Abstract
Nosocomial infections are acquired during hospital treatment or in a hospital environment. One such infecting agent, Escherichia coli, harbours many virulence genes that enable it to become pathogenic, causing damage to the host. The mechanism of the E. coli virulence factors provenance to cause infection in host environments is not clearly elucidated. We investigated the virulence and pathogenicity of E. coli affected by the host environment. For this, blood (n = 78) and faecal (n = 83) E. coli isolates were collected from patients with and without sepsis, respectively, who had been admitted to the intensive care unit. The E. coli genomic DNA was isolated; the phylogenetic grouping was conducted by triplex PCR. The occurrence of nine virulence genes among the all the isolates was confirmed by gene-specific PCR. The prevalence of E. coli in blood isolates was more in phylogenetic groups B2 and D compared to groups A and B1. However, in faecal isolates, there was no significant difference. The prevalence of adhesin and toxin (papG, sfa, afa, cnf1, hlyA) genes was higher in blood compared to faecal E. coli isolates. However, the prevalence of aer, traT and PAI was similar as well as higher among both of these groups. These observations indicate a role of external environment (hospital setting) on host susceptibility (development of infection) in the faecal E. coli isolates, thereby making the patient prone to a sepsis condition.
Keywords: Escherichia coli, host environment, phylogenetic grouping, sepsis, virulence factors
Introduction
Nosocomial infections are the major cause of death and increased morbidity among hospitalized patients. The incidence of such infections is estimated to be 5–10% of patients admitted to tertiary care hospitals, although this may go up to 28% in the intensive care unit (ICU). Nosocomial infections contribute to 0.7–10% of deaths compared to 0.1–4.4% of all the deaths occurring in hospitals [1]. Alarmingly, 10–30% of patients in the Indian population acquire such infections [1]. Nosocomial infections are most commonly acquired as a result of surgical wounds, urinary tract infections (UTIs) and lower respiratory tract infections and may be cross-infections or endogenous. Modern diagnostic procedures such as biopsies, endoscopic examinations, catheterization, intubation/ventilation and surgical procedures increase the risk of infection by microorganisms like Escherichia coli that are normally innocuous but may become pathogenic when the body's immunological defenses are compromised [1].
E. coli is the most common pathogen causing diarrhoea, neonatal septicemia, UTI, bacteraemia and urosepsis [2]. It is responsible for 80% of community-acquired UTIs and 30% of nosocomial infections [3]. E. coli is one of the leading causes of bloodstream infections and comprises 17–37% of all bacteria isolated from patients with bloodstream infections [4]. Such bloodstream infections with extraintestinal E. coli are frequently associated with patients who have undergone major surgeries; who were admitted to hospitals for long durations; or who had a peripheral or urinary catheter [1]. E. coli in the bloodstream can trigger vigorous host inflammatory response, leading to sepsis associated with high morbidity and mortality [5].
A phylogenetic analysis indicates discrete origins of a diverse natural populations of the pathogenic E. coli[6] that can be classified into major phylogenetic groups A, B1, B2 and D [7]. The commensal strains usually belong to groups A and B1, whereas the extraintestinal pathogenic strains belong to groups B2 and D [8,9]. The intestinal E. coli are mixture of all the phylogenetic groups and may act as a reservoir for the pathogenic isolates. The pathogenicity of intestinal or extraintestinal E. coli may be attributed to its genetic virulence markers [10]. Strains of groups B2 and D often carry virulence factors (VFs) that are lacking in A and B1 [8,11,12]. The reason for a commensal strain becoming virulent may be attributed to the multiple strategies of genome plasticity wherein the random point mutations were incorporated for adaptive pathogenic environments [13]. The VFs in E. coli are required to overcome host defenses, invade host tissues and trigger a local inflammatory response. E. coli virulence and phylogeny are intertwined because VFs from both the host and the environment shape its genetic structure [14].
To date, no single virulence factor has been demonstrated as being specific, unique or definitive to cause a particular disease. Virulence is multifactorial because it depends on both the characteristics of microbe and the susceptibility of the host [15]. On the basis of functional groups, the E. coli VFs can be categorized as adhesins, such as P fimbriae (papG), type 1 fimbriae (fimH), S fimbriae (sfa) and A fimbriae (afa); toxins, such as hemolysin A (hlyA) and cytotoxic necrotizing factor 1 (cnf1); iron uptake, such as aerobactin (aer); protectins, such as serum resistance (traT); and others, such as pathogenicity-associated islands (PAIs) and Tir-containing protein of E. coli (tcpc) [16–18]. PAIs have previously been investigated in pathogenic bacteria. However, to our knowledge, no study has been conducted for commensal strains [19]. Houdouin et al. [20] reported a susceptibility of the E. coli isolates from blood of urosepsis patients to diverse antibiotics according to the prevalence of VFs and phylogenetic groups.
The E. coli phylogenetic grouping and their correlation with the VFs in disease conditions is well established [11,15,21]; however, a correlation between phylogenetic groups and virulence profile of the blood and faecal E. coli isolates is not known. Most of the previous studies compared the virulence properties of E. coli isolates from disease condition with those from healthy individuals [11,15,22]; however, the potential of faecal E. coli isolates (usually commensals) to cause the disease in a compromised host is unknown. Therefore, in order to understand the host susceptibility, we investigated the blood E. coli isolates from the patients diagnosed with sepsis and compared them with the faecal E. coli isolates from patients without sepsis admitted to the ICU. We found that the prevalence of the E. coli isolates among the pathogenic groups B2 and D was significantly higher than the commensal groups A and B1 in faecal E. coli isolates. The overall prevalence of E. coli isolates among pathogenic groups was similar to commensal groups faecal E. coli isolates, indicating that this group of patients has a higher chance of contracting a severe form of infection. In addition, an overall prevalence of VFs among all the phylogenetic groups was higher in the blood E. coli isolates. However, the high prevalence of aer, traT and PAI among faecal E. coli isolates indicated that the host environment may have an important role to play for a differential expression of these virulence genes to induce pathogenicity in the hospital setting. A knowledge of such virulence patterns and their correlation with phylogenetic groups is critical for our understanding of bacterial infection and is indispensable to the development of devising related novel therapeutic strategies.
