Abstract
flbA encodes an Aspergillus nidulans RGS (regulator of G protein signaling) domain protein that is required for control of mycelial proliferation and activation of asexual sporulation. We identified a dominant mutation in a second gene, fadA, that resulted in a very similar phenotype to flbA loss-of-function mutants. Analysis of fadA showed that it encodes the alpha-subunit of a heterotrimeric G protein, and the dominant phenotype resulted from conversion of glycine 42 to arginine (fadA(G42R)). This mutation is predicted to result in a loss of intrinsic GTPase activity leading to constitutive signaling, indicating that activation of this pathway leads to proliferation and blocks sporulation. By contrast, a fadA deletion and a fadA dominant-interfering mutation (fadA(G203R)) resulted in reduced growth without impairing sporulation. In fact, the fadA(G203R) mutant was a hyperactive asexual sporulator and produced elaborate sporulation structures, called conidiophores, under environmental conditions that blocked wild-type sporulation. Both the fadA(G203R) and the fadA deletion mutations suppressed the flbA mutant phenotype as predicted if the primary role of FlbA in sporulation is in blocking activation of FadA signaling. Because overexpression of flbA could not suppress the fadA(G42R) mutant phenotype, we propose that FlbA's role in modulating the FadA proliferation signal is dependent upon the intrinsic GTPase activity of wild-type FadA.
Full text
PDF



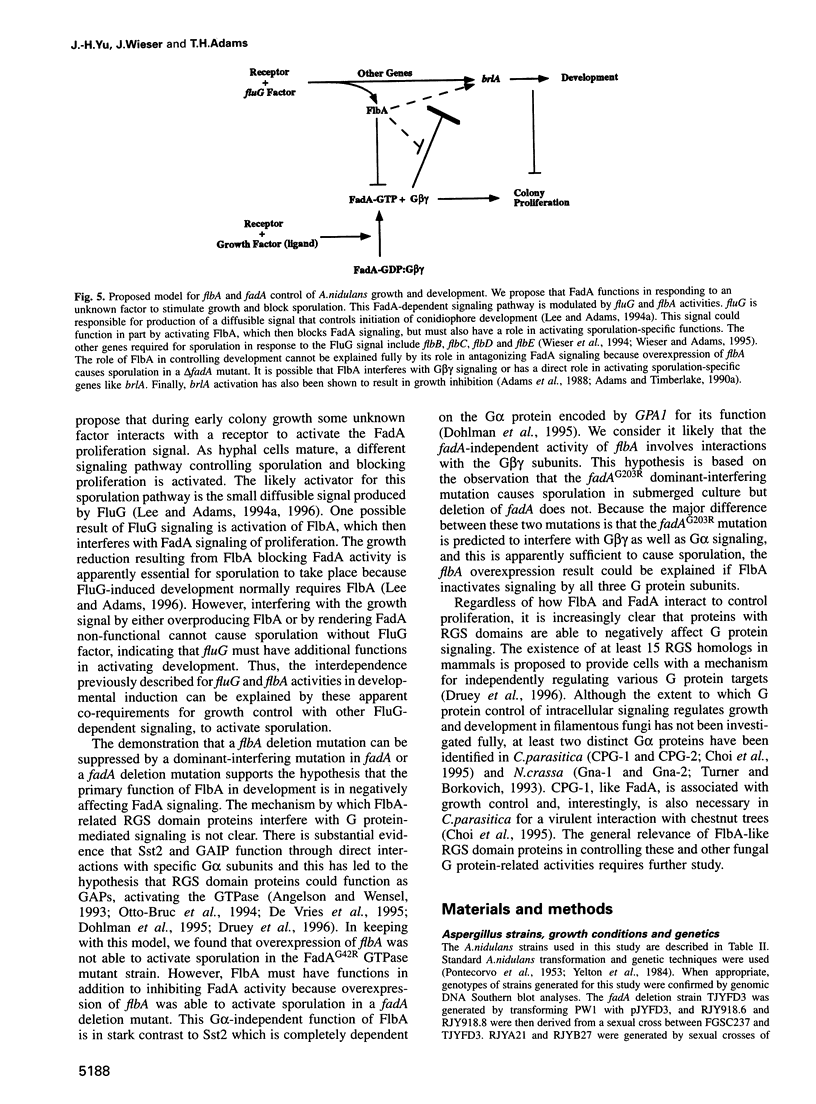


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams T. H., Boylan M. T., Timberlake W. E. brlA is necessary and sufficient to direct conidiophore development in Aspergillus nidulans. Cell. 1988 Jul 29;54(3):353–362. doi: 10.1016/0092-8674(88)90198-5. [DOI] [PubMed] [Google Scholar]
- Adams T. H., Timberlake W. E. Developmental repression of growth and gene expression in Aspergillus. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5405–5409. doi: 10.1073/pnas.87.14.5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams T. H., Timberlake W. E. Upstream elements repress premature expression of an Aspergillus developmental regulatory gene. Mol Cell Biol. 1990 Sep;10(9):4912–4919. doi: 10.1128/mcb.10.9.4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angleson J. K., Wensel T. G. A GTPase-accelerating factor for transducin, distinct from its effector cGMP phosphodiesterase, in rod outer segment membranes. Neuron. 1993 Nov;11(5):939–949. doi: 10.1016/0896-6273(93)90123-9. [DOI] [PubMed] [Google Scholar]
- Brody H., Griffith J., Cuticchia A. J., Arnold J., Timberlake W. E. Chromosome-specific recombinant DNA libraries from the fungus Aspergillus nidulans. Nucleic Acids Res. 1991 Jun 11;19(11):3105–3109. doi: 10.1093/nar/19.11.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss J. E., Mumby S. M., Casey P. J., Gilman A. G., Sefton B. M. Myristoylated alpha subunits of guanine nucleotide-binding regulatory proteins. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7493–7497. doi: 10.1073/pnas.84.21.7493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi G. H., Chen B., Nuss D. L. Virus-mediated or transgenic suppression of a G-protein alpha subunit and attenuation of fungal virulence. Proc Natl Acad Sci U S A. 1995 Jan 3;92(1):305–309. doi: 10.1073/pnas.92.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham D. E., Neer E. J. New roles for G-protein beta gamma-dimers in transmembrane signalling. Nature. 1993 Sep 30;365(6445):403–406. doi: 10.1038/365403a0. [DOI] [PubMed] [Google Scholar]
