Abstract
Aberrant activation of intracellular signalling pathways confers malignant properties on cancer cells. Targeting intracellular signalling pathways has been a productive strategy for drug development, with several drugs acting on signalling pathways already in use and more continually being developed. The JAK/STAT signalling pathway provides an example of this paradigm in haematological malignancies, with the identification of JAK2 mutations in myeloproliferative neoplasms leading to the development of specific clinically effective JAK2 inhibitors, such as ruxolitinib. It is now clear that many solid tumours also show activation of JAK/STAT signalling. In this review, we focus on the role of JAK/STAT signalling in solid tumours, examining the molecular mechanisms that cause inappropriate pathway activation and their cellular consequences. We also discuss the degree to which activated JAK/STAT signalling contributes to oncogenesis. Studies showing the effect of activation of JAK/STAT signalling upon prognosis in several tumour types are summarised. Finally, we discuss the prospects for treating solid tumours using strategies targeting JAK/STAT signalling, including what can be learned from haematological malignancies and the extent to which results in solid tumours might be expected to differ.
Keywords: STAT transcription factors, janus kinases, intracellular signalling peptides and proteins, molecular targeted therapy
The JAK/STAT pathway regulates embryonic development and is involved in the control of processes such as stem cell maintenance, haematopoiesis and the inflammatory response. The pathway transduces signals from cytokines, interleukins and growth factors that act through a number of transmembrane receptor families. Type I receptors include the erythropoietin receptor and the granulocyte colony-stimulating factor (G-CSF) receptor. The granulocyte-macrophage colony-stimulating factor receptor is a type IIa receptor and the type IIb subfamily includes the receptors for interleukin-6 and leukaemia inhibitory factor. The intracellular tails of these receptors are constitutively associated with inactive kinases named janus kinases (JAKs). Ligand binding produces conformational changes in receptors that alters the alignment of receptor-associated JAKs, enabling phosphorylation of specific tyrosine residues that converts inactive JAKs into a catalytically active tyrosine kinases (Brooks et al, 2014). In some cases, signalling through STATs can be activated by receptors with intrinsic tyrosine kinase activity such as epidermal growth factor receptor and platelet-derived growth factor receptor (Silvennoinen et al, 1993), although this sometimes also involves the cytoplasmic tyrosine kinase Src (Olayioye et al, 1999; Niu et al, 2002).
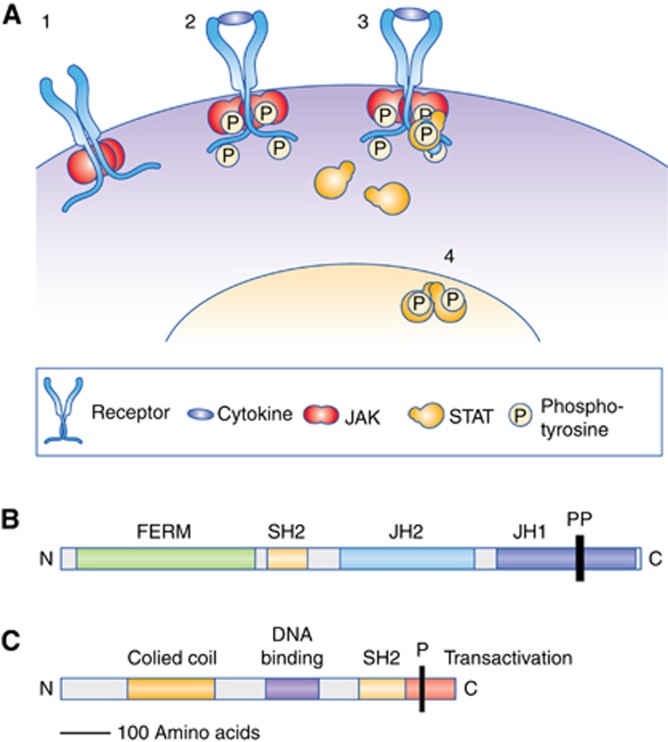
Active JAKs phosphorylate tyrosine residues in the cytoplasmic region of the receptor creating binding sites that recruit signal transducers and activators of transcriptions (STATs). The STATs form dimers that translocate to the nucleus when phosphorylated on highly conserved tyrosine residues (termed pSTAT) by JAKs or other tyrosine kinases. The STAT dimers bind specific promoter sequences and modulate transcription of genes controlling cellular processes including proliferation, differentiation and apoptosis (Figure 1A).
Figure 1.
(A) An overview of the JAK/STAT signalling pathway. (1) Receptor complexes at the cell surface are associated with inactive JAK dimers, which bind close to the transmembrane region of the receptors. (2) Binding of ligand produces a conformational change in the receptor complex that changes the relative position of the JAKs, leading to phosphorylation and activation of their tyrosine kinase activity. The activated JAKs phosphorylate tyrosine residues in the cytoplasmic tails of receptors. (3) Cytoplasmic STATs bind to the phosphorylated receptors, becoming substrates for JAKs. (4) Phosphorylated STATs form dimers and accumulate in the nucleus, where they activate transcription of specific genes. (B) Schematic structure of JAK proteins. The FERM domain (4.1 protein, ezrin, radixin and moesin) mediates the interaction with receptor complexes. The SH2 domain is a protein domain that binds to phosphorylated tyrosine residues. The JH2 pseudokinase domain regulates kinase activity of the JH1 kinase domain. P marks conserved tyrosine residues in JH1 whose phosphorylation is essential for JAK activation. N and C indicate the amino terminus and carboxy terminus. (C) Schematic structure of STAT proteins. The SH2 domain binds phosphorylated tyrosines. The carboxy terminus transactivation domain is required for full transcriptional activation. P marks the conserved tyrosine residue whose phosphorylation is essential for STAT activation.
There are four JAK family members in humans, JAK1, JAK2, JAK3 and TYK2 (Figure 1B). The family is defined by the presence of two adjacent kinase domains, JH1 and JH2, resembling the two faces of the Roman god Janus from which their name is derived. JH1 performs the phosphorylation involved in pathway activation, whereas JH2 regulates JH1 function. JH2 lacks amino acids previously thought to be essential for catalytic activity so has been termed a pseudokinase, but in fact retains kinase activity, which may mediate its regulatory functions. Janus kinases are able to form homodimers and heterodimers.
Seven STAT family members are found in humans, STAT 1-4, STAT5A, STAT5B and STAT6 (Figure 1C). Across the diversity of receptors that act via the JAK/STAT pathway, there is no simple relationship between which JAK family members activate which STAT family members. In general, STAT3 and STAT5A/B promote oncogenesis, whereas STAT1 activation has opposing effects. The functional domains of STATs include an SH2 domain (Src homology 2), which mediates binding to phosphorylated tyrosine residues and a C-terminal transactivation domain required for activation of transcription.