Methods
Selection of patients and bacterial isolates
Blood samples (n = 78) were collected from patients with sepsis admitted to the ICU of Vardhman Mahavir Medical College (VMCC) and Safdarjung Hospital, New Delhi, India, from February 2011 to August 2013. The blood culture was carried out as a part of compulsory diagnostic testing. The samples were selected from patients who had shown a clinical response arising from a nonspecific insult, which include more than two of the following: multiple positive blood culture results; bacteremia associated with systemic symptoms; temperature >38.5°C; hypotension (systolic blood pressure <90 mm Hg); and leucocytosis (white blood cell count ≥13 000/mm3). In addition, randomly selected faecal samples (n = 83) were collected from patients admitted to the ICU (on day 1) for cardiovascular surgeries and road transport accidents who were not diagnosed with sepsis at VMCC and Safdarjung Hospital during the same period of time. A total of 161 blood and faecal E. coli isolates (Table 1) were screened for E. coli positivity by standard biochemical procedures. Only one isolate from each patient is included in this study.
Table 1.
Clinical diagnosis of 161 patients with and without sepsis
| Clinical diagnosis | n (%) |
|---|---|
| Sepsis patients treated in ICU | 78 (48.4) |
| Sepsis | 19 (24.3) |
| UTI | 9 (11.5) |
| Bronchopneumonia | 10 (12.8) |
| Nephritic syndrome | 8 (10.2) |
| NA (blood culture positive for Escherichia coli) | 32 (41) |
| Nonsepsis patients treated in ICU | 83 (51.6) |
| Medical device in patient's body after major surgery or patients underwent invasive procedurea | 83 (100) |
ICU, intensive care unit.
Patients with cardiovascular surgeries and patients who experienced road transport accidents who were not diagnosed with sepsis.
Subculturing of bacterial isolates and isolation of DNA
The E. coli isolates were grown as lactose-positive colonies on MacConkey blood agar medium in the Department of Microbiology at VMCC and Safdarjung Hospital. Thereafter, the samples were subcultured on tryptone soy broth by incubating at 37°C for 18 hours. A portion of the broth containing bacterial isolates was pooled and processed for isolation of the bacterial genomic DNA by the standard sodium acetate precipitation method. DNA was quantified using NanoDrop (NanoDrop ND 100).
Triplex PCR
A triplex PCR was performed using a bacterial genomic DNA as template and primers specific to chuA and yjaA genes and the TSPE4.C2 DNA fragment, as described elsewhere [7] (Table 2). For PCR amplification, a 25 μL reaction containing 1 μL template DNA, 2 mM MgCl2, 0.5 mM dNTPs, 0.5YU of Taq polymerase (Promega) and 0.3 pmol/μL of forward and reverse primers each in 1× PCR buffer was set up. The reaction conditions included one cycle of initial denaturation for 5 minutes at 94°C and thereafter 35 cycles of denaturation for 30 seconds at 94°C, annealing for 30 seconds at 55°C and an extension of 5 minutes at 72°C. A final extension of 10 minutes at 72°C was carried out before termination. Thereafter the PCR products were run on 1.8% agarose gel (Saekem Lonza). All the strains were tested in triplicate with positive and negative controls. The positive control was E. coli strain CFTO73 obtained from ATCC (no. 700928), and the negative control was PCR master mix without DNA template.
Table 2.
Primers for amplification of Escherichia coli genes related to phylogenetic grouping and virulence genes
| Functional Category | Gene | Oligonucleotide sequence (5′–3′) | Annealing temperature (°C) | Product size (bp) | Reference |
|---|---|---|---|---|---|
| Phylogenetic grouping | |||||
| chuA | f GACGAACCAACGGTCAGGAT r TGCCGCCAGTACCAAAGACA |
55 | 279 | Clermont et al. 2000 [7] | |
| YjaA | f TGAAGTGTCAGGAGACGCTG r ATGGAGAATGCGTTCCTCAAC |
55 | 211 | ||
| TspE4.C2 | f GAGTAATGTCGGGGCATTCA r CGCGCCAACAAAGTATTACG |
55 | 152 | ||
| Virulence factors | |||||
| Adhesins | papG | f GCATTTCTACGGTAACC r TCGTCAAATTTCTCAGTCAGA |
50 | 295 | Norinder et al. 2012 [40] |
| Sfa | f CTCCGGAGAACTGGGTGCATCTTAC r CGGAGGAGTAATTACAAACCTGGCA |
54 | 408 | Le Bouguenec et al 1992 [41] | |
| Afa | f GCTGGGCAGCAAACTGATAACTCTC r CATCAAGCTGTTTGTTCGTCCGCCG |
60 | 793 | Le Bouguenec et al. 1992 [41] | |
| fimH | f TGCAGAACGGATAAGCCGTGG r GCAGTCACCTGCCCTCCGGTA |
55 | 506 | Johnson & Stell 2000 [22] | |
| Toxins | hlyA | f AACAAGGATAAGCACTGTTCTGGCT r ACCATATAAGCGGTCATTCCCGTCA |
55 | 1177 | Yamamoto et al 1995 [42] |
| cnf1 | f AAGATGGAGTTTCCTATGCAGGAG r CATTCAGAGTCCTGCCCTCATTATT |
52 | 498 | Kuhar et al 1998 [43] | |
| Iron uptake | aer | f TACCGGATTGTCATATGCAGACCGT r AATATCTTCCTCCAGTCCGGAGAAG |
56 | 602 | Yamammoto et al 1995 [44] |
| Protectins/serum resistance | traT | f GGTGTGGTGCGATGAGCACAG r CACGGTTCAGCCATCCCTGAG |
57 | 288 | Johnson and Stell 2000 [22] |
| Pathogen associated island | PAI | f GGACATCCTGTTACAGCGCGCA r TCGCCACCAATCACAGCCGAAC |
57 | 922 | Johnson and Stell 2000 [22] |
Phylogenetic grouping
A phylogenetic grouping (A, B1, B2 and D) of E. coli isolates from patients with and without sepsis was determined on the basis of triplex PCR data by making a dichotomous decision tree based on an amplification of chuA and yjaA genes and the TSPE4.C2 DNA fragment, as previously reported [7]. The fragment size of the chuA and yjaA genes and the TSPE4.C2 DNA fragment was 279, 211 and 152 bp, respectively. Group B2 was designated with a positivity of chuA and yjaA genes; group D with a positivity of chuA gene and a negativity of yjaA gene; group B1 with a positivity of TspE4.C2 DNA fragment and a negativity of chuA gene; and group A with a negativity of chuA gene and TspE4.C2 DNA fragment.
