Abstract
Silicon(Si) is the only element which can enhance the resistance to multiple stresses. However, the role of silicon in medicinal plants under salt stress is not yet understood. This experiment was conducted to study the effects of silicon addition on the growth, osmotic adjustments, photosynthetic characteristics, chloroplast ultrastructure and Chlorogenic acid (CGA) production of Honeysuckle plant (Lonicera japonica L.) under salt-stressed conditions. Salinity exerted an adverse effect on the plant fresh weight and dry weight, whilst 0.5 g L−1 K2SiO3·nH2O addition obviously improved the plant growth. Although Na+ concentration in plant organs was drastically increased with increasing salinity, higher levels of K+/Na+ ratio was obtained after K2SiO3·nH2O addition. Salinity stress induced the destruction of the chloroplast envelope; however, K2SiO3·nH2O addition counteracted the adverse effect by salinity on the structure of the photosynthetic apparatus. K2SiO3·nH2O addition also enhanced the activities of superoxide dismutase and catalase. To sum up, exogenous Si plays a key role in enhancing its resistance to salt stresses in physiological base, thereby improving the growth and CGA production of Honeysuckle plant.
Most of the environmental constraints drastically decrease plant growth1. Among abiotic threats, salinity is of special concern. Salinization is a global problem, and has attracted more attention of the international academic community. It is most common in arid and semi-arid areas all over the world and responsible for a substantial decline in crop yield2. Therefore, the efforts to screen economically salt-tolerant crops or plants bear remarkable importance for sustainable agriculture. Honeysuckle (Lonicera japonica L.), as an economic plant, is widely appreciated as tea beverage in Asia and Europe because of its unique aroma and flavour3. Moreover, it is commonly used in traditional Chinese medicine (TCM) for the treatment of arthritis, diabetes mellitus, enteritis and fever4,5. Pharmacological studies have shown that the constituents of honeysuckle have a broad spectrum of biological activity, such as antiviral, antipyretic and hepatoprotective effects6,7. For this reason, honeysuckle is being cultivated in salt-affected areas, such as tidal flats in coastal zones, for its specific therapeutic benefits.
Generally, plants adopt a number of strategies to cope with adverse effects of salt stress, in which osmotic adjustment is an important one8. Plants undergo osmotic adjustments under abiotic stress condition by avoiding Na+ and Cl− uptake which causes ion toxicity and accumulating K+ , Ca2+ , enabling plants to withstand salinization9. The amount of Na+ absorbed exceeds that of Na+ extruded through the plasma membrane (PM) cation channels, leading to excessive Na+ -toxicity events, such as damage to these systems that absorb and utilize Ca2+ and K+ ions. Damage to these systems negatively affects plant growth and development, since Ca2+ and K+ are essential for various physiological and biochemical processes10,11,12,13.
Additionally, a decrease in photosynthesis capacity is very common in salt stressed plant, mostly due to a low osmotic potential of the soil solution (osmotic stress), specific ion effects (salt stress), nutritional imbalances or more usually, a combination of these factors14. Salinity influences photosynthetic capacity and its effects vary with the salt concentration, duration of stress and the cultivar used. Photosynthetic ability of some plant species reduced in the presence of salinity15,16, while that of other species reduced only at high stress and long term stress17.
Also, salinity is one of the major stresses responsible for changes in metabolic activity of plants. Plants have evolved several adaptive mechanisms to handle with the salinity in their environments, but the understanding of these mechanisms still remains incomplete. It has been shown that reactions to salt and drought stress might be responsible for the increase or decrease in the content of relevant natural products; however, scientific background in this field is still rare18. Medicinal plants under salt stress conditions accumulate higher concentrations of secondary compounds than control plants which are cultivated under standard conditions. Chlorogenic acid (CGA) is a type of polyphenol and one kind of secondary metabolites, which has anti-inflammatory, anti-oxidative and anticancer properties19,20,21. CGA could inhibit inflammatory cell infiltration, notably neutrophil recruitment into lung22 and inhibit inflammatory cytokines release through suppressing nuclear factor kappa B (NF-kB) activation23. Keeping this in view, accumulation of CGA in the Honeysuckle plant under salt stress conditions was studied in this experiment.
Several investigators have reported that silicon enhanced the salt tolerance of wheat24, barley25,26, maize27 and tomato (Solanum lycopersicum L. cv. Hong mei)28. However, its role in plant biology has been poorly understood and the attempts to associate Si with metabolic or physiological activities have been inconclusive29.
The objective of this study was to elucidate the salt-tolerant mechanisms of the Honeysukle plant on physiological bases, therefore revealing the role of Silicon in the amelioration of salt hazards and enhancement of secondary metabolite in the medicinal plant.
Materials and methods
Experimental layout
The experiment was conducted in a greenhouse at College of Resources and Environmental Sciences, Nanjing Agricultural University, during September 14 to October 25, 2013. Honeysuckle (Lonicera japonica L.) seedlings were obtained from BoZhou city, AnHui Province. Nearly 30-day-old nursery seedlings were transplanted into plastic pots (24 cm in diameter, 18 cm in depth), one seedling per pot. The pots were filled with clean sand and each pot was watered with 1.5 L Hoagland Solution. The experiment was arranged in a completely randomized design (CRD) and each treatment had three replications.
Plants were subjected to salt stress by adding sodium chloride (NaCl) solutions, with the calculated amount of NaCl and K2SiO3·nH2O dissolved in Hoagland Solution. The experiment consisted of three treatments involving varying salinity levels and three levels of K2SiO3·nH2O in sand (Table 1).To minimize salt shock, NaCl concentration was raised stepwise in aliquots of 50 mM every other day until the final salinity levels were achieved. The greenhouse temperature and relative humidity varied 25–30 °C and 50–80%, respectively for the entire growth period. Photosynthesis is determined at the 30th day after salt treatment, respectively. Plants were sampled (the whole plant) at the 40th day after salt treatment to assess the growth, osmotic adjustments, chloroplast ultrastructrue and chlorogenic acid (CGA) accumulation. After washing and drying, leaves were frozen in liquid N2 and stored at −80 °C until biochemical analysis.
