Graphical abstract
Keywords: Flavobacterium, Chryseobacterium, Fish disease, Coldwater disease, Flavobacteriosis
Abstract
Flavobacterial diseases in fish are caused by multiple bacterial species within the family Flavobacteriaceae and are responsible for devastating losses in wild and farmed fish stocks around the world. In addition to directly imposing negative economic and ecological effects, flavobacterial disease outbreaks are also notoriously difficult to prevent and control despite nearly 100 years of scientific research. The emergence of recent reports linking previously uncharacterized flavobacteria to systemic infections and mortality events in fish stocks of Europe, South America, Asia, Africa, and North America is also of major concern and has highlighted some of the difficulties surrounding the diagnosis and chemotherapeutic treatment of flavobacterial fish diseases. Herein, we provide a review of the literature that focuses on Flavobacterium and Chryseobacterium spp. and emphasizes those associated with fish.
Introduction
Flavobacterial diseases were first reported by Davis in 1922 and have since been recognized as a serious threat to wild and propagated fish stocks alike. Originally, these diseases were attributed to three bacteria within the family Flavobacteriaceae [1]; namely, Flavobacterium psychrophilum, the etiological agent of bacterial cold water disease and rainbow trout fry syndrome [2,3]; Flavobacterium columnare, the causative agent of columnaris disease [4,5]; and Flavobacterium branchiophilum, the putative agent of bacterial gill disease [5,6]. Others have reported additional Flavobacterium spp. associated with diseased fish, including Flavobacterium johnsoniae [7], Flavobacterium succinicans [8], Flavobacterium hydatis [9], as well as other uncharacterized yellow-pigmented bacteria (Austin and Austin [10] and references therein). In acute flavobacteriosis, cumulative mortality upwards of 70% can occur among affected fish stocks, while survivors may suffer poor growth and spinal abnormalities (reviewed in Austin and Austin [10]). In subacute and chronic infections, flavobacteriosis elicits lingering mortalities that can lead to continuous economic losses [11]. Taxonomy and speciation of this family has undergone many revisions; therefore, throughout this review, the most currently recognized terminology of genera and species will be used.
With the recent advances in molecular biology and biotechnology, several novel genera within the family Flavobacteriaceae (e.g., Chryseobacterium, Elizabethkingia, Tenacibaculum, and Ornithobacterium) have emerged that encompass pathogens of fish, amphibians, reptiles, birds, and mammals, including humans [1]. Among these, Tenacibaculum spp. are important pathogens of marine fishes that have been reviewed elsewhere [12,13] and will not be discussed further herein. Rather, this review predominantly focuses on the genera Flavobacterium and Chryseobacterium, within which multiple novel species have been described over the last decade in association with diseased fishes from around the world [14–26].
History of flavobacterial diseases in fish
In the process of studying protozoan parasites at the U.S. Fisheries biological station in Fairport, Iowa, Davis [27] observed multiple fish mortality events during the summers of 1917–1919 that he associated with an unidentified bacterium. The affected fish, which included buffalofish (Ictiobus bubalus), sunfish (Leopomis spp.), common carp (Cyprinus carpio), largemouth and smallmouth bass (Micropterus salmoides and Micropterus dolomieu), crappie (Pomixis spp.), warmouth (Leopomis gulcosus), yellow perch (Perca flavescens), white bass (Morone chrysops), brook trout (Salvelinus fontinalis), bluntnose minnow (Pimephalus notatus), channel catfish (Ictalurus punctatus), and bullhead catfish (Ameiurus spp.), were cultured in aquaria and earthen ponds. Davis [27] noted that affected fish displayed “dirty-white or yellowish areas” on the body, whereby lesions developed and caused death within 24–72 h. Fins (especially the caudal fin) were eroded and, in more severe cases, only “mere stubs” remained. There was also necrosis of the gills visible as white patches that spread rapidly, causing death. The author also observed mortalities in wild fishes of the Mississippi River associated with this bacterium. Although he was unable to isolate the bacterium in any of these outbreaks, he observed large numbers of long, slender, flexible rods associated with the necrotic lesions of the skin and gills of affected fish that formed “column-like masses”; thus, he named the bacterium Bacillus columnaris. Two decades later, Ordal and Rucker [28] successfully isolated the yellow-pigmented bacterium and named it Chondrococcus columnaris due to its association with cartilage and what they characterized as the production of fruiting bodies and microcysts. However, Garnjobst [29] showed that C. columnaris did not produce such structures and reclassified the bacterium within the genus Cytophaga, as Cytophaga columnaris. While agreement on the negative impacts this bacterium had on fish stocks was well accepted, as evidenced by its inclusion in the list of notifiable fish diseases outlined in the British Diseases of Fish Act of 1937 [10], the disagreement on its taxonomy continued when it was provisionally placed into the genus Flexibacter [30,31]. Extensive molecular and phylogenetic studies by Bernardet et al. [32] placed the bacterium in the genus Flavobacterium as F. columnare, where it has remained to the present day and is recognized as the etiologic agent of columnaris disease.
Another yellow-pigmented bacterium also has a long history in association with freshwater fish diseases. In 1926 and 1927, Davis [33,34] reported multiple disease outbreaks in fingerling brook trout and steelhead (Oncorhynchus mykiss) reared in Vermont and New York, which he attributed to an unknown bacterium that was associated with damage to the gills. He noted slow chronic mortalities that increased with time when temperatures began to rise. Davis [34] noted that the bacteria formed “luxuriant growth over the surface of the gills” that coincided with increased mucus production, clubbing of the gill lamellae, and proliferation of gill epithelium causing fusion of the secondary lamellae. Otherwise, fish appeared normal until death. Other researchers observed similar disease outbreaks and isolated yellow pigmented bacteria from affected fish, but they were unable to reproduce the disease experimentally [35–37]. Wakabayashi [38] successfully recovered a yellow pigmented bacterium from hatchery-reared salmonids from Japan and Oregon that was distinct from those used in the aforementioned studies of Rucker and Bullock and successfully reproduced the disease. This bacterium was classified as Flavobacterium branchiophila [6], which became F. branchiophilum [32]. It is now widely believed that F. branchiophilum is a causative agent of bacterial gill disease (BGD; [39]); however, environmental parameters and other bacteria are also believed to play a role in some outbreaks of BGD.
A third unidentified yellow-pigmented bacterium was associated with serious disease in rainbow trout (O. mykiss) fingerlings reared at the national fish hatchery in Leetown, West Virginia [40]. Although Davis was unable to isolate this bacterium, he observed huge numbers of non-motile bacterial rods in scrapings taken from deep ulcerations present on the caudal peduncle of affected fish. These bacteria did not form the characteristic “columns” associated with F. columnare. Soon thereafter, Borg [41] reported a similar pathological condition among diseased hatchery-reared juvenile coho salmon (O. kisutch) from Washington. In this case, a bacterium was successfully isolated from the kidneys and external lesions of systemically infected fish. Borg [41,42] reproduced the disease in experimentally challenged fish, with signs that included ulcerations at the caudal peduncle that went so deep so as to almost detach the tail from the body. While this bacterium was initially placed in the order Myxobacterales and named Cytophaga psychrophila [42,43], it was reclassified as Flexibacter psychrophilus [30] and later as Flavobacterium psychrophilum [32]. As its name implies, F. psychrophilum grows best at low temperatures (∼15 °C) and frequently causes disease when water temperatures are below 10 °C. In North America, the terms low temperature disease [42] and bacterial cold water disease [44] are used to describe outbreaks associated with this bacterium, whereas outbreaks in Europe are commonly referred to as rainbow trout fry syndrome [45,46].
As is quite clear from the aforementioned history of fish-pathogenic flavobacteria, their taxonomy has been quite tumultuous. While the fish health literature is replete with reports of fish diseases associated with unidentified or partially characterized yellow-pigmented bacteria referred to as Cytophaga spp. and Cytophaga-like bacteria, Flexibacter spp., Flavobacterium-like spp., and “myxobacteria” e.g., [10,47–53], many now belong to the family Flavobacteriaceae.
The family Flavobacteriaceae
The family Flavobacteriaceae (Phylum Bacteroidetes; Class Flavobacteriia; Order Flavobacteriales; [54]) was first suggested by Jooste [55]. Despite being mentioned by Reichenbach [56], the family was not validated until 1992 by Reichenbach and its formal description published by Bernardet et al. [32]. Included within the family Flavobacteriaceae at that time was the type genus, Flavobacterium [32], along with the genera Chryseobacterium [57,58], Bergeyella [58,59], Empedobacter [58,60], Capnocytophaga [61,62], Ornithobacterium [58], Weeksella [63], and Riemerella [64,65], along with the species that would eventually comprise the genera Myroides [66,67], and Tenacibaculum [32,68]. Bernardet et al. [69] published minimal standards for describing new taxa within the family Flavobacteriaceae, which in addition to the genera mentioned above also included Coenonia [70], Psychroserpens and Gelidibacter [71], Polaribacter [72], Psychroflexus [73], Salegentibacter [74], Cellulophaga [75], and Zobellia [76], along with two generically misclassified taxa [69]. In subsequent years, the family has rapidly expanded from <20 genera to >100 described and candidate genera [77,78].
Characteristics of this family according to Bernardet [77] are as follows: Nonspore-forming short to long filamentous rods that stain Gram negative, are motile via gliding or non-motile, and are non-flagellated with rare exceptions (i.e., Polaribacter irgensii) [72]. Colony shape can vary from round and convex to rhizoid and flat, and can strongly adhere to the surface of the agar (i.e., F. columnare). Although some colonies do not pigment, most contain a non-diffusible yellowish to orange pigment due to the presence of carotenoid and/or flexirubin. Growth is typically aerobic, but some genera exhibit micro-aerophillic to anaerobic growth. Nitrates are not typically reduced, whereas oxidase and catalase activities are common. Most genera contain species that degrade organic substrates, including proteins (e.g., casein, gelatin, etc.), simple and complex carbohydrates (e.g., starch, esculin, pectin, chitin, carboxymethylcellulose), and lipids (e.g., Tween). Many species are halophilic and mesophilic, while others are halotolerant and psychrophilic. Branched saturated, branched monounsaturated, and branched hydroxy C15 to C17 fatty acids are frequently present in large amounts. The term flavobacteria will be used throughout this literature review to encompass members of the family Flavobacteriaceae.
Flavobacteria commonly reside in extremely diverse habitats, ranging from fresh and marine aquatic environments, soils, foods, beverages and their processing plants, as well as human and veterinary hospitals (reviewed in Bernardet and Nakagawa [1]). Many flavobacteria are pathogenic to a multitude of organisms, including plants [79], invertebrates [80], amphibians [81], reptiles [82], birds [64], and mammals [83], including humans [84]. Because the research presented herein focused on the genera Flavobacterium and Chryseobacterium, the remainder of this literature review will be comprised of information pertaining to them specifically.
The genus Flavobacterium
The genus Flavobacterium was described in the first edition of Bergey’s Manual of Determinative Bacteriology [85] in 1923, at which time it consisted of 46 species that included Gram negative, Gram positive, Gram variable, and flagellated bacteria [86]. By the publication of the eighth edition of Bergey’s Manual in 1974, the majority of the flagellated and Gram positive strains were systematically removed, leaving only 12 species within the genus [86]. This taxonomic upheaval continued, whereby only 7 species remained in 1984 [87]; then, the extensive work published by Bernardet et al. [32] in 1996 resulted in the type species, F. aquatile [88], being the only original member to be retained within the genus Flavobacterium. Other species assimilated into the genus Flavobacterium at that time included F. psychrophilum, F. branchiophilum, F. columnare, F. hydatis, F. succinicans, F. johnsoniae, F. flevense, F. pectinovorum, and F. saccharophilum [32]. With much of the taxonomy settled, minimal standards for describing novel flavobacteria were published [69] in 2002, at which point the number of Flavobacterium spp. was 15. Over the next 4 years, 11 more Flavobacterium spp. were described [79], bringing the total to 26, and the number of formally described Flavobacterium spp. has rapidly expanded to over 100 published and proposed species at the time this review was written [http://www.ezbiocloud.net/eztaxon; 78].
Members of the genus Flavobacterium are Gram negative rods that range from 0.3 to 0.5 μm in diameter and from 1.0 to 40.0 μm in length. All species are non-motile or display gliding motility (the presence of flagella has not been reported). It should be noted that the degree by which flavobacteria move by gliding is optimal in media with low nutrient and high moisture contents. Colonies contain non-diffusible, non-fluorescent, flexirubin and/or carotenoid – type pigments, giving them a pale to bright yellow appearance. Optimal growth occurs from 20 to 30 °C for most species, though growth is better at 15–20 °C for the more psychrophilic species. The majority of Flavobacterium spp. grow on nutrient and trypticase soy agars (TSA) without the need for growth factors, though the dominant fish pathogenic species (i.e., F. psychrophilum, F. columnare, F. branchiophilum) are not able to grow on TSA. Some species are able to oxidize carbohydrates, but strong proteolytic activity is almost universally present. The predominant fatty acids present within members of the genus are iso-C15:0, C16:1 ω6c and/or C16:1 ω7c, C15:0, iso-C17:0 3-OH, iso-C15:0 3-OH, C15:1 ω6c, iso-C 16:0 3-OH, iso-C 15:1 G, iso-C15:0 2-OH, and anteiso-C15:0. The type species is F. aquatile [88].
Flavobacterium spp. vary in the ease with which they are cultured on microbiological media. For example, many of the species that inhabit soil and freshwater grow readily on agar plates made from commercially available media (e.g., nutrient agar and TSA), as is also the case for the marine species (e.g., marine agar; reviewed in Bernardet and Bowman [79]). However, a portion of the freshwater fish-pathogenic species (see Flavobacterium spp. as pathogens below) is fastidious and requires specialized culture media, such as cytophaga agar [89], Shieh’s medium [90], Hsu-Shotts medium [91], tryptone yeast extract salts medium (TYES; [44]), and medium # 2 [92], to name a few. Some of these media and their derivatives were made more selective by incorporation of polymyxin-B, neomycin, tobramycin, and/or vancomycin to avoid overgrowth by less fastidious bacteria that may also be present in an inoculum, especially from external lesions of fish. Optimal incubation temperatures vary widely and depend upon the species, but most of the fish-pathogenic species grow best from 15 to 25 °C [79].
The morphological, physiological, and biochemical characteristics that help to differentiate Flavobacterium from other genera within the family Flavobacteriaceae were extensively reviewed by Bernardet [77], and differential characters among Flavobacterium spp. are presented in Bernardet and Bowman [54]. In addition to bacterial culture and subsequent identification via phenotypic tests [10], many other means for detection and identification were developed. Whole-cell agglutination [43,93], fluorescent antibody tests (FAT; [94,95]), enzyme-linked immunosorbent assays [95,96], in situ hybridization [97], loop-mediated isothermal amplification (LAMP; [98,99]), polymerase chain reaction (PCR; [100–106]), immunomagnetic separation in conjunction with flow cytometry [107], quantitative PCR [108,109], and DNA array-based multiplex assay [110] are used to detect and identify F. psychrophilum, F. columnare, and/or F. branchiophilum. It is noteworthy, however, that few diagnostic reagents specific for the lesser-known fish associated flavobacteria exist, which makes their identification more difficult and laborious.
Ecology of Flavobacterium spp.
Although usually considered psychrophilic or psychrotolerant, some flavobacteria are also able to grow at temperatures of 37 °C (e.g., F. granuli, F. columnare, F. suncheonse, and F. succinicans), or even 40–45 °C (e.g., F. defluvi, F. indicum, F. croceum) [54]. However, the vast majority are recovered from cool, cold, or even polar habitats (reviewed in Bernardet and Bowman [54]). Flavobacterium spp. reside in diverse habitats, including freshwater streams, lakes, and sediments (e.g., [111,112]), deep wells [57], glaciers and arctic ice (e.g., [113,114]), plants and plant material (e.g., [115,116]), soils (e.g., [117]), freshwater shrimp ponds [118], marine sediments (e.g., [119]), seawater (e.g., [120]), wastewater treatment systems (e.g., [121,122]), on marine algae (e.g., [123]), and even within air-conditioning units [79].
Flavobacterium spp. have also been detected intracellularly in amoebae [124] and from the guts of earthworms (Aporrectodea caliginosa; [125]), butterflies [126], mosquitos [127], nematodes [128], leeches [129], and in association with corals (e.g., [130]), marine sponges [131], and marine mammals, such as beaked whales (Ziphius cavirostris; [132]). Caution should be exercised when referring to the primary literature documenting Flavobacterium spp. from many other animals, including humans, because many taxa originally belonging to the genus Flavobacterium were subsequently moved to other genera (e.g. Flavobacterium breve, now Empedobacter brevis, [58]; Flavobacterium meningosepticum, now Elizabethkingia meningoseptica, [133]). By far and away, the most plentiful reports of Flavobacterium spp. in associated with animals are those that document their presence on fish and fish products. For example, Flavobacterium spp. have been detected within the intestines [134,135], on the gills [136], fins [8], in the mucus [79], from eggs [137] and reproductive fluids [138], and from internal organs of cool, cold, and warm-water fishes (reviewed in Shotts and Starliper [5], Bernardet and Bowman [79], and Austin and Austin [10]). Although some Flavobacterium spp. are commensal [43,139,140], a number are major pathogens of freshwater and marine fishes.
