Abstract
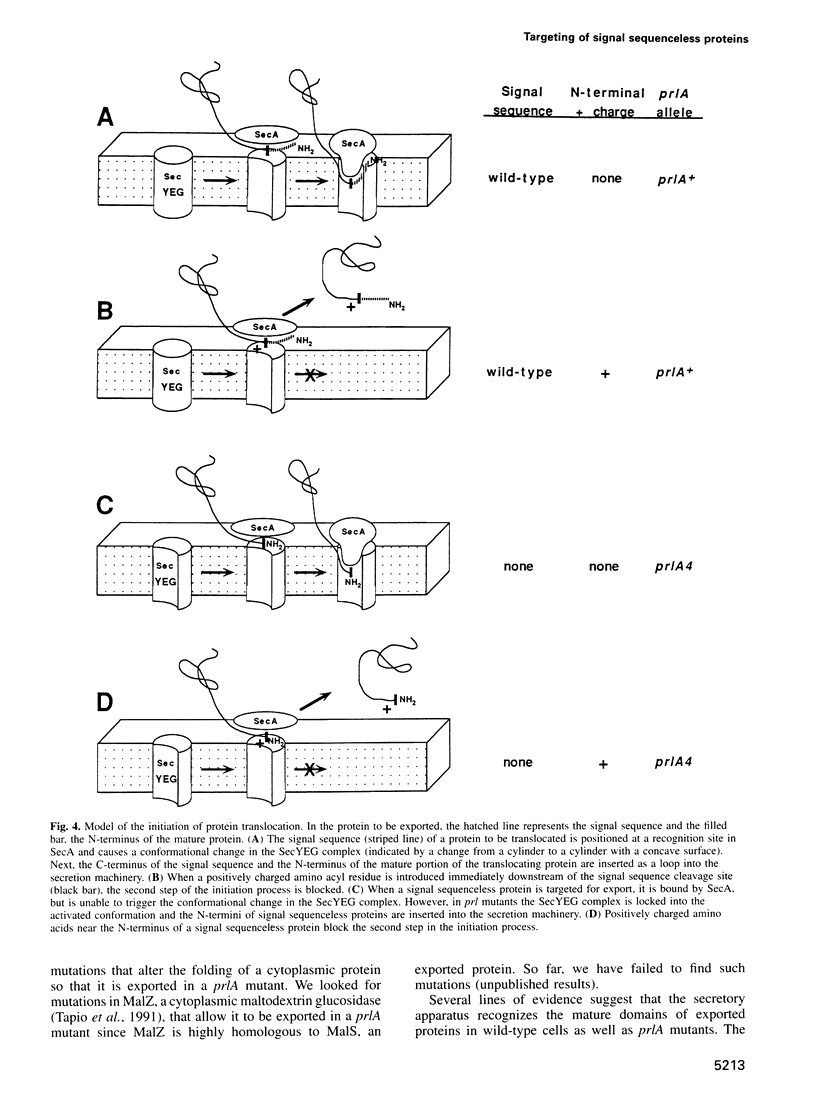
Most extracytoplasmic proteins are synthesized with an N-terminal signal sequence that targets them to the export apparatus. Escherichia coli prlA mutants (altered in the secY gene) are able to export cell envelope proteins lacking any signal sequence. In order to understand how such proteins are targeted for export, we isolated mutations in a signal sequenceless version of alkaline phosphatase that block its export in a prlA mutant. The mutations introduce basic amino acyl residues near the N-terminus of alkaline phosphatase. These changes do not disrupt an N-terminal export signal in this protein since the first 25 amino acids can be removed without affecting its export competence. These findings suggest that signal sequenceless alkaline phosphatase does not contain a discrete domain that targets it for export and may be targeted simply because it remains unfolded in the cytoplasm. We propose that basic amino acids near the N-terminus of a signal sequenceless protein affect its insertion into the translocation apparatus after it has been targeted for export. These findings allow the formulation of a model for the entry of proteins into the membrane-embedded export machinery.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman E., Emr S. D., Kumamoto C. A. The presence of both the signal sequence and a region of mature LamB protein is required for the interaction of LamB with the export factor SecB. J Biol Chem. 1990 Oct 25;265(30):18154–18160. [PubMed] [Google Scholar]
- Belin D., Bost S., Vassalli J. D., Strub K. A two-step recognition of signal sequences determines the translocation efficiency of proteins. EMBO J. 1996 Feb 1;15(3):468–478. [PMC free article] [PubMed] [Google Scholar]
- Bieker K. L., Phillips G. J., Silhavy T. J. The sec and prl genes of Escherichia coli. J Bioenerg Biomembr. 1990 Jun;22(3):291–310. doi: 10.1007/BF00763169. [DOI] [PubMed] [Google Scholar]
- Bowden G. A., Baneyx F., Georgiou G. Abnormal fractionation of beta-lactamase in Escherichia coli: evidence for an interaction with the inner membrane in the absence of a leader peptide. J Bacteriol. 1992 May;174(10):3407–3410. doi: 10.1128/jb.174.10.3407-3410.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier D. N., Bankaitis V. A., Weiss J. B., Bassford P. J., Jr The antifolding activity of SecB promotes the export of the E. coli maltose-binding protein. Cell. 1988 Apr 22;53(2):273–283. doi: 10.1016/0092-8674(88)90389-3. [DOI] [PubMed] [Google Scholar]
- Cunningham K., Wickner W. Specific recognition of the leader region of precursor proteins is required for the activation of translocation ATPase of Escherichia coli. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8630–8634. doi: 10.1073/pnas.86.22.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derman A. I., Beckwith J. Escherichia coli alkaline phosphatase fails to acquire disulfide bonds when retained in the cytoplasm. J Bacteriol. 1991 Dec;173(23):7719–7722. doi: 10.1128/jb.173.23.7719-7722.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derman A. I., Prinz W. A., Belin D., Beckwith J. Mutations that allow disulfide bond formation in the cytoplasm of Escherichia coli. Science. 1993 Dec 10;262(5140):1744–1747. doi: 10.1126/science.8259521. [DOI] [PubMed] [Google Scholar]