PCR for virulence markers
The presence of virulence genes encoding P fimbriae (papG), type 1 fimbriae (fimH), S fimbriae (sfa), A fimbriae (afa), cytotoxic necrotizing factor 1 (cnf1), hemolysin (hlyA), aerobactin (aer), serum resistance (traT) and pathogenicity-associated island marker (PAI) was evaluated by performing a PCR using gene-specific primers (Table 2). Using a bacterial genomic DNA from patients with and without sepsis as the template, a PCR amplification was performed for each gene in a standard 25 μL reaction. The reaction conditions included one cycle of initial denaturation for 5 minutes at 94°C and thereafter 35 cycles of denaturation for 30 seconds at 94°C, annealing for 30 seconds at specific temperature (Table 2) and an extension of 5 minutes at 72°C. A final extension of 10 minutes at 72°C was carried out before termination. Thereafter the PCR products were run on 1.8% agarose gel (Saekem Lonza). All the strains were tested in triplicate with positive and negative controls. The positive control was E. coli strain CFTO73 obtained from ATCC (no. 700928), and the negative control was PCR master mix without DNA template.
Statistical analysis
The Z test was used to compare the virulence factor's prevalence among the blood and faecal E. coli isolates. A one-way ANOVA nonparametric test was performed to analyse the virulence profile among various E. coli phylogenetic groups. A p value of <0.05 was considered significant.
Ethical clearance
The isolates were previously approved by the Institutional Ethical Committee of Vardhman Mahavir Medical College (VMCC) and Safdarjung Hospital, New Delhi, India (S.No-VMMC/SJH/Ethics/SEP-11/29). As per the guidelines, an informed written consent was taken from all the adult subjects included in this study.
Results
Phylogenetic grouping of blood and faecal E. coli isolates
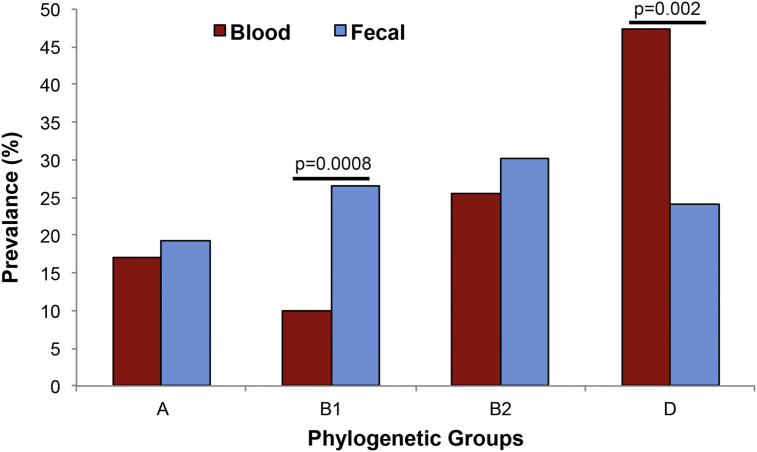
E. coli commensal strains belong to groups A and B1, whereas extraintestinal pathogenic strains belong to groups B2 and D [8,11]. We observed no significant difference in the prevalence of phylogenetic groups A (19.2%), B1 (26.5%), B2 (30%) and D (24%) (p 0.47) (Fig. 1, Table 3) in the faecal E. coli isolates, whereas phylogenetic groups B2 (25.6%) and D (47.4%) were found to be more prevalent (p 0.0001) among the blood E. coli isolates (Fig. 1, Table 3). Individually, we did not find any significant difference in the prevalence of phylogenetic group A in blood (17%) and faecal (19.2%) E. coli isolates; however, group B1 was found to be significantly higher in the faecal E. coli isolates (p 0.0008) (Fig. 1, Table 3), as previously described [23]. Contrary to expectation, we did not find any significant difference in the prevalence of phylogenetic group B2 from blood and faecal E. coli isolates. However, phylogenetic group D (47.4%) was more prevalent in blood (p 0.002) compared to faecal E. coli isolates (Fig. 1, Table 3), thus corroborating earlier observations of extraintestinal pathogenic strains belonging to group D [8,24,25].
Fig. 1.
Triplex PCR–based comparison of prevalence of phylogenetic groups between blood (n = 78) and faecal (n = 83) Escherichia coli isolates. The p value is calculated by Z test and indicate significance between blood and faecal E. coli isolates.
Table 3.
Distribution of phylogenetic groups and virulence genes in blood and faecal Escherichia coli isolates from patients with and without sepsis
| Category/variable | No. (%) of isolates for: |
p | ||
|---|---|---|---|---|
| Total (n = 161) | Sepsis, blood (n = 78) | Nonsepsis, faecal (n = 83) | ||
| Phylogenetic groups | ||||
| Group A | 29 (18) | 13 (17) | 16 (19.2) | NS |
| Group B1 | 30 (18.6) | 8 (10) | 22 (26.5) | 0.0008 |
| Group B2 | 45 (27.9) | 20 (25.6) | 25 (30) | NS |
| Group D | 57 (35.4) | 37 (47.4) | 20 (24) | 0.002 |
| Virulence factors | ||||
| Adhesins | ||||
| P fimbriae (papG) | 114 (70.8) | 75 (96) | 39 (47) | 0.0001 |
| Type 1 fimbriae (fimH) | 150 (93.2) | 70 (90) | 80 (96) | NS |
| S fimbriae (sfa) | 92 (57.14) | 65 (83) | 27 (32.5) | 0.0001 |
| A fimbriae (afa) | 52 (32.2) | 38 (49) | 14 (17) | 0.0001 |
| Toxins | ||||
| Hemolysin A (hlyA) | 19 (11.8) | 14 (18) | 5 (6) | 0.02 |
| Cytotoxic necrotizing factor (cnf1) | 46 (28.6) | 38 (49) | 8 (10) | 0.0001 |
| Iron uptake | ||||
| Aerobactin (aer) | 110 (68.3) | 53 (68) | 57 (69) | NS |
| Protectins | ||||
| Serum resistance (traT) | 136 (84.5) | 63 (81) | 73 (88) | NS |
| Other | ||||
| Pathogenicity associated island (PAI) | 80 (49.7) | 36 (46) | 44 (53) | NS |
NS = nonsignificant.