Flavobacterium spp. as pathogens
A few reports have documented Flavobacterium spp., such as F. johnsoniae, with diseases in plants [79,141,142]. Flavobacterial infections were also occasionally reported in amphibians [27,137,143], and occasionally the bacteria were associated with disease in humans. For instance, an outbreak of respiratory disease in a group of workers from a textile plant occurred in the 1980s and was attributed to a Flavobacterium sp. strain that proliferated within an air-conditioning unit in the facility [79,144,145]. Most recently, F. lindanitolerans was recovered from the ascites of a child in China that died of fatal pulmonary edema and hemorrhage [146].
Flavobacterium spp. as fish pathogens
Since the initial report of Davis [27], multiple members of the genus Flavobacterium have been recognized as serious fish pathogens worldwide; namely, F. columnare, [27,28,91]; F. psychrophilum [3,40,45,46,147,148]; and F. branchiophilum [6,38,39]. Extensive reviews have been written on fish-pathogenic Flavobacterium spp. (e.g., [5,10,79]), which highlight the predominance of F. psychrophilum, F. columnare, and F. branchiophilum in fish health literature. The three aforementioned reviews described the impacts that the “big three” fish-pathogenic Flavobacterium spp. have on fish stocks worldwide and additional publications provided even greater depth concerning the impacts of F. psychrophilum (e.g., [2,3,44,148,149]), F. columnare (e.g., [91,150–152]), and F. branchiophilum (e.g., [6,39,153–156]). Thus, while the importance of F. psychrophilum, F. columnare, and F. branchiophilum as etiologic agents of fish disease is undeniable, the remainder of the review focuses on the “less well-known” freshwater fish-associated flavobacteria.
“Other” Flavobacterium spp. associated with diseased fishes
F. johnsoniae is occasionally associated with disease in fish. Christensen [7] first mentioned F. johnsoniae in association with diseased fish, followed by the report of Carson et al. [157] of F. johnsoniae causing shallow ulcerative lesions on the skins, fins, and jaws of juvenile aquacultured barramundi (Lates calcarifer) in Australia. In an attempt to fulfill Koch’s postulates, Soltani et al. [158] utilized an isolate of F. johnsoniae from the disease outbreak in barramundi and challenged various fishes via bath exposure. Juvenile barramundi were the only studied species that were susceptible to infection with F. johnsoniae, and infections ensued only after fish were stressed thermally [158]. Rintamäki-Kinnunen et al. [159] also found an F. johnsoniae-like bacterium associated with external lesions on the gills, jaws, skin, and fins in multiple aquacultured salmonids in Finland. Most recently, F. johnsoniae and F. johnsoniae-like isolates were associated with disease in aquacultured longfin eels (Anguilla mossambica), rainbow trout, and koi (C. carpio) in South Africa [15], in cultured Russian sturgeon (Acipenser gueldenstaedtii) in Turkey [160], and in farmed rainbow trout in Korea [161].
Another Flavobacterium sp. implicated as a putative agent of fish disease is F. hydatis (formerly Cytophaga aquatilis; [9,32]). This bacterium was first isolated from the gills of propagated salmonids displaying “signs of bacterial gill disease” in Michigan by Strohl and Tait [9], but its pathogenicity was not assessed. Others have also recovered a similar bacterium from the gills of fish suffering from bacterial gill disease epizootics [10,79], thus suggesting a role as an opportunistic fish pathogen. Similarly, F. succinicans was originally isolated from the water and eroded caudal fin of a Chinook salmon (Oncorhynchus tshawytscha) fingerling at the University of Washington School of Fisheries (Seattle, Washington) and from a “lesion” on an adult Chinook salmon collected from the Brownlee Dam on the Snake River in Hells Canyon, Idaho [8]. However, the original author suggested that this bacterium was not a fish pathogen but rather a commensal, and further studies were not conducted.
Most recently, a number of novel Flavobacterium spp. were isolated from diseased fish in South America, Europe, and North America. Flavobacterium chilense was originally recovered from an external lesion of a farmed rainbow trout in a mixed culture with F. psychrophilum, while F. araucananum was recovered from the kidneys and external lesions of farmed Atlantic salmon (Salmo salar) in a mixed culture with F. psychrophilum [18], both of which were cultured in Chile. The original isolations of F. oncorhynchi took place in Spain, whereby the bacterium was recovered from the livers and gills of farmed rainbow trout with disease signs that were suggestive of an F. psychrophilum infection [19]. Likewise, F. plurextorum was originally recovered from the livers and eggs of farmed rainbow trout in Spain that were suffering from a septicemia [22], while F. tructae and F. piscis were recovered from the liver and gills and kidney and gills, respectively, of farmed diseased rainbow trout in Spain [23]. Three proposed Flavobacterium spp. (e.g., F. collinsii, F. branchiarum, and F. branchiicola) were also recovered from farmed rainbow trout in Spain in 2008 and 2008, though no signs of disease were reported [162]. In North America, F. spartansii was recovered from the kidneys of feral spawning Chinook salmon, as well as from the gills of hatchery-reared Chinook salmon, in the Great Lakes basin [26].
In addition to the aforementioned fish-associated Flavobacterium spp., other partially characterized and/or unidentified Flavobacterium spp. have periodically been reported from diseased fishes. Indeed, in their review, Shotts and Starliper [5] make brief mention of “other poorly defined Flavobacterium-like organisms” that have been implicated as facultative fish pathogens, while a similar statement that implicates partially characterized yellow-pigmented bacteria as agents of fish disease is made by Austin and Austin [10]. For example, Holliman et al. [163] documented a disease outbreak in captive-reared rainbow trout (O. mykiss) supplied with unfiltered lake water in Windermere, England, that was attributed to an unidentified “Cytophaga-like” bacterium, which generated a 16% mortality over 10-days and mortality abated after administration of oxytetracycline. On cytophaga agar, nearly pure bacterial growth was cultured from external lesions. Holliman et al. [163] found that they possessed the characters of “Cytophaga-like bacteria” that were distinct from F. aquatile, F. psychrophilum, F. columnare and indicated that this bacterium had never been previously reported in association with fish disease. Additionally, the authors confirmed pathogenicity via immersion, intraperitoneal, and intramuscular routes of transmission in rainbow trout and Atlantic salmon.
Similarly, Bowman and Nowak [164] detected a Flavobacterium sp. from the gills of net-penned Atlantic salmon in Tasmania that concurrently suffered from amoebic gill disease. Their isolate was most similar to F. frigidarium according to 16S rDNA sequences. It was not clear, however, what role this bacterium had played in the disease process. Indeed, a number of unidentified “Cytophaga-like bacteria” were also reported in association with disease outbreaks in marine fishes (e.g., [50–52]).
The genus Chryseobacterium
The genus Chryseobacterium was originally created by Vandamme et al. [58] for six bacterial taxa that, at that time, were classified as members of the genus Flavobacterium; F. balustinum, F. indologenes, F. gleum, F. meningosepticum, F. indoltheticum, and F. scophthalmum. In keeping with the taxonomic upheaval that many of the taxa within the family Flavobacteriaceae endured, an additional genus, Elizabethkingia, was subsequently created for two species within the genus Chryseobacterium; namely, C. meningosepticum and C. miricola [133]. Since then, more taxonomic clarity has been achieved through the widespread use of improved molecular techniques and guidelines for description of novel Chryseobacterium species [69]. By 2006, the genus Chryseobacterium had expanded to 10 species, along with one unvalidated species (e.g., C. proteolyticum; [1]), but the genus contained >60 species when this review was written.
Members of the genus Chryseobacterium are non-motile, Gram negative, straight rods that are usually 1–3 μm in length and ∼0.5 μm in width. None of the species within this genus have flagella, nor do they display gliding motility or swarming growth. Colonies range from pale to a bright golden yellow color due to the presence of a non-diffusible flexirubin-type pigment. Chryseobacterium spp. grow well on commercial media (i.e. TSA, brain heart infusion, marine, blood, nutrient, and Mueller Hinton agars [reviewed in 77,165]]) at 4–42 °C (ideal incubation temperature of 20–30 °C for most species [165]) and at salinities of up to 5%, depending upon the species. Catalase and oxidase activities are present, isolates are strongly proteolytic, and most are resistant to numerous antibiotics. The predominant fatty acids contained by members of this genus are iso-C15:0, iso-C 17:1 ω9c, iso-C17:0 3-OH, and iso-C15:0 2-OH/C16:1 ω6c and/or C16:1 ω7c. The type species is Chryseobacterium gleum [Holmes et al. [87]].
The phenotypic and biochemical characteristics that differentiate Chryseobacterium spp. from other closely related members of the family Flavobacteriaceae were reviewed by Bernardet [77], while differential characters among the various Chryseobacterium spp. were presented in Bernardet et al. [166]. Presumptive identification of a Chryseobacterium sp. is often based upon phenotypic characters (e.g., Gram negative, non-motile rods that produce bright yellow colonies due to the presence of flexirubin-type pigments; possess oxidase and catalase activities; produce a Chryseobacterium spp. profile on commercial galleries; [14]), after which a definitive identification is based upon polyphasic characterization, including biochemical, morphological, and physiological characterization, fatty acid profiling, and sequence/phylogenetic analyses [69]. Additional techniques that have most recently been utilized to identify Chryseobacterium spp. include matrix assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS; [167]) and PCR amplification of the 16S and internal transcribed spacer (ITS) rDNA and subsequent sequence analysis [168].
Ecology of Chryseobacterium spp.
Akin to the genus Flavobacterium, members of the genus Chryseobacterium inhabit diverse habitats. For example, Chryseobacterium spp. were recovered from soils (e.g., [169–171]), plant roots (e.g., [172]), flowers [173], decaying plant material [174], and maple sap [175]. Interestingly, some plant-associated Chryseobacterium strains inhibited plant-pathogenic fungi [172]. Chryseobacterium spp. were also recovered from freshwater creeks [176], lakes [177], their sediments [178], water cooling systems [179], drinking water [180], lactic acid beverages [181], beer bottling plants [182], bioreactor sludge [183], polluted soil [184], marine sediment [185], and permafrost [186]. In contrast to Flavobacterium spp., multiple studies that examined the bacterial assemblages of glaciers and Antarctic ice have not detected Chryseobacterium spp. ([165] and references therein).
Chryseobacterium spp. are associated with a multitude of animals. For instance, they have been detected from the midgut of mosquitos (Culicoides sonorensis, [127]; Culex quinquefasciatus, [187]), within cockroach guts (Periplaneta americana, [188]), millipede feces (Arthrosphaera magna, [189]), and penguin guano (Pygoscelis adeliae, [190]), gut homogenates of freshwater copepods (Eudiaptomus gracilis, [191]), bird feathers [192], cow’s milk (e.g., [193–196]), and from raw meats and chicken [197–199]. Chryseobacterium spp. were recovered from the mucus of apparently healthy fish [165,200] and thus, they may be commensalistic. However, Chryseobacterium balustinum was first isolated from the scales of halibut (Hippoglossus hippoglossus) freshly harvested from the Pacific Ocean [201] where it was considered as a spoilage organism [10]. In this context, Engelbrecht et al. [202] recovered multiple Chryseobacterium spp. from marine fish caught in South Africa that produced H2S and proteolyzed multiple substrates (e.g., casein, gelatin). Engelbrecht et al. [202] reported pungent and stale odors in muscle extracts, suggesting involvement in fish spoilage. Similarly, C. piscium, which was first isolated from fish in the south Atlantic Ocean of South Africa [203], was suggested to be a spoilage organism due to the presence of urease and phenylalanine deaminase activities. However, González et al. [204] suggested that Chryseobacterium spp. were not an important cause of spoilage in fish, because they comprised less than 1% of the bacterial communities of the fish that they sampled.
Chryseobacterium spp. as pathogens
As was mentioned for Flavobacterium spp., caution should be exercised when referring to the literature regarding Chryseobacterium spp. as pathogens, because Chryseobacterium meningosepticum, which caused numerous infections, was reclassified to the genus Elizabethkingia [133]. Nevertheless, Chryseobacterium spp. were recovered from multiple diseased organisms. For example, C. indologenes is pathogenic to the soft tick (Ornithodoros moubata, [205]), whereas other Chryseobacterium spp. were recovered from diseased turtles [82] and frogs [206,207]. In addition, multiple Chryseobacterium spp. were recovered from human clinical sources [57,208–211]. For example, Chryseobacterium indologenes has caused bacteremia in humans on numerous occasions [212–214] with multiple clinical manifestations, such as hospital-acquired pneumonia, peritonitis, surgical wound infections [212], and cellulitis [215]. Chryseobacterium spp. were also isolated from burn wounds [216], eye infections [168], and pneumonia in newborns [217]. Such infections are nosocomial in nature and frequently associated with indwelling devices (e.g., catheters, tracheal tubes, ventilators; [165]).
Chryseobacterium infections in fish
The first isolation of Chryseobacterium balustinum was reported in 1929 from the scales of halibut (Hippoglossus hippoglossus) freshly harvested from the Pacific Ocean [201]. However, these isolates are no longer available in culture collections [165], and the current type strain was isolated from the heart blood of a freshwater dace (Leuciscus leuciscus) that had signs of septicemia [218] as reported in 1959. When this isolate was experimentally injected into multiple fish species, it caused mortality [218], thus suggesting its pathogenic potential [165].
Mudarris and Austin [219] reported a disease outbreak in farmed turbot (Scophthalmus maximus) that occurred in Scotland in 1987 associated with a novel Chryseobacterium sp. that they later named C. scophthalmum [58,220]. They recovered “dense pure cultures” of this bacterium from the gills and viscera of fish that exhibited hyperplasia of the gills, hemorrhage of the eyes, skin, and jaw, necrosis and hemorrhage of the brain, stomach, intestine, liver, and kidney, and ascites within the peritoneum [219]. They also recovered the same bacterium from apparently healthy adult and juvenile wild turbot. The same authors then experimentally assessed the pathogenicity of C. scophthalmum in juvenile turbot in seawater and rainbow trout in freshwater using both immersion exposure and intraperitoneal injection and found similar disease signs as what was seen in naturally infected fish. The authors recovered the same bacterium from infected fish, and fulfilled Koch’s postulates. Moreover, injection of bacterial cell homogenates resulted in the same disease signs, but injections of either lipopolysaccharide (LPS) or cell supernatant did not produce any lesions [219]. The same authors later examined the histopathological changes associated with the natural and experimental C. scophthalmum infections [221] and reported swelling, necrosis, and edema within the gills, as well as epithelial hyperplasia of the secondary lamellae and proliferation of the interlamellar cells that “filled up the spaces between the secondary lamellae.” Degeneration and necrosis of the renal tubular epithelial cells, dilatation and necrosis of the glomeruli, necrosis of interstitial renal tissue, focal necrosis of hepatic cells, edema of the bile ducts, and necrosis of the mucosal epithelial cells of the intestine were also noted. Pathological changes were not observed in either the heart or spleen.
More recently, Bernardet et al. [14] performed polyphasic characterization on 52 Chryseobacterium spp. isolates recovered from diseased fish in Belgium, Finland, France, Spain, Taiwan, Singapore, and Cambodia. While the majority of these isolates could not be ascribed to a definitive species, 14 clusters were defined and 3 of these were believed to represent novel Chryseobacterium spp. [14]. In addition, two of the isolates recovered from external lesions of farmed Atlantic salmon in Finland were identified as C. joostei, which was originally isolated from raw cow’s milk in South Africa [193]; however, experiments investigating the pathogenicity of these strains were not reported. The authors did suggest that many of the Chryseobacterium spp. they studied represented facultative fish pathogens, because many were recovered from imported fish that were stressed [14].
Most recently, a number of novel Chryseobacterium spp. were recovered from fish, some of which were diseased. For example, C. piscicola was isolated from diseased Atlantic salmon and rainbow trout in Chile [16], diseased Atlantic salmon in Finland [222], and was later suggested to be moderately virulent in experimental challenges [222]. Likewise, C. chaponense was recovered from the fins, gills, and external lesions of diseased Atlantic salmon being farmed in Chile, in mixed culture with F. psychrophilum [17]. Chryseobacterium viscerum and C. oncorhynchi were originally isolated from the livers and gills of diseased rainbow trout (O. mykiss) in Spain [20,21]. In addition, Chryseobacterium aahli was described from the kidneys of systemically-infected, hatchery-reared lake trout (Salvelinus namaycush) and from the necrotic fins of hatchery-reared brown trout (Salmo trutta) of the Great Lakes of North America [25].
