin glycation induced by glucose [33]; the reasons for this discrepancy are unclear but the lack of effect of exogenous H2O2 observed in this study is consistent with the lack of effect of CAT. The lack of augmentation of glycoxidation by FeCl3 is puzzling and may be attributed mainly to low availability of iron in the reaction medium (0.1 M phosphate buffer). Moreover, reduction of Fe3+ by an equimolar concentration of ascorbate produces Fe2+ which is a reductant and can act as an antioxidant.
Augmentation of glycoxidation by superoxide dismutase is also not easy to explain. SOD could be a source of copper which is an efficient oxidant of AA; however, 10 µg/ml CuZnSOD can provide about 0.6 µM copper even if losing all its copper. SOD has been shown to have a thiol oxidase activity [34] but no analogous activity of this enzyme has been reported with respect to AA oxidation.
An alternative explanation for the effect of SOD may base on the idea that ascorbyl radical plays a role in initiating glycation and that SOD affects the rate of formation of the ascorbyl radical. If AA autoxidizes by one-electron reaction with oxygen,
SOD, by dismutating the superoxide radical, drives the reaction forward. An analogous mechanism has been proposed for the role of SOD in quinone autoxidation [35].
Protection by antioxidants can be ascribed to their keeping AA in the reduced state or reducing its oxidations product(s).
Ascorbyl radical is generally thought to be inert and its inertness due to delocalization of the odd electron is believed to be the basis of the antioxidant activity. However, it can be postulated that it can still enter into reactions with proteins. leading to attachment of the ascorbyl of AA. One-electron redox potential of the ascorbyl radical/AA redox pair ascorbyl radical/AA is low (+282 mV) [36] but, nevertheless, it can enter both one-electron oxidation and reduction reactions with proteins or ascorbyl radical can attach to a protein.
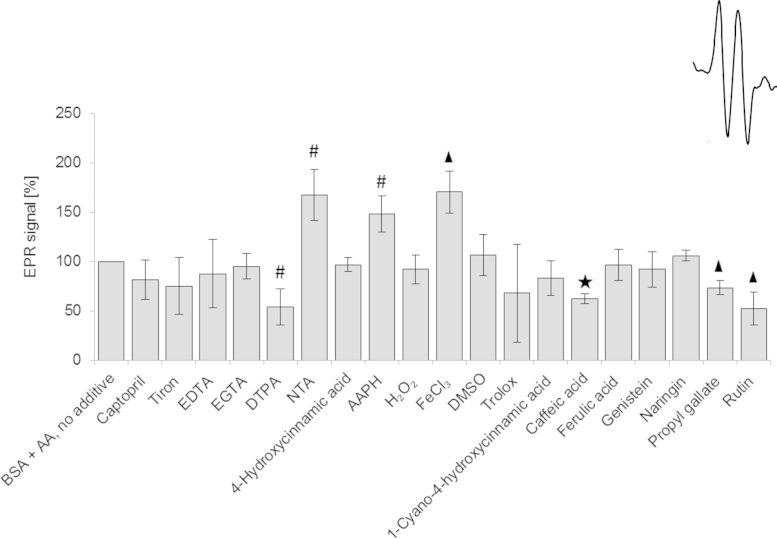
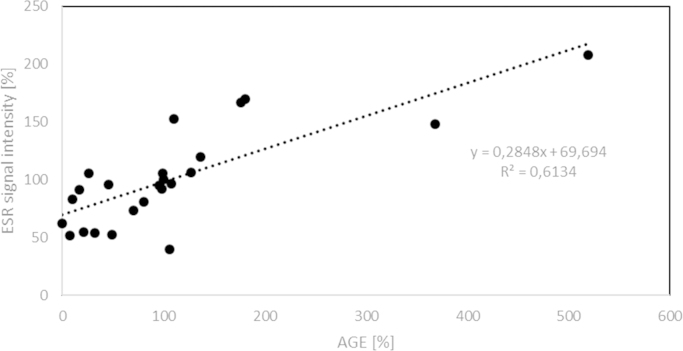
If ascorbyl radical plays a role in the AA-induced glycation, a relationship between the steady-state level of this radical in the presence of glycation inhibitors and their inhibitory activity should be observed. We studied the relationship between the AGE level and the steady-state concentration of ascorbyl radical for 20 substances affecting the rate of glycation yielded a highly significant (p<0.001) correlation coefficient (R=0.78) (Figs. 3 and 4). Thus, it can be suggested that ascorbyl radical is the primary agent inducing glycation by AA and decrease in the steady-state concentration of this radical determines the protective effect against AA-induced glycation. The lack of effects of SOD and catalase suggests that the ascorbyl radical and not reactive oxygen species produced during AA autoxidation are crucial in the process of AA-induce protein glycation. Flavonoids and many other antioxidants can regenerate AA from the ascorbyl radical [37] while compounds lacking this activity did not affect glycation.
