Abstract
Growing evidence suggests serum C-reactive protein (CRP) can serve as a prognostic marker in urological cancers. However, some studies yield contradictory results. Our objective was to determine the relationship between baseline serum CRP and survival outcome in urological cancers. We searched PubMed and EMBASE databases until October 2014 without language restrictions. 44 independent studies investigating the association between baseline serum CRP and cancer-specific survival (CSS) or overall survival (OS) were selected. High CRP yielded a worse survival in renal cell carcinoma, prostate cancer, bladder cancer, and upper urinary tract urothelial carcinoma. Combined results of meta-analyses indicated that CRP was a prognostic factor in urological cancers (CSS: p < 0.01; OS: p < 0.01). Subgroup analyses confirmed the significant association between CRP and prognosis, regardless of race and cutoff value of CRP. Specifically, prognostic impact of CRP was also noted in patients with localized RCC treated with nephrectomy (CSS: p < 0.01) and metastatic RCC treated with molecular-targeted therapy (OS: p < 0.01). In conclusion, serum CRP is an independent prognostic factor in urological cancers and risk stratification by serum CRP level could be helpful for prognostic assessment.
Urological cancer comprises of the highest prevalence groups in the world and represents a growing burden on human healthcare1,2. Renal cell carcinoma (RCC) accounts for approximately 2–3% of all adult malignancies1,3. Prostate cancer is one of the most common cancers in men and the fifth most common cancer overall4. In bladder cancer, which ranks as the ninth most frequent malignancy, an estimated 386,300 new cases and 150,200 deaths from bladder cancer occurred in 2008 worldwide1. Despite the great progress in treatment of urological cancers, such as chemotherapy and molecular therapy, the clinical outcome remains not promising due to low objective response rate, tumor local recurrence or distal metastasis. In this condition, identification of patients who are prone to have a poor prognosis has become increasingly important for treatment selection and prognostic estimation. TNM classification and histological grade are clinically most-used prognostic indicators in malignancies. However, the accuracy of TNM classification remains poor in cancers and pathologic analysis for prognosis would miss any information associated patient-related factors5. This leaves a considerable space for the development of supplementary biomarkers to predict the prognosis of patients.
It is well documented that inflammation is a hallmark feature in the development and progression of cancer6. Increasing evidence suggests that inflammation can help incipient neoplasias to acquire hallmark capabilities and promote tumour growth and metastatic dissemination by sustaining a microenvironment7,8. C-reactive protein is a representative acute-phase protein and serves as a definitive marker for systemic inflammation9. In fact, a remarkable association between CRP and the poor survival in malignancies has been demonstrated, such as colorectal and lung cancers6,8.
During the last decade, numerous studies explored the prognostic impact of CRP in urological cancers, including renal cell cancer, prostate cancer, bladder cancer and upper urinary urothelial carcinoma. Saito and Kihara concluded that CRP is a useful biomarker for urological cancers10. However, some studies failed to draw similar conclusions. Recently, several meta-analyses focused on the association between CRP and the survival of renal cell carcinoma or prostate cancer11,12,13, but urothelial cancer and bladder cancer were not taken into account. More importantly, those reports included a relatively small number of studies. Many researchers have done a lot of studies on the prognostic role of CRP in urological cancers so that a growing number of related studies were published. Therefore, to confirm the prognostic significance of baseline serum CRP in urologic cancers, we gathered the available clinical research evidence and carried out a comprehensive meta-analysis. In this study, we applied a stricter inclusion/exclusion criteria, included much more recent studies, and discussed other important factors, especially the cutoff of CRP level.
Results
Study selection
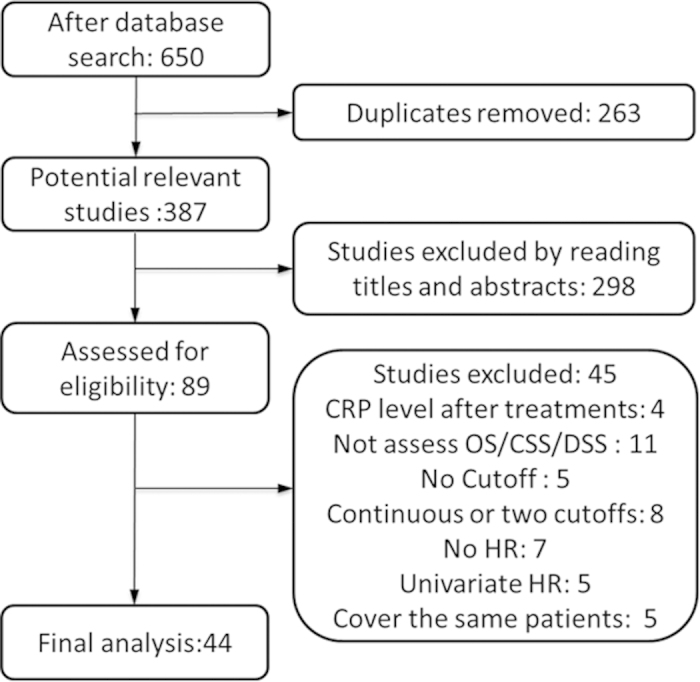
Figure 1 shows the process of study selection. We identified 650 possibly eligible articles through searching the Pubmed (n = 229) and EMBASE databases (n = 421) but 263 articles were excluded due to duplication. After carefully reviewing the titles and abstracts of 387 articles by the two authors (L.Z, X.C), 89 articles were retained for further examination. Based on the detailed information in full-texts, 45 articles were excluded as documented in Figure 1. Finally, a total of 44 studies comprised 9174 cases were included for our quantitatively analysis.
Figure 1. Flow chart of study selection for the meta-analysis.
Characteristics of included studies
Characteristics of included studies were summarized in Table 1. 44 observational studies were published from 1997 to 2014. All studies met all six points for quality evaluation. Most of these studies were conducted in Asia (n = 31), Europe (n = 11) and North America (n = 2). Of these studies, 6 explored the CRP in the prognosis of prostate cancer14,15,16,17,18,19, 27 of renal cell carcinoma20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46 and 11 of urothelial carcinoma originating from bladder (n = 6) and upper urinary tract (n = 5)47,48,49,50,51,52,53,54,55,56,57. The number of patients ranged from 30 to 1161 among the studies. As for the survival outcomes, 21 articles evaluated the association of CRP and OS. Prognostic role of CRP in CSS or disease specific survival (DSS) was evaluted in 25 articles.