- Derman A. I., Puziss J. W., Bassford P. J., Jr, Beckwith J. A signal sequence is not required for protein export in prlA mutants of Escherichia coli. EMBO J. 1993 Mar;12(3):879–888. doi: 10.1002/j.1460-2075.1993.tb05728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economou A., Pogliano J. A., Beckwith J., Oliver D. B., Wickner W. SecA membrane cycling at SecYEG is driven by distinct ATP binding and hydrolysis events and is regulated by SecD and SecF. Cell. 1995 Dec 29;83(7):1171–1181. doi: 10.1016/0092-8674(95)90143-4. [DOI] [PubMed] [Google Scholar]
- Economou A., Wickner W. SecA promotes preprotein translocation by undergoing ATP-driven cycles of membrane insertion and deinsertion. Cell. 1994 Sep 9;78(5):835–843. doi: 10.1016/s0092-8674(94)90582-7. [DOI] [PubMed] [Google Scholar]
- Emr S. D., Bassford P. J., Jr Localization and processing of outer membrane and periplasmic proteins in Escherichia coli strains harboring export-specific suppressor mutations. J Biol Chem. 1982 May 25;257(10):5852–5860. [PubMed] [Google Scholar]
- Emr S. D., Hanley-Way S., Silhavy T. J. Suppressor mutations that restore export of a protein with a defective signal sequence. Cell. 1981 Jan;23(1):79–88. doi: 10.1016/0092-8674(81)90272-5. [DOI] [PubMed] [Google Scholar]
- Flower A. M., Doebele R. C., Silhavy T. J. PrlA and PrlG suppressors reduce the requirement for signal sequence recognition. J Bacteriol. 1994 Sep;176(18):5607–5614. doi: 10.1128/jb.176.18.5607-5614.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennity J., Goldstein J., Inouye M. Signal peptide mutants of Escherichia coli. J Bioenerg Biomembr. 1990 Jun;22(3):233–269. doi: 10.1007/BF00763167. [DOI] [PubMed] [Google Scholar]
- Guzman L. M., Belin D., Carson M. J., Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995 Jul;177(14):4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy S. J., Randall L. L. Recognition of ligands by SecB, a molecular chaperone involved in bacterial protein export. Philos Trans R Soc Lond B Biol Sci. 1993 Mar 29;339(1289):343–354. doi: 10.1098/rstb.1993.0033. [DOI] [PubMed] [Google Scholar]
- High S. Protein translocation at the membrane of the endoplasmic reticulum. Prog Biophys Mol Biol. 1995;63(2):233–250. doi: 10.1016/0079-6107(95)00005-8. [DOI] [PubMed] [Google Scholar]
- Izard J. W., Kendall D. A. Signal peptides: exquisitely designed transport promoters. Mol Microbiol. 1994 Sep;13(5):765–773. doi: 10.1111/j.1365-2958.1994.tb00469.x. [DOI] [PubMed] [Google Scholar]
- Jones J. D., McKnight C. J., Gierasch L. M. Biophysical studies of signal peptides: implications for signal sequence functions and the involvement of lipid in protein export. J Bioenerg Biomembr. 1990 Jun;22(3):213–232. doi: 10.1007/BF00763166. [DOI] [PubMed] [Google Scholar]