Table 1. Nine treatments (NaCl × K2SiO3·nH2O) designed in this experiment.
| NO. | NaCl (mM) | K2SiO3·nH2O (g/L) |
|---|---|---|
| 1 | 0 | 0 |
| 2 | 0 | 0.5 |
| 3 | 0 | 1.0 |
| 4 | 100 | 0 |
| 5 | 100 | 0.5 |
| 6 | 100 | 1.0 |
| 7 | 200 | 0 |
| 8 | 200 | 0.5 |
| 9 | 200 | 1.0 |
Growth status analysis
FW is the fresh weight, and DW is the dry weight after oven-drying samples at 70 °C until constant weight.
Elemental analysis
Leaves, stems and roots were separated and washed with ultra-pure water to eliminate salts in the surface of these samples. Subsequently they were dried at 70 °C until constant weight and grinded in a stoneware mortar, from which were taken 100 mg for acid digestion. This procedure was carried out in Teflon reactors using an acid mixture of HNO3:HClO4 (3:1, v/v) during 2 h at 280 °C until the liquid was almost transparent. After cooling overnight, the digestion products were diluted with distilled water to a total volume of 100 ml. Elemental concentrations in the extracts were determined by ICP-AES (Inductively Coupled Plasma Atomic Emission Spectrometry, Optima 2100DV, Perkin Elmer, USA). Si was determined following the method of Nayar et al.30.
Transmission electron microscope assay
The fifth fully expanded leaves, numbered basipetally, were sampled for ultramicroscopic observation on day 30 after the onset of the final concentration of salt treatment. For transmission electron microscope, fresh leaves were cut into 2 segments of 1 mm each and placed immediately in a freshly prepared the stationary liquid of 2.5% (w/v) glutaraldehyde in 50 mM sodium phosphate buffer (PH 7.4). The segments were then de-gassed and fixed under vacuum for 4 h at room temperature. After washing in the same buffer, they were post-fixed in 1% (w/v) osmium tetroxide (in the same buffer) for 1 h. After fixation, the samples were rinsed three with the same buffer for 15 min. Rapid dehydration was accomplished by placing the sample in 50% ethanol for 30 min, followed by changes in 70%, 90%, 95%, 100% ethanol (30 min each). The specimens were then placed in low-viscosity epoxy resin. UL-trathin sections (80 nm) were mounted on uncoated nickel grids (300 meshes) and sequentially stained with uranyl acetate and lead citrate before being examined at 80 kV under a transmission electron microscope (H-7650, JEOL, Japan).
The determination of leaf photosynthesis
The tested photosynthetic parameters included photosynthetic rate (A), Transpiration rate (E), stomatal conductance (gs) and Intercellular CO2 concentration (Ci). They were determined by portable photosynthesis system Li-6400XT (Li-cor Inc., Lincolin, NE, USA). The PAR was provided by a red/blue light-emitting diode (model Li 6400-02B; Li-cor Inc., NE, USA) and was set at 400 or 700 umol photons m-2s-1 to match the PAR used when exposing plants to control or high light conditions, respectively. The atmospheric CO2 concentration was controlled with a CO2 injection system controlled by the Li-6400 (Li-cor Inc., Lincolin, NE, USA). The cuvette temperature was maintained at 25 °C with a relative humidity of 65%.
Pre-segmentation of CGA
Leaves washed with ultra-pure water to eliminate salts in the surface of these samples. Subsequently were dried at 70 °C until constant weight and grinded in a stoneware mortar, from which were taken 1.00 g for pre-segmentation. 1.0 g of leaf tissue of both control and stressed plants was homogenized in 14 mL 60% (v/v) ethylalcohol, and the pH of homogenate was 4.0. Homogenate was extracted in an ultrasonic cleaner (Xokeji, NJ, CHN) 15 min, then leached by Buchner funnel using a 0.45 um filter membrane.
Estimation of CGA in the Honeysuckle plant (Lonicera japonica L.) was done using HPLC. Chromatography was done in RP 18 columns with a flow rate of 1.0 mL min−1 using 1% phosphate buffer in HPLC acetonitrile (87:13) as carrier solvent. The above step was repeated for three times, and then collected and amalgamated the filter liquor for HPLC analysis. The sample load was 20 uL. Column temperature was maintained at 25 °C and absorbance was measured at 327 nm.
Measurement of Antioxidant enzyme activities
The activity of SOD was determined following Giannopolitis and Ries31 by measuring its ability to inhibit the photoreduction of nitroblue tetrazolium (NBT). Activities of catalase (CAT) and peroxidase (POD) were appraised using the method of Chance and Maehly32 with some modification. The CAT reaction solution (3 ml) contained 50 mM phosphate buffer (PH 7.0), 5.9 mM H2O2, and 50 ul enzyme extract. Then the reaction was initiated by adding the enzyme extract. Changes in absorbance of the reaction solution at 240 nm were read every 40 s. One unit CAT activity was defined as an absorbance change of 0.01 units per min. The POD reaction solution (3 ml) contained 50 mM phosphate buffer (PH 6.0), 20 mM guaiacol, 40 mM H2O2, and 30 ul enzyme extract. Changes in absorbance of the reaction solution at 470 nm were determined after every 15 s. The activity of each enzyme was expressed on protein basis.
Statistical analysis
Data were statistically analyzed using analysis of variance (ANOVA) by SPSS v.20 for Windows, and presented as treatment mean ± SE of three measurements. Tukey’s multiple range tests were calculated for the significant data at P < 0.05.
Results
Plant growth status
The effect of K2SiO3·nH2O addition on the fresh weight and dry weight of Honeysuckle plant (Lonicera japonica L.) was shown in Table 2. The highest value of the fresh weight and dry weight for no salt treatments was obtained in 0.5 g L−1 K2SiO3·nH2O addition treatments, implying that the optimum dose of K2SiO3·nH2O addition (0.5 g L−1 K2SiO3·nH2O) did enhance the plant growth. The same trends, however, were observed in 0.5 g L−1 K2SiO3·nH2O × 100 mM NaCl treatment and only for plant fresh weight. Additionally, higher concentration of K2SiO3·nH2O addition (1.0 g L−1) exerted no effect on the plant fresh weight and dry weight. As a whole, NaCl concentration of <200 mM did not affect the plant growth, suggesting the tested variety of Honeysuckle itself was a more salt-tolerant one. These results implied that K2SiO3·nH2O addition with 0.5 mg L−1 promoted the growth of the Honeysuckle, therefore increasing salt tolerance of the plant to a certain extent.