Interestingly, a number of Chryseobacterium spp. that are more frequently associated with human infections or human consumables were recently recovered from fish. For example, C. hominis, so named for its penchant for causing infections in humans [209], was isolated from the kidneys of a pufferfish (Arothron hispidus) [223,224] in 2005. In addition, C. shigense, which was originally isolated from a beverage in Japan [181], was recovered from farmed rainbow trout fry undergoing multiple disease outbreaks in Spain [225] in 2008 and 2009. In 2012, C. indologenes, which is increasingly associated with human infections [212–215], was identified as a cause of disease and mortality in farmed yellow perch (P. flavescens) in the United States and confirmed to be pathogenic to perch via experimental challenge [226].
Chryseobacterium spp. are inherently resistant to a wide spectrum of antibiotics, including tetracyclines, erythromycin, linezolid, polymyxins, aminoglycosides, chloramphenicol, and many β-lactams, while also being intermediately sensitive to vancomycin and clindamycin and vary in their sensitivity to trimethoprim-sulfamethoxazole [165,214,227,228]. Michel et al. [229] found that among the 65 isolates they obtained from aquatic habitats, 89% were resistant to polymyxin-B, 97% were resistant to ampicillin, 62% were resistant to erythromycin, and 54% were resistant to oxytetracycline, while 21.5% and 41.5% were resistant and moderately resistant to florfenicol. Additionally, 69% of the isolates were sensitive to trimethoprim-sulfamethoxazole.
Emergence of flavobacteria in the Laurentian Great Lakes
Recently, multiple wild fish kills in the Great Lakes Basin have been attributed to infectious agents, such as viral hemorrhagic septicemia virus VHSv; [230], Renibacterium salmoninarum [231], and F. columnare (Faisal and Loch unpublished). Indeed, as a group, flavobacteria have historically accounted for more fish mortality in the hatcheries of the State of Michigan than all other pathogens combined [232,233], though it was unclear which Flavobacterium spp. were involved. In this context, the Michigan Department of Natural Resources (MDNR) and the Aquatic Animal Health Laboratory at Michigan State University (MSU-AAHL) undertook extensive field and laboratory studies to identify pathogens threatening conservation efforts in wild fish stocks, as well as those reared within State Fish Hatcheries.
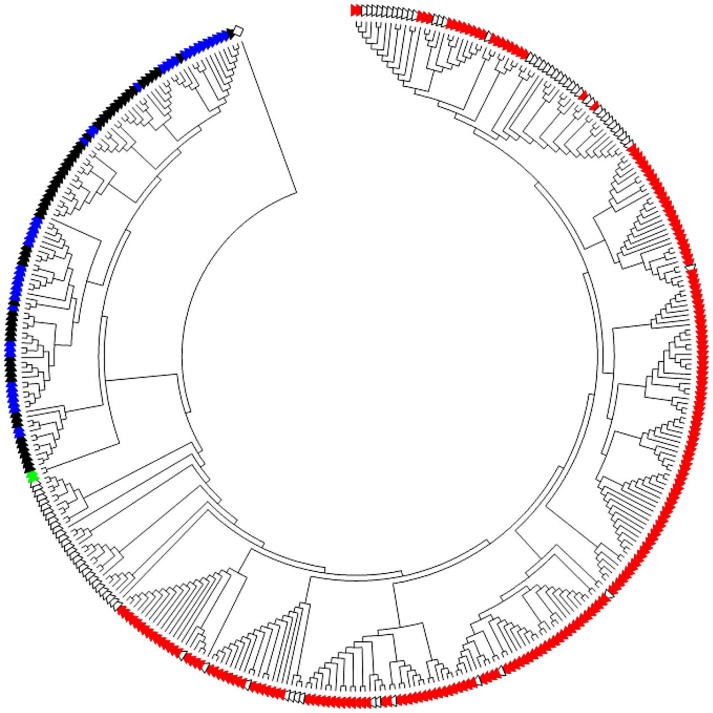
These studies revealed that a diverse assemblage of Flavobacterium and Chryseobacterium spp. were associated with diseased, as well as apparently healthy wild, feral, and farmed Great Lakes fishes [25,26,234–236]. Among 254 fish-associated flavobacterial isolates that were recovered from 21 fish species during 2003–2010, 211 were identified as Flavobacterium spp. and 43 as Chryseobacterium spp. according to partial 16S rRNA gene sequencing and phylogenetic analysis [235]. A dendrogram depicting this diversity is displayed in Fig. 1. Although F. psychrophilum and F. columnare were associated with multiple fish mortality events and severe gross pathology (Fig. 2a–d), many previously uncharacterized flavobacteria were recovered from systemically infected fish showing overt signs of disease (Fig. 2e and f). Indeed, the majority of the isolates were either most similar to recently described fish-associated Flavobacterium and Chryseobacterium spp. whose presence had never before been reported in North America (e.g., F. oncorhynchi, F. araucananum, C. viscerum, C. piscicola, and C. chaponense), or they were phylogenetically distinct from all of the described species and were suspected as comprising novel flavobacterial taxa [235]. Interestingly, 144 of the 254 flavobacterial isolates that were characterized in that study were recovered from fish species that were either accidentally or intentionally introduced into the Great Lakes.
Fig. 1.
Dendrogram based upon partial 16S rRNA gene sequence analysis, which was generated using the neighbor-joining method in Molecular Evolutionary Genetics Analysis (MEGA) version 5, depicting the phylogenetic relationships between Great Lakes fish-associated Flavobacterium (red triangles) and Chryseobacterium (blue triangles) spp. and described and candidate Flavobacterium (white triangles) and Chryseobacterium (black triangles) spp. The closely related Elizabethkingia meningoseptica and E. miricola (green triangles) were also included. The tree was rooted with Capnocytophaga ochracea U41350 (white diamond).
Fig. 2.
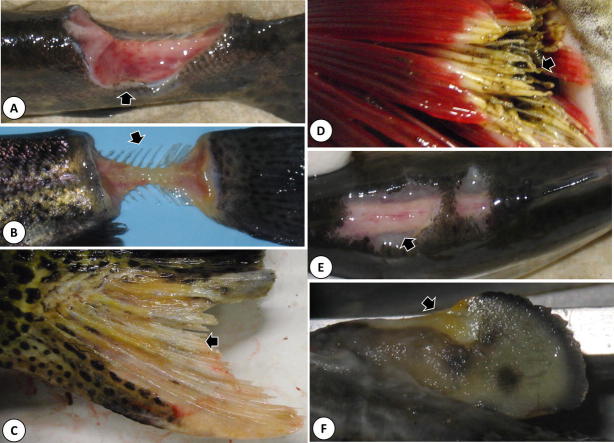
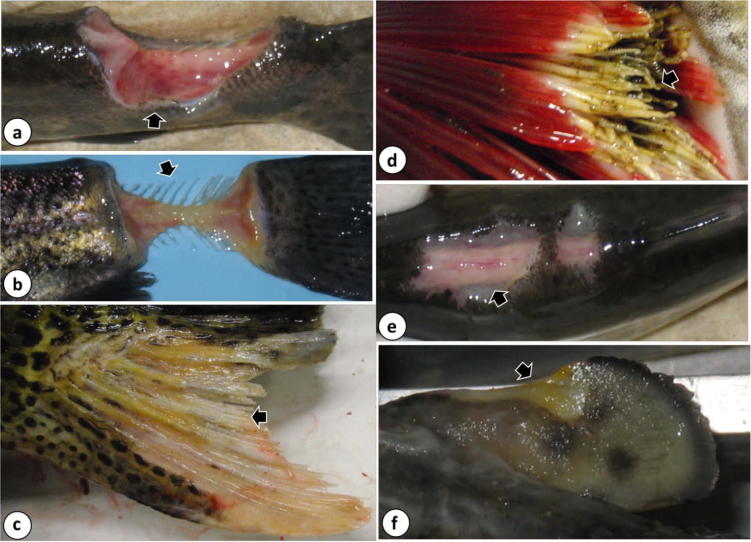
Gross pathology in Great Lakes fishes infected with flavobacteria. (a) Deep muscular ulceration and concurrent hemorrhage (arrow) of the caudal peduncle in a rainbow trout infected with F. psychrophilum, (b) Flavobacterium psychrophilum-associated ulceration of the caudal peduncle that has left the vertebral column (arrow) fully exposed. Note also the yellowish discoloration of the lesion due to mats of the yellow-pigmented bacterium, (c) extensive erosion and necrosis (arrow) of the anal fin of a Chinook salmon (O. tshawytscha) infected with F. columnare. Note also the yellowish discoloration of the lesion due to the presence of the yellow-pigmented bacterium, (d) severe necrosis of the gill lamellae (arrow), also accompanied by yellowish discoloration, of a Chinook salmon from which F. columnare was recovered, (e) multifocal ulceration (arrow) and hemorrhage on the dorsum of a rainbow trout infected with a previously uncharacterized Flavobacterium sp (note faint yellowish discoloration to the right of the arrow), (f) erosion and necrosis (arrow) of the adipose fin, which is accompanied by a yellowish discoloration, of a Chinook salmon infected with a previously uncharacterized flavobacterial species.
Further study of a subset (n = 65) of these Great Lakes fish-associated Flavobacterium spp. using 16S rRNA gene sequencing and phylogenetic analyses based upon neighbor-joining and Bayesian methodologies revealed that 13 of these isolates were highly similar to the newly described fish-associated F. plurextorum, F. spartansii, and F. tructae [236]. However, the remaining isolates did not conclusively match any described Flavobacterium spp. and were thus suspected as representing novel flavobacterial species. Further polyphasic characterization, which included morphological, physiological, biochemical, and additional phylogenetic analyses, was undertaken on 6 isolates that represented a range of genetic distinctness and association with disease signs in hatchery raised or free-ranging fish [236]. Those findings showed that at least five of the six isolates were most likely novel Flavobacterium species that had never before been reported from fish. The same study confirmed that some of these flavobacteria were capable of generating gross pathology in experimentally challenged fish (Fig. 3), where flavobacteria were re-isolated from their brains, spleens, livers, and kidneys [236]. Similarly, median lethal dose (LD50) experiments with two isolates of F. spartansii (T16T and S12) indicated that this bacterium is a facultative pathogen of its host of origin, the Great Lakes Chinook salmon, which is capable of inducing mortality and histopathological changes that include severe proliferative branchitis, lymphocytic and histiocytic myositis, multifocal hepatic necrosis, lymphocytic hepatitis, renal tubular degeneration and necrosis, and multifocal edema within the granular cell layer of the cerebellar cortex [237].
Fig. 3.
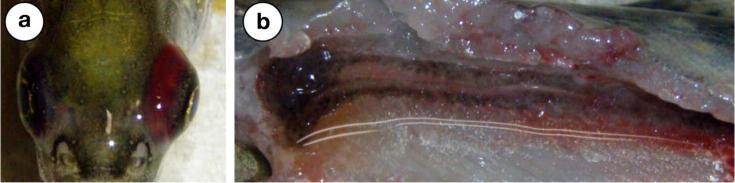
Gross pathology in fish that were experimentally challenged with previously undescribed Flavobacterium spp. isolates that were originally recovered from Great Lakes fishes. (a) Severe unilateral exophthalmia with diffuse periocular hemorrhage in a Flavobacterium sp. S21-infected brook trout fingerling, (b) pale, mottled, hemorrhagic, and edematous kidney of a Flavobacterium sp. S21-infected brook trout fingerling.
A similar study was conducted with the Great Lakes fish-associated Chryseobacterium spp. [238], which showed that multiple isolates were highly similar to isolates previously recovered from Europe, Africa, and Asia, but were never before been reported in North America. Polyphasic characterization, which also included fatty acid profiling, highlighted the diversity of Great Lakes’ fish-associated Chryseobacterium spp. and also suggested that at least two taxa represented potentially novel Chryseobacterium spp. Experimental challenges with representative Chryseobacterium spp. isolates showed they were capable of inducing varying degrees of gross pathology (Fig. 4) in multiple Great Lakes salmonids, some of which were severe and resulted in host death. LD50 experiments for one isolate (e.g., Chryseobacterium sp. T28) demonstrated that the LD50 exceeded 4.5 × 108 cfu, indicating that it is a facultative salmonid-pathogen [238]. An investigation into the histopathological changes that accompanied T28 infection revealed epithelial hyperplasia of the secondary lamellae and interlamellar space that resulted in secondary lamellar fusion and monocytic infiltrate and mucus cell hyperplasia within primary lamellae, which are consistent with a proliferative branchitis, along with monocytic myositis, hemorrhage within the muscle, liver, adipose tissue, and ovaries, and hepatic and renal degenerative changes [238]. Median lethal dose experiments with C. aahli T68T demonstrated it was mildly pathogenic to yearling brook trout in terms of induced mortality; however, histopathological changes in infected fish were at times severe, and included a proliferative branchitis, monocytic epicarditis, pancreatitis, hepatitis, and peritonitis, multifocal hemorrhage within the muscle and adipose tissue, splenic congestion and hemosiderosis, and necrosis of the interstitial tissue within the posterior kidney [237].
Fig. 4.
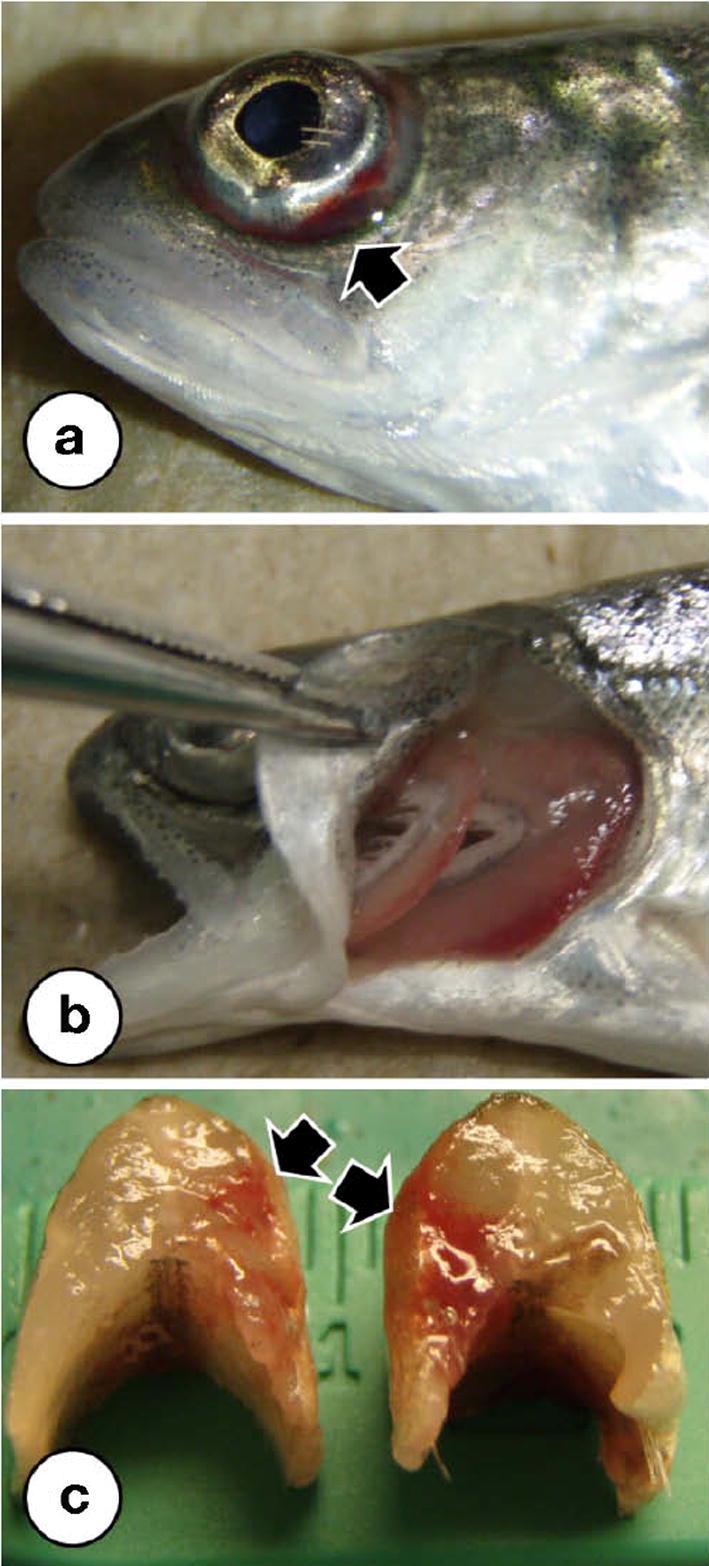
Gross lesions observed in fish experimentally challenged with Chryseobacterium spp. isolates. (a) Unilateral exophthalmia and periocular hemorrhage (arrow) in a Chinook salmon fingerling infected with Chryseobacterium sp. T31, (b) pale hemorrhagic gills of a Chinook salmon challenged with Chryseobacterium sp. T28, (c) extensive multifocal hemorrhage (arrows) within the musculature of a Chryseobacterium sp. T72 infected Chinook salmon.
