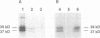Abstract
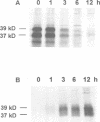
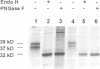
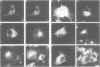
Palmitoyl protein thioesterase (PPT) is an enzyme that removes palmitate residues from various S-acylated proteins in vitro. We recently identified mutations in the human PPT gene in patients suffering from a neurodegenerative disease in childhood, infantile neuronal ceroid lipofuscinosis (INCL), with dramatic manifestations limited to the neurons of neocortical origin. Here we have expressed the human PPT cDNA in COS-1 cells and demonstrate the lysosomal targeting of the enzyme via the mannose 6-phosphate receptor-mediated pathway. The enzyme was also secreted into the growth medium and could be endocytosed by recipient cells. We further demonstrate the disturbed intracellular routing of PPT carrying the worldwide most common INCL mutation, Arg122Trp, to lysosomes. The results provide evidence that INCL represents a novel lysosomal enzyme deficiency. Further, the defect in the PPT gene causing a neurodegenerative disorder suggests that depalmitoylation of the still uncharacterized substrate(s) for PPT is critical for postnatal development or maintenance of cortical neurons.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger M., Schmidt M. F. Characterization of a protein fatty acylesterase present in microsomal membranes of diverse origin. J Biol Chem. 1986 Nov 15;261(32):14912–14918. [PubMed] [Google Scholar]
- Berthiaume L., Resh M. D. Biochemical characterization of a palmitoyl acyltransferase activity that palmitoylates myristoylated proteins. J Biol Chem. 1995 Sep 22;270(38):22399–22405. doi: 10.1074/jbc.270.38.22399. [DOI] [PubMed] [Google Scholar]
- Bizzozero O. A., Good L. K. Rapid metabolism of fatty acids covalently bound to myelin proteolipid protein. J Biol Chem. 1991 Sep 15;266(26):17092–17098. [PubMed] [Google Scholar]
- Bizzozero O. A., Leyba J., Nuñez D. J. Characterization of proteolipid protein fatty acylesterase from rat brain myelin. J Biol Chem. 1992 Apr 15;267(11):7886–7894. [PubMed] [Google Scholar]
- Camp L. A., Hofmann S. L. Purification and properties of a palmitoyl-protein thioesterase that cleaves palmitate from H-Ras. J Biol Chem. 1993 Oct 25;268(30):22566–22574. [PubMed] [Google Scholar]
- Camp L. A., Verkruyse L. A., Afendis S. J., Slaughter C. A., Hofmann S. L. Molecular cloning and expression of palmitoyl-protein thioesterase. J Biol Chem. 1994 Sep 16;269(37):23212–23219. [PubMed] [Google Scholar]
- Derewenda Z. S., Sharp A. M. News from the interface: the molecular structures of triacylglyceride lipases. Trends Biochem Sci. 1993 Jan;18(1):20–25. doi: 10.1016/0968-0004(93)90082-x. [DOI] [PubMed] [Google Scholar]
- Gutierrez L., Magee A. I. Characterization of an acyltransferase acting on p21N-ras protein in a cell-free system. Biochim Biophys Acta. 1991 Jun 24;1078(2):147–154. doi: 10.1016/0167-4838(91)99003-b. [DOI] [PubMed] [Google Scholar]
- Haltia M., Rapola J., Santavuori P., Keränen A. Infantile type of so-called neuronal ceroid-lipofuscinosis. 2. Morphological and biochemical studies. J Neurol Sci. 1973 Mar;18(3):269–285. doi: 10.1016/0022-510x(73)90076-2. [DOI] [PubMed] [Google Scholar]
- Hancock J. F., Magee A. I., Childs J. E., Marshall C. J. All ras proteins are polyisoprenylated but only some are palmitoylated. Cell. 1989 Jun 30;57(7):1167–1177. doi: 10.1016/0092-8674(89)90054-8. [DOI] [PubMed] [Google Scholar]
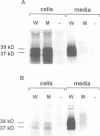
- Hasilik A., Neufeld E. F. Biosynthesis of lysosomal enzymes in fibroblasts. Synthesis as precursors of higher molecular weight. J Biol Chem. 1980 May 25;255(10):4937–4945. [PubMed] [Google Scholar]