Table 2. Effect of K2SiO3·nH2O addition on the fresh weight and dry weight of Honeysuckle plant (Lonicera japonica L.) under NaCl-stressed conditions.
| The potency of K2SiO3·nH2O (g L−1) | Plant fresh weigh (g plant−1) Salt levels (mM of NaCl) | ||||
|---|---|---|---|---|---|
| Control (0 mM) |
100 |
200 |
|||
| Mean ± S.E | Mean ± S.E | Percent of control | Mean ± S.E | Percent of control | |
| 0 | 17.34 ± 2.99aB | 15.61 ± 2.68aB | 90.02 | 13.54 ± 1.95aA | 78.09 |
| 0.5 | 25.41 ± 2.21aA | 22.01 ± 3.30aA | 86.70 | 16.45 ± 3.20aA | 64.74 |
| 1.0 | 17.00 ± 1.75aB | 13.47 ± 3.25aB | 79.24 | 13.15 ± 3.61aA | 77.35 |
| The potency of K2SiO3·nH2O (g L−1) | Plant dry weigh (g plant−1) Salt levels (mM of NaCl) | ||||
| Control (0mM) | 100 | 200 | |||
| Mean ± S.E | Mean ± S.E | Percent of control | Mean ± S.E | Percent of control | |
| 0 | 5.46 ± 0.54aB | 4.53 ± 1.03aA | 82.97 | 4.28 ± 0.51aA | 78.31 |
| 0.5 | 7.12 ± 1.02aA | 5.26 ± 1.23aA | 73.90 | 5.04 ± 2.50aA | 70.86 |
| 1.0 | 4.63 ± 0.72aB | 4.40 ± 1.14aA | 95.03 | 4.09 ± 1.76aA | 88.46 |
The values represent the means of three replicates. The means followed by the same letters (lower-case letters within lines and upper-case letters within columns, respectively) did not differ significantly at 5% probability by Tukey’s test.
Osmotic adjustments
Salt stress significantly affected the ionic uptake and distribution in the plant organs (Table 3). Na+ concentration in leaves, stems and roots was significantly increased under salt-stressed conditions as compared with the control. However, this increment in Na+ concentration was partially counteracted in 100 mM NaCl treatment by 0.5 mg L−1 K2SiO3·nH2O application. Additionally, an interesting phenomenon was investigated that Na+ concentration in plant organs was much lower than K+ concentration, indicating that selective absorption of K+ over Na+ (high SK+/Na+) could be one of the mechanism for this plant adapting to salt stress. Salt stress also caused the declined Ca2+ uptake by plant; whereas 1 g L−1 K2SiO3·nH2O application increased the Ca2+ concentration to a high level of approximately equal to that in the control treatment. Thus, it was concluded that K2SiO3·nH2O application played an important role in osmotic adjustments, subsequently enhancing the salt tolerance of the Honeysuckle plant.
Table 3. Effect of K2SiO3·nH2O addition on the ion distribution within Honeysuckle (Lonicera japonica L.) plant under NaCl-stressed conditions.
| The potency of K2SiO3·nH2O (g L−1) |
Na+
Concentration ((mg g−1
dwt) Salt levels (mM of NaCl) |
|||||
|---|---|---|---|---|---|---|
|
Control (0mM) |
100 |
200 |
||||
| Leaves and stems | Roots | Leaves and stems | Roots | Leaves and stems | Roots | |
| 0 | 0.66 ± 0.05aA | 0.45 ± 0.07bB | 5.16 ± 0.12aA | 6.65 ± 0.14aA | 4.58 ± 0.28bA | 8.95 ± 0.00aAB |
| 0.5 | 0.66 ± 0.05cA | 0.56 ± 0.06cB | 3.16 ± 0.29bB | 4.52 ± 0.18bB | 5.60 ± 0.16aA | 10.46 ± 0.08aA |
| 1.0 | 0.11 ± 0.00cB | 0.96 ± 0.08bA | 5.39 ± 0.06aA | 6.34 ± 0.21aA | 4.52 ± 0.12bA | 7.78 ± 0.52aB |
| The potency of K2SiO3·nH2O (g L−1) | K+ Concentration ((mg g−1 dwt) Salt levels (mM of NaCl) | |||||
| Control (0 mM) | 100 | 200 | ||||
| Leaves and stems | Roots | Leaves and stems | Roots | Leaves and stems | Roots | |
| 0 | 19.36 ± 1.16bA | 39.64 ± 0.88aA | 23.30 ± 0.57aA | 37.71 ± 0.92aA | 18.58 ± 1.19bB | 33.65 ± 0.34bA |
| 0.5 | 22.44 ± 0.13aA | 37.42 ± 0.89aA | 20.62 ± 2.79aA | 40.08 ± 1.25aA | 22.97 ± 0.92aA | 40.54 ± 1.07aA |
| 1.0 | 8.56 ± 0.004cB | 39.39 ± 2.92aA | 24.86 ± 0.64aA | 40.30 ± 0.69aA | 21.53 ± 1.42bAB | 38.12 ± 2.06aA |
| The potency of K2SiO3·nH2O (g L−1) | SK+/Na+ Salt levels (mM of NaCl) | |||||
| Control (0 mM) | 100 | 200 | ||||
| Leaves and stems | Roots | Leaves and stems | Roots | Leaves and stems | Roots | |
| 0 | 31.64 ± 0.16aB | 87.69 ± 9.02aA | 4.60 ± 0.04bB | 5.52 ± 0.27bC | 4.06 ± 0.05cA | 3.81 ± 0.09bB |
| 0.5 | 34.01 ± 2.23aAB | 71.45 ± 7.39aA | 6.31 ± 0.22bA | 8.88 ± 0.53bA | 4.00 ± 0.11bA | 3.90 ± 0.11bB |
| 1.0 | 43.53 ± 4.20aA | 76.47 ± 1.96aA | 4.43 ± 0.19bB | 6.66 ± 0.55bB | 4.77 ± 0.24bA | 4.89 ± 0.05bA |
| The potency of K2SiO3·nH2O (g L−1) | Ca2+ Concentration ((mg g−1 dwt) Salt levels (mM of NaCl) | |||||
| Control (0 mM) | 100 | 200 | ||||
| Leaves and stems | Roots | Leaves and stems | Roots | Leaves and stems | Roots | |
| 0 | 15.09 ± 0.62aA | 8.40 ± 0.31aA | 13.01 ± 0.54bA | 5.35 ± 0.01bB | 11.79 ± 0.75bA | 5.32 ± 0.02bA |
| 0.5 | 15.06 ± 0.58aA | 7.15 ± 0.10aB | 13.01 ± 0.09abA | 6.48 ± 0.16aA | 10.43 ± 1.28bB | 5.45 ± 0.15bA |
| 1.0 | 13.96 ± 0.13abA | 6.83 ± 0.94aB | 14.66 ± 0.25aA | 5.52 ± 0.15aB | 13.61 ± 0.62bA | 5.10 ± 0.25aA |
The values represent the means of three replicates. The means followed by the same letters (lower-case letters within lines and upper-case letters within columns, respectively) did not differ significantly at 5% probability by Tukey’s test.