The p value is calculated by Z test and indicates significance between patients with and without sepsis.
Virulence profile of blood and faecal E. coli isolates in phylogenetic groups
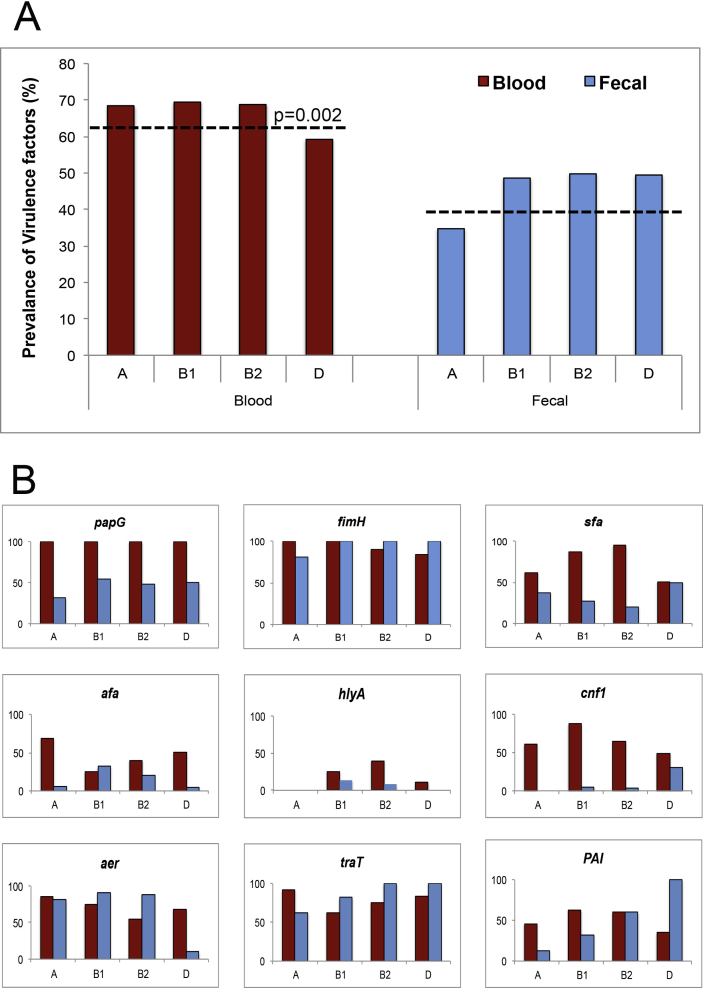
E. coli phylogenetic grouping and its correlation with the VFs in various disease conditions has been previously described [11,21,22]. The combined virulence profile of all the nine VFs (papG, fimH, sfa, afa, cnf1, hlyA, aer, traT and PAI) among the phylogenetic groups (A, B1, B2 and D) of the blood E. coli isolates was found to be significantly higher than the faecal isolates (p 0.002; Fig. 2A, dotted lines). Relatively higher levels of VFs among the faecal E. coli isolates suggest the entry of commensal E. coli into the bloodstream, thus enhancing its pathogenicity.
Fig. 2.
(A) Combined prevalence of virulence factors among phylogenetic groups of blood and faecal Escherichia coli isolates. Dotted lines indicate mean values among blood and faecal E. coli isolates. The p value is calculated by Z test and indicates significance among both groups. (B) Expression of virulence genes among phylogenetic groups in blood and faecal E. coli isolates.
We investigated a possible link between strain phylogeny and individual virulence genes among blood and faecal E. coli isolates. Our data showed that the adhesin coding genes PapG and fimH were the most prevalent (90–100%) among all the phylogenetic groups in blood E. coli isolates, and fimH was equally (80–100%) high in the faecal isolates (Fig. 2B). A significant difference (p <0.0001) was observed in the prevalence of papG between blood and faecal E. coli isolates. The overall prevalence of sfa in the blood E. coli isolates was higher in groups B1 and B2 compared to groups A and D (p <0.0001). Afa in the blood E. coli isolates was higher in groups A and D compared to groups B1 and B2, whereas it was varied for the faecal E. coli isolates. Of the toxin-coding genes studied, the overall prevalence of cnf1 in the blood E. coli isolates was higher in groups B1 and B2 compared to groups A and D (p <0.0001), whereas hlyA was very low in both groups.
A significant difference was found in the prevalence of aer in groups B1 and B2 (p <0.01) of blood and faecal E. coli isolates; however, the reverse scenario was seen for the group D. The prevalence of traT in groups B1, B2 and D was higher in faecal E. coli isolates compared to blood isolates (p <0.05). The prevalence of PAI in groups A and B1 was found to be higher in the blood E. coli isolates (p <0.05); however, the prevalence of group D was significantly higher in faecal E. coli isolates (100%) compared to the blood isolates (35%, p <0.002) (Fig. 2B). Interestingly, an unexpected high expression of the virulence genes among the faecal E. coli isolates indicates the role of these genes in the development of infection.