Conclusions and future research
Research on flavobacteria is essential due to their role as important etiological agents of disease and their importance in microbial ecology. However, researchers working with these organisms are faced with significant challenges. First, many flavobacteria, especially those that are pathogenic to fish, are fastidious and grow only on nutrient poor media [79] that, under many conditions, must be supplemented with a variety of antibiotics to prevent overgrowth by other less fastidious bacteria. The ratio and brand of ingredients incorporated into these media, as well as osmotic conditions, can affect the ability to cultivate some flavobacteria [239–241]. Even when an ideal culture medium for a particular situation is used, the slow generation time of some species and variety in preferred incubation conditions (e.g., temperature, aerobic vs. micro-aerophillic atmosphere) can impede successful culture of all organisms.
A second challenge, which was alluded to above, is the rapid pace at which members of the family Flavobacteriaceae are discovered and described. Indeed, one must constantly search the literature to include species that must be incorporated into any identification scheme and to stay abreast with numerous changes in nomenclature [210,242]. A third challenge, especially when working with fish-associated flavobacteria, is their ubiquity in aquatic habitats and on/in the skin, gills, mucus, and intestines of fish [79]. While some of the flavobacteria that are present on apparently healthy fish have also been implicated as facultative fish pathogens (i.e., F. hydatis, F. succinicans, C. scophthalmum; [8,9,219]), the role that many flavobacteria play in fish health is not well understood. It may not be clear whether external flavobacteria are transient inhabitants of fish or whether they are normal constituents of their bacterial flora and which of the newly described species are truly pathogens. Indeed, some of the bacterial flora that are present in the skin, gills, and intestine of fish inhibited known fish pathogens [243,244], while some Chryseobacterium strains present on the skin of salamanders (Hemidactylium scutatum) exhibit antifungal activity [245] and thus may play a mutualistic role with their host.
Fourth, there is a lack of specific diagnostic reagents available to detect and identify many fish-associated flavobacteria outside of those that are commonly associated with fish disease. For example, the majority of recent reports of novel or less common fish-associated flavobacteria have based their identification on extensive polyphasic characterization as outlined by Bernardet et al. [69]. These methods work well for a definitive identification and allow for a consistent comparison among the described flavobacteria, but they necessitate culture of the organism and the technical capabilities to perform an array of biochemical and molecular assays, some of which are specialized (e.g., DNA-DNA hybridization, fatty acid methyl ester analyses).
Finally, another impediment to flavobacterial research, especially fish-pathogenic flavobacteria, is the difficulty associated with experimental challenge models to study their pathogenicity. Despite the fact that F. psychrophilum causes economically depressing disease outbreaks in fish all over the world and has been studied extensively, a truly reliable experimental model to assess its pathogenicity still does not exist. For example, experimental infections conducted by numerous authors utilizing immersion challenges have yielded highly variable results and highlight that bath infections are difficult to control, standardize, and reproduce [97,246–251]. However, Madsen and Dalsgaard [247] studied the reproducibility of F. psychrophilum challenge methods and showed that, for the most part, intraperitoneal injection of the bacterium was reproducible, though some parameters (i.e., isolate used, number of fish within the tank, origin of fish, weight of fish) could introduce variability. Thus, recent research aimed at elucidating the virulence mechanisms and pathogenesis of F. psychrophilum, as well as ways to prevent and treat the diseases it causes, now use intraperitoneal [252–254], subcutaneous [255], or intramuscular injection [256–258], even though it is recognized that these methods bypass important aspects of the innate immune system.
Conflict of interest
The authors have declared no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Acknowledgments
The authors thank the Great Lakes Fishery Trust (Lansing MI, Grant # 2010-1147), USDA-APHIS (Grant #10-9100-1293), and the Michigan Department of Natural Resources for funding, and to Mr. Dan Bjorklund and Dr. Jamie Morrison for assistance with formatting.
Biographies

Thomas P. Loch M.S., Ph.D., is currently a Post-Doctoral Research Associate in the Aquatic Animal Health Laboratory, Department of Pathobiology and Diagnostic Investigation, in the College of Veterinary Medicine at Michigan State University. He has over ten years of experience as a microbiologist and aquatic animal health professional. His research focuses on the pathogenesis, ecology, and control of the microbial pathogens that affect fishes, particularly those of the Laurentian Great Lakes.

Mohamed Faisal D.V.M., Ph.D., is currently a S.F. Snieszko Endowed Scholar and Professor of Aquatic Animal Medicine at Michigan State University. He has worked in the field of aquatic animal medicine for the last 40 years, has more than 400 peer-reviewed publications on diseases of aquatic animals, from coral reefs to marine mammals, and has mentored more than 60 masters and PhD students. He established a fish disease program at Michigan State University in collaboration with the Michigan Department of Natural Resources, the College of Agriculture and Natural Resources, and the College of Veterinary Medicine. Through this program, Dr. Faisal’s lab has studied a number of emerging and resurging infections within the USA.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Bernardet J-F., Nakagawa Y. An introduction to the family Flavobacteriaceae. In: Dworkin M., Falkow S., Rosenberg E., Schleifer K.H., Stackebrandt E., editors. vol. 7. Springer-Verlag; 2006. pp. 455–480. (The prokaryotes). [Google Scholar]
- 2.Nematollahi A., Decostere A., Pasmans F., Haesebrouck F. Flavobacterium psychrophilum infections in salmonid fish. J Fish Dis. 2003;26(10):563–574. doi: 10.1046/j.1365-2761.2003.00488.x. [DOI] [PubMed] [Google Scholar]
- 3.Starliper C.E. Bacterial coldwater disease of fishes caused by Flavobacterium psychrophilum. J Adv Res. 2011;2(2):97–108. [Google Scholar]
- 4.Hawke J.P., Thune R.L. Systemic isolation and antimicrobial susceptibility of Cytophaga columnaris from commercially reared channel catfish. J Aquat Anim Heal. 1992;4(2):109–113. [Google Scholar]
- 5.Shotts E., Starliper C. Flavobacterial diseases: columnaris disease, cold-water disease and bacterial gill disease. In: Woo P.T.K., Bruno D.W., editors. vol. 3. CABI Publishing; New York (NY): 1999. pp. 559–576. (Fish diseases and disorders: viral, bacterial and fungal infections). [Google Scholar]
- 6.Wakabayashi H., Huh G., Kimura N. Flavobacterium branchiophila sp. nov., a causative agent of bacterial gill disease of freshwater fishes. Int J Syst Bacteriol. 1989;39(3):213–216. [Google Scholar]
- 7.Christensen P.J. The history, biology, and taxonomy of the Cytophaga group. Can J Microbiol. 1977;23(12):1599–1653. doi: 10.1139/m77-236. [DOI] [PubMed] [Google Scholar]
- 8.Anderson R.L., Ordal E.J. Cytophaga succinicans sp. n., a facultatively anaerobic, aquatic myxobacterium. J Bacteriol. 1961;81(1):130. doi: 10.1128/jb.81.1.130-138.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strohl W.R., Tait L.R. Cytophaga aquatilis sp. nov., a facultative anaerobe isolated from the gills of freshwater fish. Int J Syst Bacteriol. 1978;28(2):293–303. [Google Scholar]
- 10.Austin B., Austin D.A. 4th ed. Praxis Publishing Ltd.; United Kingdom: 2007. Bacterial fish pathogens: diseases of farmed and wild fish. [Google Scholar]
- 11.United States Department of Agriculture. Reference of 2002 U.S. catfish health and production practices. Fort Collins, Colorado: Center for Epidemiology and Animal Health; 2003.
- 12.Avendaño-Herrera R., Toranzo A.E., Magariños B. Tenacibaculosis infection in marine fish caused by Tenacibaculum maritimum: a review. Dis Aquat Org. 2006;71(3):255–266. doi: 10.3354/dao071255. [DOI] [PubMed] [Google Scholar]
- 13.Toranzo A.E., Magariños B., Romalde J.L. A review of the main bacterial fish diseases in mariculture systems. Aquaculture. 2005;246(1):37–61. [Google Scholar]
- 14.Bernardet J-F., Vancanneyt M., Matte-Tailliez O., Grisez L., Tailliez P., Bizet C. Polyphasic study of Chryseobacterium strains isolated from diseased aquatic animals. Syst Appl Microbiol. 2005;28(7):640–660. doi: 10.1016/j.syapm.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 15.Flemming L., Rawlings D., Chenia H. Phenotypic and molecular characterisation of fish-borne Flavobacterium johnsoniae-like isolates from aquaculture systems in South Africa. Res Microbiol. 2007;158(1):18–30. doi: 10.1016/j.resmic.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Ilardi P., Fernández J., Avendaño-Herrera R. Chryseobacterium piscicola sp. nov., isolated from diseased salmonid fish. Int J Syst Evol Microbiol. 2009;59(12):3001–3005. doi: 10.1099/ijs.0.007021-0. [DOI] [PubMed] [Google Scholar]
- 17.Kämpfer P., Fallschissel K., Avendaño-Herrera R. Chryseobacterium chaponense sp. nov., isolated from farmed Atlantic salmon (Salmo salar) Int J Syst Evol Microbiol. 2011;61(3):497–501. doi: 10.1099/ijs.0.022004-0. [DOI] [PubMed] [Google Scholar]
- 18.Kämpfer P., Lodders N., Martin K., Avendaño-Herrera R. Flavobacterium chilense sp. nov. and Flavobacterium araucananum sp. nov., isolated from farmed salmonid fish. Int J Syst Evol Microbiol. 2012;62(6):1402–1408. doi: 10.1099/ijs.0.033431-0. [DOI] [PubMed] [Google Scholar]
- 19.Zamora L., Fernández-Garayzábal J.F., Svensson-Stadler L.A., Palacios M.A., Domínguez L., Moore E.R.B. Flavobacterium oncorhynchi sp. nov., a new species isolated from rainbow trout (Oncorhynchus mykiss) Syst Appl Microbiol. 2012;35(2):86–91. doi: 10.1016/j.syapm.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Zamora L., Fernández-Garayzábal J., Palacios M., Sánchez-Porro C., Svensson-Stadler L., Domínguez L. Chryseobacterium oncorhynchi sp. nov., isolated from rainbow trout (Oncorhynchus mykiss) Syst Appl Microbiol. 2012;35(1):24–29. doi: 10.1016/j.syapm.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 21.Zamora L., Vela A.I., Palacios M.A., Sánchez-Porro C., Svensson-Stadler L.A., Domínguez L. Chryseobacterium viscerum sp. nov., isolated from diseased fish. Int J Syst Evol Microbiol. 2012;62(12):2934–2940. doi: 10.1099/ijs.0.036699-0. [DOI] [PubMed] [Google Scholar]
- 22.Zamora L., Fernandez-Garayzabal J.F., Sanchez-Porro C., Palacios M.A., Moore E.R., Domínguez L. Flavobacterium plurextorum sp. nov. isolated from farmed rainbow trout (Oncorhynchus mykiss) PLoS One. 2013;8(6):e67741. doi: 10.1371/journal.pone.0067741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zamora L., Vela A., Sánchez-Porro C., Palacios M., Moore E., Domínguez L. Flavobacterium tructae sp. nov. and Flavobacterium piscis sp. nov., isolated from farmed rainbow trout (Oncorhynchus mykiss) Int J Syst Evol Microbiol. 2014;64(2):392–399. doi: 10.1099/ijs.0.056341-0. [DOI] [PubMed] [Google Scholar]
- 24.Zamora L., Vela A., Palacios M., Sánchez-Porro C., Moore E., Domínguez L. Chryseobacterium tructae sp. nov., isolated from rainbow trout (Oncorhynchus mykiss) Syst Appl Microbiol. 2012;35(5):315–319. doi: 10.1016/j.syapm.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 25.Loch T., Faisal M. Chryseobacterium aahli sp. nov., isolated from lake trout (Salvelinus namaycush) and brown trout (Salmo trutta), and emended descriptions of Chryseobacterium ginsenosidimutans and Chryseobacterium gregarium. Int J Syst Evol Microbiol. 2014;64(5):1573–1579. doi: 10.1099/ijs.0.052373-0. [DOI] [PubMed] [Google Scholar]
- 26.Loch T.P., Faisal M. Flavobacterium spartansii sp. nov., a pathogen of fishes, and emended descriptions of Flavobacterium aquidurense and Flavobacterium araucananum. Int J Syst Evol Microbiol. 2014;64(2):406–412. doi: 10.1099/ijs.0.051433-0. [DOI] [PubMed] [Google Scholar]
- 27.Davis H.S. Government Printing Office; 1922. A new bacterial disease of fresh-water fishes. [Google Scholar]
- 28.Ordal E.J., Rucker R.R. Pathogenic myxobacteria. Proc Soc Exp Biol Med. 1944:15–18. [Google Scholar]
- 29.Garnjobst L. Cytophaga columnaris (Davis) in pure culture: a myxobacterium pathogenic to fish. J Bacteriol. 1945;49(2):113. doi: 10.1128/jb.49.2.113-128.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bernardet J-F., Grimont P.A. Deoxyribonucleic acid relatedness and phenotypic characterization of Flexibacter columnaris sp. nov., nom. rev., Flexibacter psychrophilus sp. nov., nom. rev., and Flexibacter maritimus Wakabayashi, Hikida, and Masumura 1986. Int J Syst Bacteriol. 1989;39(3):346–354. [Google Scholar]
- 31.Leadbetter E., Order I.I. Cytophagales nomen novum. In: Buchanan R., Gibbons N., editors. Bergey’s manual of determinative bacteriology. 8th ed. Williams and Wilkins; Baltimore, MD: 1974. p. 99. [Google Scholar]
- 32.Bernardet J-F., Segers P., Vancanneyt M., Berthe F., Kersters K., Vandamme P. Cutting a Gordian knot: emended classification and description of the genus Flavobacterium, emended description of the family Flavobacteriaceae, and proposal of Flavobacterium hydatis nom. nov. (basonym, Cytophaga aquatilis Strohl and Tait 1978) Int J Syst Bacteriol. 1996;46(1):128–148. [Google Scholar]
- 33.Davis H. Further observations on the gill disease of trout. Trans Am Fish Soc. 1927;57(1):210–216. [Google Scholar]
- 34.Davis H. A new gill disease of trout. Trans Am Fish Soc. 1926;56(1):156–160. [Google Scholar]
- 35.Rucker R.R., Johnson H.E., Ordal E.J. An investigation of the bactericidal action and fish toxicity of two homologous series of quaternary ammonium compounds. J Bacteriol. 1949;57(2):225. doi: 10.1128/jb.57.2.225-234.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rucker R.R., Johnson H.E., Kaydas G.M. An interim report on gill disease. Prog Fish-Cult. 1952;14(1):10–14. [Google Scholar]
- 37.Bullock G. Studies on selected myxobacteria pathogenic for fishes and on bacterial gill disease in hatchery-reared salmonids. Technical Paper of US Bureau of Sport Fisheries and Wildlife, vol. 60; 1972. p. 3–30.
- 38.Wakabayashi H. Bacterial gill disease of salmonid fish. Fish Pathol. 1980;14(4):185–189. [Google Scholar]
- 39.Bullock G. vol. 19. U.S. Fish and Wildlife Service Fish Disease Leaflet; Washington (DC): 1990. (Bacterial gill disease of freshwater fishes). [Google Scholar]
- 40.Davis HS. Care and diseases of trout United States fish and wildlife service research report 12: US Government Printing Office, Washington, DC; 1946.
- 41.Borg A.F. University of Washington; 1948. Studies on myxobacteria associated with diseases in salmonid fishes. [Google Scholar]
- 42.Borg A.F. Studies on myxobacteria associated with diseases in salmonid fishes. Wildlife Dis Ser. 1960;8:l–85. [Google Scholar]
- 43.Pacha R., Porter S. Characteristics of myxobacteria isolated from the surface of freshwater fish. Appl Microbiol. 1968;16(12):1901–1906. doi: 10.1128/am.16.12.1901-1906.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holt RA. Cytophaga psychrophila, the causative agent of bacterial cold-water disease in salmonid fish [PhD Thesis]. Oregon State University, Corvallis; 1987.