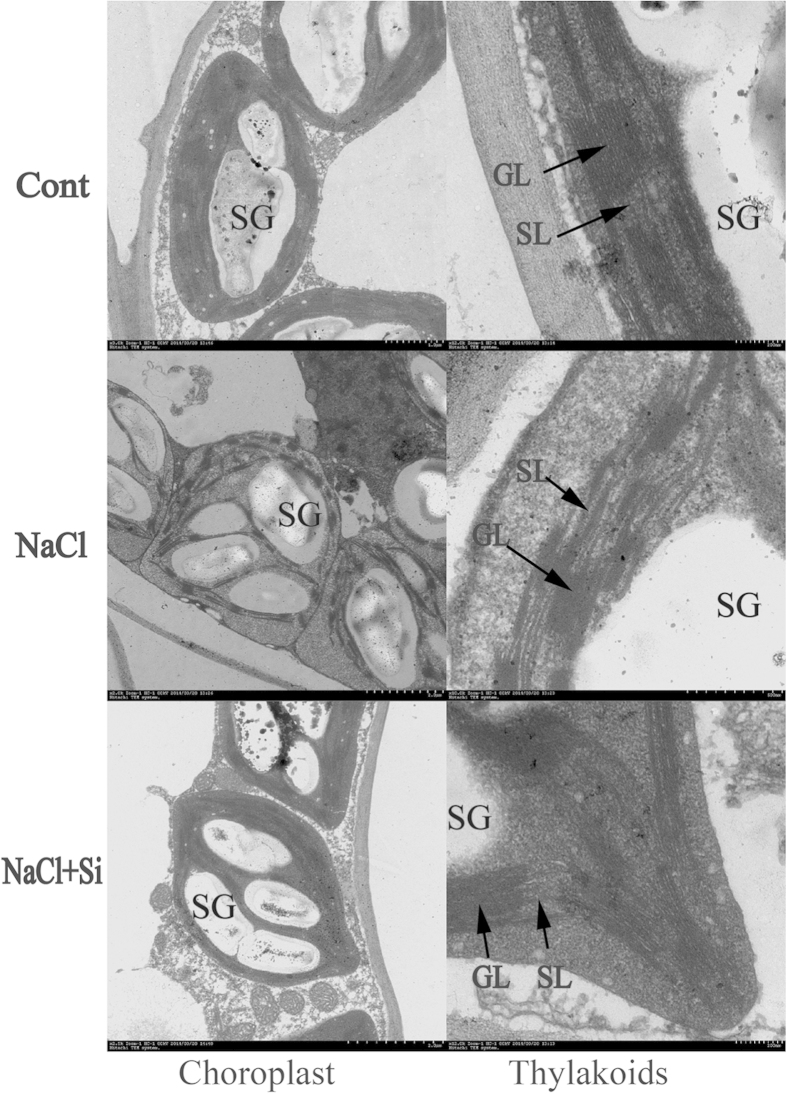
Chloroplast ultrastructure
There were no significant differences in ultrastructure of chloroplasts between the control and control plus remission of K2SiO3·nH2O (Fig. 1). Salt stress induced alterations in the structure of chloroplasts as compared to the control. The chloroplasts in salt-stressed plants were separated from the plasmamembrane, where chloroplasts were in closely contact with the membrane. The envelope membrane in the NaCl treated samples appeared to be ruptured. However, exogenous remission of K2SiO3·nH2O alleviated the structural changes of chloroplasts induced by salt stress. Remission of K2SiO3·nH2O maintained a well-preserved internal lamellar system in the chloroplasts of salt-stressed leaves and the chloroplasts contained less osmiophilic plastoglobuli. These results suggested that K2SiO3·nH2O application helped to maintain the integrity of chloroplast ultrastructrue, thus executed a normal physiological functions of the plant exposed to salt stress.
Figure 1. The effects of salt stress with and without exogenous remission of K2SiO3·nH2O on the chloroplast ultrastructure in leaves of Honeysuckle (Lonicera japonica L.).
(a) Cont; 0 mM NaCl + 0 g L−1 K2SiO3·nH2O, (b) NaCl; 200 mM NaCl + 0 g L−1 K2SiO3·nH2O, (c) NaCl + Si; 200 mM NaCl + 0.5 g L−1 K2SiO3·nH2O. SL, stroma lamella; GL, grana lamellae; SG, starch grain.
Plant photosynthesis
The changes of photosynthetic characteristics in leaf Honeysuckle were shown in Table 4. Salt stress markedly reduced the photosynthetic rate in Honeysuckle leaves, whilst K2SiO3·nH2O application with the concentration of 1.0 g L−1 unexpectedly increased the photosynthetic rate. Similarly, the transpiration rate and stomatal conductance were significantly decreased with increasing NaCl concentrations no matter what K2SiO3·nH2O was applied or not. However, 0.5 g L−1 K2SiO3·nH2O addition appeared no effect on the plant photosynthesis, transpiration rate and stomatal conductance, but even inhibited them in a great deal.