As in other signalling cascades, activation of the JAK/STAT pathway is tightly controlled by negative regulators acting at multiple levels. Several families of phosphatases remove phosphate groups from JAKs and STATs. Protein inhibitors of activated STAT (PIAS) proteins inhibit STAT–DNA binding, control STAT cellular location and facilitate post-translational modifications of STATs. Suppressor of cytokine signalling (SOCS) proteins are competitive inhibitors of STAT receptor binding and also act as ubiquitin ligases that target pathway components for proteosomal degradation. STATs positively regulate transcription of SOCS genes, creating a negative feedback loop that imposes a fine level of control on the pathway.
JAK and STAT pathway components also have effects on gene expression outside the ‘canonical' signalling phosphorylation cascade, by contributing to epigenetic modifications of chromatin. It has been reported that activated JAK2 can enter the nucleus and modify histones (Dawson et al, 2009), although this finding remains controversial (Girodon et al, 2011). Dimers of unphosphorylated STAT5A can localise to the nucleus and influence heterochromatin formation by binding heterochromatin protein 1 (Hu et al, 2013). STATs are traditionally thought of as facilitating activation of transcription, but data emerging from large-scale gene expression profiling experiments show that activated STATs also downregulate expression of a substantial number of genes. STATs have also been shown to have effects on the cell that are independent of transcription, by altering mitochondrial function. STAT3 can localise to the mitochondria when phosphorylated on a specific C-terminal serine residue, where it augments activity of the electron transport chain (Gough et al, 2009). This serine phosphorylation is dependent upon the Ras/Raf/MEK/ERK cascade, and is required for Ras-mediated transformation (Gough et al, 2013).
JAK/STAT activation in haematological malignancies
JAK/STAT activation in haematological malignancies has been comprehensively examined in recent reviews (Chen et al, 2012; Vainchenker and Constantinescu, 2013), and provides a basis for its examination in solid tumours. The pathway came to prominence with the identification that 50–95% of patients with classical myeloproliferative neoplasms (MPNs) polycythaemia vera (PV), essential thrombocytosis (ET) and primary myelofibrosis (PMF) have an activating mutation in JAK2 (a valine to phenylalanine change, V617F) in the malignant clone. The demonstration that JAK2V617F caused a PV phenotype in a mouse model, and that Stat5a/b is necessary for the development of PV in these mice provided evidence for a causative role for JAK2-STAT5 activation in MPNs. The role of STAT3 in MPNs is less well understood. Deletion of Stat3 in a mouse MPN model enhances aspects of the MPN phenotype and shortens survival, suggesting that STAT3 may be restraining malignant proliferation. This seems in part to be related to secondary changes in STAT1 activation (Grisouard et al, 2015). The exact role of JAK/STAT signalling in oncogenesis in MPNs and other cancers is also challenged by the consideration of rare families with germline mutations causing weak JAK activation (Dusa et al, 2008; Mead et al, 2013; Marty et al, 2014). The mutations cause a hereditary thrombocytosis, but haematopoiesis is polyclonal and individuals do not develop haematological malignancies or solid tumours, suggesting that JAK/STAT activation alone does not drive malignant disease.
The identification of JAK/STAT activation in MPNs led to the development of specific JAK1/2 inhibitors, one of which, ruxolitinib, has been examined in phase 3 trials (Harrison et al, 2012). Activating mutations affecting JAK/STAT signalling have been identified in other groups of patients with haematological malignancies and there have been calls for clinical trials of JAK inhibitors in these groups (Bain and Ahmad, 2014). It needs to be established, however, to what extent JAK/STAT activation is driving disease in these groups. For example, in T-cell acute lymphoblastic leukaemia activating mutations in JAK1 have been identified in a substantial proportion of patients, where they are thought to promote survival of malignant cells but JAK mutations are not thought to be the primary driver of disease (Flex et al, 2008).
Understanding the degree to which JAK/STAT activation is driving disease has implications for determining the effect of JAK inhibitors upon the malignant clone in MPNs, illustrated by comparison with chronic myeloid leukaemia (CML). In BCR–ABL-positive CML, the BCR–ABL fusion gene drives the disease. Targeted kinase inhibitors such as imatinib produce a marked reduction in the clone size and prevent progression to accelerated phase/blast crisis (Hughes et al, 2010). In contrast, ruxolitinib treatment in myelofibrosis leads to a much more modest reduction in the size of the malignant clone (Cervantes et al, 2013), and there is not yet clear evidence that it alters progression to acute myeloid leukaemia. Furthermore, ruxolitinib is also an effective treatment in patients lacking the JAK2V617F mutation. This suggests the role of JAK2 in MPNs is less fundamental than that of BCR–ABL in CML.
Myeloproliferative neoplasms were the subject of a further interesting development at the end of 2013, with whole-exome sequencing studies revealing that a substantial proportion of patients with ET and PMF lacking the JAK2V617F mutation have mutations in CALR, the gene encoding calreticulin (Klampfl et al, 2013; Nangalia et al, 2013). A diverse range of mutations have been observed, but all cause a frameshift that encodes an identical novel C-terminal peptide. There is some evidence that these CALR variants promote STAT activation, but further elucidation of their contribution to disease pathogenesis will be interesting.
Mechanisms of JAK/STAT activation in cancer
In many cancers where JAK/STAT activation is a feature, the mechanism underlying inappropriate pathway activation is not known. Examining the instances in which a mechanism has been identified shows that cancer cells employ a diverse range of strategies to activate the pathway. Defining the mechanisms causing JAK/STAT pathway activation does not, however, indicate whether the pathway activation contributes significantly to oncogenesis in these cancers.
In head and neck squamous cell carcinoma, phosphorylation of STAT3 is a consequence of increased production of IL-6 by tumour cells (Sriuranpong et al, 2003). Increased expression of the G-CSF receptor is observed in high-grade ovarian epithelial tumours, and experiments in cell culture suggest that G-CSF contributes to JAK/STAT activation in this disease (Kumar et al, 2014).
Gain-of-function mutations in JAKs have been observed to cause pathway activation in haematological malignancies. More recently, large-scale sequencing efforts have identified genetic changes affecting JAKs in certain solid tumours. Missense mutations in JAK1 have been identified in 9% of patients with Hepatitis B-associated hepatocellular carcinoma, and validation in cell culture shows that these mutations increase phosphorylation of JAK1 and STAT3 and enable cytokine-independent growth (Kan et al, 2013). In gastric adenocarcinoma, a comprehensive molecular characterisation project has revealed frequent amplification of the chromosomal locus containing JAK2. Corresponding increases in JAK2 messenger RNA suggest that this may increase JAK2 protein levels and pathway activity (The Cancer Genome Atlas Research Network, 2014).