Combined virulence profile of blood and faecal E. coli isolates
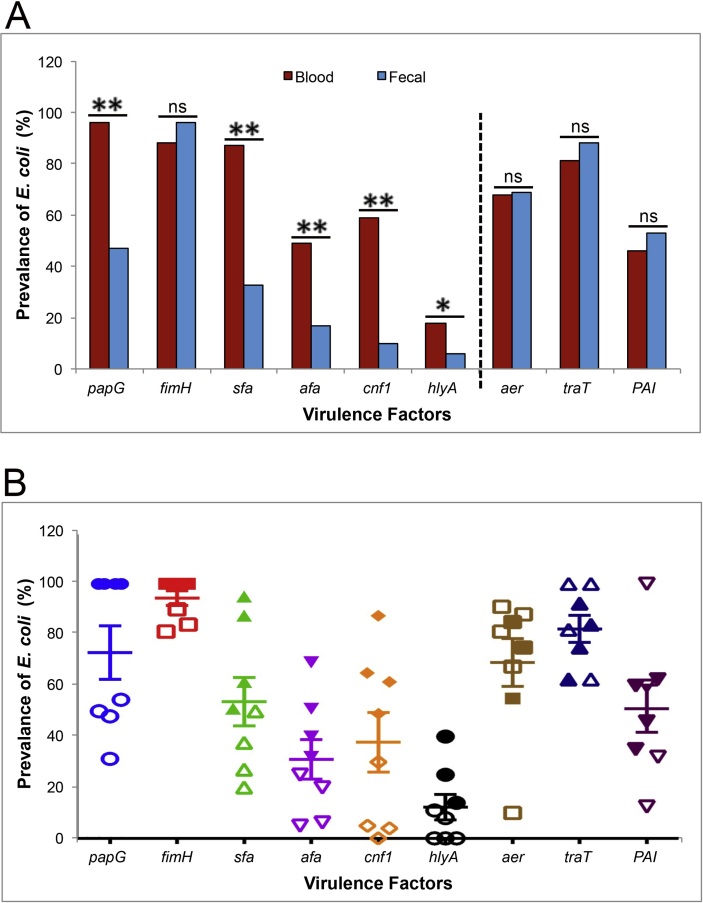
The pathogenic E. coli possesses an array of VFs leading to its pathogenesis [26]. The prevalence of the adhesin and toxin categories—that is, papG, sfa, afa, cnf1 and hlyA among blood E. coli isolates—was found to be significantly higher than faecal isolates, indicating their significant association to the pathogenic conditions (Fig. 3A, Table 3). However, no significant difference was observed in the prevalence of fimH among the blood and faecal E. coli isolates, as we expected. Surprisingly, no difference was observed in the prevalence of aer, traT and PAI among the blood and faecal E. coli isolates, indicating a role of the host environment in stimulating the bacteria to acquire these virulence genes (Fig. 3A, Table 3).
Fig. 3.
PCR-based prevalence of virulence genes (papG, fimH, sfa, afa, cnf1, hlyA, aer, traT, PAI) between blood (n = 78) and faecal (n = 83) Escherichia coli isolates (A). Solid and empty shapes represent blood and faecal E. coli isolates, respectively (B). The p value is calculated by Z test and indicates significance among both the groups. *p 0.02; **p 0.0001; ns, nonsignificant.
The prevalence of the E. coli isolates with respect to the papG, sfa, afa, cnf1 and hlyA in the phylogenetic groups (A, B1, B2 and D) belonging to the blood E. coli isolates was higher compared to faecal E. coli isolates. However, no discrete difference in the prevalence of the E. coli isolates with respect to aer, traT and PAI was observed among various phylogenetic groups (Fig. 3B). Instead, a high prevalence of fimH, aer, traT and PAI among the faecal E. coli isolates suggests an effect of external environment to induce bacterial pathogenicity [27].
Discussion
Characterization of E. coli is important for both clinical and epidemiologic implications. A community- or hospital-acquired E. coli infection is the primary cause of neonatal meningitis, UTI, urosepsis and sepsis [11,21,24,28], but reports of E. coli causing bloodstream infections are limited. The pathogenic E. coli strains belong to phylogenetic groups B2 or D, out of which B2 isolates are more prevalent among the intestinal pathogenic strains whereas the commensal one belongs to group A or group B1 [8,16]. Irrespective of the presence or absence of virulence genes or factors, the status of the host can be critical for the development of an infection [29]. Studying bacterial isolates from different diseased hosts like pyelonephritis, cystitis and asymptomatic bacteriuria (ABU) is common, but for sepsis it is not known.
Pathogenic behaviour is predicted both by virulence factor repertoire and by phylogenetic background [8,30]. Genetically, extraintestinal pathogenic E. coli harbours a variety of VFs, which gathered into pathogenicity-associated islands and enhance the capacity of E. coli to cause systemic infections [31]. However, it is still not clear how strains with apparently low virulence can cause sepsis, not only in compromised but also in noncompromised hosts [32]. It is conceivable that these strains may possess unrecognized VFs or specific VFs that may facilitate bacteraemia [20]. Previous studies described papC and aer as the minimal prerequisite for bacterial passage from kidney infection into the bloodstream [21,33,34]. The role of E. coli VFs in the pathogenesis of sepsis in relation to the site of its primary infection is virtually unknown.
Virulence properties of the isolates belonging to phylogenetic groups A and B1 were not analysed. To understand the role of commensal intestinal E. coli as a potential source for pathogenic and E. coli populations, we investigated the phylogenetic groups and virulence profile of the blood and faecal E. coli isolates from patients with and without sepsis, respectively, admitted to the ICU. The faecal isolates were not collected from the same sepsis patients because such patients will definitely have an effect on their gut flora and would not have acted as a suitable control. We established phylogenetic grouping of E. coli isolates into four groups: A, B1, B2 and D [7]. No significant difference was observed in the prevalence of the commensal and pathogenic phylogenetic groups in the blood and faecal E. coli isolates. However, group B1 was more prevalent in the faecal E. coli isolates, suggesting they may have been acquired from the hospital environment by these patients. A high prevalence of E. coli in the group D blood isolates corroborates the nature of the E. coli as an extraintestinal pathogenic strain [8]. The aberrant observation of high prevalence of groups B2 among the faecal E. coli isolates may be attributed to hospital-acquired infections. A collective high prevalence (54%) of groups B2 and D among the faecal E. coli isolates may be ascribed to the varied bacterial characteristics, antibiotic usage or genetic factors of the host. The observed difference in distribution of E. coli phylogenetic groups between pathogenic and commensal E. coli populations was similar to those in an earlier study that compared faecal and urine isolates from different host population cohorts [30].