Table 4. Photosynthetic rate (A), transpiration rate (E) and stomatal conductance (Gs) of Honeysuckle (Lonicera japonica L.) exposed to nine treatments.
| The potency of K2SiO3·nH2O (g L−1) |
A (umol CO2 m−2s−1) photosynthetic rate Salt levels (mM of NaCl) |
||||
|---|---|---|---|---|---|
| Control (0 mM) |
100 |
200 |
|||
| Mean ± S.E | Mean ± S.E | Percent of control | Mean ± S.E | Percent of control | |
| 0 | 15.13 ± 0.047aA | 9.27 ± 1.22bA | 61.27 | 3.03 ± 0.07cB | 20.03 |
| 0.5 | 10.28 ± 0.09aB | 5.74 ± 0.21bB | 55.84 | 4.03 ± 0.03cB | 39.20 |
| 1.0 | 6.98 ± 0.32cC | 8.06 ± 0.81bA | 115.47 | 9.55 ± 0.27aA | 136.82 |
| The potency of K2SiO3·nH2O (g L−1) | E (mmol m−2 s−1) transpiration rate Salt levels (mM of NaCl) | ||||
| Control (0 mM) | 100 | 200 | |||
| Mean ± S.E | Mean ± S.E | Percent of control | Mean ± S.E | Percent of control | |
| 0 | 2.82 ± 0.36aA | 0.86 ± 0.03bA | 30.50 | 0.31 ± 0.01cAB | 10.99 |
| 0.5 | 1.50 ± 0.19aB | 0.42 ± 0.20bB | 28.00 | 0.23 ± 0.01bB | 15.33 |
| 1.0 | 1.02 ± 0.01aC | 0.67 ± 0.11bAB | 65.69 | 0.45 ± 0.06cA | 44.12 |
| The potency of K2SiO3·nH2O (g L−1) | Gs (mol m−2 s−1) stomatal conductance Salt levels (mM of NaCl) | ||||
| Control (0 mM) | 100 | 200 | |||
| Mean ± S.E | Mean ± S.E | Percent of control | Mean ± S.E | Percent of control | |
| 0 | 0.16 ± 0.44aA | 0.06 ± 0.00bA | 37.5 | 0.02 ± 0.00cA | 12.5 |
| 0.5 | 0.11 ± 0.02aAB | 0.03 ± 0.00bB | 27.27 | 0.01 ± 0.00cA | 9.10 |
| 1.0 | 0.06 ± 0.00aB | 0.05 ± 0.01aA | 83.33 | 0.03 ± 0.01bA | 50.00 |
The values represent the means of three replicates. The means followed by the same letters (lower-case letters within lines and upper-case letters within columns, respectively) did not differ significantly at 5% probability by Tukey’s test.
Secondary metabolite of CGA
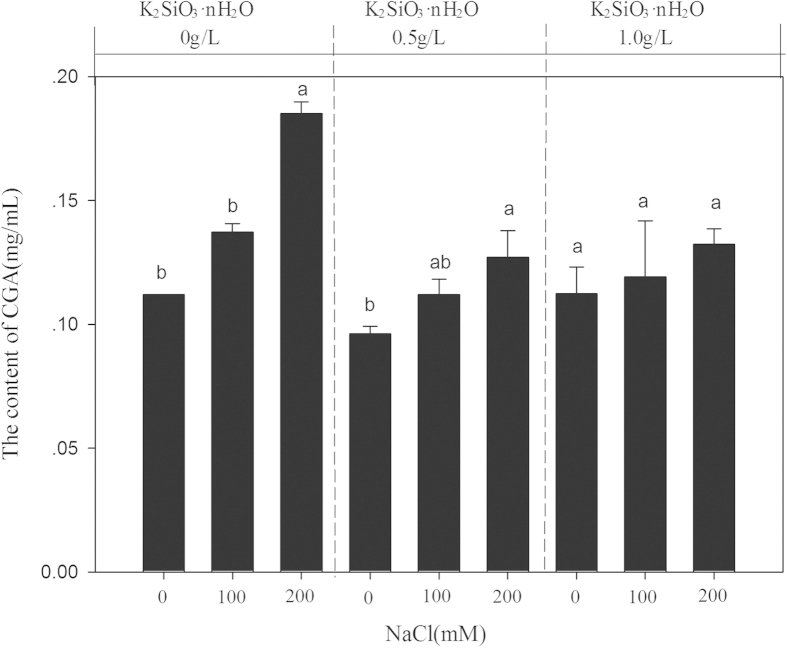
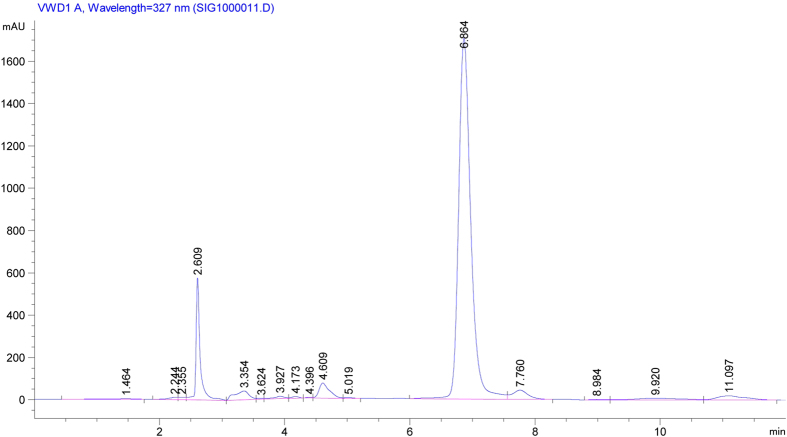
CGA content in leaf Honeysuckle increased significantly with increasing salinities, as compared with the control (Fig. 2). With exogenous application of K2SiO3·nH2O, CGA content was gradually decreased, suggesting that CGA production in the plant leaves was hindered although K2SiO3·nH2O application alleviated plant injury induced by salt stress. Moreover, no significant difference was observed in CGA content of leaf Honeysuckle among all salt-stressed treatments when 1.0 g L−1 K2SiO3·nH2O was applied. In this experiment, the representative chromatogram with retention time for CGA was 7 min (Fig. 3).
Figure 2. Secondary metabolite of CGA quantified in the leaves of Honeysuckle plant (Lonicera japonica L.) exposed to salt stress.
Different letters within each column indicated significant difference among treatments at the p < 0.05 level. Three independent HPLC profiles were run for each sample and value represents mean ± S.E.
Figure 3. Representative chromatogram of prepared extract of Honeysuckle leaves (Lonicera japonica L.) grown in 200 mM NaCl concentrations with retention time of 7 min for CGA measurement.