Activating mutations in STATs, although generally rare, have been described in cancer. In large granular lymphocytic leukaemia, 40% of patients have mutations affecting the SH2 domain of STAT3. These introduce hydrophobic residues thought to stabilise STAT dimers, and lead to increased STAT-responsive transcription (Koskela et al, 2012). Amplification of the STAT5A/B locus has been described in prostate cancer, and is associated with increased expression and nuclear localisation of STAT5 in tumour samples. These amplifications increase cell survival in culture and promote tumour growth in a xenograft model (Haddad et al, 2013).
Reduced expression of negative regulators can cause increased pathway activation. In non-small cell lung cancer (NSCLC) tumour samples, expression of SOCS3 is lost due to promoter hypermethylation, an epigenetic change that reduces gene transcription. The impact of this on pathway activation was validated using a NSCLC cell line, where restoration of SOCS3 expression reduced constitutive STAT3 phosphorylation (He et al, 2003). The PIAS3 protein levels have been shown to be reduced in glioblastoma, possibly due to increased protein degradation. In glioblastoma tissue samples, low levels of PIAS3 are associated with increased pSTAT3 and increased expression of proteins produced from STAT target genes (Brantley et al, 2008).
Effect of JAK/STAT signalling on malignant cells and the tumour microenvironment
In order for solid tumours to enlarge, cancer cells must not only increase in number but also adapt to and alter their microenvironment. Genes controlled by STATs have roles in both of these aspects of the malignant phenotype, although in many cases further study is required to establish to what extent persistent JAK/STAT activation drives these phenotypic changes and what contribution is made by other changes in malignant cells.
Preclinically, therapies that inhibit STAT activity decrease proliferation and increase apoptosis in cell culture studies and tumour xenograft models. STAT3 facilitates cell cycle progression by promoting activation of cyclin-dependent kinases (CDKs). It increases transcription of positive regulators such as cyclin D2 and downregulates transcription of CDK inhibitors such as p21. STAT5 confers protection from apoptosis, by activating transcription of Bcl-x, to produce the anti-apoptotic protein Bcl-xL.
Cancer cells undergo changes in energy metabolism, switching from mitochondrial oxidative phosphorylation to glycolysis for ATP production. STAT3 reduces expression of genes encoding mitochondrial proteins and increases expression of genes involved in glycolysis, such as pyruvate dehydrogenase kinase 1. These effects are dependent upon transcription of the hypoxia-inducible factor HIF-1α, which is induced by STAT3 (Demaria et al, 2010). STAT3 has a global role in the adaptation of tumour cells to a hypoxic environment. It physically associates with HIF1α and is required for the full transcriptional activation of HIF1α-regulated genes in hypoxia (Pawlus et al, 2014). Angiogenesis is required for tumour growth, with a key role played by vascular endothelial growth factor (VEGF). STAT3 binds to the VEGF promoter and induces VEGF expression. In tumour allograft models, expression of a constitutively active STAT3 leads to increased VEGF expression and increased vasculogenesis.
A key feature in the interaction of malignant cells with the tumour microenvironment is their ability to suppress antitumour immune responses. This is illustrated by the importance of the graft vs tumour effect in allogeneic stem cell transplantation in haematological malignancies (Andersen, 2014), and by the effects of immune checkpoint inhibitors in certain solid tumours (Victor et al, 2015). Activation of STAT1 by interferons promotes immune surveillance and antitumour immunity, partly by upregulating MHC class I-mediated antigen presentation by tumour cells. In contrast, STAT3 and STAT5 signalling in immune cells appears to suppress antitumour immunity. Antitumour immune responses in mice are enhanced by ablation of STAT3 from haematopoietic cells and by drugs that block STAT3 (Kortylewski et al, 2005). The enhanced antitumour immunity observed in these mice is in part due to a reduction in tumour-infiltrating regulatory T cells, a cell type whose development is dependent on the STAT3/5 target gene FOXP3 (Zorn et al, 2006).
JAK/STAT activation contributes to acquisition of properties required for tumour invasion and metastasis. This is in part mediated by activation of the programme of epithelial to mesenchymal transition (EMT) involved in embryonic development. The transcription factor TWIST1 is a key regulator of the induction of EMT. STAT3 is required for TWIST1 expression, as abrogation of STAT3 activity by siRNA knockdown, pharmacological inhibition of STAT3 or mutation of the STAT-binding site in the promoter of TWIST1 reduces its expression (Cho et al, 2013). STAT3 can activate transcription of matrix-degrading enzymes such as matrix metalloproteinase-2. Inhibiting STAT3 reduces invasion through an in vitro basement membrane model, and prevents the establishment of metastatic tumours in a mouse model.
Interactions between JAK/STAT signalling and other oncogenic signalling pathways
Although frequently described as discrete pathways, intracellular signalling cascades are probably more accurately considered as networks made up of multiple interactions between pathways. Physical interactions and functional effects have been described for interactions between JAK/STAT signalling and a number of other signalling pathways known to be involved in oncogenesis, including signalling downstream of the epidermal growth factor receptor and androgen receptor signalling. The recognition of interactions between pathways has implications for improving the effectiveness of targeted therapies, by combining therapies acting on interacting pathways to overcome resistance. This has been demonstrated in in vitro models in melanoma, where STAT3 is required for full activation of transcription downstream of mutant B-RAF (Becker et al, 2014). Suppression of STAT3 phosphorylation by siRNA knockdown of STAT3 or with an inhibitor of JAK2 restores sensitivity to a B-RAF inhibitor in melanoma cell lines with acquired resistance to B-RAF inhibition (Liu et al, 2013). Understanding how pathway interactions cause JAK-independent activation of STATs is also relevant for therapy as it may explain the failure of JAK inhibitors in clinical trials in solid tumours.
JAK/STAT signalling in solid tumours
Early evidence that JAK/STAT signalling is activated in solid tumours was derived from cancer cell lines. There is now substantial data demonstrating tyrosine phosphorylation and nuclear localisation of STATs, indicative of STAT activation, in tumour tissue derived from a large number of patients across a range of tumour types. A relationship between JAK/STAT activation and prognosis has been observed in many of these tumour types (Table 1). In general, activation of STAT3 or STAT5 is associated with a worse prognosis, although in breast cancer and in some studies in colorectal cancer and head and neck squamous cell carcinoma it appears to be associated with more favourable outcomes. In breast cancer, this relationship is consistent with the role of pSTAT5 in normal physiology—constitutive phosphorylation of STAT5 is a feature of normal breast epithelial cells, where it is thought to promote differentiation (Peck et al, 2011). For other tumour types, differences in the strategies used to quantify STAT phosphorylation, which vary across all the studies described below, may account for the apparently conflicting associations between STAT phosphorylation and outcome (Monnien et al, 2010). Interestingly, there is some evidence that in MPNs STAT3 may oppose malignant proliferation (Grisouard et al, 2015), suggesting this may also occur in certain situations in solid tumours. Activation of STAT1, in contrast, is generally associated with better outcomes across all tumour types.