- 45.Lorenzen E., Dalsgaard I., Bernardet J.-F. Characterization of isolates of Flavobacterium psychrophilum associated with coldwater disease or rainbow trout fry syndrome I: phenotypic and genomic studies. Dis Aquat Org. 1997;31(3):197–208. [Google Scholar]
- 46.Lorenzen E., Dalsgaard I., From J., Hansen E., Horlyck V., Korsholm H. Preliminary investigations of fry mortality syndrome in rainbow trout. Bull Eur Assoc Fish Pathol. 1991:11. [Google Scholar]
- 47.Wood J.W. State of Washington Department of Fisheries, Hatchery Division; Washington, DC: 1968. Diseases of Pacific salmon: their prevention and treatment. [Google Scholar]
- 48.Bullock G., McLaughlin J. Advances in knowledge concerning bacteria pathogenic to fishes (1954–1968) In: Snieszko S.F., editor. A symposium on diseases of fishes and shellfishes. American Fisheries Society, Special Publication; Washington (DC): 1970. pp. 231–242. [Google Scholar]
- 49.Lien SK. Relatedness of Flexibacter spp. and Flavobacterium spp. isolated from fishes [PhD Thesis]. University of Georgia; 1988.
- 50.Kent M., Dungan C., Elston R., Holt R. Cytophaga sp.(Cytophagales) infection in seawater pen-reared Atlantic salmon Salmo salar. Dis Aquat Org. 1988;4(3):173–179. [Google Scholar]
- 51.Pepin J., Emery E. Marine Cytophaga-like bacteria (CLB) isolated from diseased reared sea bass (Dicentrarchus labrax L.) from French Mediterranean coast. Bull Eur Assoc Fish Pathol. 1993;13:165–167. [Google Scholar]
- 52.Frelier P., Elston R., Loy J., Mincher C. Macroscopic and microscopic features of ulcerative stomatitis in farmed Atlantic salmon Salmo salar. Dis Aquat Org. 1994;18(3):227–231. [Google Scholar]
- 53.Starliper CE. Studies on the effects of environmental factors of fish diseases [PhD Thesis]. University of Georgia; 1992.
- 54.Bernardet J.-F., Bowman J. Genus 1. Flavobacterium. In: Krieg N., Staley J., Brown D., Hedlund B., Paster B., Ward N., editors. 2nd ed. vol. 4. Springer; Berlin: 2011. pp. 112–154. (Bergey’s manual of systematic bacteriology). [Google Scholar]
- 55.Jooste PJ. The taxonomy and significance of Flavobacterium-Cytophaga strains from dairy sources [PhD Thesis]. Bloemfontein, South Africa: University of the Orange Free State; 1985.
- 56.Reichenbach H. Order I: cytophagales leadbetter 1974. In: Staley J.T., Bryant M.P., Pfennig N., Holt J.G., editors. Bergey’s manual of systematic bacteriology. Williams and Wilkins; Baltimore, MD: 1989. [Google Scholar]
- 57.Holmes B., Owen R., Steigerwalt A., Brenner D.J. Flavobacterium gleum, a new species found in human clinical specimens. Int J Syst Bacteriol. 1984;34(1):21–25. [Google Scholar]
- 58.Vandamme P., Bernardet J.-F., Segers P., Kersters K., Holmes B. New perspectives in the classification of the Flavobacteria: description of Chryseobacterium gen. nov., Bergeyella gen. nov., and Empedobacter nom. rev. Int J Syst Bacteriol. 1994;44(4):827–831. [Google Scholar]
- 59.Holmes B., Steigerwalt A., Weaver R., Brenner D.J. Weeksella zoohelcum sp. nov. (Formerly group IIj), from human clinical specimens. Syst Appl Microbiol. 1986;8(3):191–196. [Google Scholar]
- 60.Holmes B., Snell J., Lapage S. Revised description, from clinical strains, of Flavobacterium breve (Lustig) Bergey et al. 1923 and proposal of the neotype strain. Int J Syst Bacteriol. 1978;28(2):201–208. [Google Scholar]
- 61.Holt S., Kinder S. Genus Capnocytophaga Leadbetter, Holt and Socransky 1982. In: Staley J.T., Bryant M.P., Pfennig N., Holt J.G., editors. vol. 3. Williams and Wilkins; Baltimore (MD): 1989. pp. 2050–2058. (Bergey’s manual of systematic bacteriology). [Google Scholar]
- 62.Vandamme P., Vancanneyt M., Van Belkum A., Segers P., Quint W., Kersters K. Polyphasic analysis of strains of the genus Capnocytophaga and Centers for Disease Control group DF-3. Int J Syst Bacteriol. 1996;46(3):782–791. doi: 10.1099/00207713-46-3-782. [DOI] [PubMed] [Google Scholar]
- 63.Holmes B., Steigerwalt A., Weaver R., Brenner D.J. Weeksella virosa gen. no v., sp. nov. (Formerly group IIf), found in human clinical specimens. Syst Appl Microbiol. 1986;8(3):185–190. [Google Scholar]
- 64.Segers P., Mannheim W., Vancanneyt M., De Brandt K., Hinz K.-H., Kersters K. Riemerella anatipestifer gen. nov., comb. nov., the causative agent of septicemia anserum exsudativa, and its phylogenetic affiliation within the Flavobacterium-Cytophaga rRNA homology group. Int J Syst Bacteriol. 1993;43(4):768–776. doi: 10.1099/00207713-43-4-768. [DOI] [PubMed] [Google Scholar]
- 65.Vancanneyt M., Vandamme P., Segers P., Torck U., Coopman R., Kersters K. Riemerella columbina sp. nov., a bacterium associated with respiratory disease in pigeons. Int J Syst Bacteriol. 1999;49(1):289–295. [Google Scholar]
- 66.Holmes B., Snell J., Lapage S. Revised description, from clinical isolates, of Flavobacterium odoratum Stutzer and Kwaschnina 1929, and designation of the neotype strain. Int J Syst Bacteriol. 1977;27(4):330–336. [Google Scholar]
- 67.Vancanneyt M., Segers P., Torck U., Hoste B., Bernardet J-F., Vandamme P. Reclassification of Flavobacterium odoratum (Stutzer 1929) strains to a New Genus, Myroides, as Myroides odoratus comb. nov. and Myroides odoratimimus sp. nov. Int J Syst Bacteriol. 1996;46(4):926–932. [Google Scholar]
- 68.Suzuki M., Nakagawa Y., Harayama S., Yamamoto S. Phylogenetic analysis and taxonomic study of marine Cytophaga-like bacteria: proposal for Tenacibaculum gen. nov. with Tenacibaculum maritimum comb. nov. and Tenacibaculum ovolyticum comb. nov., and description of Tenacibaculum mesophilum sp. nov. and Tenacibaculum amylolyticum sp. nov. Int J Syst Evol Microbiol. 2001;51(5):1639–1652. doi: 10.1099/00207713-51-5-1639. [DOI] [PubMed] [Google Scholar]
- 69.Bernardet J-F., Nakagawa Y., Holmes B. Proposed minimal standards for describing new taxa of the family Flavobacteriaceae and emended description of the family. Int J Syst Evol Microbiol. 2002;52(3):1049–1070. doi: 10.1099/00207713-52-3-1049. [DOI] [PubMed] [Google Scholar]
- 70.Vandamme P., Vancanneyt M., Segers P., Ryll M., Köhler B., Ludwig W. Coenonia anatina gen. nov., sp. nov., a novel bacterium associated with respiratory disease in ducks and geese. Int J Syst Bacteriol. 1999;49(2):867–874. doi: 10.1099/00207713-49-2-867. [DOI] [PubMed] [Google Scholar]
- 71.Bowman J.P., McCammon S.A., Brown J.L., Nichols P.D., McMeekin T.A. Psychroserpens burtonensis gen. nov., sp. nov., and Gelidibacter algens gen. nov., sp. nov., psychrophilic bacteria isolated from Antarctic lacustrine and sea ice habitats. Int J Syst Bacteriol. 1997;47(3):670–677. doi: 10.1099/00207713-47-3-670. [DOI] [PubMed] [Google Scholar]
- 72.Gosink J.J., Woese C.R., Staley J.T. Polaribacter gen. nov., with three new species, P. irgensii sp. nov., P. franzmannii sp. nov. and P. filamentus sp. nov., gas vacuolate polar marine bacteria of the Cytophaga-Flavobacterium-Bacteroides group and reclassification of ‘Flectobacillus glomeratus’ as Polaribacter glomeratus comb. nov. Int J Syst Bacteriol. 1998;48(1):223–235. doi: 10.1099/00207713-48-1-223. [DOI] [PubMed] [Google Scholar]
- 73.Bowman J.P., McCammon S.A., Lewis T., Skerratt J.H., Brown J.L., Nichols D.S. Psychroflexus torquis gen. nov., sp. nov. a psychrophilic species from Antarctic sea ice, and reclassification of Flavobacterium gondwanense (Dobson et al. 1993) as Psychroflexus gondwanense gen. nov. Microbiology. 1998;144(6):1601–1609. doi: 10.1099/00221287-144-6-1601. [DOI] [PubMed] [Google Scholar]
- 74.McCammon S.A., Bowman J.P. Taxonomy of Antarctic Flavobacterium species: description of Flavobacterium gillisiae sp. nov., Flavobacterium tegetincola sp. nov., and Flavobacterium xanthum sp. nov., nom. rev. and reclassification of [Flavobacterium] salegens as Salegentibacter salegens gen. nov., comb. nov. Int J Syst Evol Microbiol. 2000;50(3):1055–1063. doi: 10.1099/00207713-50-3-1055. [DOI] [PubMed] [Google Scholar]
- 75.Johansen J., Nielsen P., Sjoholm C. Description of Cellulophaga baltica gen nov, sp nov and Cellulophaga fucicola gen nov, sp nov and reclassification of (Cytophaga) lytica to Cellulophaga lytica gen nov. Int J Syst Bacteriol. 1999;49(3):1231–1240. doi: 10.1099/00207713-49-3-1231. [DOI] [PubMed] [Google Scholar]
- 76.Barbeyron T., L’Haridon S., Corre E., Kloareg B., Potin P. Zobellia galactanovorans gen. nov., sp. nov., a marine species of Flavobacteriaceae isolated from a red alga, and classification of [Cytophaga] uliginosa (ZoBell and Upham 1944) Reichenbach 1989 as Zobellia uliginosa gen. nov., comb. nov. Int J Syst Evol Microbiol. 2001;51(3):985–997. doi: 10.1099/00207713-51-3-985. [DOI] [PubMed] [Google Scholar]
- 77.Bernardet J.-F. Family I Flavobacteriaceae Reichenbach 1989. In: Whitman W., editor. vol. 4. Williams and Wilkins Co.; Baltimore (MD): 2011. pp. 106–111. (Bergey’s manual of systematic bacteriology). [Google Scholar]
- 78.Kim O-S., Cho Y-J., Lee K., Yoon S.-H., Kim M., Na H. Introducing EzTaxon-e: a prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Microbiol. 2012;62(3):716–721. doi: 10.1099/ijs.0.038075-0. [DOI] [PubMed] [Google Scholar]
- 79.Bernardet J-F., Bowman J.P. vol. 7. Springer-Verlag; New York, NY: 2006. pp. 481–531. (The genus Flavobacterium. The prokaryotes: a handbook on the biology of bacteria). [Google Scholar]
- 80.Li H., Qiao G., Gu J-Q., Zhou W., Li Q., Woo S-H. Phenotypic and genetic characterization of bacteria isolated from diseased cultured sea cucumber Apostichopus japonicus in northeastern China. Dis Aquat Org. 2010;91(3):223–235. doi: 10.3354/dao02254. [DOI] [PubMed] [Google Scholar]
- 81.Xie Z-Y., Zhou Y-C., Wang S-F., Mei B., Xu X-D., Wen W-Y. First isolation and identification of Elizabethkingia meningoseptica from cultured tiger frog, Rana tigerina rugulosa. Vet Microbiol. 2009;138(1):140–144. doi: 10.1016/j.vetmic.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 82.Hernandez-Divers S.J., Hensel P., Gladden J., Hernandez-Divers S.M., Buhlmann K.A., Hagen C. Investigation of shell disease in map turtles (Graptemys spp.) J Wildl Dis. 2009;45(3):637–652. doi: 10.7589/0090-3558-45.3.637. [DOI] [PubMed] [Google Scholar]
- 83.Haburjak J., Schubert T. Flavobacterium breve meningitis in a dog. J Am Anim Hosp Assoc. 1997;33(6):509–512. doi: 10.5326/15473317-33-6-509. [DOI] [PubMed] [Google Scholar]
- 84.Benedetti P., Rassu M., Pavan G., Sefton A., Pellizzer G. Septic shock, pneumonia, and soft tissue infection due to Myroides odoratimimus: report of a case and review of Myroides infections. Infection. 2011;39(2):161–165. doi: 10.1007/s15010-010-0077-1. [DOI] [PubMed] [Google Scholar]
- 85.Bergey D., Harrison F., Breed R., Hammer B., Huntoon F. 1st ed. Williams and Wilkins; Baltimore, MD: 1923. Bergey’s manual of determinative bacteriology. [Google Scholar]
- 86.McMeekin T., Shewan J. Taxonomic strategies for Flavobacterium and related genera. J Appl Microbiol. 1978;45(3):321–332. doi: 10.1111/j.1365-2672.1978.tb04232.x. [DOI] [PubMed] [Google Scholar]
- 87.Holmes B., Owen R., McMeekin T. Genus Flavobacterium Bergey, Harrison, Breed, Hammer and Huntoon, 1923, 97. In: Krieg N.R., Holt J.G., editors. vol. 1. Williams and Wilkins; Baltimore (MD): 1984. pp. 353–361. (Bergey’s manual of systematic bacteriology). [Google Scholar]
- 88.Frankland G.C., Frankland P.F. Ueber einige typische Mikroorganismen im Wasser und im Boden. Med Microbiol Immunol. 1889;6(1):373–400. [Google Scholar]
- 89.Anacker R.L., Ordal E.J. Study of a bacteriophage infecting the myxobacterium Chondrococcus columnaris. J Bacteriol. 1955;70(6):738. doi: 10.1128/jb.70.6.738-741.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shieh H. Studies on the nutrition of a fish pathogen, Flexibacter columnaris. Microbios Lett. 1980;13(51/52):129–133. [Google Scholar]
- 91.Bullock G, Hsu T-C, Shotts Jr E. Columnaris disease of fishes. Service USFaW; 1986.
- 92.Starliper CE, Marcquenski S, Noyes A. Development of an improved medium for primary isolation of Flavobacterium psychrophilum, cause of bacterial coldwater disease. Great Lakes Fishery Commission Research Report; 2007.