Fig. 3.
Relative concentration of ascorbyl radical in BSA incubated with AA and various additives. Inset: ESR spectrum of the ascorbyl radical.
Fig. 4.
Correlation between the level of AGEs estimated fluorimetrically and relative concentration of ascorbyl radicals in BSA samples incubated with AA and various additives.
If our hypothesis is true, it would extend the list of AA oxidation products able to initiate glycation and contribute to explanation of the differences between glycation in vivo and in vitro. In vivo ascorbyl radical can be subject to non-enzymatic or enzymatic regeneration. In the cytosol, it is reconverted to AA by cytochrome b5 reductase and thioredoxin reductase in reactions involving NADH and NADPH, respectively [38]. The transmembrane redox system may enable extracellular reduction of the ascorbyl radical [39,40]. Therefore, chances of their reactions with proteins are much lower than in in simple in vitro systems. Disproportionation of ascorbyl radical, also more probable in the latter case, leads to formation of DHA and products of its decomposition which are known glycating agents. In some regions of the body (e.g. lens), where concentration of AA is high and possibilities of regeneration of ascorbyl radical are limited, especially under stress conditions, ascorbyl radical and further oxidation products of AA may also become glycating agents. Further studies are required to demonstrate experimentally the reactivity of ascorbyl radicals for proteins and initiation of protein glycation by these radicals.
Acknowledgment
The study has been performed within the framework of the COST CM1001 action supported by Grant 2011/01/M/NZ3/02065 from the Polish National Science Center.
References
- 1.Vistoli G., De Maddis D., Cipak A., Zarkovic N., Carini M., Aldini G. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): an overview of their mechanisms of formation. Free Radic. Res. 2013;47(Suppl. 1):S3–S27. doi: 10.3109/10715762.2013.815348. [DOI] [PubMed] [Google Scholar]
- 2.Aldini G., Vistoli G., Stefek M., Chondrogianni N., Grune T., Sereikaite J., Sadowska-Bartosz I., Bartosz G. Molecular strategies to prevent, inhibit, and degrade advanced glycoxidation and advanced lipoxidation end products. Free Radic. Res. 2013;47(Suppl. 1):S93–S137. doi: 10.3109/10715762.2013.792926. [DOI] [PubMed] [Google Scholar]
- 3.Brubacher D., Moser U., Jordan P. Vitamin C concentrations in plasma as a function of intake: a meta-analysis. Int. J. Vitam. Nutr. Res. 2000;70(5):226–237. doi: 10.1024/0300-9831.70.5.226. [DOI] [PubMed] [Google Scholar]
- 4.Bensch K.G., Fleming J.E., Lohmann W. The role of ascorbic acid in senile cataract. Proc. Natl. Acad. Sci. USA. 1985;82(21):7193–7196. doi: 10.1073/pnas.82.21.7193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grandhee S.K., Monnier V.M. Mechanism of formation of the Maillard protein cross-link pentosidine. Glucose, fructose, and ascorbate as pentosidine precursors. J. Biol. Chem. 1991;266(18):11649–11653. [PubMed] [Google Scholar]
- 6.Vinson J.A., Howard T.B. Inhibition of protein glycation and advanced glycation end products by ascorbic acid and other vitamins and nutrients. J. Nutr. Biochem. 1996;7(12):659–663. [Google Scholar]
- 7.Jacques P.F., Taylor A., Hankinson S.E., Willett W., Mahnken B., Lee Y., Vaid K., Lahav M. Long-term vitamin C supplement use and prevalence of early age-related lens opacities. Am. J. Clin. Nutr. 1997;66(4):911–916. doi: 10.1093/ajcn/66.4.911. [DOI] [PubMed] [Google Scholar]