Discussion
Silicon has been extensively shown to increase crop yield and stress tolerance33. In this study, we found that K2SiO3·nH2O addition resulted in an improvement in the growth of salt-stressed Honeysuckle (Lonicera japonica L.) seedlings. These findings were comparable with observations in other crops, for example sorghum studied by Yin34, in which the application of silicon alone had no effects upon sorghum growth, while it partly reversed the salt-induced reduction in plant growth. In fact, silicon application resulted in the improvement of growth in many plants exposed to salt stresses that is often associated with many aspects, such as salt concentration, plant salt tolerance in addition to the plant species or genotypes. For rice plant, added Si can increase rigidity of the mature leaves, which have a rougher texture and are held more horizontally, delays leaf senescence and increases chlorophyll content and ribulose-bisphosphate carboxylase activity35. In this experiment, it was evident that Si uptake by the plant increased with increasing concentration of exogenous K2SiO3·nH2O, while inhibited by increasing salinity of hydroponic solution, suggesting Si may be involved in the metabolic or physiological activity in Honeysuckle exposed to salt stress (Table 5).
Table 5. Effect of K2SiO3·nH2O addition on the Si distribution within Honeysuckle (Lonicera japonica L.) plant under NaCl-stressed conditions.
| The potency of K2SiO3·nH2O (g L−1) |
Si Concentration (mg g−1 dwt) salt levels (mM of NaCl) |
|||||
|---|---|---|---|---|---|---|
|
Control (0mM) |
100 |
200 |
||||
| Leaves and stems | Roots | Leaves and stems | Roots | Leaves and stems | Roots | |
| 0 | 0.45 ± 0.00aC | 0.08 ± 0.00aC | 0.37 ± 0.00bC | 0.02 ± 0.01bC | 0.16 ± 0.00cB | 0.00 ± 0.00cC |
| 0.5 | 12.14 ± 1.08aB | 3.75 ± 0.52aB | 9.83 ± 0.76bB | 2.09 ± 0.37bB | 8.55 ± 1.02bA | 1.29 ± 0.13cB |
| 1.0 | 20.33 ± 2.96aA | 5.40 ± 0.67aA | 16.55 ± 2.41aA | 5.00 ± 0.38aA | 10.38 ± 1.23bA | 3.00 ± 0.16bA |
The values represent the means of three replicates. The means followed by the same letters (lower-case letters within lines and upper-case letters within columns, respectively) did not differ significantly at 5% probability by Tukey’s test.
Maintaining structural integrity and orderliness of chloroplast is necessary in the conversion of light energy for photosynthesis. It was reported that many stressors led to the decrease in the photochemical efficiency and electron transport activity that might be associated with the changes of the structure of photosynthetic apparatus36. In the present study, we observed that exogenous Si decreased the separation of plasma membrane from plasmolysis. Moreover, it commendably maintains the integrity of the plasma membrane. These results demonstrated that silicon was involved in protecting the photosynthetic apparatus. Our results also showed that photosynthetic activity in Honeysuckle (Lonicera japonica L.) was affected by salinity, and the extent of the reduction was dependent on the salt strength. Zollinger37 found a similar reduction in photosynthetic rate and stomatal conductance in E. purpurea irrigated with increasing concentrations of salinity up to 5 gL−1. Reduction in photosynthetic rate under salinity can be attributed to both stomatal and non stomatal limitations38. In this study, we found a strong negative correlation between stomatal conductance and Na+ concentrations both in the aboveground parts and roots, which indicates that plants tend to close their stomatal conductance as a result of specific-ion accumulations. This reduction in stomatal conductance led subsequently to a reduction in photosynthetic rate as indicated by the high correlation between stomatal conductance and photosynthetic rate. Gong et al.39 reported that silicon did not enhance the K+ content of rice under salt stress. This is in agreement with our results that silicon had no effect on the K+ concentration. It is well known that high concentrations of Na+ can inhibit K+ and Ca2+ uptake through antagonism between these ions40. This fact can be attributed to their physicochemical similarity, which promotes ionic competition for binding sites on membrane transporters41. Further studies on antioxidant systems showed that the activities of superoxide dismutase, and catalase were continuously enhanced with increasing salinity, thereby protecting the Honeysuckle plants from oxidative injury (Table 6). Moreover, 0.5 g L−1 K2SiO3·nH2O addition did increase the activities of superoxide dismutase and catalase, demonstrating exogenous Si played an important role in these two enzymes.
Table 6. Effect of K2SiO3·nH2O addition on superoxide dismutase (SOD), peroxide (POD), and catalase (CAT) contents of Honeysuckle (Lonicera japonica L.) plant under NaCl-stressed conditions.
| The potency of K2SiO3·nH2O (g L−1) |
SOD (Uμg-1 protein) Salt levels (mM of NaCl) |
|||||
|---|---|---|---|---|---|---|
| Control (0mM) |
100 |
200 |
||||
| Mean ± S.E | Mean ± S.E | Percent of control | Mean ± S.E | Percent of control | ||
| 0 | 43.05 ± 7.13bA | 49.91 ± 2.65bC | 115.9 | 61.91 ± 1.26aA | 143.8 | |
| 0.5 | 43.77 ± 2.37bA | 59.56 ± 1.87aA | 136.1 | 62.82 ± 4.72aA | 143.5 | |
| 1.0 | 46.25 ± 1.31cA | 54.32 ± 0.73bB | 117.4 | 60.78 ± 5.76aA | 131.4 | |
| The potency of K2SiO3·nH2O (g L−1) | POD (μg g−1 FW min−1) Salt levels (mM of NaCl) | |||||
| Control (0 mM) | 100 | 200 | ||||
| Mean ± S.E | Mean ± S.E | Percent of control | Mean ± S.E | Percent of control | ||
| 0 | 82.22 ± 1.93cB | 97.04 ± 0.14bA | 118.0 | 137.0 ± 8.98aA | 166.6 | |
| 0.5 | 63.89 ± 0.28cC | 82.96 ± 6.42bB | 129.8 | 93.23 ± 1.68aB | 145.9 | |
| 1.0 | 86.67 ± 6.42bA | 97.78 ± 3.58aA | 112.8 | 100.0 ± 1.50aB | 115.4 | |
| The potency of K2SiO3·nH2O (g L−1) | CAT (U mg−1 min−1) Salt levels (mM of NaCl) | |||||
| Control (0 mM) | 100 | 200 | ||||
| Mean ± S.E | Mean ± S.E | Percent of control | Mean ± S.E | Percent of control | ||
| 0 | 0.37 ± 0.03cA | 0.75 ± 0.03aC | 202.7 | 0.61 ± 0.03bB | 164.9 | |
| 0.5 | 0.37 ± 0.03bA | 0.96 ± 0.06aA | 259.5 | 0.80 ± 0.11aA | 216.2 | |
| 1.0 | 0.37 ± 0.03bA | 0.83 ± 0.02aB | 224.3 | 0.77 ± 0.02aA | 208.1 | |
The values represent the means of three replicates. The means followed by the same letters (lower-case letters within lines and upper-case letters within columns, respectively) did not differ significantly at 5% probability by Tukey’s test.