Table 1. Characteristics of all identified studies.
| Author | Year | Country | Tumor | N | Male | Metastatic | Outcome | Cut-off (mg/dl) |
|---|---|---|---|---|---|---|---|---|
| Nakagawa | 2014 | Japan | BC | 267 | 212 | 267 | CSS | 0.5 |
| Kramer | 2014 | Germany | BC | 194 | 151 | 25 | CSS | 0.5 |
| Tanaka | 2014 | Japan | UUT-UC | 564 | 409 | 122 | CSS | 0.5 |
| Sakano | 2013 | Japan | UUT-UC | 536 | 370 | 0 | DSS | 0.13 |
| Kruck | 2013 | Germany | RCC | 327 | 219 | NA | CSS | 0.25 |
| Kamba | 2013 | Japan | RCC | 144 | 112 | 144 | OS | 0.3 |
| Shinohara | 2013 | Japan | RCC | 473 | 329 | 473 | OS | 0.3 |
| Nakagawa | 2013 | Japan | BC | 114 | 92 | 95 | OS | 0.5 |
| Beuselinck | 2013 | Belgium | RCC | 200 | 142 | 200 | OS | 0.5 |
| Kwon | 2013 | Korea | RCC | 106 | 78 | 106 | OS | 0.5 |
| Stein | 2013 | Germany | UUT-UC | 115 | 83 | 26 | CSS | 0.5 |
| Kumano | 2013 | Japan | RCC | 83 | 61 | 83 | OS | 0.8 |
| Yasuda | 2013 | Japan | RCC | 52 | 42 | 52 | OS | 0.8 |
| Naito | 2013 | Japan | RCC | 556 | 411 | 556 | OS | 1 |
| Herrel | 2013 | America | RCC | 284 | 176 | 0 | CSS | 2 |
| Sakai | 2013 | Japan | RCC | 164 | 130 | 164 | OS | 4 |
| Eggers | 2013 | Germany | BC | 34 | 28 | 34 | OS | 0.8 |
| Steffens | 2012 | Germany | RCC | 1,161 | 761 | 226 | CSS | 0.4 |
| Kinoshita | 2012 | Japan | RCC | 559 | NR | 559 | CSS | 1 |
| Ito | 2012 | Japan | RCC | 181 | 133 | 181 | OS | 1.8 |
| Komura | 2011 | Japan | RCC | 170 | 114 | 0 | CSS,OS | 0.3 |
| Kume | 2011 | Japan | RCC | 94 | NR | 94 | OS | 0.3 |
| Ito | 2011 | Japan | PC | 80 | 80 | 80 | OS | 0.5 |
| Hotta | 2011 | Japan | RCC | 105 | 76 | 18 | OS | 0.5 |
| Morizane | 2011 | Japan | UUT-UC | 30 | 23 | 30 | CSS | 1 |
| Tomioka | 2010 | Japan | PC | 287 | 287 | 265 | OS | 0.3 |
| Naya | 2010 | Japan | RCC | 117 | NR | 0 | CSS | 1 |
| McArdle | 2010 | UK | PC | 98 | 98 | 0 | CSS,OS | 1 |
| Iimura | 2009 | Japan | RCC | 249 | 170 | 27 | CSS | 0.5 |
| Miyake | 2009 | Japan | RCC | 52 | 35 | 52 | CSS | 0.5 |
| Saito | 2009 | Japan | RCC | 108 | 80 | 108 | OS | 0.5 |
| Nakashima | 2008 | Japan | PC | 126 | 126 | 126 | DSS | 0.15 |