- Jungnickel B., Rapoport T. A. A posttargeting signal sequence recognition event in the endoplasmic reticulum membrane. Cell. 1995 Jul 28;82(2):261–270. doi: 10.1016/0092-8674(95)90313-5. [DOI] [PubMed] [Google Scholar]
- Kim Y. J., Rajapandi T., Oliver D. SecA protein is exposed to the periplasmic surface of the E. coli inner membrane in its active state. Cell. 1994 Sep 9;78(5):845–853. doi: 10.1016/s0092-8674(94)90602-5. [DOI] [PubMed] [Google Scholar]
- Kumamoto C. A. SecB protein: a cytosolic export factor that associates with nascent exported proteins. J Bioenerg Biomembr. 1990 Jun;22(3):337–351. doi: 10.1007/BF00763171. [DOI] [PubMed] [Google Scholar]
- Lauring B., Kreibich G., Weidmann M. The intrinsic ability of ribosomes to bind to endoplasmic reticulum membranes is regulated by signal recognition particle and nascent-polypeptide-associated complex. Proc Natl Acad Sci U S A. 1995 Oct 10;92(21):9435–9439. doi: 10.1073/pnas.92.21.9435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lill R., Dowhan W., Wickner W. The ATPase activity of SecA is regulated by acidic phospholipids, SecY, and the leader and mature domains of precursor proteins. Cell. 1990 Jan 26;60(2):271–280. doi: 10.1016/0092-8674(90)90742-w. [DOI] [PubMed] [Google Scholar]
- Luirink J., High S., Wood H., Giner A., Tollervey D., Dobberstein B. Signal-sequence recognition by an Escherichia coli ribonucleoprotein complex. Nature. 1992 Oct 22;359(6397):741–743. doi: 10.1038/359741a0. [DOI] [PubMed] [Google Scholar]
- Mendenhall M. D., Richardson H. E., Reed S. I. Dominant negative protein kinase mutations that confer a G1 arrest phenotype. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4426–4430. doi: 10.1073/pnas.85.12.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis S., Hunt J. F., Beckwith J. Effects of signal sequence mutations on the kinetics of alkaline phosphatase export to the periplasm in Escherichia coli. J Bacteriol. 1986 Jul;167(1):160–167. doi: 10.1128/jb.167.1.160-167.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne R. S., Silhavy T. J. PrlA suppressor mutations cluster in regions corresponding to three distinct topological domains. EMBO J. 1993 Sep;12(9):3391–3398. doi: 10.1002/j.1460-2075.1993.tb06013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panzner S., Dreier L., Hartmann E., Kostka S., Rapoport T. A. Posttranslational protein transport in yeast reconstituted with a purified complex of Sec proteins and Kar2p. Cell. 1995 May 19;81(4):561–570. doi: 10.1016/0092-8674(95)90077-2. [DOI] [PubMed] [Google Scholar]
- Park S., Liu G., Topping T. B., Cover W. H., Randall L. L. Modulation of folding pathways of exported proteins by the leader sequence. Science. 1988 Feb 26;239(4843):1033–1035. doi: 10.1126/science.3278378. [DOI] [PubMed] [Google Scholar]
- Phillips G. J., Silhavy T. J. The E. coli ffh gene is necessary for viability and efficient protein export. Nature. 1992 Oct 22;359(6397):744–746. doi: 10.1038/359744a0. [DOI] [PubMed] [Google Scholar]