The data recorded elevated CGA content under both non-silicon kinds of salinity when compared to the with-silicon saline conditions plants suggesting their probable role in the secondary metabolite of CGA. Many hypotheses suggest that the synthesis of secondary metabolites, including saponins, a large diverse group of secondary metabolites defined as amphipathic glycosides in which glycosyl residues are attached to tritrtpenoid (triterpeneor or teroidal) aglycon42, is a plant response to environmental factors and part of an adaptative strategy leading to tolerance to abiotic stresses. Just showed as our results, the content of secondary metabolite of CGA is highest at 200 Mm NaCl level without any remission of silicon, indicating that fluctuations in the content of CGA resulting from salinity changes in environmental circumstances might be expected. In fact, the level of CGA like other plant secondary metabolites, can be significantly influenced by the physiological status of the plant as well as the external factors, both abiotic and biotic, including temperature, availability of water and interactions with pathogens and parasites. However, CGA biosynthesis and accumulation by plant tissues in response to saline conditions in plant habitat are still not clear, so the study on Honeysuckle plant (Lonicera japonica L.) might have considerable merit in it.
In this paper, the salt-tolerant mechanisms of the Honeysukle plant on physiological and biochemical bases have been elucidated, which is well consistent with the study aims. The role of silicon in genomics and proteome of the medicinal plant (Lonicera japonica L.) under salt stress will be studied further in future.
Conclusions
Plant salt tolerance is a multifaceted physiological trait. In line with physiological observations, medicinal Honeysuckle plant (Lonicera japonica L.) adapted to salt stress under K2SiO3·nH2O addition is mainly relied on ionic osmotic adjustment, improvement of superoxide dismutase and catalase activities and stability of chloroplasts ultrastructures. These results clearly highlighted the role of Si in protecting the plant against the hazardous effect of salinity. Also, this study provided the evidence that high salt stress affected the entire quantity of secondary metabolite of CGA produced. Thus, regulation of salinity through K2SiO3·nH2O addition could be a promising way to obtain better growth and considerable secondary metabolite of the medicinal plant.
Additional Information
How to cite this article: Gengmao, Z. et al. The role of silicon in physiology of the medicinal plant (Lonicera japonica L.) under salt stress. Sci. Rep. 5, 12696; doi: 10.1038/srep12696 (2015).
Acknowledgments
This work was partially supported by the National Natural Science Foundation of China (No. 31370422), the National Key 948 Project from Ministry of Agriculture (N0. 2013-Z22), and Jiangsu Collaborative Innovation Center for Solid Organic Waste Resource Utilization. We also express our sincere thanks to the students Liu Rongyan, Zhang Qi and Li Mei who participate in Honeysuckle research in the SRT programme of Nanjing Agricultural University.
Footnotes
Author Contributions Z.G., L.S. and W.Y. performed the research, analyzed the data and wrote the paper. S.X. contributed to the TEM characterization. Z.G. guided the experimental processes and C.Z. revised the manuscript. Z.G., L.S. and S.X. equally contributed to this work.
References
- Shahbaz M., Ashraf M., Al-Qurainy F. & Harris P. J. C. Salt tolerance in selected vegetable crops. Crit Rev Plant Sci 31, 303–320 (2012). [Google Scholar]
- Bybordi A. The influence of salt stress on seed germination, growth and yield of canola cultivars. Not Bot Horti Agrobo 38, 128–133 (2010). [Google Scholar]
- Wang Z., Clifford M. N. & Sharp P. Analysis of chlorogenic acids in beverages prepared from Chinese health foods and investigation, in vitro, of effects on glucose absorption in cultured Caco-2 cells. Food Chem 108, 369–373 (2008). [Google Scholar]
- Zhang B., Yang R., Zhao Y. & Liu C. Z. Separation of chlorogenic acid from honeysuckle crude extracts by macroporous resins. J Chromatogr B 867, 253–258 (2008). [DOI] [PubMed] [Google Scholar]
- Shang X., Pan H., Li M., Miao X. & Ding H. Lonicera japonic Thunb.: Ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J Ethnopharmacol 138, 1–21 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung H. W. C. et al. P38-associated pathway involvement in apoptosis induced by photodynamic therapy with Lonicera japonica in human lung squamous carcinoma CH27 cells. Food Chem Toxicol 46, 3389–3400 (2008). [DOI] [PubMed] [Google Scholar]
- Yoo H. J., Kang H. J., Song Y. S., Park E. H. & Lim C. J. Anti‐angiogenic, antinociceptive and anti‐inflammatory activities of Lonicera japonica extract. J Pharm Pharmacol 60, 779–786 (2008). [DOI] [PubMed] [Google Scholar]
- Chen H. & Jiang J. G. Osmotic responses of Dunaliella to the changes of salinity. J Cell Physiol 219, 251–258 (2009). [DOI] [PubMed] [Google Scholar]
- Sushmita S., Priyanka D., Mamata R. & Surendra Chandra S. Osmolyte modulated enhanced rice leaf catalase activity under salt-stress. Adv Biosci Biotech 1, 39–46 (2010). [Google Scholar]
- Demidchik V. & Tester M. Sodium fluxes through nonselective cation channels in the plasma membrane of protoplasts from Arabidopsis roots. Plant Physiol 128, 379–387 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidchik V. & Maathuis F. J. Physiological roles of nonselective cation channels in plants: from salt stress to signalling and development. New Phytol 175, 387–404 (2007). [DOI] [PubMed] [Google Scholar]
- Ebrahimi R. & Bhatla S. C. Ion distribution measured by electron probe X-ray microanalysis in apoplastic and symplastic pathways in root cells in sunflower plants grown in saline medium. J bioscience 37, 713–721 (2012). [DOI] [PubMed] [Google Scholar]