- 8.Hegde K.R., Varma S.D. Protective effect of ascorbate against oxidative stress in the mouse lens. Biochim. Biophys. Acta. 2004;1670(1):12–18. doi: 10.1016/j.bbagen.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Fan X., Reneker L.W., Obrenovich M.E., Strauch C., Cheng R., Jarvis S.M., Ortwerth B.J., Monnier V.M. Vitamin C mediates chemical aging of lens crystallins by the Maillard reaction in a humanized mouse model. Proc. Natl. Acad. Sci. USA. 2006;103(45):16912–16917. doi: 10.1073/pnas.0605101103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Linetsky M., Shipova E., Cheng R., Ortwerth B.J. Glycation by ascorbic acid oxidation products leads to the aggregation of lens proteins. Biochim. Biophys. Acta. 2008;1782(1):22–34. doi: 10.1016/j.bbadis.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tupe R.S., Agte V.V. Role of zinc along with ascorbic acid and folic acid during long-term in vitro albumin glycation. Br. J. Nutr. 2010;103(3):370–377. doi: 10.1017/S0007114509991929. [DOI] [PubMed] [Google Scholar]
- 12.Kisic B., Miric D., Zoric L., Ilic A., Dragojevic I. Antioxidant capacity of lenses with age-related cataract. Oxid. Med. Cell. Longev. 2012:467130. doi: 10.1155/2012/467130. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sinclair A.J., Girling A.J., Gray L., Le Guen C., Lunec J., Barnett A.H. Disturbed handling of ascorbic acid in diabetic patients with and without microangiopathy during high dose ascorbate supplementation. Diabetologia. 1991;34(3):171–175. doi: 10.1007/BF00418271. [DOI] [PubMed] [Google Scholar]
- 14.Toohey J.I. Mercaptopropionaldehyde from homocysteine: implications for Alzheimer's disease. J. Alzheimer's Dis. 2007;12(3):241–243. doi: 10.3233/jad-2007-12305. [DOI] [PubMed] [Google Scholar]
- 15.Lee P., Wu X. Review: modifications of human serum albumin and their binding effect. Curr. Pharm. Des. 2015;21(14):1862–1865. doi: 10.2174/1381612821666150302115025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao H., Chen T., Shi Y. Glycation of human serum albumin in diabetes: impacts on the structure and function. Curr. Med. Chem. 2015;22(1):4–13. doi: 10.2174/0929867321666140912155738. [DOI] [PubMed] [Google Scholar]
- 17.Sadowska-Bartosz I., Bartosz G. Ascorbic acid and protein glycation in vitro. Chem. Biol. Interact. 2015 doi: 10.1016/j.cbi.2015.07.006. in press. [DOI] [PubMed] [Google Scholar]
- 18.Sadowska-Bartosz I., Galiniak S., Bartosz G. Kinetics of glycoxidation of bovine serum albumin by methylglyoxal and glyoxal and its prevention by various compounds. Molecules. 2014;19(4):4880–4896. doi: 10.3390/molecules19044880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sadowska-Bartosz I., Galiniak S., Bartosz G. Kinetics of glycoxidation of bovine serum albumin by glucose, fructose and ribose and its prevention by food components. Molecules. 2014;19(11):18828–18849. doi: 10.3390/molecules191118828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sadowska-Bartosz I., Galiniak S., Skolimowski J., Stefaniuk I., Bartosz G. Nitroxides prevent protein glycoxidation in vitro. Free Radic. Res. 2015;49(2):113–121. doi: 10.3109/10715762.2014.982113. [DOI] [PubMed] [Google Scholar]
- 21.Henle T., Deppisch R., Beck W., Hergesell O., Hänsch G.M., Ritz E. Advanced glycated end-products (AGE) during haemodialysis treatment: discrepant results with different methodologies reflecting the heterogeneity of AGE compounds. Nephrol. Dial. Transplant. 1999;14(8):1968–1975. doi: 10.1093/ndt/14.8.1968. [DOI] [PubMed] [Google Scholar]
- 22.Münch G., Keis R., Wessels A., Riederer P., Bahner U., Heidland A., Niwa T., Lemke H.D., Schinzel R. Determination of advanced glycation end products in serum by fluorescence spectroscopy and competitive ELISA. Eur. J. Clin. Chem. Clin. Biochem. 1997;35(9):669–677. doi: 10.1515/cclm.1997.35.9.669. [DOI] [PubMed] [Google Scholar]
- 23.Robaszkiewicz A., Bartosz G., Soszyński M. N-chloroamino acids cause oxidative protein modifications in the erythrocyte membrane. Mech. Ageing Dev. 2008;129(10):572–579. doi: 10.1016/j.mad.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Rice-Evans C., Diplock A.T., Symons M.C.R. Elsevier; Amsterdam,London: 1991. Techniques in Free Radical Research. ISBN 0-444-81304-7. [Google Scholar]