Because faecal flora is considered to be the natural reservoir of pathogenic strains in extraintestinal infections [35], the phylogenetic distribution of commensal E. coli isolates from healthy individuals could provide an important comparison of and insight into the spread of the potential pathogenic lineage. Previous reports indicated that group B2 E. coli strains are rare in faecal samples [30], which is contrary to our data. This implies that acquiring the group B2 strain in faecal samples is important in the risk of infection. In a similar phylogenetic study among patients with UTIs, group B2 dominated in the uropathogenic strains, while they also accounted for about 50% of the rectal specimen [35]. The high percentage of group B2 and group D in the faecal E. coli isolates prompted us to ask how frequently the virulent B2 pathogens that are routinely carried by healthy humans would affect the disease dynamic in this population.
In order to find possible link between strain phylogeny and individual virulence genes, we analysed an overall virulence profile (papG, fimH, sfa, afa, cnf1, hlyA, aer, traT and PAI) of blood and faecal E. coli isolates. The aggregate score of the virulence was found to be higher in blood than in faecal E. coli isolates. We observed a variable expression of virulence genes among the commensal (A and B1) and pathogenic (B2 and D) groups of blood and faecal E. coli isolates. An unexpected high expression of the virulence genes among the faecal E. coli isolates indicated a role of these genes in the development of infection and suggested that the challenged host environment (such as the presence of catheters or hospital-acquired infections, or even poor hygiene due to infrequent urination of the patient, leading to a high vulnerability) may have altered the bacterial pathogenicity [1].
In E. coli extraintestinal infections, phylogenetic group B2 was found to be predominant and more virulent than other phylogenetic groups. An ascending route was proposed to be the major pathway of E. coli causing UTIs [36]. In addition to the virulence factors, host factors such as obstructions and immune-compromising conditions may favour the development of UTI among such patients [37]. Out of the VFs examined in this study, papG, sfa, afa, cnf1 and hlyA were found to be more prevalent in the blood than the faecal E. coli isolates. Our data suggest that the adhesin coding gene fimH was the most prevalent among all the phylogenetic groups of blood and faecal E. coli isolates. FimH-mediated biofilm formation is known to facilitate bacterial colonization of urinary catheters and other medical implants—an unfortunately common problem for hospitalized individuals [38]. An unexpected high expression of the virulence genes aer, traT and PAI among the faecal E. coli isolates indicates their role in the development of infection. A high prevalence of these virulence genes may have led to the entry of commensal E. coli into the bloodstream, leading to sepsis [21,33,34]. hlyA and traT are known to predict the bacterial pathogenicity [39] among both compromised and noncompromised hosts [22].
The prevalence of adhesin and toxin (papG, sfa, afa, cnf1 and hlyA) genes was significantly higher in the E. coli isolates from the blood compared to faecal E. coli isolates, indicating a possible association to the pathogenic conditions [22]. The high prevalence of adhesins in blood and faecal E. coli isolates than the expression of toxins or other virulence factors is arguably the most important determinant of pathogenicity. Surprisingly, no difference was observed in the prevalence of fimH, Aer, traT and PAI among the E. coli isolates of both these groups, indicating a role of the host environment in stimulating the bacteria to acquire these VFs and thus inducing the bacterial pathogenicity [26]. The analysis of virulence gene expression suggested a variable prevalence of aer, traT and PAI in the phylogenetic groups among blood and faecal E. coli isolates. The unusual high prevalence of PAI in the faecal E. coli isolates in our study indicates its role in making these patients vulnerable to severe infection. These observations suggest that the faecal E. coli isolates may transform a nonsepsis condition into sepsis in a hospital environment. In addition, the similar prevalence of aer, traT, and PAI in blood and faecal E. coli isolates is contrary to the findings of previous studies in E. coli strains from urosepsis [22].
In summary, we report for the first time a correlation of phylogenetic groups with important virulence markers in blood and faecal E. coli isolates from patients with and without sepsis admitted to the ICU. The high prevalence of all the VFs studied in the blood and faecal E. coli isolates and a similar prevalence of aer, traT and PAI indicate their role in the sustenance and development of infection. The specific association of hlyA and cnf1 in the blood and faecal E. coli isolates indicate that the host environment may have an important role to play for a differential expression of virulence genes, thereby causing pathogenicity among these isolates in hospital settings. Therefore, our data suggest that faecal isolates could become pathogenic in the immunocompromised patients under challenging host conditions. Our study also indicates that patients prone to a severe form of infection in the hospital environment may be identified on the basis of their virulence profile. Further investigation is required to determine the interplay of these VFs and concurrently identifying the mechanisms regulating the expression of these traits in sepsis and nonsepsis E. coli isolates in order to improve the management of infectious diseases.
Conflict of Interest
None declared.
Acknowledgements
This work was supported by the Dean Research Grant, University of Delhi, India to MY [Dean(R)/R&D/2012/917] and by Indian Council of Medical Research Thesis Grant to GM (3/2/2012/PG-Thesis-HRD).