Table 1. Summary of studies describing STAT activation and its clinical implications in solid tumours.
| Cancer type | Reference | STAT activation, tissue sample | Number of patients | Clinical implications of STAT activation |
|---|---|---|---|---|
| NSCLC | Xu and Lu, 2014 | STAT3 and pSTAT3 detection with immunohistochemistry | 1793 (meta-analysis of 17 studies) | Positivity for STAT3 or pSTAT3 associated with reduced overall survival (HR=0.67, P<0.0001) |
| Prostate | Mirtti et al, 2013 | Nuclear STAT5A/B, immunohistochemistry on tissue microarrays from prostatectomy or TURP | 562 radical prostatectomy | Presence of nuclear STAT5 associated with early recurrence (HR=1.6, P=0.012) |
| 106 palliative treatment | Presence of nuclear STAT5 associated with prostate cancer-specific death (HR=1.59, P=0.034) | |||
| Breast | Sonnenblick et al, 2013 | Immunohistochemistry for pSTAT3 on tissue microarrays | 137 out of 375 positive (36%) | Presence of pSTAT3 associated with improved overall survival in patients receiving adjuvant chemotherapy (10 year survival 79% for pSTAT3 positive, vs 61.5% for pSTAT3 negative, HR=0.48, P=0.01) |
| Peck et al, 2011 | Immunohistochemistry and immunofluorescence for nuclear pSTAT5 on tissue microarrays | 208 out of 421 positive (49%). Node negative, with no adjuvant therapy | Absence of activated STAT5 associated with decreased cancer-specific survival (HR=2.39, P=0.023) | |
| Rectal/colorectal | Monnien et al, 2010 | Immunohistochemistry for nuclear pSTAT3 | 39 out of 104 (37.5%) positive. 104 rectal, T3 or resectable T4M0 | Presence of activated STAT3 associated with better overall survival (HR=0.3, P=0.01) |
| Kusaba et al, 2006 | Immunohistochemistry for pSTAT3 | 62 out of 108 (57%) positive. Colorectal adenocarcinoma | Presence of activated STAT3 associated with worse overall survival (P<0.001) | |
| Oral squamous cell carcinoma | Macha et al, 2011 | Immunohistochemistry for nuclear pSTAT3 | 63 out of 94 (67%) positive (follow-up data for 71) | Nuclear pSTAT3 associated with shorter median disease-free survival (13months vs 64 months, P=0.019). |
| Pectasides et al, 2010 | AQUA immunohistochemistry for nuclear STAT3 | High nuclear STAT3 associated with improved overall survival (Mean 119 months vs 57.3 months, P=0.009) | ||
| Cervical squamous cell carcinoma | Takemoto et al, 2009 | Immunohistochemistry for nuclear pSTAT3 | 71 out of 125 (56.8%) positive | Nuclear pSTAT3 associated with reduced overall survival (5 year survival 79.2 months vs 95.3 months, P=0.006) |
| Malignant melanoma | Messina et al, 2008 | Immunohistochemistry for pSTAT1 and pSTAT3 | 6 out of 14 primary tumours positive for nuclear pSTAT3, 16 out of 26 lymph node metastases positive for pSTAT3, 6 out of 23 positive for STAT1 | In patients with lymph node metastases, higher rates of recurrence with high pSTAT3 staining compared with low-grade staining (9 out of 16 vs 3 out of 10). Lower rates of recurrence with high pSTAT1 staining (8 out of 23 vs 2 out of 6) in lymph node and brain metastases |
| Renal cell carcinoma | Horiguchi et al, 2002 | Immunohistochemistry for nuclear pSTAT3 | 24 out of 48 (50%) positive | Nuclear pSTAT3 associated with shortened cancer-specific survival (P=0.0439) |
| Glioblastoma | Birner et al, 2010 | Immunohistochemistry for pSTAT3 on tissue microarrays | 58.8% of 111 positive | High or very high numbers of cells positive for pSTAT3 associated with reduced overall survival (P=0.001) |
Abbreviations: AQUA=automated quantitative analysis; HR=hazard ratio; NSCLC=non-small cell lung cancer; STAT=signal transducers and activators of transcription; TURP=transurethral resection of prostate.
Although STAT activation has now been observed in a wide range of tumour types, in many cases the mechanisms causing STAT activation have not been well defined. More importantly, these studies describe associations between JAK/STAT activation and outcomes but do not indicate whether the observed JAK/STAT activation has a causal role in the diseases. Further study is needed to establish this, and is particularly relevant for considering the JAK/STAT pathway as a therapeutic target.
The JAK/STAT signalling pathway as a therapeutic target in cancer
The evidence that the JAK/STAT pathway is activated in a large proportion of solid tumours and that its activation contributes to the malignant properties of cancer cells makes the JAK/STAT pathway a promising target for the development of new therapies. The effectiveness of targeting JAK/STAT signalling has been demonstrated in phase 3 trials in patients with PMF and myelofibrosis secondary to PV and ET. The JAK1/2 inhibitor ruxolitinib improves symptoms and prolongs survival (Harrison et al, 2012). JAK inhibitors have also been developed for the treatment of autoimmune diseases, in particular tofacitinib, a JAK3 inhibitor, has been shown in phase 3 trials in patients with rheumatoid arthritis to improve joint signs and symptoms and reduce joint damage (Lee et al, 2014).
In contrast to myelofibrosis, where JAK/STAT activation is frequently associated with a gain-of-function mutation in JAK2, the cause of JAK/STAT activation in solid malignancies is less well defined. Indeed, in a phase 1 trial of a JAK1/2 inhibitor in patients with solid tumours no response was seen, when assessed according to Response Evaluation Criteria in Solid Tumours. There was, however, a reduction in pSTAT3 in granulocytes and in tumour tissue in one patient who had pre- and post-treatment biopsies (Plimack et al, 2013).