- De Vries L., Mousli M., Wurmser A., Farquhar M. G. GAIP, a protein that specifically interacts with the trimeric G protein G alpha i3, is a member of a protein family with a highly conserved core domain. Proc Natl Acad Sci U S A. 1995 Dec 5;92(25):11916–11920. doi: 10.1073/pnas.92.25.11916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzel C., Kurjan J. Pheromonal regulation and sequence of the Saccharomyces cerevisiae SST2 gene: a model for desensitization to pheromone. Mol Cell Biol. 1987 Dec;7(12):4169–4177. doi: 10.1128/mcb.7.12.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohlman H. G., Apaniesk D., Chen Y., Song J., Nusskern D. Inhibition of G-protein signaling by dominant gain-of-function mutations in Sst2p, a pheromone desensitization factor in Saccharomyces cerevisiae. Mol Cell Biol. 1995 Jul;15(7):3635–3643. doi: 10.1128/mcb.15.7.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druey K. M., Blumer K. J., Kang V. H., Kehrl J. H. Inhibition of G-protein-mediated MAP kinase activation by a new mammalian gene family. Nature. 1996 Feb 22;379(6567):742–746. doi: 10.1038/379742a0. [DOI] [PubMed] [Google Scholar]
- Hasson M. S., Blinder D., Thorner J., Jenness D. D. Mutational activation of the STE5 gene product bypasses the requirement for G protein beta and gamma subunits in the yeast pheromone response pathway. Mol Cell Biol. 1994 Feb;14(2):1054–1065. doi: 10.1128/mcb.14.2.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelle M. R., Horvitz H. R. EGL-10 regulates G protein signaling in the C. elegans nervous system and shares a conserved domain with many mammalian proteins. Cell. 1996 Jan 12;84(1):115–125. doi: 10.1016/s0092-8674(00)80998-8. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurjan J. Pheromone response in yeast. Annu Rev Biochem. 1992;61:1097–1129. doi: 10.1146/annurev.bi.61.070192.005313. [DOI] [PubMed] [Google Scholar]
- Käfer E. Meiotic and mitotic recombination in Aspergillus and its chromosomal aberrations. Adv Genet. 1977;19:33–131. doi: 10.1016/s0065-2660(08)60245-x. [DOI] [PubMed] [Google Scholar]
- Lee B. N., Adams T. H. FluG and flbA function interdependently to initiate conidiophore development in Aspergillus nidulans through brlA beta activation. EMBO J. 1996 Jan 15;15(2):299–309. [PMC free article] [PubMed] [Google Scholar]
- Lee B. N., Adams T. H. Overexpression of flbA, an early regulator of Aspergillus asexual sporulation, leads to activation of brlA and premature initiation of development. Mol Microbiol. 1994 Oct;14(2):323–334. doi: 10.1111/j.1365-2958.1994.tb01293.x. [DOI] [PubMed] [Google Scholar]