| Kawata | 2008 | Japan | RCC | 252 | 196 | NA | CSS | 0.3 |
| Yoshida | 2008 | Japan | BC | 88 | 63 | NA | CSS | 0.5 |
| Beer | 2008 | Canada | PC | 160 | 160 | 160 | OS | 0.8 |
| Ramsey | 2008 | UK | RCC | 83 | 55 | NA | CSS | 1 |
| Komai | 2007 | Japan | RCC | 101 | 63 | 0 | DSS | 0.5 |
| Saito | 2007 | Japan | UUT-UC | 130 | 88 | NA | DSS | 0.5 |
| Vogl | 2006 | Japan | RCC | 99 | 74 | 99 | OS | 0.8 |
| Hilmy | 2006 | UK | BC | 103 | 70 | 0 | CSS | 1 |
| McArdle | 2006 | UK | PC | 62 | 62 | 62 | CSS | 1 |
| Ito | 2006 | Japan | RCC | 178 | 38 | 0 | CSS | 1 |
| Miyata | 2001 | Japan | RCC | 92 | 71 | 19 | CSS | 0.5 |
| Ljungberg | 1997 | Sweden | RCC | 196 | 119 | 66 | OS | 1 |
N, number of patients. Metastatic, number of patients with metastatic cancer. Cut-off: cut-off value of c-reactive protein (CRP) applied in each study. BC, bladder cancer. RCC, renal cell carcinoma. UUT-UC, upper urinary tract - urothelial carcinoma. DSS, disease specific survival. CSS, cancer specific survival. OS, overall survival. NA, not available.
Outcome
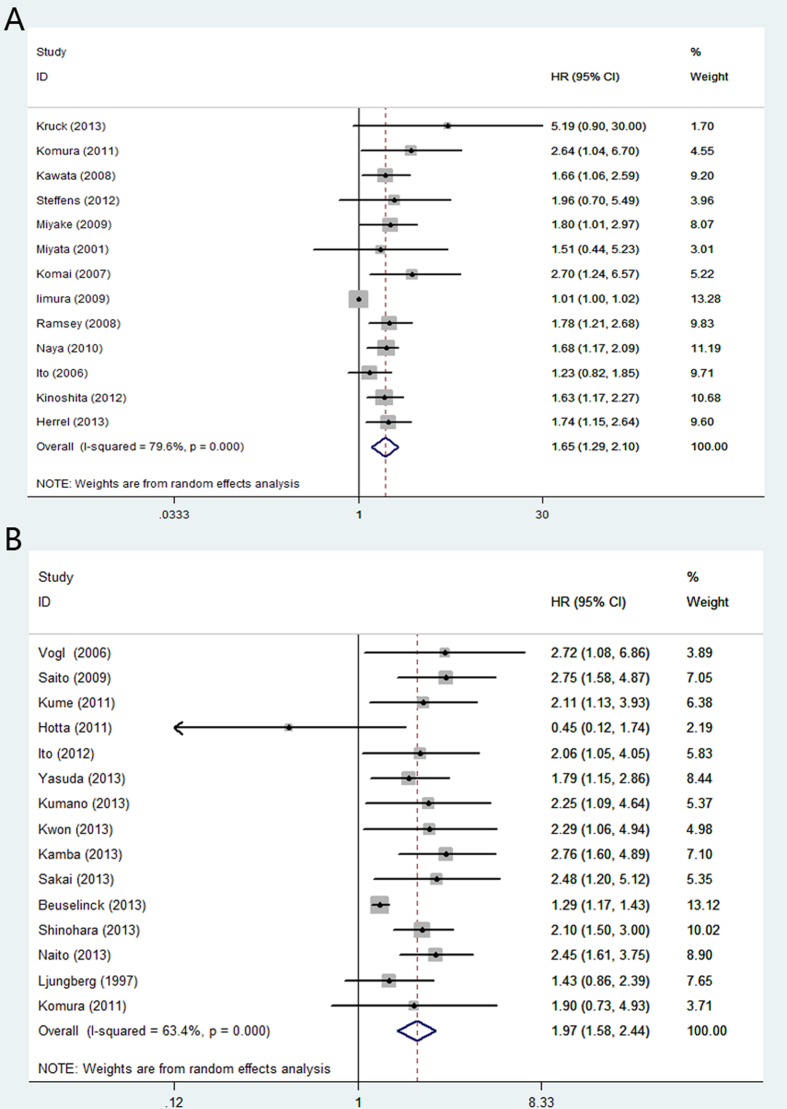
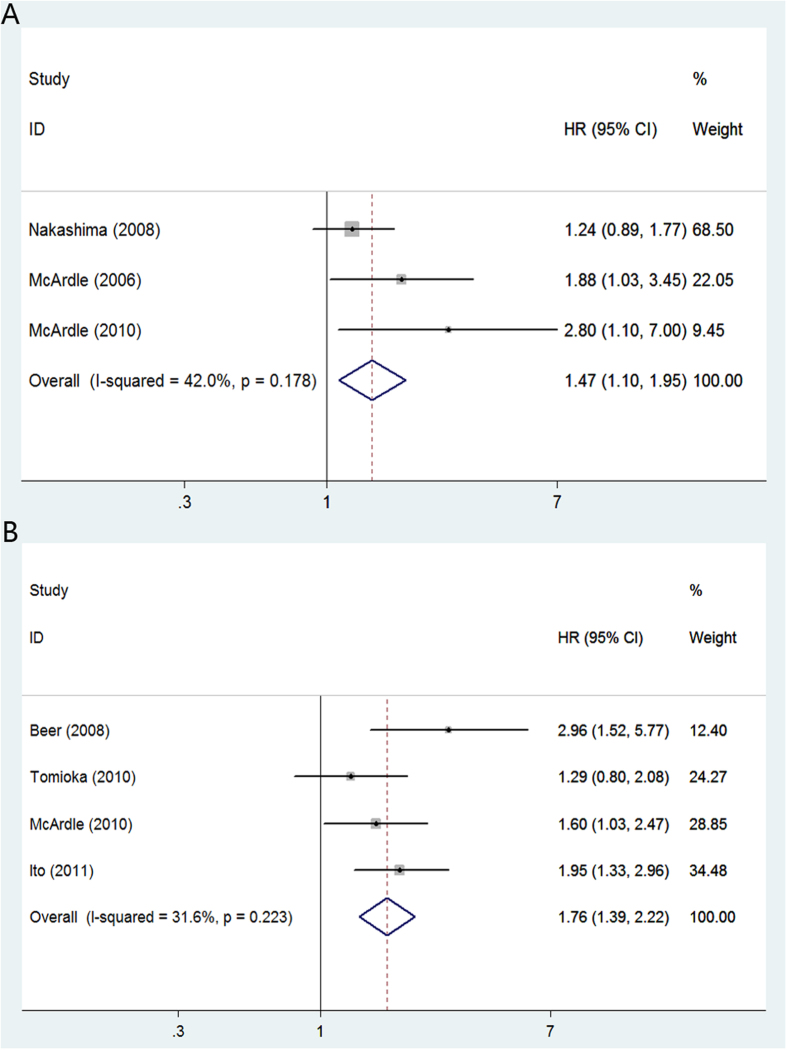
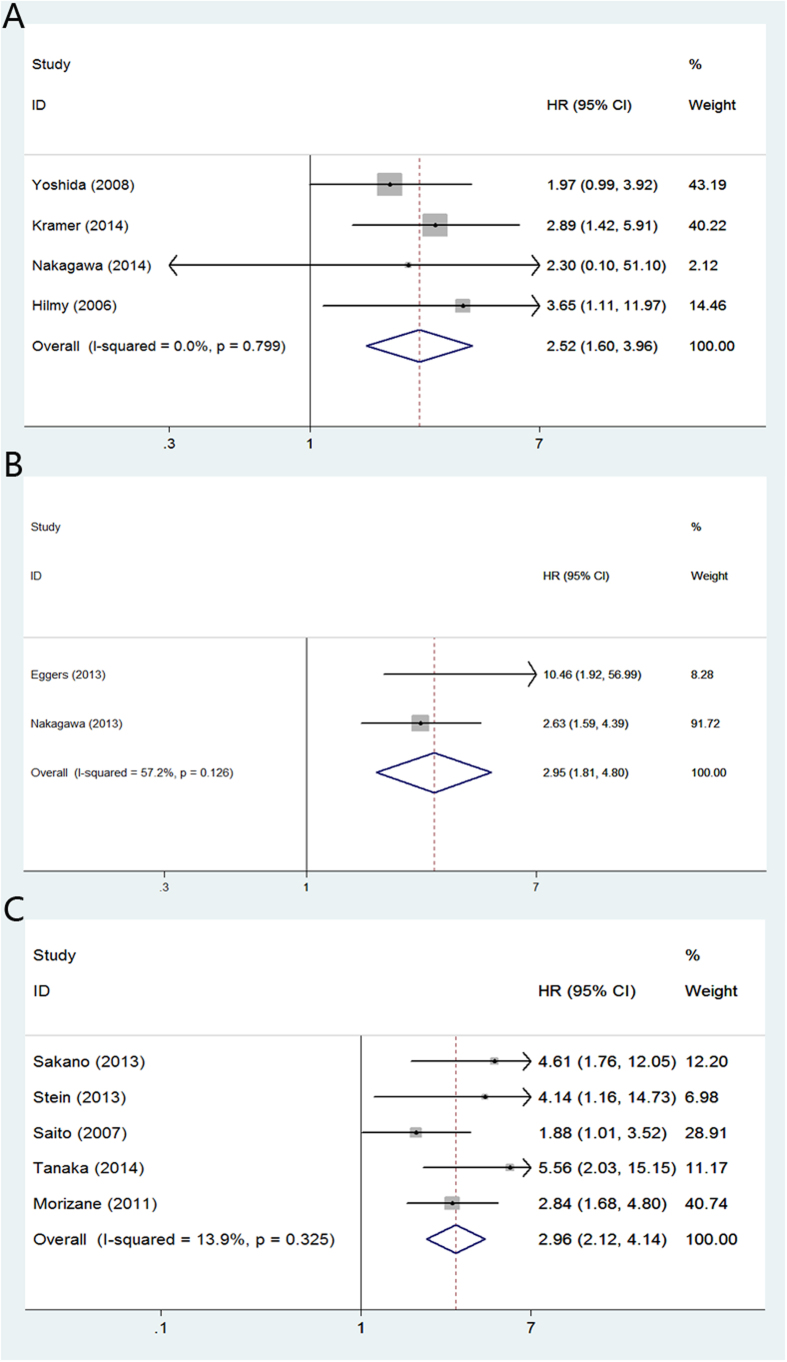
In renal cell carcinoma, high CRP yielded a worse CSS (random-effect model; [HR] = 1.65, 95% [CI] = 1.29–2.10; p < 0.01) (Fig. 2A) and OS (random-effects model; [HR] = 1.97, 95% [CI] = 1.58–2.44; p < 0.01) (Fig. 2B). The multivariable HRs (95% CI) of prostate cancer for CSS and OS were 1.91 (fixed-effect model; [HR] = 1.47; 95% CI = 1.10–1.95; p < 0.01) (Fig. 3A) and 1.77 (fixed-effects model; [HR] = 1.76, 95%CI = 1.39–2.22; p < 0.01) (Fig. 3B), respectively. For bladder cancer, the multivariable HRs (95% CI) for CSS and OS were 2.52 (fixed-effects model; [HR] = 2.52, 95% CI = 1.60–3.96; p < 0.01) (Fig. 4A) and 2.95 (fixed-effects model; [HR] = 2.95, 95% CI = 1.81–4.80; p = 0.03) (Fig. 4B). Five studies reported the prognostic role of CRP in upper urinary tract urothelial carcinoma and the multivariable HR for CSS was 2.96 (fixed-effects model; [HR] = 2.96, 95% CI = 2.12–4.14; p < 0.01) (Fig. 4C).