Approaches to develop therapies have also focussed on suppressing the activity of STATs. The beneficial effect of reducing STAT3 and STAT5 activity in cancer has been demonstrated in cell culture and animal models using overexpression of dominant negative STATs and knockdown of STAT expression with siRNAs. Translating findings based on experimental genetic techniques into therapies that can be administered to patients has been challenging, but approaches that disrupt protein–protein interactions needed for STAT phosphorylation or inhibit STAT–DNA binding have shown promise in preclinical studies. Both strategies have been shown to affect cancer cell proliferation and apoptosis, and decrease tumour growth in mouse models. The natural product withacnistin inhibits the binding of STAT3 and STAT5 to the cytoplasmic region of receptors, thus preventing the recruitment of STATs for phosphorylation (Zhang et al, 2014). High-throughput methods to develop peptide drugs with the capacity to enter cells have been employed to create an inhibitor of STAT3 activation, which acts by binding the SH2 domain (Kim et al, 2014). A small-molecule inhibitor of STAT3 that is thought to interact with the SH2 domain has been examined in a phase 1 trial in patients with solid tumours. No further investigation of this molecule is planned, however, as pharmacokinetic studies showed that plasma concentrations in patients were extremely variable and substantially lower than those observed in preclinical models (Bendell et al, 2014). A synthetic inhibitor of STAT–DNA binding has shown promise in cell and animal models, including in vitro effects on tumour cells from the ascites of patients with ovarian cancer (Rath et al, 2014). The STAT–DNA interaction has also been disrupted by ‘decoy oligonucleotides' that contain STAT-binding sequences and competitively inhibit STAT binding to genomic DNA. A decoy oligonucleotide-targeting STAT3 has been shown to decrease expression of STAT target genes in head and neck squamous cell carcinoma when injected into tumours intraoperatively (Sen et al, 2012).
It is hoped that some of the JAK/STAT-targeted therapies shown to be beneficial in preclinical models will soon enter clinical trials. In order for these therapies to be examined in appropriate patient groups, a better understanding of the mechanisms causing JAK/STAT activation and the degree to which pathway activation drives disease in different tumour types is required. Combining strategies to target STAT activation with those affecting other signalling pathways, such as B-RAF and BCR–ABL may be particularly effective, as has already been demonstrated in preclinical models of melanoma and CML (Liu et al, 2013; Gallipoli et al, 2014).
Conclusion
Direct examination of tissue samples has shown that a large number of solid tumours exhibit activation of the JAK/STAT signalling pathway. The central position of JAK/STAT signalling in a network of signalling pathways whose deregulation contributes to cancer suggests that targeted inhibition of JAK/STAT signalling could be harnessed therapeutically to treat patients with solid tumours. However, further work is required to establish to what extent the observed activation of JAK/STAT signalling is driving disease. This may determine the extent to which inhibition of the pathway brings therapeutic benefits. The near future should see the transition of drugs that inhibit JAK/STAT signalling from preclinical models into early phase clinical trials in solid tumours. Large-scale sequencing projects are starting to reveal subgroups of patients whose tumours harbour mutations in JAK/STAT components. Targeting drugs that inhibit JAK/STAT activation to these groups of patients is likely to be particularly promising.
Acknowledgments
SJT is funded by Cancer Research UK and Yorkshire Cancer Research through a Sheffield Cancer Centre Fellowship. JAS acknowledges the support of Sheffield Hospitals Charity.
The authors declare no conflict of interest.
References
- Andersen MH. The targeting of immunosuppressive mechanisms in hematological malignancies. Leukemia. 2014;28 (9:1784–1792. doi: 10.1038/leu.2014.108. [DOI] [PubMed] [Google Scholar]
- Bain BJ, Ahmad S. Should myeloid and lymphoid neoplasms with PCM1-JAK2 and other rearrangements of JAK2 be recognized as specific entities. Br J Haematol. 2014;166 (6:809–817. doi: 10.1111/bjh.12963. [DOI] [PubMed] [Google Scholar]
- Becker TM, Boyd SC, Mijatov B, Gowrishankar K, Snoyman S, Pupo GM, Scolyer RA, Mann GJ, Kefford RF, Zhang XD, Rizos H. Mutant B-RAF-Mcl-1 survival signaling depends on the STAT3 transcription factor. Oncogene. 2014;33 (9:1158–1166. doi: 10.1038/onc.2013.45. [DOI] [PubMed] [Google Scholar]
- Bendell JC, Hong DS, Burris HA, 3rd, Naing A, Jones SF, Falchook G, Bricmont P, Elekes A, Rock EP, Kurzrock R. Phase 1, open-label, dose-escalation, and pharmacokinetic study of STAT3 inhibitor OPB-31121 in subjects with advanced solid tumors. Cancer Chemother Pharmacol. 2014;74 (1:125–130. doi: 10.1007/s00280-014-2480-2. [DOI] [PubMed] [Google Scholar]
- Birner P, Toumangelova-Uzeir K, Natchev S, Guentchev M. STAT3 tyrosine phosphorylation influences survival in glioblastoma. J Neurooncol. 2010;100 (3:339–343. doi: 10.1007/s11060-010-0195-8. [DOI] [PubMed] [Google Scholar]
- Brantley EC, Nabors LB, Gillespie GY, Choi YH, Palmer CA, Harrison K, Roarty K, Benveniste EN. Loss of protein inhibitors of activated STAT-3 expression in glioblastoma multiforme tumors: implications for STAT-3 activation and gene expression. Clin Cancer Res. 2008;14 (15:4694–4704. doi: 10.1158/1078-0432.CCR-08-0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks AJ, Dai W, O'Mara ML, Abankwa D, Chhabra Y, Pelekanos RA, Gardon O, Tunny KA, Blucher KM, Morton CJ, Parker MW, Sierecki E, Gambin Y, Gomez GA, Alexandrov K, Wilson IA, Doxastakis M, Mark AE, Waters MJ. Mechanism of activation of protein kinase JAK2 by the growth hormone receptor. Science. 2014;344 (6185:1249783. doi: 10.1126/science.1249783. [DOI] [PubMed] [Google Scholar]