- 25.LeVine H., 3rd Quantification of beta-sheet amyloid fibril structures with thioflavin T. Methods Enzymol. 1999;309:274–284. doi: 10.1016/s0076-6879(99)09020-5. [DOI] [PubMed] [Google Scholar]
- 26.Bouma B., Kroon-Batenburg L.M., Wu Y.P., Brünjes B., Posthuma G., Kranenburg O., de Groot P.G., Voest E.E., Gebbink M.F. Glycation induces formation of amyloid cross-beta structure in albumin. J. Biol. Chem. 2003;278(43):41810–41819. doi: 10.1074/jbc.M303925200. [DOI] [PubMed] [Google Scholar]
- 27.Dehghan M., Akhtar-Danesh N., McMillan C.R., Thabane L. Is plasma vitamin C an appropriate biomarker of vitamin C intake? A systematic review and meta-analysis. Nutr. J. 2007;6:41. doi: 10.1186/1475-2891-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corti A., Casini A.F., Pompella A. Cellular pathways for transport and efflux of ascorbate and dehydroascorbate. Arch. Biochem. Biophys. 2010;500(2):107–115. doi: 10.1016/j.abb.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 29.Bürzle M., Hediger M.A. Functional and physiological role of vitamin C transporters. Curr. Top. Membr. 2012;70:357–375. doi: 10.1016/B978-0-12-394316-3.00011-9. [DOI] [PubMed] [Google Scholar]
- 30.Niki E. Free radical initiators as source of water- or lipid-soluble peroxyl radicals. Methods Enzymol. 1990;186:100–108. doi: 10.1016/0076-6879(90)86095-d. [DOI] [PubMed] [Google Scholar]
- 31.Mehlhorn R.J. Ascorbate- and dehydroascorbic acid-mediated reduction of free radicals in the human erythrocyte. J. Biol. Chem. 1991;266(5):2724–2731. [PubMed] [Google Scholar]
- 32.Flamm E.S., Demopoulos H.B., Seligman M.L., Poser R.G., Ransohoff J. Free radicals in cerebral ischemia. Stroke. 1978;9(5):445–447. doi: 10.1161/01.str.9.5.445. [DOI] [PubMed] [Google Scholar]
- 33.Vlassopoulos A., Lean M.E., Combet E. Role of oxidative stress in physiological albumin glycation: a neglected interaction. Free Radic. Biol. Med. 2013;60:318–324. doi: 10.1016/j.freeradbiomed.2013.03.010. 2013. [DOI] [PubMed] [Google Scholar]
- 34.Winterbourn C.C., Peskin A.V., Parsons-Mair H.N. Thiol oxidase activity of copper, zinc superoxide dismutase. J. Biol. Chem. 2002;277(3):1906–1911. doi: 10.1074/jbc.M107256200. [DOI] [PubMed] [Google Scholar]
- 35.Song Y., Buettner G.R. Thermodynamic and kinetic considerations for the reaction of semiquinone radicals to form superoxide and hydrogen peroxide. Free Radic. Biol. Med. 2010;49(6):919–962. doi: 10.1016/j.freeradbiomed.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Du J., Cullen J.J., Buettner G.R. Ascorbic acid: chemistry, biology and the treatment of cancer. Biochim. Biophys. Acta. 2012;1826(2):443–457. doi: 10.1016/j.bbcan.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cossins E., Lee R., Packer L. ESR studies of vitamin C regeneration, order of reactivity of natural source phytochemical preparations. Biochem. Mol. Biol. Int. 1998;45(3):583–597. doi: 10.1080/15216549800202982. [DOI] [PubMed] [Google Scholar]
- 38.Linster C.L., Van Schaftingen E., Biosynthesis Vitamin C. recycling and degradation in mammals. Biochem. J. 2007;274(1):1–22. doi: 10.1111/j.1742-4658.2006.05607.x. [DOI] [PubMed] [Google Scholar]
- 39.May J.M., Qu Z.C., Cobb C.E. Extracellular reduction of the ascorbate free radical by human erythrocytes. Biochem. Biophys. Res. Commun. 2000;267(1):118–123. doi: 10.1006/bbrc.1999.1906. [DOI] [PubMed] [Google Scholar]
- 40.VanDuijn M.M., Tijssen K., VanSteveninck J., Van Den Broek P.J., Van Der Zee J. Erythrocytes reduce extracellular ascorbate free radicals using intracellular ascorbate as an electron donor. J. Biol. Chem. 2000;275(36):27720–27725. doi: 10.1074/jbc.M910281199. [DOI] [PubMed] [Google Scholar]