References
- 1.Ducel G. 2nd ed. World Health Organization; Lyon, France: 2002. Prevention of hospital-acquired infections. A practical guide. WHO/CDS/CSR/EPH/200212. [Google Scholar]
- 2.Camins B.C., Marschall J., DeVader S.R., Maker D.E., Hoffman M.W., Fraser V.J. The clinical impact of fluoroquinolone resistance in patients with E. coli bacteremia. J Hosp Med. 2011;6(6):344–349. doi: 10.1002/jhm.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferry S.A., Holm S.E., Stenlund H., Lundholm R., Monsen T.J. The natural course of uncomplicated lower urinary tract infection in women illustrated by a randomized placebo controlled study. Scand J Infect Dis. 2004;36(4):296–301. doi: 10.1080/00365540410019642. [DOI] [PubMed] [Google Scholar]
- 4.Russo T.A., Johnson J.R. Medical and economic impact of extraintestinal infections due to Escherichia coli: focus on an increasingly important endemic problem. Microbes Infect. 2003;5:449–456. doi: 10.1016/s1286-4579(03)00049-2. [DOI] [PubMed] [Google Scholar]
- 5.Marx G., Reinhart K. Urosepsis: from the intensive care viewpoint. Int J Antimicrob Agents. 2008;31(Suppl. 1):S79–S84. doi: 10.1016/j.ijantimicag.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 6.Pupo G.M., Karaolis D.K., Lan R., Reeves P.R. Evolutionary relationships among pathogenic and nonpathogenic Escherichia coli strains inferred from multilocus enzyme electrophoresis and mdh sequence studies. Infect Immun. 1997;65:2685–2692. doi: 10.1128/iai.65.7.2685-2692.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clermont O., Bonacorsi S., Bingen E. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl Environ Microbiol. 2000;66:4555–4558. doi: 10.1128/aem.66.10.4555-4558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Picard B., Garcia J.S., Gouriou S., Duriez P., Brahimi N., Bingen E. The link between phylogeny and virulence in Escherichia coli extraintestinal infection. Infect Immun. 1999;67(2):546–553. doi: 10.1128/iai.67.2.546-553.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlos C., Pires M.M., Stoppe N.C., Hachich E.M., Sato M.I., Gomes T.A. Escherichia coli phylogenetic group determination and its application in the identification of the major animal source of fecal contamination. BMC Microbiol. 2010;10:161. doi: 10.1186/1471-2180-10-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muhldorfer I., Hacker J. Genetic aspects of Escherichia coli virulence. Microb Pathog. 1994;16:171–181. doi: 10.1006/mpat.1994.1018. [DOI] [PubMed] [Google Scholar]
- 11.Johnson J.R., Delavari P., Kuskowski M., Stell A.L. Phylogenetic distribution of extraintestinal virulence-associated traits in Escherichia coli. J Infect Dis. 2001;183:78–88. doi: 10.1086/317656. [DOI] [PubMed] [Google Scholar]
- 12.Katouli M. Population structure of gut Escherichia coli and its role in development of extra-intestinal infections. Iran J Microbiol. 2010;2:59–72. [PMC free article] [PubMed] [Google Scholar]
- 13.Chattopadhyay S., Weissman S.J., Minin V.N., Russo T.A., Dykhuizen D.E., Sokurenko E.V. High frequency of hotspot mutations in core genes of Escherichia coli due to short-term positive selection. Proc Natl Acad Sci U S A. 2009;106(30):12412–12417. doi: 10.1073/pnas.0906217106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tenaillon O., Skurnik D., Picard B., Denamur E. The population genetics of commensal Escherichia coli. Nat Rev Microbiol. 2010;8:207–217. doi: 10.1038/nrmicro2298. [DOI] [PubMed] [Google Scholar]
- 15.Tarchouna M., Ferjani A., Ben-Selma W., Boukadida J. Distribution of uropathogenic virulence genes in Escherichia coli isolated from patients with urinary tract infection. Int J Infect Dis. 2013;17:e450–e453. doi: 10.1016/j.ijid.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 16.Johnson J.R., Russo T.A. Extraintestinal pathogenic Escherichia coli: ‘the other bad E. coli.’. J Lab Clin Med. 2002;139:155–162. doi: 10.1067/mlc.2002.121550. [DOI] [PubMed] [Google Scholar]
- 17.Cirl C., Wieser A., Yadav M., Duerr S., Schubert S., Fischer H. Subversion of Toll-like receptor signaling by a unique family of bacterial Toll/interleukin-1 receptor domain–containing proteins. Nat Med. 2008;14(4):399–406. doi: 10.1038/nm1734. [DOI] [PubMed] [Google Scholar]
- 18.Yadav M., Zhang J., Fischer H., Huang W., Lutay N., Cirl C. Inhibition of TIR domain signaling by TcpC: MyD88-dependent and independent effects on Escherichia coli virulence. PLoS Pathog. 2010;6(9):e1001120. doi: 10.1371/journal.ppat.1001120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sabate M., Moreno E., Perez T., Andreu A., Prats G. Pathogenicity island markers in commensal and uropathogenic Escherichia coli isolates. Clin Microbiol Infect. 2006;12:880–886. doi: 10.1111/j.1469-0691.2006.01461.x. [DOI] [PubMed] [Google Scholar]
- 20.Houdouin V., Bonacorsi S., Bidet P., Bingen-Bidois M., Barraud D., Bingen E. Phylogenetic background and carriage of pathogenicity island-like domains in relation to antibiotic resistance profiles among Escherichia coli urosepsis isolates. J Antimicrob Chemother. 2006;58(4):748–751. doi: 10.1093/jac/dkl326. [DOI] [PubMed] [Google Scholar]
- 21.Bingen-Bidois M., Clermont O., Bonacorsi S., Terki M., Brahimi N., Loukil C. Phylogenetic analysis and prevalence of urosepsis strains of Escherichia coli bearing pathogenicity island-like domains. Infect Immun. 2002;70(6):3216–3226. doi: 10.1128/IAI.70.6.3216-3226.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson J.R., Stell A.L. Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. J Infect Dis. 2000;181:261–272. doi: 10.1086/315217. [DOI] [PubMed] [Google Scholar]
- 23.Walk S.T., Alm E.W., Calhoun L.M., Mladonicky J.M., Whittam T.S. Genetic diversity and population structure of Escherichia coli isolated from freshwater beaches. Environ Microbiol. 2007;9:2274–2288. doi: 10.1111/j.1462-2920.2007.01341.x. [DOI] [PubMed] [Google Scholar]