- Cervantes F, Vannucchi AM, Kiladjian JJ, Al-Ali HK, Sirulnik A, Stalbovskaya V, McQuitty M, Hunter DS, Levy RS, Passamonti F, Barbui T, Barosi G, Harrison CN, Knoops L, Gisslinger H. Three-year efficacy, safety, and survival findings from COMFORT-II, a phase 3 study comparing ruxolitinib with best available therapy for myelofibrosis. Blood. 2013;122 (25:4047–4053. doi: 10.1182/blood-2013-02-485888. [DOI] [PubMed] [Google Scholar]
- Chen E, Staudt LM, Green AR. Janus kinase deregulation in leukemia and lymphoma. Immunity. 2012;36 (4:529–541. doi: 10.1016/j.immuni.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KH, Jeong KJ, Shin SC, Kang J, Park CG, Lee HY. STAT3 mediates TGF-beta1-induced TWIST1 expression and prostate cancer invasion. Cancer Lett. 2013;336 (1:167–173. doi: 10.1016/j.canlet.2013.04.024. [DOI] [PubMed] [Google Scholar]
- Dawson MA, Bannister AJ, Gottgens B, Foster SD, Bartke T, Green AR, Kouzarides T. JAK2 phosphorylates histone H3Y41 and excludes HP1alpha from chromatin. Nature. 2009;461 (7265:819–822. doi: 10.1038/nature08448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaria M, Giorgi C, Lebiedzinska M, Esposito G, D'Angeli L, Bartoli A, Gough DJ, Turkson J, Levy DE, Watson CJ, Wieckowski MR, Provero P, Pinton P, Poli V. A STAT3-mediated metabolic switch is involved in tumour transformation and STAT3 addiction. Aging (Albany NY) 2010;2 (11:823–842. doi: 10.18632/aging.100232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusa A, Staerk J, Elliott J, Pecquet C, Poirel HA, Johnston JA, Constantinescu SN. Substitution of pseudokinase domain residue Val-617 by large non-polar amino acids causes activation of JAK2. J Biol Chem. 2008;283 (19:12941–12948. doi: 10.1074/jbc.M709302200. [DOI] [PubMed] [Google Scholar]
- Flex E, Petrangeli V, Stella L, Chiaretti S, Hornakova T, Knoops L, Ariola C, Fodale V, Clappier E, Paoloni F, Martinelli S, Fragale A, Sanchez M, Tavolaro S, Messina M, Cazzaniga G, Camera A, Pizzolo G, Tornesello A, Vignetti M, Battistini A, Cave H, Gelb BD, Renauld JC, Biondi A, Constantinescu SN, Foa R, Tartaglia M. Somatically acquired JAK1 mutations in adult acute lymphoblastic leukemia. J Exp Med. 2008;205 (4:751–758. doi: 10.1084/jem.20072182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallipoli P, Cook A, Rhodes S, Hopcroft L, Wheadon H, Whetton AD, Jorgensen HG, Bhatia R, Holyoake TL. JAK2/STAT5 inhibition by nilotinib with ruxolitinib contributes to the elimination of CML CD34+ cells in vitro and in vivo. Blood. 2014;124 (9:1492–1501. doi: 10.1182/blood-2013-12-545640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girodon F, Steinkamp MP, Cleyrat C, Hermouet S, Wilson BS. Confocal imaging studies cast doubt on nuclear localization of JAK2V617F. Blood. 2011;118 (9:2633–2634. doi: 10.1182/blood-2011-02-336479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough DJ, Corlett A, Schlessinger K, Wegrzyn J, Larner AC, Levy DE. Mitochondrial STAT3 supports Ras-dependent oncogenic transformation. Science. 2009;324 (5935:1713–1716. doi: 10.1126/science.1171721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough DJ, Koetz L, Levy DE. The MEK-ERK pathway is necessary for serine phosphorylation of mitochondrial STAT3 and Ras-mediated transformation. PLoS One. 2013;8 (11:e83395. doi: 10.1371/journal.pone.0083395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisouard J, Shimizu T, Duek A, Kubovcakova L, Hao-Shen H, Dirnhofer S, Skoda RC. Deletion of Stat3 in hematopoietic cells enhances thrombocytosis and shortens survival in a JAK2-V617F mouse model of MPN. Blood. 2015;125 (13:2131–2140. doi: 10.1182/blood-2014-08-594572. [DOI] [PubMed] [Google Scholar]
- Haddad BR, Gu L, Mirtti T, Dagvadorj A, Vogiatzi P, Hoang DT, Bajaj R, Leiby B, Ellsworth E, Blackmon S, Ruiz C, Curtis M, Fortina P, Ertel A, Liu C, Rui H, Visakorpi T, Bubendorf L, Lallas CD, Trabulsi EJ, McCue P, Gomella L, Nevalainen MT. STAT5A/B gene locus undergoes amplification during human prostate cancer progression. Am J Pathol. 2013;182 (6:2264–2275. doi: 10.1016/j.ajpath.2013.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V, McQuitty M, Hunter DS, Levy R, Knoops L, Cervantes F, Vannucchi AM, Barbui T, Barosi G. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366 (9:787–798. doi: 10.1056/NEJMoa1110556. [DOI] [PubMed] [Google Scholar]
- He B, You L, Uematsu K, Zang K, Xu Z, Lee AY, Costello JF, McCormick F, Jablons DM. SOCS-3 is frequently silenced by hypermethylation and suppresses cell growth in human lung cancer. Proc Natl Acad Sci USA. 2003;100 (24:14133–14138. doi: 10.1073/pnas.2232790100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi A, Oya M, Shimada T, Uchida A, Marumo K, Murai M. Activation of signal transducer and activator of transcription 3 in renal cell carcinoma: a study of incidence and its association with pathological features and clinical outcome. J Urol. 2002;168 (2:762–765. [PubMed] [Google Scholar]