- 93.Morrison C., Cornick J., Shum G., Zwicker B. Microbiology and histopathology of ‘saddleback’disease of underyearling Atlantic salmon, Salmo salar L. J Fish Dis. 1981;4(3):243–258. [Google Scholar]
- 94.Panangalal V., Shelby R.A., Shoemaker C.A., Klesius P.H., Mitra A., Morrison E.E. Immunofluorescent test for simultaneous detection of Edwardsiella ictaluri and Flavobacterium columnare. Dis Aquat Org. 2006;68(3):197–207. doi: 10.3354/dao068197. [DOI] [PubMed] [Google Scholar]
- 95.Lindstrom N.M., Call D.R., House M.L., Moffitt C.M., Cain K.D. A quantitative enzyme-linked immunosorbent assay and filtration-based fluorescent antibody test as potential tools to screen broodstock for infection with Flavobacterium psychrophilum. J Aquat Anim Heal. 2009;21(1):43–56. doi: 10.1577/H08-031.1. [DOI] [PubMed] [Google Scholar]
- 96.MacPhee D., Ostland V., Lumsden J., Ferguson H. Development of an enzyme-linked immunosorbent assay (ELISA) to estimate the quantity of Flavobacterium branchiophilum on the gills of rainbow trout Oncorhynchus mykiss. Dis Aquat Org. 1995;21(1):13–23. [Google Scholar]
- 97.Liu H., Izumi S., Wakabayashi H. Detection of Flavobacterium psychrophilum in various organs of ayu Plecoglossus altivelis by in situ hybridization. Fish Pathol. 2001 [Google Scholar]
- 98.Yeh H.Y., Shoemaker C., Klesius P. Sensitive and rapid detection of Flavobacterium columnare in channel catfish Ictalurus punctatus by a loop-mediated isothermal amplification method. J Appl Microbiol. 2006;100(5):919–925. doi: 10.1111/j.1365-2672.2006.02853.x. [DOI] [PubMed] [Google Scholar]
- 99.Fujiwara-Nagata E., Eguchi M. Development and evaluation of a loop-mediated isothermal amplification assay for rapid and simple detection of Flavobacterium psychrophilum. J Fish Dis. 2009;32(10):873–881. doi: 10.1111/j.1365-2761.2009.01066.x. [DOI] [PubMed] [Google Scholar]
- 100.Toyama T., Kita-Tsukamoto K., Wakabayashi H. Identification of Cytophaga psychrophila by PCR targeted 16S ribosomal RNA. Fish Pathol. 1994;29(4):271–275. [Google Scholar]
- 101.Toyama T., Kita-Tsukamoto K., Wakabayashi H. Identification of Flexibacter maritimus, Flavobacterium branchiophilum and Cytophaga columnaris by PCR targeted 16S ribosomal DNA. Fish Pathol. 1996;31(1):25–31. [Google Scholar]
- 102.Bader J.A., Shotts E.B., Jr Identification of Flavobacterium and Flexibacter species by species-specific polymerase chain reaction primers to the 16S ribosomal RNA gene. J Aquat Anim Heal. 1998;10(4):311–319. [Google Scholar]
- 103.Triyanto, Kumamaru A., Wakabayashi H. The use of PCR targeted 16S rDNA for identification of genomovars of Flavobacterium columnare. Fish Pathol. 1999;34(4):217–218. [Google Scholar]
- 104.Wiklund T., Madsen L., Bruun M.S., Dalsgaard I. Detection of Flavobacterium psychrophilum from fish tissue and water samples by PCR amplification. J Appl Microbiol. 2000;88(2):299–307. doi: 10.1046/j.1365-2672.2000.00959.x. [DOI] [PubMed] [Google Scholar]
- 105.Bader J.A., Shoemaker C.A., Klesius P.H. Rapid detection of columnaris disease in channel catfish (Ictalurus punctatus) with a new species-specific 16S rRNA gene-based PCR primer for Flavobacterium columnare. J Microbiol Methods. 2003;52(2):209–220. doi: 10.1016/s0167-7012(02)00208-7. [DOI] [PubMed] [Google Scholar]
- 106.Crumlish M., Diab A., George S., Ferguson H. Detection of the bacterium Flavobacterium psychrophilum from a natural infection in rainbow trout, Oncorhynchus mykiss (Walbaum), using formalin-fixed, wax-embedded fish tissues. J Fish Dis. 2007;30(1):37–41. doi: 10.1111/j.1365-2761.2007.00779.x. [DOI] [PubMed] [Google Scholar]
- 107.Hibi K., Ushio H., Fukuda H., Mitsubayashi K., Hayashi T., Ren H. Immunomagnetic separation using carbonyl iron powder and flow cytometry for rapid detection of Flavobacterium psychrophilum. Anal Bioanal Chem. 2008;391(4):1147–1152. doi: 10.1007/s00216-008-2110-0. [DOI] [PubMed] [Google Scholar]
- 108.Orieux N., Bourdineaud J.P., Douet D.G., Daniel P., Le Henaff M. Quantification of Flavobacterium psychrophilum in rainbow trout, Oncorhynchus mykiss (Walbaum), tissues by qPCR. J Fish Dis. 2011;34(11):811–821. doi: 10.1111/j.1365-2761.2011.01296.x. [DOI] [PubMed] [Google Scholar]
- 109.Marancik D.P., Wiens G.D. A real-time polymerase chain reaction assay for identification and quantification of Flavobacterium psychrophilum and application to disease resistance studies in selectively bred rainbow trout Oncorhynchus mykiss. FEMS Microbiol Lett. 2013;339(2):122–129. doi: 10.1111/1574-6968.12061. [DOI] [PubMed] [Google Scholar]
- 110.Lievens B., Frans I., Heusdens C., Justé A., Jonstrup S.P., Lieffrig F. Rapid detection and identification of viral and bacterial fish pathogens using a DNA array-based multiplex assay. J Fish Dis. 2011;34(11):861–875. doi: 10.1111/j.1365-2761.2011.01304.x. [DOI] [PubMed] [Google Scholar]
- 111.Qu J-H., Yuan H-L., Li H-F., Deng C-P. Flavobacterium cauense sp. nov., isolated from sediment of a eutrophic lake. Int J Syst Evol Microbiol. 2009;59(11):2666–2669. doi: 10.1099/ijs.0.009688-0. [DOI] [PubMed] [Google Scholar]
- 112.Lee S.H., Kim J.M., Lee J.R., Park W., Jeon C.O. Flavobacterium fluvii sp. nov., isolated from stream sediment. Int J Syst Evol Microbiol. 2010;60(2):353–357. doi: 10.1099/ijs.0.010850-0. [DOI] [PubMed] [Google Scholar]
- 113.Xu M., Xin Y., Tian J., Dong K., Yu Y., Zhang J. Flavobacterium sinopsychrotolerans sp. nov., isolated from a glacier. Int J Syst Evol Microbiol. 2011;61(1):20–24. doi: 10.1099/ijs.0.014126-0. [DOI] [PubMed] [Google Scholar]
- 114.Dong K., Liu H., Zhang J., Zhou Y., Xin Y. Flavobacterium xueshanense sp. nov. and Flavobacterium urumqiense sp. nov., two psychrophilic bacteria isolated from glacier ice. Int J Syst Evol Microbiol. 2012;62(5):1151–1157. doi: 10.1099/ijs.0.030049-0. [DOI] [PubMed] [Google Scholar]
- 115.Kim J.-J., Jin H.M., Lee H.J., Jeon C.O., Kanaya E., Koga Y. Flavobacterium banpakuense sp. nov., isolated from leaf-and-branch compost. Int J Syst Evol Microbiol. 2011;61(7):1595–1600. doi: 10.1099/ijs.0.022467-0. [DOI] [PubMed] [Google Scholar]
- 116.Liu M., Li Y.H., Liu Y., Zhu J.N., Liu Q.F., Liu Y. Flavobacterium phragmitis sp. nov., an endophyte of reed (Phragmites australis) Int J Syst Evol Microbiol. 2011;61(11):2717–2721. doi: 10.1099/ijs.0.027417-0. [DOI] [PubMed] [Google Scholar]
- 117.Lim C-S., Oh Y-S., Lee J-K., Park A-R., Yoo J-S., Rhee S-K. Flavobacterium chungbukense sp. nov., isolated from soil. Int J Syst Evol Microbiol. 2011;61(11):2734–2739. doi: 10.1099/ijs.0.028563-0. [DOI] [PubMed] [Google Scholar]
- 118.Sheu S-Y., Chiu T-F., Young C-C., Arun A., Chen W-M. Flavobacterium macrobrachii sp. nov., isolated from a freshwater shrimp culture pond. Int J Syst Evol Microbiol. 2011;61(6):1402–1407. doi: 10.1099/ijs.0.025403-0. [DOI] [PubMed] [Google Scholar]
- 119.Fu Y., Tang X., Lai Q., Zhang C., Zhong H., Li W. Flavobacterium beibuense sp. nov., isolated from marine sediment. Int J Syst Evol Microbiol. 2011;61(1):205–209. doi: 10.1099/ijs.0.018846-0. [DOI] [PubMed] [Google Scholar]
- 120.Yoon J-H., Park S., Kang S-J., Oh S-J., Myung S.C., Kim W. Flavobacterium ponti sp. nov., isolated from seawater. Int J Syst Evol Microbiol. 2011;61(1):81–85. doi: 10.1099/ijs.0.017582-0. [DOI] [PubMed] [Google Scholar]
- 121.Ryu S.H., Park M., Jeon Y., Lee J.R., Park W., Jeon C.O. Flavobacterium filum sp. nov., isolated from a wastewater treatment plant in Korea. Int J Syst Evol Microbiol. 2007;57(9):2026–2030. doi: 10.1099/ijs.0.65138-0. [DOI] [PubMed] [Google Scholar]
- 122.Zhang J., Jiang R-B., Zhang X.-X., Hang B-J., He J., Li S-P. Flavobacterium haoranii sp. nov., a cypermethrin-degrading bacterium isolated from a wastewater treatment system. Int J Syst Evol Microbiol. 2010;60(12):2882–2886. doi: 10.1099/ijs.0.020776-0. [DOI] [PubMed] [Google Scholar]
- 123.Miyashita M., Fujimura S., Nakagawa Y., Nishizawa M., Tomizuka N., Nakagawa T. Flavobacterium algicola sp. nov., isolated from marine algae. Int J Syst Evol Microbiol. 2010;60(2):344–348. doi: 10.1099/ijs.0.009365-0. [DOI] [PubMed] [Google Scholar]
- 124.Horn M., Harzenetter M.D., Linner T., Schmid E.N., Müller K.D., Michel R. Members of the Cytophaga–Flavobacterium–Bacteroides phylum as intracellular bacteria of acanthamoebae: proposal of ‘Candidatus Amoebophilus asiaticus’. Environ Microbiol. 2001;3(7):440–449. doi: 10.1046/j.1462-2920.2001.00210.x. [DOI] [PubMed] [Google Scholar]
- 125.Horn M.A., Ihssen J., Matthies C., Schramm A., Acker G., Drake H.L. Dechloromonas denitrificans sp. nov., Flavobacterium denitrificans sp. nov., Paenibacillus anaericanus sp. nov. and Paenibacillus terrae strain MH72, N2O-producing bacteria isolated from the gut of the earthworm Aporrectodea caliginosa. Int J Syst Evol Microbiol. 2005;55(3):1255–1265. doi: 10.1099/ijs.0.63484-0. [DOI] [PubMed] [Google Scholar]
- 126.Xiang H., Wei G-F., Jia S., Huang J., Miao X-X., Zhou Z. Microbial communities in the larval midgut of laboratory and field populations of cotton bollworm (Helicoverpa armigera) Can J Microbiol. 2006;52(11):1085–1092. doi: 10.1139/w06-064. [DOI] [PubMed] [Google Scholar]
- 127.Campbell C.L., Mummey D.L., Schmidtmann E.T., Wilson W.C. Culture-independent analysis of midgut microbiota in the arbovirus vector Culicoides sonorensis (Diptera: Ceratopogonidae) J Med Entomol. 2004;41(3):340–348. doi: 10.1603/0022-2585-41.3.340. [DOI] [PubMed] [Google Scholar]
- 128.Park H.W., Kim Y.O., Ha J-S., Youn S.H., Kim H.H., Bilgrami A.L. Effects of associated bacteria on the pathogenicity and reproduction of the insect-parasitic nematode Rhabditis blumi (Nematoda: Rhabditida) Can J Microbiol. 2011;57(9):750–758. doi: 10.1139/w11-067. [DOI] [PubMed] [Google Scholar]
- 129.Schulz C.A., Thomas M.V., Fitzgerald S., Faisal M. Leeches (Annelida: Hirudinida) parasitizing fish of Lake St. Clair, Michigan, U.S.A. Comp Parasitol. 2011;78(1):73–83. [Google Scholar]
- 130.Kannapiran E., Ravindran J. Dynamics and diversity of phosphate mineralizing bacteria in the coral reefs of Gulf of Mannar. J Basic Microbiol. 2012;52(1):91–98. doi: 10.1002/jobm.201100095. [DOI] [PubMed] [Google Scholar]
- 131.Imhoff J.F., Stöhr R. Springer; Sponges (Porifera): 2003. Sponge-associated bacteria: general overview and special aspects of bacteria associated with Halichondria panicea. p. 35–57. [DOI] [PubMed] [Google Scholar]
- 132.Vela A., Fernandez A., Sánchez-Porro C., Sierra E., Mendez M., Arbelo M. Flavobacterium ceti sp. nov., isolated from beaked whales (Ziphius cavirostris) Int J Syst Evol Microbiol. 2007;57(11):2604–2608. doi: 10.1099/ijs.0.65154-0. [DOI] [PubMed] [Google Scholar]
- 133.Kim K.K., Kim M.K., Lim J.H., Park H.Y., Lee S.-T. Transfer of Chryseobacterium meningosepticum and Chryseobacterium miricola to Elizabethkingia gen. nov. as Elizabethkingia meningoseptica comb. nov. and Elizabethkingia miricola comb. nov. Int J Syst Evol Microbiol. 2005;55(3):1287–1293. doi: 10.1099/ijs.0.63541-0. [DOI] [PubMed] [Google Scholar]
- 134.Huber I., Spanggaard B., Appel K., Rossen L., Nielsen T., Gram L. Phylogenetic analysis and in situ identification of the intestinal microbial community of rainbow trout (Oncorhynchus mykiss, Walbaum) J Appl Microbiol. 2004;96(1):117–132. doi: 10.1046/j.1365-2672.2003.02109.x. [DOI] [PubMed] [Google Scholar]
- 135.Hu C., Xu Y., Xia M., Xiong L., Xu Z. Effects of Cu 2+-exchanged montmorillonite on growth performance, microbial ecology and intestinal morphology of Nile tilapia (Oreochromis niloticus) Aquaculture. 2007;270(1):200–206. [Google Scholar]
- 136.Farkas J. Filamentous Flavobacterium sp. isolated from fish with gill diseases in cold water. Aquaculture. 1985;44(1):1–10. [Google Scholar]
- 137.Brown L., Cox W., Levine R. Evidence that the causal agent of bacterial cold-water disease Flavobacterium psychrophilum is transmitted within salmonid eggs. Dis Aquat Org. 1997;29(3):213–218. [Google Scholar]
- 138.Rangdale R., Richards R., Alderman D. Isolation of Cytophaga psychrophila, causal agent of rainbow trout fry syndrome (RTFS) from reproductive fluids and egg surfaces of rainbow trout (Oncorhynchus mykiss) Bull Eur Assoc Fish Pathol. 1996;16(2):63–67. [Google Scholar]
- 139.Shewan J. The microbiology of sea-water fish. In: Borgstrom G., editor. vol. 1. Academic Press; New York: 1961. pp. 487–560. (Fish as food). [Google Scholar]
- 140.Trust T. Bacteria associated with the gills of salmonid fishes in freshwater. J Appl Bacteriol. 1975;38(3):225–233. doi: 10.1111/j.1365-2672.1975.tb00527.x. [DOI] [PubMed] [Google Scholar]
- 141.Liao C-H., Wells J.M. Properties of Cytophaga johnsonae strains causing spoilage of fresh produce at food markets. Appl Environ Microbiol. 1986;52(6):1261–1265. doi: 10.1128/aem.52.6.1261-1265.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lelliott R.A., Stead D.E. Blackwell Scientific Publications; Oxford (UK): 1987. Methods for the diagnosis of bacterial diseases of plants. [Google Scholar]
- 143.Bullock G.L., Conroy D.A., Snieszko S.F. Bacterial diseases of fishes. In: Snieszko S.F., Axelrod H.R., editors. Diseases of fish. TFH Publications; Jersey City (NJ): 1971. [Google Scholar]
- 144.Flaherty D.K., Deck F.H., Hood M.A., Liebert C., Singleton F., Winzenburger P. A Cytophaga species endotoxin as a putative agent of occupation-related lung disease. Infect Immun. 1984;43(1):213–216. doi: 10.1128/iai.43.1.213-216.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liebert C., Hood M., Deck F., Bishop K., Flaherty D. Isolation and characterization of a new Cytophaga species implicated in a work-related lung disease. Appl Environ Microbiol. 1984;48(5):936–943. doi: 10.1128/aem.48.5.936-943.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tian G.Z., Piao D.R., Zhao H.Y., Jiang H., Cui B.Y., Li J.Y. A Flavobacterium lindanitolerans strain isolated from the ascites sample of a Chinese patient with EV71 virus infection. Biomed Environ Sci. 2011;24(6):694–696. doi: 10.3967/0895-3988.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 147.Wood J. Washington Department of Fisheries; Olympia (WA): 1974. Diseases of Pacific salmon, their prevention and treatment. [Google Scholar]
- 148.Cipriano RC, Holt RA. Flavobacterium psychrophilum, cause of bacterial cold-water disease and rainbow trout fry syndrome: US Department of the Interior, US Geological Survey, National Fish Health Research Laboratory; 2005.
- 149.Barnes M.E., Brown M.L. A review of Flavobacterium psychrophilum biology, clinical signs, and bacterial cold water disease prevention and treatment. Open Fish Sci J. 2011:4. [Google Scholar]
- 150.Amend D. Columnaris (Flexibacter columnaris) disease of freshwater fishes and a brief review of other flexibacterial diseases of fish. In: Anderson D.P., Dorson M.M., Dubourget P., editors. Antigens of fish pathogens. Collection Foundation Marcel Mérieux; Lyon, France: 1983. pp. 139–161. [Google Scholar]
- 151.Kunttu H. Characterizing the bacterial fish pathogen Flavobacterium columnare, and some factors affecting its pathogenicity. Biol Environ Sci. 2010;206:1–69. [Google Scholar]
- 152.Eissa A.E., Zaki M.M., Aziz A.A. Flavobacterium columnare/Myxobolus tilapiae concurrent infection in the earthen pond reared Nile Tilapia (Oreochromis niloticus) during the early summer. Interdisc Bio Cent. 2010;2(1):5. [Google Scholar]
- 153.Snieszko SF. Bacterial gill disease of freshwater fishes. U.S. Fish Wildlife Service Fish Disease Leaflet, vol. 62; 1981.