Figure 2. Meta-analysis of the association of CRP and clinical outcomes of patients with renal cell carcinoma.
(A): CRP and CSS; (B): CRP and OS.
Figure 3. Meta-analysis of the association of CRP and clinical outcomes of patients with prostate cancer.
(A): CRP and CSS; (B): CRP and OS.
Figure 4. Meta-analysis of the association of CRP and clinical outcomes of patients with urothelial carcinoma or bladder cancer.
(A): CRP and CSS in patients with bladder cancer; (B) CRP and OS in patients with bladder cancer; (C) CRP and CSS in patients with upper urinary tract urothelial carcinoma.
In order to explore whether CRP predicts the outcome of specific patients, we extracted the data on treatment and tumor stage. Among the 44 studies, only five studies including 763 localized RCC treated with nephrectomy evaluated the association between pretreatment CRP and CSS22,24,30,38,58. The multivariable HR (95% CI) for CSS was 3.66 (fixed-effect model; [HR] = 3.66, 95% CI = 2.20–6.09; p < 0.01) (Supplementary Figure S1A online). Another five studies included 585 cases and summarized that elevated pretreatment CRP was a poor predictor for OS in patients with metastatic RCC treated with molecular-targeted therapy37,39,41,42,46 (random-effects model; [HR] = 1.86, 95% CI = 1.30–2.66; p < 0.01) (Supplementary Figure S1B online). In addition, CRP also predicted a poor survival in metastatic prostate cancer no matter treated with chemotherapy15,19 (OS: fixed-effect model; [HR] = 2.18, 95% CI = 1.55–3.07; p < 0.01) (Supplementary Figure S1C online)or endocrine therapy14,16 (CSS: fixed-effect model; [HR] = 1.92, 95% CI = 1.22–3.03; p < 0.01) (Supplementary Figure S1D online). We identified two studies that reported the effect of CRP on CSS in patients with localized upper urinary tract urothelial carcinoma (UUT-UC) treated with nephroureterectomy and their combined HR was 1.46 (fixed-effect model; [HR] = 1.45, 95% CI = 1.12–1.88; p < 0.01) (Supplementary Figure S1E online)48,53.
Subgroup analysis
As Table 2 showed, the meta-analysis of all studies suggested a significant association between baseline serum CRP and CSS (random-effects model; [HR] = 1.97, 95% [CI] = 1.59–2.45; p < 0.01). For overall survival, the multivariable HR (95% CI) of all studies was 1.99 [random-effects model; [HR] = 1.99, 95% [CI] = 1.66–2.38; p < 0.01).
Table 2. Main results of subgroup analyses.
| Outcome | Variable | Number of studies | Model | HR(95%,CI) | I2 | Pheterogeneity |
|---|---|---|---|---|---|---|
| CSS | All | 25 | Random | 1.97(1.59,2.45) | 81.5% | <0.01 |
| Race | ||||||
| Caucasian | 9 | Random | 1.92(1.34,2.75) | 82.7% | <0.01 | |
| Asian | 16 | Random | 1.87(1.55,2.26) | 33.7% | <0.01 | |
| Cut-off | ||||||
| ≤0.5 | 16 | Random | 1.65(1.33,2.04) | 78.2% | <0.01 | |
| >0.5 | 9 | Fixed | 2.80(2.14,3.66) | 0.0% | 0.65 | |
| OS | All | 21 | Random | 1.99(1.66,2.38) | 62.6% | <0.01 |
| Race | ||||||
| Caucasian | 5 | Random | 1.74(1.20,2.51) | 67.5% | 0.02 | |
| Asian | 16 | Fixed | 2.10(1.83,2.41) | 0.0% | 0.57 | |
| Cut-off | ||||||
| ≤0.5 | 11 | Random | 1.88(1.47,2.42) | 69.9% | <0.01 | |
| >0.5 | 10 | Fixed | 2.06(1.70,2.49) | 5.7% | 0.39 |
Random: random-effects model. Fixed: fixed-effects model. Pheterogeneity, P value for Cochran’s Q test of heterogeneity.
Subgroup analyses by race showed that high CRP predicted a worse CSS (Caucasian: random-effects model; [HR] = 1.92, 95% CI = 1.34–2.75; p < 0.01; Asian: random -effects model; [HR] = 1.87, 95% CI = 1.55–2.26; p < 0.01). Similar role was shown for OS (Caucasian: random-effects model; [HR] = 1.74, 95% CI = 1.20–2.51; p < 0.01; Asian: fixed-effect model; [HR] = 2.10, 95% CI = 1.83–2.41; p < 0.01).
Studies were divided into two groups according to cutoff value. High CRP was shown to be a worse prognostic marker for CSS (cutoff ≤ 0.5: random-effects model; [HR] = 1.65, 95% CI = 1.33–2.04; p < 0.01; cutoff > 0.5: fixed-effect model; [HR] = 2.80, 95% CI = 2.14–3.66; p < 0.01) and OS (cutoff ≤ 0.5: random-effects model; [HR] = 1.88, 95% CI = 1.47–2.42; p < 0.01; cutoff > 0.5: fixed-effect model; [HR] = 2.06, 95% CI = 1.70–2.49; p < 0.01).