- 24.Bashir S., Haque A., Sarwar Y., Ali A., Anwar M.I. Virulence profile of different phylogenetic groups of locally isolated community acquired uropathogenic E. coli from Faisalabad region of Pakistan. Ann Clin Microbiol Antimicrob. 2012;11:23. doi: 10.1186/1476-0711-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bailey J.K., Pinyon J.L., Anantham S., Hall R.M. Distribution of human commensal Escherichia coli phylogenetic groups. J Clin Microbiol. 2010;48:3455–3456. doi: 10.1128/JCM.00760-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmidt H., Hensel M. Pathogenicity islands in bacterial pathogenesis. Clin Microbiol Rev. 2004;17:14–56. doi: 10.1128/CMR.17.1.14-56.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nandanwar N., Janssen T., Kuhl M., Ahmed N., Ewers C., Wieler L.H. Extraintestinal pathogenic Escherichia coli (ExPEC) of human and avian origin belonging to sequence type complex 95 (STC95) portray indistinguishable virulence features. Int J Med Microbiol. 2014;304:835–842. doi: 10.1016/j.ijmm.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 28.Ramos N.L., Sekikubo M., Dzung D.T., Kosnopfel C., Kironde F., Mirembe F. Uropathogenic Escherichia coli isolates from pregnant women in different countries. J Clin Microbiol. 2012;50(11):3569–3574. doi: 10.1128/JCM.01647-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson J.R., Orskov I., Orskov F., Goullet P., Picard B., Moseley S.L. O, K, and H antigens predict virulence factors, carboxylesterase B pattern, antimicrobial resistance, and host compromise among Escherichia coli strains causing urosepsis. J Infect Dis. 1994;169(1):119–126. doi: 10.1093/infdis/169.1.119. [DOI] [PubMed] [Google Scholar]
- 30.Duriez P., Clermont O., Bonacorsi S., Bingen E., Chaventre A., Elion J. Commensal Escherichia coli isolates are phylogenetically distributed among geographically distinct human populations. Microbiology. 2001;147(pt 6):1671–1676. doi: 10.1099/00221287-147-6-1671. [DOI] [PubMed] [Google Scholar]
- 31.Houdouin V., Bonacorsi S., Brahimi N., Clermont O., Nassif X., Bingen E. A uropathogenicity island contributes to the pathogenicity of Escherichia coli strains that cause neonatal meningitis. Infect Immun. 2002;70(10):5865–5869. doi: 10.1128/IAI.70.10.5865-5869.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peterson J.W. Bacterial pathogenesis. In: Baron S., editor. Medical microbiology. 4th ed. University of Texas Medical Branch at Galveston; Galveston, TX: 1996. http://www.ncbi.nlm.nih.gov/books/NBK8526/ Chapter 7. Available at: [Google Scholar]
- 33.Torres A.G., Redford P., Welch R.A., Payne S.M. TonB-dependent systems of uropathogenic Escherichia coli: aerobactin and heme transport and TonB are required for virulence in the mouse. Infect Immun. 2001;69:6179–6185. doi: 10.1128/IAI.69.10.6179-6185.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schubert S., Rakin A., Karch H., Carniel E., Heesemann J. Prevalence of the ‘high-pathogenicity island’ of Yersinia species among Escherichia coli strains that are pathogenic to humans. Infect Immun. 1998;66:480–485. doi: 10.1128/iai.66.2.480-485.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang L., Foxman B., Marrs C. Both urinary and rectal Escherichia coli isolates are dominated by strains of phylogenetic group B2. J Clin Microbiol. 2002;40:3951–3955. doi: 10.1128/JCM.40.11.3951-3955.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Handley M.A., Reingold A.L., Shiboski S., Padian N.S. Incidence of acute urinary tract infection in young women and use of male condoms with and without nonoxynol-9 spermicides. Epidemiology. 2002;13:431–436. doi: 10.1097/00001648-200207000-00011. [DOI] [PubMed] [Google Scholar]
- 37.Wang M.C., Tseng C.C., Chen C.Y., Wu J.J., Huang J.J. The role of bacterial virulence and host factors in patients with Escherichia coli bacteremia who have acute cholangitis or upper urinary tract infection. Clin Infect Dis. 2002;35:1161–1166. doi: 10.1086/343828. [DOI] [PubMed] [Google Scholar]
- 38.Sokurenko E.V., Chesnokova V., Dykhuizen D.E., Ofek I., Wu X.R., Krogfelt K.A. Pathogenic adaptation of Escherichia coli by natural variation of the FimH adhesin. Proc Natl Acad Sci U S A. 1998;95(15):8922–8926. doi: 10.1073/pnas.95.15.8922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee S., Yu J.K., Park K., Oh E.J., Kim S.Y., Park Y.J. Phylogenetic groups and virulence factors in pathogenic and commensal strains of Escherichia coli and their association with blaCTX-M. Ann Clin Lab Sci. 2010;40(4):361–367. [PubMed] [Google Scholar]
- 40.Norinder B.S., Koves B., Yadav M., Brauner A., Svanborg C. Do Escherichia coli strains causing acute cystitis have a distinct virulence repertoire? Microb Pathog. 2012;52(1):10–16. doi: 10.1016/j.micpath.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 41.Le Bouguenec C., Archambaud M., Labigne A. Rapid and specific detection of the pap, afa, and sfa adhesin-encoding operons in uropathogenic Escherichia coli strains by polymerase chain reaction. J Clin Microbiol. 1992;30(5):1189–1193. doi: 10.1128/jcm.30.5.1189-1193.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamamoto S., Terai A., Yuri K., Kurazono H., Takeda Y., Yoshida O. Detection of urovirulence factors in Escherichia coli by multiplex polymerase chain reaction. FEMS Immunol Med Microbiol. 1995;12(2):85–90. doi: 10.1111/j.1574-695X.1995.tb00179.x. [DOI] [PubMed] [Google Scholar]
- 43.Kuhar I., Grabnar M., Zgur-Bertok D. Virulence determinants of uropathogenic Escherichia coli in fecal strains from intestinal infections and healthy individuals. FEMS Microbiol Lett. 1998;164(2):243–248. doi: 10.1111/j.1574-6968.1998.tb13093.x. [DOI] [PubMed] [Google Scholar]
- 44.Yamamoto S., Tsukamoto T., Terai A., Kurazono H., Takeda Y., Yoshida O. Distribution of virulence factors in Escherichia coli isolated from urine of cystitis patients. Microbiol Immunol. 1995;39(6):401–404. doi: 10.1111/j.1348-0421.1995.tb02219.x. [DOI] [PubMed] [Google Scholar]