- 154.Ferguson H., Ostland V., Byrne P., Lumsdsen J. Experimental production of bacterial gill disease in trout by horizontal transmission and by bath challenge. J Aquat Anim Heal. 1991;3(2):118–123. [Google Scholar]
- 155.Ostland V., Lumsden J., MacPhee D., Ferguson H. Characteristics of Flavobacterium branchiophilum, the cause of salmonid bacterial gill disease in Ontario. J Aquat Anim Heal. 1994;6(1):13–26. [Google Scholar]
- 156.Good C.M., Thorburn M.A., Stevenson R.M. Factors associated with the incidence of bacterial gill disease in salmonid lots reared in Ontario, Canada government hatcheries. Prev Vet Med. 2008;83(3):297–307. doi: 10.1016/j.prevetmed.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 157.Carson J., Schmidtke L., Munday B. Cytophaga johnsonae: a putative skin pathogen of juvenile farmed barramundi, Lates calcarifer Bloch. J Fish Dis. 1993;16(3):209–218. [Google Scholar]
- 158.Soltani M., Munday B., Carson J. Susceptibility of some freshwater species of fish to infection by Cytophaga johnsonae. Bull Eur Assoc Fish Pathol. 1994;14:133–135. [Google Scholar]
- 159.Rintamäki-Kinnunen P., Bernardet J.-F., Bloigu A. Yellow pigmented filamentous bacteria connected with farmed salmonid fish mortality. Aquaculture. 1997;149(1):1–14. [Google Scholar]
- 160.Karatas S., Ercan D., Steinum T.M., Turgay E., Memis D., Candan A. First isolation of a Flavobacterium johnsoniae like bacteria from cultured Russian sturgeon in Turkey. J Anim Vet Adv. 2010;9(14):1943–1946. [Google Scholar]
- 161.Suebsing R.K., Kim J.H. Isolation and Characterization of Flavobacterium johnsoniae from Farmed Rainbow Trout Oncorhynchus mykiss. Can J Fish Aquat Sci. 2012;15(1):83–89. [Google Scholar]
- 162.Zamora L., Vela A., Sánchez-Porro C., Palacios M., Domínguez L., Moore E. Characterization of flavobacteria possibly associated with fish and fish farm environment: description of three novel Flavobacterium species: Flavobacterium collinsii sp. nov., Flavobacterium branchiarum sp. nov., and Flavobacterium branchiicola sp. nov. Aquaculture. 2013;416:346–353. [Google Scholar]
- 163.Holliman A., Austin B., Pottinger T. A previously unrecognised Cytophage-like bacterium (CLB) causing jaw erosion in farmed rainbow trout (Oncorhynchus mykiss) Bull Eur Assoc Fish Pathol. 1991;11:114–115. [Google Scholar]
- 164.Bowman J., Nowak B. Salmonid gill bacteria and their relationship to amoebic gill disease. J Fish Dis. 2004;27(8):483–492. doi: 10.1111/j.1365-2761.2004.00569.x. [DOI] [PubMed] [Google Scholar]
- 165.Bernardet J-F., Hugo C., Bruun B. 3rd ed. Springer; 2006. The genera Chryseobacterium and Elizabethkingia The prokaryotes: a handbook on the biology of bacteria. p. 638–76. [Google Scholar]
- 166.Bernardet J.-F., Hugo C.J., Bruun B. Genus VII. Chryseobacterium Vandamme et al. 1994. In: Krieg N.R., Staley J.T., Brown D.R., Hedlund B.P., Paster B.J., Ward N.L., Ludwig W., Whitman W., editors. vol. 4. Springer; New York: 2011. pp. 180–196. (Bergey’s manual of systematic bacteriology). [Google Scholar]
- 167.Fernández-Olmos A., García-Castillo M., Morosini M-I., Lamas A., Máiz L., Cantón R. MALDI-TOF MS improves routine identification of non-fermenting Gram negative isolates from cystic fibrosis patients. J Cyst Fib. 2012;11(1):59–62. doi: 10.1016/j.jcf.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 168.Daroy M., Lopez J., Torres B., Loy M., Tuaño P., Matias R. Identification of unknown ocular pathogens in clinically suspected eye infections using ribosomal RNA gene sequence analysis. Clin Microbiol Infect. 2011;17(5):776–779. doi: 10.1111/j.1469-0691.2010.03369.x. [DOI] [PubMed] [Google Scholar]
- 169.Benmalek Y., Cayol J-L., Bouanane N.A., Hacene H., Fauque G., Fardeau M.-L. Chryseobacterium solincola sp. nov., isolated from soil. Int J Syst Evol Microbiol. 2010;60(8):1876–1880. doi: 10.1099/ijs.0.008631-0. [DOI] [PubMed] [Google Scholar]
- 170.Im W-T., Yang J-E., Kim S-Y., Yi T-H. Chryseobacterium ginsenosidimutans sp. nov., a bacterium with ginsenoside-converting activity isolated from soil of a Rhus vernicifera-cultivated field. Int J Syst Evol Microbiol. 2011;61(6):1430–1435. doi: 10.1099/ijs.0.023614-0. [DOI] [PubMed] [Google Scholar]
- 171.Li Z., Zhu H. Chryseobacterium vietnamense sp. nov., isolated from forest soil. Int J Syst Evol Microbiol. 2012;62(4):827–831. doi: 10.1099/ijs.0.027201-0. [DOI] [PubMed] [Google Scholar]
- 172.Park M.S., Jung S.R., Lee K.H., Lee M-S., Do J.O., Kim S.B. Chryseobacterium soldanellicola sp. nov. and Chryseobacterium taeanense sp. nov., isolated from roots of sand-dune plants. Int J Syst Evol Microbiol. 2006;56(2):433–438. doi: 10.1099/ijs.0.63825-0. [DOI] [PubMed] [Google Scholar]
- 173.Luis N-B.J., Hugo J-I., Enrique B-A., Ramiro R-M., Octavio P-L. An optimization study of solid-state fermentation: xanthophylls extraction from marigold flowers. Appl Microbiol Biotechnol. 2004;65(4):383–390. doi: 10.1007/s00253-004-1615-5. [DOI] [PubMed] [Google Scholar]
- 174.Behrendt U., Ulrich A., Schumann P. Chryseobacterium gregarium sp. nov., isolated from decaying plant material. Int J Syst Evol Microbiol. 2008;58(5):1069–1074. doi: 10.1099/ijs.0.65544-0. [DOI] [PubMed] [Google Scholar]
- 175.Lagacé L., Pitre M., Jacques M., Roy D. Identification of the bacterial community of maple sap by using amplified ribosomal DNA (rDNA) restriction analysis and rDNA sequencing. Appl Environ Microbiol. 2004;70(4):2052–2060. doi: 10.1128/AEM.70.4.2052-2060.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Strahan B.L., Failor K.C., Batties A.M., Hayes P.S., Cicconi K.M., Mason C.T. Chryseobacterium piperi sp. nov., isolated from a freshwater creek. Int J Syst Evol Microbiol. 2011;61(9):2162–2166. doi: 10.1099/ijs.0.027805-0. [DOI] [PubMed] [Google Scholar]
- 177.Joung Y., Joh K. Chryseobacterium yonginense sp. nov., isolated from a mesotrophic artificial lake. Int J Syst Evol Microbiol. 2011;61(6):1413–1417. doi: 10.1099/ijs.0.022590-0. [DOI] [PubMed] [Google Scholar]
- 178.Kim K.K., Bae H-S., Schumann P., Lee S-T. Chryseobacterium daecheongense sp. nov., isolated from freshwater lake sediment. Int J Syst Evol Microbiol. 2005;55(1):133–138. doi: 10.1099/ijs.0.02931-0. [DOI] [PubMed] [Google Scholar]
- 179.Park S.C., Kim M.S., Baik K.S., Kim E.M., Rhee M.S., Seong C.N. Chryseobacterium aquifrigidense sp. nov., isolated from a water-cooling system. Int J Syst Evol Microbiol. 2008;58(3):607–611. doi: 10.1099/ijs.0.65475-0. [DOI] [PubMed] [Google Scholar]
- 180.Gallego V., García M.T., Ventosa A. Chryseobacterium hispanicum sp. nov., isolated from the drinking water distribution system of Sevilla, Spain. Int J Syst Evol Microbiol. 2006;56(7):1589–1592. doi: 10.1099/ijs.0.64264-0. [DOI] [PubMed] [Google Scholar]
- 181.Shimomura K., Kaji S., Hiraishi A. Chryseobacterium shigense sp. nov., a yellow-pigmented, aerobic bacterium isolated from a lactic acid beverage. Int J Syst Evol Microbiol. 2005;55(5):1903–1906. doi: 10.1099/ijs.0.63690-0. [DOI] [PubMed] [Google Scholar]
- 182.Herzog P., Winkler I., Wolking D., Kämpfer P., Lipski A. Chryseobacterium ureilyticum sp. nov., Chryseobacterium gambrini sp. nov., Chryseobacterium pallidum sp. nov. and Chryseobacterium molle sp. nov., isolated from beer-bottling plants. Int J Syst Evol Microbiol. 2008;58(1):26–33. doi: 10.1099/ijs.0.65362-0. [DOI] [PubMed] [Google Scholar]
- 183.Quan Z-X., Kim K.K., Kim M-K., Jin L., Lee S-T. Chryseobacterium caeni sp. nov., isolated from bioreactor sludge. Int J Syst Evol Microbiol. 2007;57(1):141–145. doi: 10.1099/ijs.0.64599-0. [DOI] [PubMed] [Google Scholar]
- 184.Zhou Y., Dong J., Wang X., Huang X., Zhang K-Y., Zhang Y-Q. Chryseobacterium flavum sp. nov., isolated from polluted soil. Int J Syst Evol Microbiol. 2007;57(8):1765–1769. doi: 10.1099/ijs.0.65046-0. [DOI] [PubMed] [Google Scholar]
- 185.Campbell L., Williams O. A study of chitin-decomposing micro-organisms of marine origin. J Gen Microbiol. 1951;5(5):894–905. doi: 10.1099/00221287-5-5-894. [DOI] [PubMed] [Google Scholar]
- 186.Loveland-Curtze J., Miteva V., Brenchley J. Novel ultramicrobacterial isolates from a deep Greenland ice core represent a proposed new species, Chryseobacterium greenlandense sp. nov. Extremophiles. 2010;14(1):61–69. doi: 10.1007/s00792-009-0287-6. [DOI] [PubMed] [Google Scholar]
- 187.Kämpfer P., Chandel K., Prasad G., Shouche Y., Veer V. Chryseobacterium culicis sp. nov., isolated from the midgut of the mosquito Culex quinquefasciatus. Int J Syst Evol Microbiol. 2010;60(10):2387–2391. doi: 10.1099/ijs.0.019794-0. [DOI] [PubMed] [Google Scholar]
- 188.Dugas J., Zurek L., Paster B., Keddie B., Leadbetter E. Isolation and characterization of a Chryseobacterium strain from the gut of the American cockroach, Periplaneta americana. Arch Microbiol. 2001;175(4):259–262. doi: 10.1007/s002030000243. [DOI] [PubMed] [Google Scholar]
- 189.Kämpfer P., Arun A., Young C-C., Chen W-M., Sridhar K., Rekha P. Chryseobacterium arthrosphaerae sp. nov., isolated from the faeces of the pill millipede Arthrosphaera magna Attems. Int J Syst Evol Microbiol. 2010;60(8):1765–1769. doi: 10.1099/ijs.0.016840-0. [DOI] [PubMed] [Google Scholar]
- 190.Zdanowski M.K., Weglenski P., Golik P., Sasin J.M., Borsuk P., Zmuda M.J. Bacterial diversity in Adélie penguin, Pygoscelis adeliae, guano: molecular and morpho-physiological approaches. FEMS Microbiol Ecol. 2004;50(3):163–173. doi: 10.1016/j.femsec.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 191.Homonnay Z.G., Kéki Z., Márialigeti K., Tóth E.M. Bacterial communities in the gut of the freshwater copepod Eudiaptomus gracilis. J Basic Microbiol. 2012;52(1):86–90. doi: 10.1002/jobm.201100052. [DOI] [PubMed] [Google Scholar]
- 192.Riffel A., Lucas F., Heeb P., Brandelli A. Characterization of a new keratinolytic bacterium that completely degrades native feather keratin. Arch Microbiol. 2003;179(4):258–265. doi: 10.1007/s00203-003-0525-8. [DOI] [PubMed] [Google Scholar]
- 193.Hugo C.J., Segers P., Hoste B., Vancanneyt M., Kersters K. Chryseobacterium joostei sp. nov., isolated from the dairy environment. Int J Syst Evol Microbiol. 2003;53(3):771–777. doi: 10.1099/ijs.0.02232-0. [DOI] [PubMed] [Google Scholar]
- 194.Hantsis-Zacharov E., Halpern M. Chryseobacterium haifense sp. nov., a psychrotolerant bacterium isolated from raw milk. Int J Syst Evol Microbiol. 2007;57(10):2344–2348. doi: 10.1099/ijs.0.65115-0. [DOI] [PubMed] [Google Scholar]
- 195.Hantsis-Zacharov E., Shakéd T., Senderovich Y., Halpern M. Chryseobacterium oranimense sp. nov., a psychrotolerant, proteolytic and lipolytic bacterium isolated from raw cow’s milk. Int J Syst Evol Microbiol. 2008;58(11):2635–2639. doi: 10.1099/ijs.0.65819-0. [DOI] [PubMed] [Google Scholar]
- 196.Hantsis-Zacharov E., Senderovich Y., Halpern M. Chryseobacterium bovis sp. nov., isolated from raw cow’s milk. Int J Syst Evol Microbiol. 2008;58(4):1024–1028. doi: 10.1099/ijs.0.65500-0. [DOI] [PubMed] [Google Scholar]
- 197.Garcia-Lopez M., Prieto M., Otero A. The physiological attributes of Gram-negative bacteria associated with spoilage of meat and meat products. In: Davies A., Board R., editors. The microbiology of meat and poultry. Blackie Academic and Professional; London (UK): 1998. pp. 1–34. [Google Scholar]
- 198.de Beer H., Hugo C.J., Jooste P.J., Willems A., Vancanneyt M., Coenye T. Chryseobacterium vrystaatense sp. nov., isolated from raw chicken in a chicken-processing plant. Int J Syst Evol Microbiol. 2005;55(5):2149–2153. doi: 10.1099/ijs.0.63746-0. [DOI] [PubMed] [Google Scholar]
- 199.Olofsson T., Ahrné S., Molin G. Composition of the bacterial population of refrigerated beef, identified with direct 16S rRNA gene analysis and pure culture technique. Int J Food Microbiol. 2007;118(3):233–240. doi: 10.1016/j.ijfoodmicro.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 200.Lijnen H.R., Van Hoef B., Ugwu F., Collen D., Roelants I. Specific proteolysis of human plasminogen by a 24 kDa endopeptidase from a novel Chryseobacterium sp. Biochemistry. 2000;39(2):479–488. doi: 10.1021/bi992014r. [DOI] [PubMed] [Google Scholar]
- 201.Harrison F.C. The discoloration of halibut. Can J Res. 1929;1(3):214–239. [Google Scholar]
- 202.Engelbrecht K., Jooste P., Prior B. Spoilage characteristics of Gram-negative genera and species isolated from Cape marine fish. S Afr J Food Sci Nutr. 1996;8:66–71. [Google Scholar]
- 203.De Beer H., Hugo C.J., Jooste P.J., Vancanneyt M., Coenye T., Vandamme P. Chryseobacterium piscium sp. nov., isolated from fish of the South Atlantic Ocean off South Africa. Int J Syst Evol Microbiol. 2006;56(6):1317–1322. doi: 10.1099/ijs.0.64014-0. [DOI] [PubMed] [Google Scholar]
- 204.González C.J., Santos J.A., García-López M-L., Otero A. Psychrobacters and related bacteria in freshwater fish. J Food Prot. 2000;63(3):315–321. doi: 10.4315/0362-028x-63.3.315. [DOI] [PubMed] [Google Scholar]
- 205.Burešová V., Franta Z., Kopáček P. A comparison of Chryseobacterium indologenes pathogenicity to the soft tick Ornithodoros moubata and hard tick Ixodes ricinus. J Invertebr Pathol. 2006;93(2):96–104. doi: 10.1016/j.jip.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 206.Olson M., Gard S., Brown M., Hampton R., Morck D. Flavobacterium indologenes infection in leopard frogs. J Am Vet Med Assoc. 1992;201(11):1766–1770. [PubMed] [Google Scholar]
- 207.Mauel M.J., Miller D.L., Frazier K.S., Hines M.E. Bacterial pathogens isolated from cultured bullfrogs (Rana castesbeiana) J Vet Diag Invest. 2002;14(5):431–433. doi: 10.1177/104063870201400515. [DOI] [PubMed] [Google Scholar]