- Lee B. N., Adams T. H. The Aspergillus nidulans fluG gene is required for production of an extracellular developmental signal and is related to prokaryotic glutamine synthetase I. Genes Dev. 1994 Mar 15;8(6):641–651. doi: 10.1101/gad.8.6.641. [DOI] [PubMed] [Google Scholar]
- May G. S., Tsang M. L., Smith H., Fidel S., Morris N. R. Aspergillus nidulans beta-tubulin genes are unusually divergent. Gene. 1987;55(2-3):231–243. doi: 10.1016/0378-1119(87)90283-6. [DOI] [PubMed] [Google Scholar]
- Neer E. J. Heterotrimeric G proteins: organizers of transmembrane signals. Cell. 1995 Jan 27;80(2):249–257. doi: 10.1016/0092-8674(95)90407-7. [DOI] [PubMed] [Google Scholar]
- O'Donnell K. L., Osmani A. H., Osmani S. A., Morris N. R. bimA encodes a member of the tetratricopeptide repeat family of proteins and is required for the completion of mitosis in Aspergillus nidulans. J Cell Sci. 1991 Aug;99(Pt 4):711–719. doi: 10.1242/jcs.99.4.711. [DOI] [PubMed] [Google Scholar]
- Otto-Bruc A., Antonny B., Vuong T. M. Modulation of the GTPase activity of transducin. Kinetic studies of reconstituted systems. Biochemistry. 1994 Dec 27;33(51):15215–15222. doi: 10.1021/bi00255a001. [DOI] [PubMed] [Google Scholar]
- PONTECORVO G., ROPER J. A., HEMMONS L. M., MACDONALD K. D., BUFTON A. W. J. The genetics of Aspergillus nidulans. Adv Genet. 1953;5:141–238. doi: 10.1016/s0065-2660(08)60408-3. [DOI] [PubMed] [Google Scholar]
- Rens-Domiano S., Hamm H. E. Structural and functional relationships of heterotrimeric G-proteins. FASEB J. 1995 Aug;9(11):1059–1066. doi: 10.1096/fasebj.9.11.7649405. [DOI] [PubMed] [Google Scholar]
- Simon M. I., Strathmann M. P., Gautam N. Diversity of G proteins in signal transduction. Science. 1991 May 10;252(5007):802–808. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- Turner G. E., Borkovich K. A. Identification of a G protein alpha subunit from Neurospora crassa that is a member of the Gi family. J Biol Chem. 1993 Jul 15;268(20):14805–14811. [PubMed] [Google Scholar]
- Weiner J. L., Guttierez-Steil C., Blumer K. J. Disruption of receptor-G protein coupling in yeast promotes the function of an SST2-dependent adaptation pathway. J Biol Chem. 1993 Apr 15;268(11):8070–8077. [PubMed] [Google Scholar]
- West R. E., Jr, Moss J., Vaughan M., Liu T., Liu T. Y. Pertussis toxin-catalyzed ADP-ribosylation of transducin. Cysteine 347 is the ADP-ribose acceptor site. J Biol Chem. 1985 Nov 25;260(27):14428–14430. [PubMed] [Google Scholar]
- Wieser J., Adams T. H. flbD encodes a Myb-like DNA-binding protein that coordinates initiation of Aspergillus nidulans conidiophore development. Genes Dev. 1995 Feb 15;9(4):491–502. doi: 10.1101/gad.9.4.491. [DOI] [PubMed] [Google Scholar]
- Wieser J., Lee B. N., Fondon J. w., 3rd, Adams T. H. Genetic requirements for initiating asexual development in Aspergillus nidulans. Curr Genet. 1994 Dec;27(1):62–69. doi: 10.1007/BF00326580. [DOI] [PubMed] [Google Scholar]
- Yelton M. M., Hamer J. E., Timberlake W. E. Transformation of Aspergillus nidulans by using a trpC plasmid. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1470–1474. doi: 10.1073/pnas.81.5.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]