- Hu X, Dutta P, Tsurumi A, Li J, Wang J, Land H, Li WX. Unphosphorylated STAT5A stabilizes heterochromatin and suppresses tumor growth. Proc Natl Acad Sci USA. 2013;110 (25:10213–10218. doi: 10.1073/pnas.1221243110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes TP, Hochhaus A, Branford S, Muller MC, Kaeda JS, Foroni L, Druker BJ, Guilhot F, Larson RA, O'Brien SG, Rudoltz MS, Mone M, Wehrle E, Modur V, Goldman JM, Radich JP. Long-term prognostic significance of early molecular response to imatinib in newly diagnosed chronic myeloid leukemia: an analysis from the International Randomized Study of Interferon and STI571 (IRIS) Blood. 2010;116 (19:3758–3765. doi: 10.1182/blood-2010-03-273979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan Z, Zheng H, Liu X, Li S, Barber TD, Gong Z, Gao H, Hao K, Willard MD, Xu J, Hauptschein R, Rejto PA, Fernandez J, Wang G, Zhang Q, Wang B, Chen R, Wang J, Lee NP, Zhou W, Lin Z, Peng Z, Yi K, Chen S, Li L, Fan X, Yang J, Ye R, Ju J, Wang K, Estrella H, Deng S, Wei P, Qiu M, Wulur IH, Liu J, Ehsani ME, Zhang C, Loboda A, Sung WK, Aggarwal A, Poon RT, Fan ST, Hardwick J, Reinhard C, Dai H, Li Y, Luk JM, Mao M. Whole-genome sequencing identifies recurrent mutations in hepatocellular carcinoma. Genome Res. 2013;23 (9:1422–1433. doi: 10.1101/gr.154492.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Lee IH, Kim S, Choi M, Kim H, Ahn S, Saw PE, Jeon H, Lee Y, Jon S. A specific STAT3-binding peptide exerts antiproliferative effects and antitumor activity by inhibiting STAT3 phosphorylation and signaling. Cancer Res. 2014;74 (8:2144–2151. doi: 10.1158/0008-5472.CAN-13-2187. [DOI] [PubMed] [Google Scholar]
- Klampfl T, Gisslinger H, Harutyunyan AS, Nivarthi H, Rumi E, Milosevic JD, Them NC, Berg T, Gisslinger B, Pietra D, Chen D, Vladimer GI, Bagienski K, Milanesi C, Casetti IC, Sant'Antonio E, Ferretti V, Elena C, Schischlik F, Cleary C, Six M, Schalling M, Schonegger A, Bock C, Malcovati L, Pascutto C, Superti-Furga G, Cazzola M, Kralovics R. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369 (25:2379–2390. doi: 10.1056/NEJMoa1311347. [DOI] [PubMed] [Google Scholar]
- Kortylewski M, Kujawski M, Wang T, Wei S, Zhang S, Pilon-Thomas S, Niu G, Kay H, Mule J, Kerr WG, Jove R, Pardoll D, Yu H. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat Med. 2005;11 (12:1314–1321. doi: 10.1038/nm1325. [DOI] [PubMed] [Google Scholar]
- Koskela HLM, Eldfors S, Ellonen P, van Adrichem AJ, Kuusanmäki H, Andersson EI, Lagström S, Clemente MJ, Olson T, Jalkanen SE, Majumder MM, Almusa H, Edgren H, Lepistö M, Mattila P, Guinta K, Koistinen P, Kuittinen T, Penttinen K, Parsons A, Knowles J, Saarela J, Wennerberg K, Kallioniemi O, Porkka K, Loughran TP, Heckman CA, Maciejewski JP, Mustjoki S. Somatic STAT3 Mutations in Large Granular Lymphocytic Leukemia. N Engl J Med. 2012;366 (20:1905–1913. doi: 10.1056/NEJMoa1114885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar J, Fraser FW, Riley C, Ahmed N, McCulloch DR, Ward AC. Granulocyte colony-stimulating factor receptor signalling via Janus kinase 2/signal transducer and activator of transcription 3 in ovarian cancer. Br J Cancer. 2014;110 (1:133–145. doi: 10.1038/bjc.2013.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusaba T, Nakayama T, Yamazumi K, Yakata Y, Yoshizaki A, Inoue K, Nagayasu T, Sekine I. Activation of STAT3 is a marker of poor prognosis in human colorectal cancer. Oncol Rep. 2006;15 (6:1445–1451. [PubMed] [Google Scholar]
- Lee EB, Fleischmann R, Hall S, Wilkinson B, Bradley JD, Gruben D, Koncz T, Krishnaswami S, Wallenstein GV, Zang C, Zwillich SH, van Vollenhoven RF. Tofacitinib versus Methotrexate in Rheumatoid Arthritis. N Engl J Med. 2014;370 (25:2377–2386. doi: 10.1056/NEJMoa1310476. [DOI] [PubMed] [Google Scholar]
- Liu F, Cao J, Wu J, Sullivan K, Shen J, Ryu B, Xu Z, Wei W, Cui R. Stat3-targeted therapies overcome the acquired resistance to vemurafenib in melanomas. J Invest Dermatol. 2013;133 (8:2041–2049. doi: 10.1038/jid.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macha MA, Matta A, Kaur J, Chauhan SS, Thakar A, Shukla NK, Gupta SD, Ralhan R. Prognostic significance of nuclear pSTAT3 in oral cancer. Head Neck. 2011;33 (4:482–489. doi: 10.1002/hed.21468. [DOI] [PubMed] [Google Scholar]
- Marty C, Saint-Martin C, Pecquet C, Grosjean S, Saliba J, Mouton C, Leroy E, Harutyunyan AS, Abgrall JF, Favier R, Toussaint A, Solary E, Kralovics R, Constantinescu SN, Najman A, Vainchenker W, Plo I, Bellanne-Chantelot C. Germ-line JAK2 mutations in the kinase domain are responsible for hereditary thrombocytosis and are resistant to JAK2 and HSP90 inhibitors. Blood. 2014;123 (9:1372–1383. doi: 10.1182/blood-2013-05-504555. [DOI] [PubMed] [Google Scholar]
- Mead AJ, Chowdhury O, Pecquet C, Dusa A, Woll P, Atkinson D, Burns A, Score J, Rugless M, Clifford R, Moule S, Bienz N, Vyas P, Cross N, Gale RE, Henderson S, Constantinescu SN, Schuh A, Jacobsen SE. Impact of isolated germline JAK2V617I mutation on human hematopoiesis. Blood. 2013;121 (20:4156–4165. doi: 10.1182/blood-2012-05-430926. [DOI] [PubMed] [Google Scholar]
- Messina JL, Yu H, Riker AI, Munster PN, Jove RL, Daud AI. Activated stat-3 in melanoma. Cancer Control. 2008;15 (3:196–201. doi: 10.1177/107327480801500302. [DOI] [PubMed] [Google Scholar]
- Mirtti T, Leiby BE, Abdulghani J, Aaltonen E, Pavela M, Mamtani A, Alanen K, Egevad L, Granfors T, Josefsson A, Stattin P, Bergh A, Nevalainen MT. Nuclear Stat5a/b predicts early recurrence and prostate cancer-specific death in patients treated by radical prostatectomy. Hum Pathol. 2013;44 (3:310–319. doi: 10.1016/j.humpath.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnien F, Zaki H, Borg C, Mougin C, Bosset JF, Mercier M, Arbez-Gindre F, Kantelip B. Prognostic value of phosphorylated STAT3 in advanced rectal cancer: a study from 104 French patients included in the EORTC 22921 trial. J Clin Pathol. 2010;63 (10:873–878. doi: 10.1136/jcp.2010.076414. [DOI] [PubMed] [Google Scholar]