- 208.Yabuuchi E., Kaneko T., Yano I., Moss C.W., Miyoshi N. Sphingobacterium gen. nov., Sphingobacterium spiritivorum comb. nov., Sphingobacterium multivorum comb. nov., Sphingobacterium mizutae sp. nov., and Flavobacterium indologenes sp. nov.: glucose-nonfermenting Gram-negative rods in CDC groups IIK-2 and IIb. Int J Syst Bacteriol. 1983;33(3):580–598. [Google Scholar]
- 209.Vaneechoutte M., Kämpfer P., De Baere T., Avesani V., Janssens M., Wauters G. Chryseobacterium hominis sp. nov., to accommodate clinical isolates biochemically similar to CDC groups II-h and II-c. Int J Syst Evol Microbiol. 2007;57(11):2623–2628. doi: 10.1099/ijs.0.65158-0. [DOI] [PubMed] [Google Scholar]
- 210.Kämpfer P., Vaneechoutte M., Lodders N., De Baere T., Avesani V., Janssens M. Description of Chryseobacterium anthropi sp. nov. to accommodate clinical isolates biochemically similar to Kaistella koreensis and Chryseobacterium haifense, proposal to reclassify Kaistella koreensis as Chryseobacterium koreense comb. nov. and emended description of the genus Chryseobacterium. Int J Syst Evol Microbiol. 2009;59(10):2421–2428. doi: 10.1099/ijs.0.008250-0. [DOI] [PubMed] [Google Scholar]
- 211.Yassin A., Hupfer H., Siering C., Busse H.-J. Chryseobacterium treverense sp. nov., isolated from a human clinical source. Int J Syst Evol Microbiol. 2010;60(9):1993–1998. doi: 10.1099/ijs.0.017327-0. [DOI] [PubMed] [Google Scholar]
- 212.Hsueh P-R., Hsiue T-R., Wu J-J., Teng L.-J., Ho S-W., Hsieh W-C. Flavobacterium indologenes bacteremia: clinical and microbiological characteristics. Clin Infect Dis. 1996;23(3):550–555. doi: 10.1093/clinids/23.3.550. [DOI] [PubMed] [Google Scholar]
- 213.Lin Y-T., Jeng Y-Y., Lin M-L., Yu K-W., Wang F-D., Liu C-Y. Clinical and microbiological characteristics of Chryseobacterium indologenes bacteremia. J Microbiol Immunol Infect. 2010;43(6):498–505. doi: 10.1016/S1684-1182(10)60077-1. [DOI] [PubMed] [Google Scholar]
- 214.Chou D-W., Wu S-L., Lee C-T., Tai F-T., Yu W-L. Clinical characteristics, antimicrobial susceptibilities, and outcomes of patients with Chryseobacterium indologenes bacteremia in an intensive care unit. Jpn J Infect Dis. 2011;64(6):520–524. [PubMed] [Google Scholar]
- 215.Green B., Nolan P. Cellulitis and bacteraemia due to Chryseobacterium indologenes. J Infect. 2001;42(3):219–220. doi: 10.1053/jinf.2001.0822. [DOI] [PubMed] [Google Scholar]
- 216.Azzopardi E.A., Azzopardi S.M., Boyce D.E., Dickson W.A. Emerging gram-negative infections in burn wounds. J Burn Care Res. 2011;32(5):570–576. doi: 10.1097/BCR.0b013e31822ac7e6. [DOI] [PubMed] [Google Scholar]
- 217.Calderón G., García E., Rojas P., García E., Rosso M., Losada A. Chryseobacterium indologenes infection in a newborn: a case report. J Med Case Rep. 2011;5:10–13. doi: 10.1186/1752-1947-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Brisou J., Tysset C., Vacher B. Etude de trois souches microbiennes, famille des Pseudomonadaceae, dont la synergie provoque une maladie de caractère septicémique chez les poissons blancs de la Dordogne, du Lot et de leurs affluents. Ann l’Inst Pasteur/Actual. 1959;96:689–696. [PubMed] [Google Scholar]
- 219.Mudarris M., Austin B. Systemic disease in turbot Scophthalmus maximus caused by a previously unrecognised Cytophaga-like bacterium. Dis Aquat Org. 1989;6(3):161–166. [Google Scholar]
- 220.Mudarris M., Austin B., Segers P., Vancanneyt M., Hoste B., Bernardet J. Flavobacterium scophthalmum sp. nov., a pathogen of turbot (Scophthalmus maximus L.) Int J Syst Bacteriol. 1994;44(3):447–453. doi: 10.1099/00207713-44-3-447. [DOI] [PubMed] [Google Scholar]
- 221.Mudarris M., Austin B. Histopathology of a gill and systemic disease of turbot (Scophthalmus maximus) caused by Cytophaga-like bacterium (CLB) Bull Eur Assoc Fish Pathol. 1992;12:120–123. [Google Scholar]
- 222.Ilardi P., Abad J., Rintamäki P., Bernardet J.F., Avendaño-Herrera R. Phenotypic, serological and molecular evidence of Chryseobacterium piscicola in farmed Atlantic salmon, Salmo salar L., in Finland. J Fish Dis. 2010;33(2):179–181. doi: 10.1111/j.1365-2761.2009.01091.x. [DOI] [PubMed] [Google Scholar]
- 223.Campbell S., Harada R.M., Li Q.X. Chryseobacterium arothri sp. nov., isolated from the kidneys of a pufferfish. Int J Syst Evol Microbiol. 2008;58(1):290–293. doi: 10.1099/ijs.0.65276-0. [DOI] [PubMed] [Google Scholar]
- 224.Kämpfer P., Vaneechoutte M., Wauters G. Chryseobacterium arothri Campbell et al. 2008 is a later heterotypic synonym of Chryseobacterium hominis Vaneechoutte et al. 2007. Int J Syst Evol Microbiol. 2009;59(4):695–697. doi: 10.1099/ijs.0.004093-0. [DOI] [PubMed] [Google Scholar]
- 225.Zamora L., Vela A.I., Palacios M.A., Domínguez L., Fernández-Garayzábal J.F. First isolation and characterization of Chryseobacterium shigense from rainbow trout. BMC Veter Res. 2012;8(1):77. doi: 10.1186/1746-6148-8-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Pridgeon J.W., Klesius P.H., Garcia J.C. Identification and virulence of Chryseobacterium indologenes isolated from diseased yellow perch (Perca flavescens) J Appl Microbiol. 2012 doi: 10.1111/jam.12070. [DOI] [PubMed] [Google Scholar]
- 227.Fraser S.L., Jorgensen J.H. Reappraisal of the antimicrobial susceptibilities of Chryseobacterium and Flavobacterium species and methods for reliable susceptibility testing. Antimicrob Agents Chemother. 1997;41(12):2738–2741. doi: 10.1128/aac.41.12.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kirby J.T., Sader H.S., Walsh T.R., Jones R.N. Antimicrobial susceptibility and epidemiology of a worldwide collection of Chryseobacterium spp.: report from the SENTRY antimicrobial surveillance program (1997–2001) J Clin Microbiol. 2004;42(1):445–448. doi: 10.1128/JCM.42.1.445-448.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Michel C., Matte-Tailliez O., Kerouault B., Bernardet J.F. Resistance pattern and assessment of phenicol agents’ minimum inhibitory concentration in multiple drug resistant Chryseobacterium isolates from fish and aquatic habitats. J Appl Microbiol. 2005;99(2):323–332. doi: 10.1111/j.1365-2672.2005.02592.x. [DOI] [PubMed] [Google Scholar]
- 230.Faisal M., Shavalier M., Kim R.K., Millard E.V., Gunn M.R., Winters A.D. Spread of the emerging viral hemorrhagic septicemia virus strain, genotype IVb, in Michigan, USA. Viruses. 2012;4(5):734–760. doi: 10.3390/v4050734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Holey M.E., Elliott R.F., Marcquenski S.V., Hnath J.G., Smith K.D. Chinook salmon epizootics in Lake Michigan: possible contributing factors and management implications. J Aquat Anim Heal. 1998;10(2):202–210. [Google Scholar]
- 232.Faisal M., Hnath J. Michigan State University Press; East Lansing, Michigan: 2005. Fish health and diseases issues in the Laurentian Great Lakes. Health and diseases of aquatic organisms: bilateral perspectives. p. 331–49. [Google Scholar]
- 233.Faisal M., Schulz C.A., Loch T.P., Kim R.K., Hnath J., Whelan G. Great Lakes fish diseases: past, present, and future. In: Taylor W., Leonard N., Lynch A., editors. Great lakes fisheries. Michigan State University Press; East Lansing (MI): 2011. pp. 259–302. [Google Scholar]
- 234.Faisal M., Loch T., Fujimoto M., Woodiga S., Honeyfield D.C., Wolgamod M. Characterization of novel Flavobacterium spp. Involved in the mortality of Coho salmon (Oncorhynchus kisutch) in their early life stages. J Aquacult Res Dev. 2011;2(S2):8. [Google Scholar]
- 235.Loch T.P., Fujimoto M., Woodiga S.A., Walker E.D., Marsh T.L., Faisal M. Diversity of fish-associated flavobacteria of Michigan. J Aquat Anim Heal. 2013;25(3):149–164. doi: 10.1080/08997659.2012.758189. [DOI] [PubMed] [Google Scholar]
- 236.Loch T., Faisal M. Deciphering the biodiversity of fish-pathogenic Flavobacterium spp. recovered from the Great Lakes basin. Dis Aquat Org. 2014;112(1):45–57. doi: 10.3354/dao02791. [DOI] [PubMed] [Google Scholar]
- 237.Loch TP. Identification of novel flavobacteria from Michigan and assessment of their impacts on fish health [PhD Thesis]. East Lansing: Michigan State University; 2012.
- 238.Loch TP, Faisal, M. Polyphasic characterization reveals the presence of novel fish-associated Chryseobacterium spp. in the Great Lakes of North America. Dis Aquat Org; accepted for publication. [DOI] [PubMed]
- 239.Lorenzen E. The importance of the brand of the beef extract in relation to the growth of Flexibacter psychrophilus in Anacker and Ordals medium. Bull Eur Assoc Fish Pathol. 1993;13:64–65. [Google Scholar]
- 240.Cipriano R.C., Teska J. Effects of medium composition on the growth of two fish pathogens, Cytophaga columnaris and Cytophaga psychrophila. Biomed Lett. 1994;49(193):7–12. [Google Scholar]
- 241.Michel C., Antonio D., Hedrick R.P. Production of viable cultures of Flavobacterium psychrophilum: approach and control. Res Microbiol. 1999;150(5):351–358. doi: 10.1016/s0923-2508(99)80061-8. [DOI] [PubMed] [Google Scholar]
- 242.Kämpfer P., Lodders N., Vaneechoutte M., Wauters G. Transfer of Sejongia antarctica, Sejongia jeonii and Sejongia marina to the genus Chryseobacterium as Chryseobacterium antarcticum comb. nov., Chryseobacterium jeonii comb. nov. and Chryseobacterium marinum comb. nov. Int J Syst Evol Microbiol. 2009;59(9):2238–2240. doi: 10.1099/ijs.0.009142-0. [DOI] [PubMed] [Google Scholar]
- 243.Smith P., Davey S. Evidence for the competitive exclusion of Aeromonas salmonicida from fish with stress-inducible furunculosis by a fluorescent pseudomonad. J Fish Dis. 1993;16(5):521–524. [Google Scholar]
- 244.Spanggaard B., Huber I., Nielsen J., Sick E.B., Pipper C.B., Martinussen T. The probiotic potential against vibriosis of the indigenous microflora of rainbow trout. Environ Microbiol. 2001;3(12):755–765. doi: 10.1046/j.1462-2920.2001.00240.x. [DOI] [PubMed] [Google Scholar]
- 245.Lauer A., Simon M.A., Banning J.L., Lam B.A., Harris R.N. Diversity of cutaneous bacteria with antifungal activity isolated from female four-toed salamanders. ISME J. 2007;2(2):145–157. doi: 10.1038/ismej.2007.110. [DOI] [PubMed] [Google Scholar]
- 246.Rangdale RE. Studies on rainbow trout fry syndrome (RTFS) [PhD Thesis]. University of Stirling; 1995.
- 247.Madsen L., Dalsgaard I. Reproducible methods for experimental infection with Flavobacterium psychrophilum in rainbow trout Oncorhynchus mykiss. Dis Aquat Org. 1999;36(3):169–176. doi: 10.3354/dao036169. [DOI] [PubMed] [Google Scholar]
- 248.Decostere A., Lammens M., Haesebrouck F. Difficulties in experimental infection studies with Flavobacterium psychrophilum in rainbow trout (Oncorhynchus mykiss) using immersion, oral and anal challenges. Res Vet Sci. 2000;69(2):165–169. doi: 10.1053/rvsc.2000.0408. [DOI] [PubMed] [Google Scholar]
- 249.Garcia C., Pozet F., Michel C. Standardization of experimental infection with Flavobacterium psychrophilum, the agent of rainbow trout Oncorhynchus mykiss fry syndrome. Dis Aquat Org. 2000;42(3):191–197. doi: 10.3354/dao042191. [DOI] [PubMed] [Google Scholar]
- 250.Madetoja J., Nyman P., Wiklund T. Flavobacterium psychrophilum, invasion into and shedding by rainbow trout Oncorhynchus mykiss. Dis Aquat Org. 2000;43(1):27–38. doi: 10.3354/dao043027. [DOI] [PubMed] [Google Scholar]
- 251.Busch S., Dalsgaard I., Buchmann K. Concomitant exposure of rainbow trout fry to Gyrodactylus derjavini and Flavobacterium psychrophilum: effects on infection and mortality of host. Vet Parasitol. 2003;117(1):117–122. doi: 10.1016/j.vetpar.2003.07.018. [DOI] [PubMed] [Google Scholar]
- 252.Korkea-aho T., Heikkinen J., Thompson K., Von Wright A., Austin B. Pseudomonas sp. M174 inhibits the fish pathogen Flavobacterium psychrophilum. J Appl Microbiol. 2011;111(2):266–277. doi: 10.1111/j.1365-2672.2011.05044.x. [DOI] [PubMed] [Google Scholar]
- 253.Nagai T., Nakai T. Growth of Flavobacterium psychrophilum in fish serum correlates with pathogenicity. J Fish Dis. 2011;34(4):303–310. doi: 10.1111/j.1365-2761.2011.01245.x. [DOI] [PubMed] [Google Scholar]
- 254.Castillo D., Higuera G., Villa M., Middelboe M., Dalsgaard I., Madsen L. Diversity of Flavobacterium psychrophilum and the potential use of its phages for protection against bacterial cold water disease in salmonids. J Fish Dis. 2012;35(3):193–201. doi: 10.1111/j.1365-2761.2011.01336.x. [DOI] [PubMed] [Google Scholar]
- 255.Plant K., LaPatra S., Call D., Cain K. Immunization of rainbow trout, Oncorhynchus mykiss (Walbaum), with Flavobacterium psychrophilum proteins elongation factor-Tu, SufB Fe-S assembly protein and ATP synthaseβ. J Fish Dis. 2011;34(3):247–250. doi: 10.1111/j.1365-2761.2010.01235.x. [DOI] [PubMed] [Google Scholar]
- 256.Hadidi S., Glenney G.W., Welch T.J., Silverstein J.T., Wiens G.D. Spleen size predicts resistance of rainbow trout to Flavobacterium psychrophilum challenge. J Immunol. 2008;180(6):4156–4165. doi: 10.4049/jimmunol.180.6.4156. [DOI] [PubMed] [Google Scholar]
- 257.Silverstein J.T., Vallejo R.L., Palti Y., Leeds T.D., Rexroad C.E., Welch T.J. Rainbow trout resistance to bacterial cold-water disease is moderately heritable and is not adversely correlated with growth. J Anim Sci. 2009;87(3):860–867. doi: 10.2527/jas.2008-1157. [DOI] [PubMed] [Google Scholar]
- 258.Leeds T., Silverstein J., Weber G., Vallejo R., Palti Y., Rexroad C. Response to selection for bacterial cold water disease resistance in rainbow trout. J Anim Sci. 2010;88(6):1936–1946. doi: 10.2527/jas.2009-2538. [DOI] [PubMed] [Google Scholar]