- Poritz M. A., Bernstein H. D., Strub K., Zopf D., Wilhelm H., Walter P. An E. coli ribonucleoprotein containing 4.5S RNA resembles mammalian signal recognition particle. Science. 1990 Nov 23;250(4984):1111–1117. doi: 10.1126/science.1701272. [DOI] [PubMed] [Google Scholar]
- Randall L. L., Hardy S. J., Thom J. R. Export of protein: a biochemical view. Annu Rev Microbiol. 1987;41:507–541. doi: 10.1146/annurev.mi.41.100187.002451. [DOI] [PubMed] [Google Scholar]
- Randall L. L., Topping T. B., Hardy S. J. No specific recognition of leader peptide by SecB, a chaperone involved in protein export. Science. 1990 May 18;248(4957):860–863. doi: 10.1126/science.2188362. [DOI] [PubMed] [Google Scholar]
- Ribes V., Römisch K., Giner A., Dobberstein B., Tollervey D. E. coli 4.5S RNA is part of a ribonucleoprotein particle that has properties related to signal recognition particle. Cell. 1990 Nov 2;63(3):591–600. doi: 10.1016/0092-8674(90)90454-m. [DOI] [PubMed] [Google Scholar]
- Schatz P. J., Beckwith J. Genetic analysis of protein export in Escherichia coli. Annu Rev Genet. 1990;24:215–248. doi: 10.1146/annurev.ge.24.120190.001243. [DOI] [PubMed] [Google Scholar]
- Schiebel E., Driessen A. J., Hartl F. U., Wickner W. Delta mu H+ and ATP function at different steps of the catalytic cycle of preprotein translocase. Cell. 1991 Mar 8;64(5):927–939. doi: 10.1016/0092-8674(91)90317-r. [DOI] [PubMed] [Google Scholar]
- Schneider E., Freundlieb S., Tapio S., Boos W. Molecular characterization of the MalT-dependent periplasmic alpha-amylase of Escherichia coli encoded by malS. J Biol Chem. 1992 Mar 15;267(8):5148–5154. [PubMed] [Google Scholar]
- Tapio S., Yeh F., Shuman H. A., Boos W. The malZ gene of Escherichia coli, a member of the maltose regulon, encodes a maltodextrin glucosidase. J Biol Chem. 1991 Oct 15;266(29):19450–19458. [PubMed] [Google Scholar]
- Walter P., Johnson A. E. Signal sequence recognition and protein targeting to the endoplasmic reticulum membrane. Annu Rev Cell Biol. 1994;10:87–119. doi: 10.1146/annurev.cb.10.110194.000511. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Blobel G. High-affinity binding of Escherichia coli SecB to the signal sequence region of a presecretory protein. Proc Natl Acad Sci U S A. 1995 Oct 24;92(22):10133–10136. doi: 10.1073/pnas.92.22.10133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M., Blobel G. SecB functions as a cytosolic signal recognition factor for protein export in E. coli. Cell. 1989 Aug 25;58(4):695–705. doi: 10.1016/0092-8674(89)90104-9. [DOI] [PubMed] [Google Scholar]
- Wickner W., Driessen A. J., Hartl F. U. The enzymology of protein translocation across the Escherichia coli plasma membrane. Annu Rev Biochem. 1991;60:101–124. doi: 10.1146/annurev.bi.60.070191.000533. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985 Jul 5;184(1):99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]