Meta-regression analysis
We performed a meta-regression analysis to see the association between different cut-off values and HR (Supplementary Figure S2 online). For CSS, the results indicated that the association is greater when the cut-off value was higher (coefficient 0.68; p = 0.01). But no similar trend was observed in OS (coefficient 0.07; p = 0.56), which confirmed the results of subgroup analyses. To investigate the source of heterogeneity among studies, we conducted univariate meta-regression analyses by using variables as year of publication, race, tumor type, cutoff value and sample size suggested that the major sources of significant heterogeneity in studies on CSS were year of publication (coefficient = 0.07, p = 0.05, adjusted R2 = 0.13), tumor type (coefficient = 0.34, p = 0.02, adjusted R2 = 0.27) and cutoff value (coefficient = 0.68, p = 0.01, adjusted R2 = 0.33). For OS, only race (coefficient = −0.31, p = 0.06, adjusted R2 = 0.65) contributed to the heterogeneity. Then, a multiple meta-regression was carried out by using the five variables and we found these variables together could only explain heterogeneity in part (CSS: adjusted R2 = 42.4%; OS: adjusted R2 = 4.74%).
Publication bias
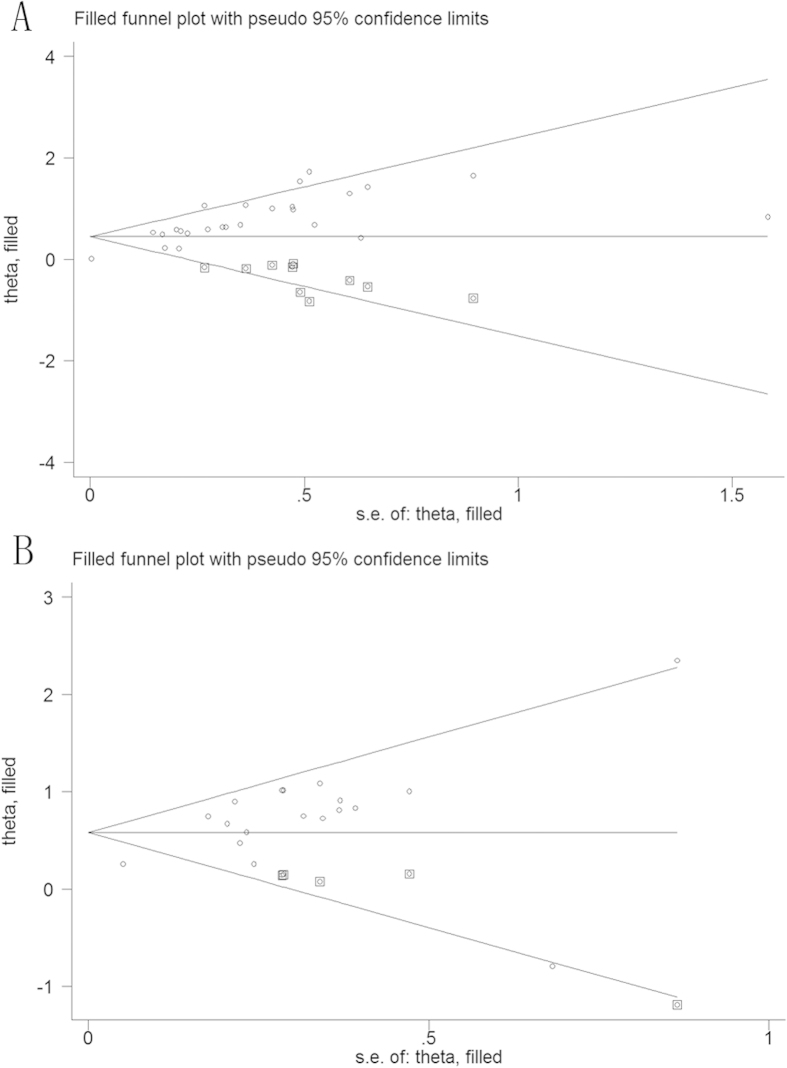
Publication bias was evaluated using Begg’s funnel plot and the Egger’s linear regression test. However, significant publication bias was found for OS (p = 0.36 for Begg’s test and P < 0.01 for Egger’s test) and CSS (p = 0.09 for Begg’s test and p < 0.01 for Egger’s test). Then, we conducted funnel plots adjusted with trim and fill method. As shown in Fig. 5, ten theoretical studies were added in analysis of CSS and four in OS. The recalculated results did not change significantly for CSS (random-effects model; [HR] = 1.41, 95% CI = 1.26–1.58; p < 0.01) (Fig. 5A) and OS (random-effects model; [HR] = 1.49, 95% CI = 1.34–1.66; p < 0.01) (Fig. 5B), indicating the stability of the results.
Figure 5.
Funnel plot adjusted with trim and fill method for cancer specific survival (A) and overall survival (B). Circles: included studies. Diamonds: presumed missing studies.
Discussion
To our knowledge, this is the most comprehensive meta-analysis studying the prognostic impact of CRP on urological cancers. Meanwhile, it is the first meta-analysis to evaluate the prognostic role of CRP in urothelial carcinoma/bladder cancer. Our results showed that elevated CRP was an independent prognostic marker in urological cancers, regardless of the tumor type, ethnicity background and cutoff value in the studies. Different pathological stages and treatment modalities could be correlated with the prognosis of cancers. However, few studies included in our meta-analysis analyzed these variables. In our meta-analysis, we evaluated the association between CRP and survival outcome of patients with different tumor stages or treated with different therapies. As we expected, the prognostic role of CRP was also confirmed in localized RCC treated with surgery, metastatic RCC treated with targeted therapy. Interestingly, the cutoff value of CRP should be carefully selected based on our results of meta-regression analysis. For CSS, the higher the cutoff value of CRP was, the higher HR was. However, there was no similar association between cutoff and overall survival in urological cancers.
The acute-phase reactant C-reactive protein is a member of the pentaxin protein family, mainly synthesized by hepatocytes, secreted into the circulation and mostly influenced by pro-inflammatory cytokines, particularly interleukin 659. Apart from the liver, other tissues were able to synthesize the CRP. CRP is one of the major acute-phase proteins and is considered as a definitive marker of systemic inflammation. In clinical practice, it is commonly used to evaluate the severity of the systemic inflammation or outcomes of a variety of inflammation-related disorders9. In normal population, 70%–90% of samples have a CRP concentration of less than 0.3 mg/dl60, while serum CRP level of cancer patients is significantly higher61. A possible explanation is that inflammatory cytokines secreted by tumor cells could strongly stimulate the CRP production in liver62,63. Additionally, some tumor cells could also secret CRP and that may contribute to the serum CRP level64. Therefore, serum CRP level of cancer patients could be an indirect indicator of cancer related inflammation.
Previous studies reported that elevated CRP was associated with poor prognosis in several kinds of malignancies6,8. Since the links between cancer and inflammation were reported and accepted, clinical value of CRP in various malignancies was noticed by more and more researchers. However, the mechanism is not yet fully understood. As mentioned above, elevated CRP in serum represents a systemic inflammatory response, which may indicate much inflammatory mediators from cancer tissue. Meanwhile, inflammatory microenvironment in tumor could activate some specific signaling pathways critical for proliferation, angiogenesis and metastasis of cancer65. Therefore, a systematic inflammatory response defined by elevated serum CRP could be associated with prognosis of urological cancers.