- Nangalia J, Massie CE, Baxter EJ, Nice FL, Gundem G, Wedge DC, Avezov E, Li J, Kollmann K, Kent DG, Aziz A, Godfrey AL, Hinton J, Martincorena I, Van Loo P, Jones AV, Guglielmelli P, Tarpey P, Harding HP, Fitzpatrick JD, Goudie CT, Ortmann CA, Loughran SJ, Raine K, Jones DR, Butler AP, Teague JW, O'Meara S, McLaren S, Bianchi M, Silber Y, Dimitropoulou D, Bloxham D, Mudie L, Maddison M, Robinson B, Keohane C, Maclean C, Hill K, Orchard K, Tauro S, Du MQ, Greaves M, Bowen D, Huntly BJ, Harrison CN, Cross NC, Ron D, Vannucchi AM, Papaemmanuil E, Campbell PJ, Green AR. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med. 2013;369 (25:2391–2405. doi: 10.1056/NEJMoa1312542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu G, Bowman T, Huang M, Shivers S, Reintgen D, Daud A, Chang A, Kraker A, Jove R, Yu H. Roles of activated Src and Stat3 signaling in melanoma tumor cell growth. Oncogene. 2002;21 (46:7001–7010. doi: 10.1038/sj.onc.1205859. [DOI] [PubMed] [Google Scholar]
- Olayioye MA, Beuvink I, Horsch K, Daly JM, Hynes NE. ErbB receptor-induced activation of stat transcription factors is mediated by Src tyrosine kinases. J Biol Chem. 1999;274 (24:17209–17218. doi: 10.1074/jbc.274.24.17209. [DOI] [PubMed] [Google Scholar]
- Pawlus MR, Wang L, Hu CJ. STAT3 and HIF1alpha cooperatively activate HIF1 target genes in MDA-MB-231 and RCC4 cells. Oncogene. 2014;33 (13:1670–1679. doi: 10.1038/onc.2013.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck AR, Witkiewicz AK, Liu C, Stringer GA, Klimowicz AC, Pequignot E, Freydin B, Tran TH, Yang N, Rosenberg AL, Hooke JA, Kovatich AJ, Nevalainen MT, Shriver CD, Hyslop T, Sauter G, Rimm DL, Magliocco AM, Rui H. Loss of nuclear localized and tyrosine phosphorylated Stat5 in breast cancer predicts poor clinical outcome and increased risk of antiestrogen therapy failure. J Clin Oncol. 2011;29 (18:2448–2458. doi: 10.1200/JCO.2010.30.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pectasides E, Egloff AM, Sasaki C, Kountourakis P, Burtness B, Fountzilas G, Dafni U, Zaramboukas T, Rampias T, Rimm D, Grandis J, Psyrri A. Nuclear localization of signal transducer and activator of transcription 3 in head and neck squamous cell carcinoma is associated with a better prognosis. Clin Cancer Res. 2010;16 (8:2427–2434. doi: 10.1158/1078-0432.CCR-09-2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plimack ER, Lorusso PM, McCoon P, Tang W, Krebs AD, Curt G, Eckhardt SG. AZD1480: a phase I study of a novel JAK2 inhibitor in solid tumors. Oncologist. 2013;18 (7:819–820. doi: 10.1634/theoncologist.2013-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rath KS, Naidu SK, Lata P, Bid HK, Rivera BK, McCann GA, Tierney BJ, Elnaggar AC, Bravo V, Leone G, Houghton P, Hideg K, Kuppusamy P, Cohn DE, Selvendiran K. HO-3867, a safe STAT3 inhibitor, is selectively cytotoxic to ovarian cancer. Cancer Res. 2014;74 (8:2316–2327. doi: 10.1158/0008-5472.CAN-13-2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen M, Thomas SM, Kim S, Yeh JI, Ferris RL, Johnson JT, Duvvuri U, Lee J, Sahu N, Joyce S, Freilino ML, Shi H, Li C, Ly D, Rapireddy S, Etter JP, Li PK, Wang L, Chiosea S, Seethala RR, Gooding WE, Chen X, Kaminski N, Pandit K, Johnson DE, Grandis JR. First-in-human trial of a STAT3 decoy oligonucleotide in head and neck tumors: implications for cancer therapy. Cancer Discov. 2012;2 (8:694–705. doi: 10.1158/2159-8290.CD-12-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvennoinen O, Schindler C, Schlessinger J, Levy DE. Ras-independent growth factor signaling by transcription factor tyrosine phosphorylation. Science. 1993;261 (5129:1736–1739. doi: 10.1126/science.8378775. [DOI] [PubMed] [Google Scholar]
- Sonnenblick A, Uziely B, Nechushtan H, Kadouri L, Galun E, Axelrod JH, Katz D, Daum H, Hamburger T, Maly B, Allweis TM, Peretz T. Tumor STAT3 tyrosine phosphorylation status, as a predictor of benefit from adjuvant chemotherapy for breast cancer. Breast Cancer Res Treat. 2013;138 (2:407–413. doi: 10.1007/s10549-013-2453-x. [DOI] [PubMed] [Google Scholar]
- Sriuranpong V, Park JI, Amornphimoltham P, Patel V, Nelkin BD, Gutkind JS. Epidermal growth factor receptor-independent constitutive activation of STAT3 in head and neck squamous cell carcinoma is mediated by the autocrine/paracrine stimulation of the interleukin 6/gp130 cytokine system. Cancer Res. 2003;63 (11:2948–2956. [PubMed] [Google Scholar]
- Takemoto S, Ushijima K, Kawano K, Yamaguchi T, Terada A, Fujiyoshi N, Nishio S, Tsuda N, Ijichi M, Kakuma T, Kage M, Hori D, Kamura T. Expression of activated signal transducer and activator of transcription-3 predicts poor prognosis in cervical squamous-cell carcinoma. Br J Cancer. 2009;101 (6:967–972. doi: 10.1038/sj.bjc.6605212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Cancer Genome Atlas Research Network Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513 (7517:202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainchenker W, Constantinescu SN. JAK/STAT signaling in hematological malignancies. Oncogene. 2013;32 (21:2601–2613. doi: 10.1038/onc.2012.347. [DOI] [PubMed] [Google Scholar]
- Victor CT-S, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi PM, Herati RS, Mansfield KD, Patsch D, Amaravadi RK, Schuchter LM, Ishwaran H, Mick R, Pryma DA, Xu X, Feldman MD, Gangadhar TC, Hahn SM, Wherry EJ, Vonderheide RH, Minn AJ. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520 (7547:373–377. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu YH, Lu S. A meta-analysis of STAT3 and phospho-STAT3 expression and survival of patients with non-small-cell lung cancer. Eur J Surg Oncol. 2014;40 (3:311–317. doi: 10.1016/j.ejso.2013.11.012. [DOI] [PubMed] [Google Scholar]
- Zhang X, Blaskovich MA, Forinash KD, Sebti SM. Withacnistin inhibits recruitment of STAT3 and STAT5 to growth factor and cytokine receptors and induces regression of breast tumours. Br J Cancer. 2014;111 (5:894–902. doi: 10.1038/bjc.2014.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorn E, Nelson EA, Mohseni M, Porcheray F, Kim H, Litsa D, Bellucci R, Raderschall E, Canning C, Soiffer RJ, Frank DA, Ritz J. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood. 2006;108 (5:1571–1579. doi: 10.1182/blood-2006-02-004747. [DOI] [PMC free article] [PubMed] [Google Scholar]