- Hilge M. Ca2+ regulation of ion transport in the Na+/Ca2+ exchanger. J Biol Chem 287, 31641–31649 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaleel C. A., Gopi R., Manivannan P. & Panneerselvam R. Antioxidative Potentials as a Protective Mechanism in Catharanthus roseus (L.) G. Don. Plants under Salinity Stress. Turk J Bot 31, 245–251 (2007). [Google Scholar]
- Parida A. K., Das A. B. & Mittra B. Effects of NaCl stress on the structure, pigment complex composition, and photosynthetic activity of mangrove Bruguiera parviflora chloroplasts. Photosynthetica 41, 191–200 (2003). [Google Scholar]
- Stepien P. & Johnson G. N. Contrasting responses of photosynthesis to salt stress in the glycophyte Arabidopsis and the halophyte Thellungiella: role of the plastid terminal oxidase as an alternative electron sink. Plant Physiol 149, 1154–1165 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyro H. W., Hussain T., Huchzermeyer B. & Khan M. A. Photosynthetic and growth responses of a perennial halophytic grass Panicum turgidum to increasing NaCl concentrations. Environ Exp Bot 91, 22–29 (2013). [Google Scholar]
- Selmar D. Potential of salt and drought stress to increase pharmaceutical significant secondary compounds in plants. Landbauforsch Volk 58, 139–144 (2008). [Google Scholar]
- Feng R. et al. Inhibition of activator protein-1, NF-κB, and MAPKs and induction of phase 2 detoxifying enzyme activity by chlorogenic acid. J Biol Chem 280, 27888–27895 (2005). [DOI] [PubMed] [Google Scholar]
- Lambert J. D., Hong J., Yang G. Y., Liao J. & Yang C. S. Inhibition of carcinogenesis by polyphenols: evidence from laboratory investigations. Am J Clin Nutr 81, 284S–291S (2005). [DOI] [PubMed] [Google Scholar]
- Roche M., Dufour C., Mora N. & Dangles O. Antioxidant activity of olive phenols: mechanistic investigation and characterization of oxidation products by mass spectrometry. Org Biomol Chem 3, 423–430 (2005). [DOI] [PubMed] [Google Scholar]
- Zhang X. et al. Chlorogenic acid protects mice against lipopolysaccharide-induced acute lung injury. Injury 41, 746–752 (2010). [DOI] [PubMed] [Google Scholar]
- Shan J. et al. Chlorogenic acid inhibits lipopolysaccharide-induced cyclooxygenase-2 expression in RAW264. 7 cells through suppressing NF-κB and JNK/AP-1 activation. Int Immunopharmacol 9, 1042–1048 (2009). [DOI] [PubMed] [Google Scholar]
- Ahmad R., Zaheer S. H. & Ismail S. Role of silicon in salt tolerance of wheat (Triticum aestivum L.). Plant Sci 85, 43–50 (1992). [Google Scholar]
- Liang Y., Shen Q., Shen Z. & Ma T. Effects of silicon on salinity tolerance of two barley cultivars. J Plant Nutr 19, 173–183 (1996). [Google Scholar]
- Liang Y. Effect of silicon on leaf ultrastructure, chlorophyll content and photosynthetic activity of barley under salt stress. Pedosphere 8, 289–296 (1997). [Google Scholar]
- Parveen N. & Ashraf M. Role of silicon in mitigating the adverse effects of salt stress on growth and photosynthetic attributes of two maize (Zea mays L.) cultivars grown hydroponically. Pak J Bot 42, 1675–1684 (2010). [Google Scholar]
- Al-aghabary K., Zhu Z. & Shi Q. Influence of silicon supply on chlorophyll content, chlorophyll fluorescence, and antioxidative enzyme activities in tomato plants under salt stress. J Plant Nutr 27, 2101–2115 (2005). [Google Scholar]
- Epstein E. The anomaly of silicon in plant biology. P Natl Acad Sci 91, 11–17 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayar P. K., Misra A. K. & Patnaik S. Rapid microdetermination of silicon in rice plant. Plant Soil 42, 491–494 (1975). [Google Scholar]
- Giannopolitis C. N. & Ries S. K. Superoxide dismutases I. Occurrence in higher plants. Plant physiol 59, 309–314 (1977). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B. & Maehly A. C. Assay of catalases and peroxidases. Methods Enzymol 2, 764–775 (1955). [DOI] [PubMed] [Google Scholar]
- Gonzalo M. J., Lucena J. J. & Hernández-Apaolaza L. Effect of silicon addition on soybean (Glycine max) and cucumber (Cucumis sativus) plants grown under iron deficiency. Plant Physiol Bioch 70, 455–461 (2013). [DOI] [PubMed] [Google Scholar]
- Yin L., Wang S., Li J., Tanaka K. & Oka M. Application of silicon improves salt tolerance through ameliorating osmotic and ionic stresses in the seedling of Sorghum bicolor. Acta Physiol Plant 35, 3099–3107 (2013). [Google Scholar]
- Fadzilla N. M. & Burdon R. H. Salinity, oxidative stress and antioxidant responses in shoot cultures of rice. J Exp Bot 48, 325–331 (1997) [Google Scholar]
- Mittal S., Kumari N. & Sharma V. Differential response of salt stress on Brassica juncea: Photosynthetic performance, pigment, proline, D1 and antioxidant enzymes. Plant Physiol Bioch 54, 17–26 (2012). [DOI] [PubMed] [Google Scholar]
- Zollinger S. A. Performance constraints and vocal complexity in birdsong: Evidence from a vocal mimic. Ph.D thesis, Indiana University (2007).
- Paranychianakis N. V. & Chartzoulakis K. S. Irrigation of Mediterranean crops with saline water: from physiology to management practices. Agr Ecosyst Environ 106, 171–187 (2005). [Google Scholar]
- Gong H. J., Randall D. P. & Flowers T. J. Silicon deposition in the root reduces sodium uptake in rice (Oryza sativa L.) seedlings by reducing bypass flow. Plant Cell Environ 29, 1970–1979 (2006). [DOI] [PubMed] [Google Scholar]
- Lemos Alves F. A., Ferreira Da Silva S. L., Da Silva E. N. & Gomes Da Silveira J. A. Clones of dwarf-precocious cashew submitted to salt stress and the accumulation of potassium and sodium. Rev Cienc Agron 39, 422–428 (2008). [Google Scholar]
- Zhu J. K. Regulation of ion homeostasis under salt stress. Curr opin plant biol 6, 441–445 (2003). [DOI] [PubMed] [Google Scholar]
- Szakiel A., Pączkowski C. & Henry M. Influence of environmental abiotic factors on the content of saponins in plants. Phytochem Rev 10, 471–491 (2011). [Google Scholar]