Here, we wish to emphasize several limitations of our study. First, even though the amount of included studies is large, the heterogeneity is relatively high and could not be eliminated completely. The five variables included in our meta-regression analysis partly explained heterogeneity. Other factors, such as age and histological grade, is likely to affect the prognosis. Second, some studies of urothelial carcinoma contained both bladder cancer and urothelial carcinoma of upper urinary tract so that we have to combine the bladder cancer and urothelial carcinoma into one group. Third, owing to lack of data, the association between CRP and other clinical parameters such as pathological stage and prostate-specific antigen (PSA) was not explored. Above all, additional well-designed studies are necessary to present more reliable results in urological cancers.
Conclusions
In conclusion, current evidence from the meta-analysis of published studies identified elevated baseline serum CRP as a strong prognostic biomarker in urological cancers. However, limitations listed above should be noted and more large-scale and standard investigations should be carried out.
Methods
Inclusion/exclusion criteria
Clinical studies were considered eligible if they investigated the association between pretreatment CRP in serum and survival outcome (OS, DSS or CSS) in patients who were diagnosed as urological cancer pathologically. No language or publication status restrictions were imposed. Studies were excluded based on any of the following criteria: (i) studies were review articles, laboratory articles or letters; (ii) HR was calculated with univariate rather than multivariable logistic regression analyses; (iii) papers lacked key information for calculation; (iv) a definite cutoff value of CRP wasn’t given; (v) if two studies were published by the same group with overlapping patient populations, the most recent one was selected.
Literature search
Studies were identified by searching electronic databases. A systematic literature search was carried out using PubMed and Embase databases. The literature search was undertaken in May 2014, and updated in October 2014. No language restriction was applied. We used the following search terms to search related studies: CRP; C-reactive protein; C reactive protein; renal cell carcinoma; bladder cancer; prostate cancer; urothelial cancer; transitional cell carcinoma. Search strategies are as following: Pubmed: C-reactive protein [mesh] AND (urinary bladder neoplasms [mesh] OR prostatic neoplasms [mesh] OR kidney neoplasms [mesh] OR carcinoma, transitional Cell [mesh]); EMBASE: C-reactive protein AND (bladder cancer OR prostate cancer OR renal cell carcinoma OR urothelial cancer OR urothelial carcinoma OR transitional cell carcinoma).
Study selection and quality assessment
After primary search, duplications are removed by screening the titles. Then, the titles and abstracts are reviewed for further evaluation. Finally, we will read the full-text for eligibility. If eligible, the study is included for the meta-analysis. Eligibility assessment was performed independently by two authors (L.Z. and X.C.). Disagreements were resolved by consensus. According to the review checklist of the Dutch Cochrane Centre proposed by Meta-analysis of Observational Studies in Epidemiology (MOOSE) group, we assessed the quality of all studies with the same method in Wu’s work12,66. The quality criteria include (1) clear definition of study population, (2) clear definition of study design, (3) enough sample size more than 30, (4) clear definition of outcome assessment, OS or CSS, (5) clear definition of cut-off of CRP level and (6) sufficient period of follow-up.
Data extraction
Primary information, including hazard ratio (HR) and 95% confidence interval (CI) and p value, was extracted directly from articles by two investigators independently. Additional data were extracted from the studies, including the first author, year of publication, sample size, race, cutoff value and other clinical characteristics.
Statistical analysis
The primary outcome measure was the hazard ratios of CRP predicting CSS, DSS or OS, which were obtained from each study. We combined CSS and DSS in an analysis due to similar definitions. Meta-analysis was performed to calculate the estimated hazard ratio and its variance. Results were shown in forest plot graphs. HR greater than 1 and 95% CI for the aggregated HR not crossing 1 indicates a prognostic role of elevated CRP. P < 0.05 was considered statistically significant and all P values were two-sided. Heterogeneity was defined as p < 0.10 or I2 > 50%. Random effects analysis of the Mantel Haenszel Model was utilized when statistical heterogeneity was suggested (p < 0.10 or I2 > 50%). If not, a fixed effect model was used. If statistical heterogeneity was found (p < 0.10 or I2 > 50%), subgroup analysis and meta-regression were conducted to explore the potential source of heterogeneity among studies. In our study, subgroup analyses and meta-regression were conducted for tumor, race and cutoff value. Egger’s test and Begg’s test were performed to test for publication bias. Statistical analyses were done with Stata version 12.0 (STATA Corporation, College Station, TX, USA).
Additional Information
How to cite this article: Zhou, L. et al. Prognostic Role of C-Reactive Protein In Urological Cancers: A Meta-Analysis. Sci. Rep. 5, 12733; doi: 10.1038/srep12733 (2015).
Supplementary Material
Acknowledgments
This work was supported by the Ph.D. Programs Foundation of Ministry of Education of China (Priority Area) (Grant No. 20110181130003), and the Science and Technology Bureau of Chengdu City (Grant No. 12PPYB030SF-002).
Footnotes
Author Contributions L.Z. conceived of the study, participated in the data collection and wrote the main manuscript. X.C. performed the statistical analysis and assisted in the data collection. Q.L. and Z.Y.J. prepared figures 1, 2, 3 and table1–3. H.L. and K.J.W. participated in its design and helped to draft the manuscript. All authors reviewed and approved the final manuscript.
References
- Ferlay J. et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International journal of cancer. Int J Cancer 127, 2893–2917 (2010). [DOI] [PubMed] [Google Scholar]
- Soerjomataram I. et al. Global burden of cancer in 2008: a systematic analysis of disability-adjusted life-years in 12 world regions. Lancet 380, 1840–1850 (2012). [DOI] [PubMed] [Google Scholar]
- Bex A. et al. Integrating Surgery with Targeted Therapies for Renal Cell Carcinoma: Current Evidence and Ongoing Trials. Eur Urol 58, 819–828 (2010). [DOI] [PubMed] [Google Scholar]
- Cancer Research UK. Cancer Statistics Report: Cancer Incidence and Mortality in the UK. (2014) Available at: www.cancerresearchuk.org. (Accessed: 4th October 2014) [Google Scholar]
- Eisenberg M. S. et al. The SPARC score: a multifactorial outcome prediction model for patients undergoing radical cystectomy for bladder cancer. J Urol 190, 2005–2010 (2013). [DOI] [PubMed] [Google Scholar]
- Mantovani A., Allavena P., Sica A. & Balkwill F. Cancer-related inflammation. Nature 454, 436–444 (2008). [DOI] [PubMed] [Google Scholar]
- Allavena P., Sica A., Solinas G., Porta C. & Mantovani A. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol 66, 1–9 (2008). [DOI] [PubMed] [Google Scholar]
- Diakos C. I., Charles K. A., McMillan D. C. & Clarke S. J. Cancer-related inflammation and treatment effectiveness. Lancet Oncol 15, e493–503 (2014). [DOI] [PubMed] [Google Scholar]
- van Leeuwen M. A. et al. Interrelationship of outcome measures and process variables in early rheumatoid arthritis. A comparison of radiologic damage, physical disability, joint counts, and acute phase reactants. J Rheumatol 21, 425–429 (1994). [PubMed] [Google Scholar]
- Saito K. & Kihara K. Role of C-reactive protein in urological cancers: a useful biomarker for predicting outcomes. Int J Urol 20, 161–171 (2013). [DOI] [PubMed] [Google Scholar]
- Hu Q. et al. The prognostic value of C-reactive protein in renal cell carcinoma: a systematic review and meta-analysis. Urol Oncol 32, e51–58 (2014). [DOI] [PubMed] [Google Scholar]
- Wu Y. et al. Prognostic role of systemic inflammatory response in renal cell carcinoma: a systematic review and meta-analysis. J Cancer Res Clin Oncol 137, 887–896 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha P. et al. Prognostic impact of C-reactive protein (CRP) in metastatic prostate cancer (MPC): A systematic review and meta-analysis. Oncol Res Treat 37, 772–776 (2014). [DOI] [PubMed] [Google Scholar]
- McArdle P. A. et al. Systemic inflammatory response, prostate-specific antigen and survival in patients with metastatic prostate cancer. Urol Int 77, 127–129 (2006). [DOI] [PubMed] [Google Scholar]
- Beer T. M. et al. C-reactive protein as a prognostic marker for men with androgen-independent prostate cancer: Eesults from the ASCENT trial. Cancer 112, 2377–2383 (2008). [DOI] [PubMed] [Google Scholar]
- Nakashima J. et al. Simple stratification of survival using bone scan and serum C-reactive protein in prostate cancer patients with metastases. Urol Int 80, 129–133 (2008). [DOI] [PubMed] [Google Scholar]
- McArdle P. A., Qayyum T. & McMillan D. C. Systemic inflammatory response and survival in patients with localised prostate cancer: 10-year follow-up. Urol Int 84, 430–435 (2010). [DOI] [PubMed] [Google Scholar]
- Tomioka A. et al. Nadir PSA and time to PSA progression predict overall survival in patients with metastatic prostate cancer. Eur Urol Suppl 9, 77 (2010). [Google Scholar]
- Ito M. et al. Prognostic impact of C-reactive protein for determining overall survival of patients with castration-resistant prostate cancer treated with docetaxel. Urology 78, 1131–1135 (2011). [DOI] [PubMed] [Google Scholar]
- Ljungberg B., Grankvist K. & Rasmuson T. Serum Interleukin-6 in Relation to Acute-phase Reactants and Survival in Patients with Renal Cell Carcinoma. Eur J Cancer 33, 1794–1798 (1997). [DOI] [PubMed] [Google Scholar]
- Miyata Y. et al. Predictive values of acute phase reactants, basic fetoprotein, and immunosuppressive acidic protein for staging and survival in renal cell carcinoma. Urology 58, 161–164 (2001). [DOI] [PubMed] [Google Scholar]
- Ito K. et al. Impact of thrombocytosis and C-reactive protein elevation on the prognosis for patients with renal cell carcinoma. Int J Urol 13, 1365–1370 (2006). [DOI] [PubMed] [Google Scholar]
- Vogl U. M. et al. Prognostic factors in metastatic renal cell carcinoma: metastasectomy as independent prognostic variable. Brit J Cancer 95, 691–698 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komai Y., Saito K., Sakai K. & Morimoto S. Increased preoperative serum C-reactive protein level predicts a poor prognosis in patients with localized renal cell carcinoma. BJU Int 99, 77–80 (2007). [DOI] [PubMed] [Google Scholar]
- Kawata N. et al. How do symptoms have an impact on the prognosis of renal cell carcinoma? Int J Urol 15, 299–303 (2008). [DOI] [PubMed] [Google Scholar]
- Ramsey S., Lamb G. W., Aitchison M. & McMillan D. C. Prospective study of the relationship between the systemic inflammatory response, prognostic scoring systems and relapse-free and cancer-specific survival in patients undergoing potentially curative resection for renal cancer. BJU Int 101, 959–963 (2008). [DOI] [PubMed] [Google Scholar]
- Iimura Y. et al. Development and external validation of a new outcome prediction model for patients with clear cell renal cell carcinoma treated with nephrectomy based on preoperative serum C-reactive protein and TNM classification: the TNM-C score. J Urol 181, 1004–1012 (2009). [DOI] [PubMed] [Google Scholar]
- Miyake H., Kurahashi T., Takenaka A., Inoue T. A. & Fujisawa M. Clinical outcome of combined immunotherapy with interferon-alpha and low-dose interleukine-2 for Japanese patients with metastatic renal cell carcinoma. Urol Oncol 27, 598–603 (2009). [DOI] [PubMed] [Google Scholar]
- Saito K. et al. Impact of C-reactive protein kinetics on survival of patients with metastatic renal cell carcinoma. Eur Urol 55, 1145–1153 (2009). [DOI] [PubMed] [Google Scholar]
- Naya Y. et al. Influence of visceral obesity on oncologic outcome in patients with renal cell carcinoma. Urol Int 85, 30–36 (2010). [DOI] [PubMed] [Google Scholar]
- Hotta K. et al. Prognostic significance of CD45RO+ memory T cells in renal cell carcinoma. Brit J Cancer 105, 1191–1196 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komura K. et al. Prognostic significance of body mass index in Asian patients with localized renal cell carcinoma. Nutr Cancer 63, 908–915 (2011). [DOI] [PubMed] [Google Scholar]
- Kume H. et al. Prognostic factors for renal cell carcinoma with bone metastasis: who are the long-term survivors? J Urol 185, 1611–1614 (2011). [DOI] [PubMed] [Google Scholar]
- Ito H. et al. C-reactive protein in patients with advanced metastatic renal cell carcinoma: usefulness in identifying patients most likely to benefit from initial nephrectomy. BMC cancer 12, 337 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita H. et al. Prognostic factors of metastatic renal cell carcinoma patients with removed metastases: A multicenter study of 559 patients. J Urol 1), S726 (2012). [DOI] [PubMed] [Google Scholar]
- Steffens S. et al. Validation of CRP as prognostic marker for renal cell carcinoma in a large series of patients. BMC cancer 12, 399 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuselinck B. et al. Prognostic impact of baseline serum C-reactive protein in patients with metastatic renal cell carcinoma (RCC) treated with sunitinib. BJU Int 114, 81–89 (2014). [DOI] [PubMed] [Google Scholar]
- Herrel L. A. et al. Preoperative C-reactive protein combined with SSIGN score as an independent predictor of cancer-specific survival in patients with clear cell renal cell carcinoma. J Clin Oncol 1), S451 (2013). [Google Scholar]
- Kamba T. et al. Improvement of prognosis in patients with metastatic renal cell carcinoma and Memorial Sloan-Kettering Cancer Center intermediate risk features by modern strategy including molecular-targeted therapy in clinical practice. Int J Clin Oncol 19, 505–515 (2014). [DOI] [PubMed] [Google Scholar]
- Kruck S. et al. Use of preoperative C-reactive protein to improve predictive accuracy in clear cell renal cell carcinoma. J Clin Oncol 1), S457 (2013). [Google Scholar]
- Kumano M., Miyake H., Harada K. & Fujisawa M. Sequential use of mammalian target of rapamycin inhibitors in patients with metastatic renal cell carcinoma following failure of tyrosine kinase inhibitors. Med Oncol 30, 745–753 (2013). [DOI] [PubMed] [Google Scholar]
- Kwon W. A. et al. Validation of the mskcc and heng risk criteria models for predicting survival in patients with metastatic renal cell carcinoma treated with sunitinib. Ann Surg Oncol 20, 4397–4404 (2013). [DOI] [PubMed] [Google Scholar]
- Naito S. et al. Prognostic factors of patients with metastatic renal cell carcinoma with removed metastases: a multicenter study of 556 patients. Urology 82, 846–851 (2013). [DOI] [PubMed] [Google Scholar]
- Sakai I., Miyake H., Hinata N. & Fujisawa M. Improved survival in patients with metastatic renal cell carcinoma undergoing cytoreductive nephrectomy in the era of targeted therapy. Int J Clin Oncol 19, 674–678 (2014). [DOI] [PubMed] [Google Scholar]
- Shinohara N. et al. Is Memorial Sloan-Kettering Cancer Center risk classification appropriate for Japanese patients with metastatic renal cell carcinoma in the cytokine era? Urol Oncol 31, 1276–1282 (2013). [DOI] [PubMed] [Google Scholar]
- Yasuda Y. et al. Prognostic impact of pretreatment C-reactive protein for patients with metastatic renal cell carcinoma treated with tyrosine kinase inhibitors. Int J Clin Oncol 18, 884–889 (2013). [DOI] [PubMed] [Google Scholar]
- Hilmy M. et al. The relationship between the systemic inflammatory response, tumour proliferative activity, T-lymphocytic infiltration and COX-2 expression and survival in patients with transitional cell carcinoma of the urinary bladder. Brit J Cancer 95, 1234–1238 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K. et al. The impact of preoperative serum C-reactive protein on the prognosis of patients with upper urinary tract urothelial carcinoma treated surgically. BJU Int 100, 269–273 (2007). [DOI] [PubMed] [Google Scholar]
- Yoshida S. et al. C-reactive protein level predicts prognosis in patients with muscle-invasive bladder cancer treated with chemoradiotherapy. BJU Int 101, 978–981 (2008). [DOI] [PubMed] [Google Scholar]
- Morizane S. et al. Significance of hemoglobin value before gemcitabine/cisplatin or carboplatin treatment as an independent prognostic biomarker in advanced urothelial cancer. Urology 1), S394 (2011). [Google Scholar]
- Eggers H. et al. Serum C-reactive protein: A prognostic factor in metastatic urothelial cancer of the bladder. Med Oncol 30, 705 (2013). [DOI] [PubMed] [Google Scholar]
- Nakagawa T. et al. Prognostic risk stratification of patients with urothelial carcinoma of the bladder with recurrence after radical cystectomy. J Urol 189, 1275–1281 (2013). [DOI] [PubMed] [Google Scholar]
- Sakano S. et al. Risk group stratification based on preoperative factors to predict survival after nephroureterectomy in patients with upper urinary tract urothelial carcinoma. Ann Surg Oncol 20, 4389–4396 (2013). [DOI] [PubMed] [Google Scholar]
- Stein B. et al. Preoperative serum C- reactive protein: a prognostic marker in patients with upper urinary tract urothelial carcinoma. BMC cancer 13, 101 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer M. W. et al. [C-reactive protein prior to radical cystectomy: preoperative determination of CRP]. Der Urologe. Ausg. A 53, 222–227 (2014). [DOI] [PubMed] [Google Scholar]
- Nakagawa T. et al. Cancer-specific survival in patients with urothelial carcinoma of the bladder with recurrence after radical cystectomy: External validation of the prognostic risk stratification model. J Clin Oncol 1), S325 (2014). [Google Scholar]
- Tanaka N. et al. The predictive value of C-reactive protein for prognosis in patients with upper tract urothelial carcinoma treated with radical nephroureterectomy: a multi-institutional study. Eur Urol 65, 227–234 (2014). [DOI] [PubMed] [Google Scholar]
- Ramsey S., Lamb G. W., Aitchison M., Graham J. & McMillan D. C. Evaluation of an inflammation-based prognostic score in patients with metastatic renal cancer. Cancer 109, 205–212 (2007). [DOI] [PubMed] [Google Scholar]
- Guthrie G. J., Roxburgh C. S., Horgan P. G. & McMillan D. C. Does interleukin-6 link explain the link between tumour necrosis, local and systemic inflammatory responses and outcome in patients with colorectal cancer? Cancer Treat Rev 39, 89–96 (2013). [DOI] [PubMed] [Google Scholar]
- Kushner I. The phenomenon of the acute phase response. Ann N Y Acad Sci 389, 39–48 (1982). [DOI] [PubMed] [Google Scholar]
- Trichopoulos D., Psaltopoulou T., Orfanos P., Trichopoulou A. & Boffetta P. Plasma C-reactive protein and risk of cancer: a prospective study from Greece. Cancer Epidemiol Biomarkers Prev 15, 381–384 (2006). [DOI] [PubMed] [Google Scholar]
- Kinoshita T., Ito H. & Miki C. Serum interleukin-6 level reflects the tumor proliferative activity in patients with colorectal carcinoma. Cancer 85, 2526–2531 (1999). [DOI] [PubMed] [Google Scholar]
- Okamoto M., Hattori K. & Oyasu R. Interleukin-6 functions as an autocrine growth factor in human bladder carcinoma cell lines in vitro. International journal of cancer. Int J Cancer 72, 149–154 (1997). [DOI] [PubMed] [Google Scholar]
- Johnson T. V. et al. Intratumor C-reactive protein as a biomarker of prognosis in localized renal cell carcinoma. J Urol 186, 1213–1217 (2011). [DOI] [PubMed] [Google Scholar]
- Gueron G., De Siervi A. & Vazquez E. Advanced prostate cancer: reinforcing the strings between inflammation and the metastatic behavior. Prostate Cancer Prostatic Dis 15, 213–221 (2012). [DOI] [PubMed] [Google Scholar]
- Stroup D. F. et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Jama 283, 2008–2012 (2000). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.