- Hess D. T., Slater T. M., Wilson M. C., Skene J. H. The 25 kDa synaptosomal-associated protein SNAP-25 is the major methionine-rich polypeptide in rapid axonal transport and a major substrate for palmitoylation in adult CNS. J Neurosci. 1992 Dec;12(12):4634–4641. doi: 10.1523/JNEUROSCI.12-12-04634.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannou Y. A., Bishop D. F., Desnick R. J. Overexpression of human alpha-galactosidase A results in its intracellular aggregation, crystallization in lysosomes, and selective secretion. J Cell Biol. 1992 Dec;119(5):1137–1150. doi: 10.1083/jcb.119.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing S. Q., Trowbridge I. S. Identification of the intermolecular disulfide bonds of the human transferrin receptor and its lipid-attachment site. EMBO J. 1987 Feb;6(2):327–331. doi: 10.1002/j.1460-2075.1987.tb04758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ludwig T., Munier-Lehmann H., Bauer U., Hollinshead M., Ovitt C., Lobel P., Hoflack B. Differential sorting of lysosomal enzymes in mannose 6-phosphate receptor-deficient fibroblasts. EMBO J. 1994 Aug 1;13(15):3430–3437. doi: 10.1002/j.1460-2075.1994.tb06648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee A. I., Gutierrez L., McKay I. A., Marshall C. J., Hall A. Dynamic fatty acylation of p21N-ras. EMBO J. 1987 Nov;6(11):3353–3357. doi: 10.1002/j.1460-2075.1987.tb02656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omary M. B., Trowbridge I. S. Biosynthesis of the human transferrin receptor in cultured cells. J Biol Chem. 1981 Dec 25;256(24):12888–12892. [PubMed] [Google Scholar]
- Pepperkok R., Scheel J., Horstmann H., Hauri H. P., Griffiths G., Kreis T. E. Beta-COP is essential for biosynthetic membrane transport from the endoplasmic reticulum to the Golgi complex in vivo. Cell. 1993 Jul 16;74(1):71–82. doi: 10.1016/0092-8674(93)90295-2. [DOI] [PubMed] [Google Scholar]
- Proia R. L., d'Azzo A., Neufeld E. F. Association of alpha- and beta-subunits during the biosynthesis of beta-hexosaminidase in cultured human fibroblasts. J Biol Chem. 1984 Mar 10;259(5):3350–3354. [PubMed] [Google Scholar]
- Randall W. R. Cellular expression of a cloned, hydrophilic, murine acetylcholinesterase. Evidence of palmitoylated membrane-bound forms. J Biol Chem. 1994 Apr 22;269(16):12367–12374. [PubMed] [Google Scholar]
- Rapola J., Haltia M. Cytoplasmic inclusions in the vermiform appendix and skeletal muscle in two types of so-called neuronal ceroid-lipofuscinosis. Brain. 1973 Dec;96(4):833–840. doi: 10.1093/brain/96.4.833. [DOI] [PubMed] [Google Scholar]
- Robinson L. J., Busconi L., Michel T. Agonist-modulated palmitoylation of endothelial nitric oxide synthase. J Biol Chem. 1995 Jan 20;270(3):995–998. doi: 10.1074/jbc.270.3.995. [DOI] [PubMed] [Google Scholar]
- Santavuori P., Haltia M., Rapola J. Infantile type of so-called neuronal ceroid-lipofuscinosis. Dev Med Child Neurol. 1974 Oct;16(5):644–653. doi: 10.1111/j.1469-8749.1974.tb04183.x. [DOI] [PubMed] [Google Scholar]
- Schmidt M. F. Fatty acylation of proteins. Biochim Biophys Acta. 1989 Dec 6;988(3):411–426. doi: 10.1016/0304-4157(89)90013-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepp-Lorenzino L., Coleman P. S., Larocca J. N. Isoprenylated proteins in myelin. J Neurochem. 1994 Apr;62(4):1539–1545. doi: 10.1046/j.1471-4159.1994.62041539.x. [DOI] [PubMed] [Google Scholar]
- Sudo Y., Valenzuela D., Beck-Sickinger A. G., Fishman M. C., Strittmatter S. M. Palmitoylation alters protein activity: blockade of G(o) stimulation by GAP-43. EMBO J. 1992 Jun;11(6):2095–2102. doi: 10.1002/j.1460-2075.1992.tb05268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman D. J., Milman G. Short-term, high-efficiency expression of transfected DNA. Mol Cell Biol. 1984 Aug;4(8):1641–1643. doi: 10.1128/mcb.4.8.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarkkanen A., Haltai M., Merenmies L. Ocular pathology in infantile type of neuronal ceroid-lipofuscinosis. J Pediatr Ophthalmol. 1977 Jul-Aug;14(4):235–241. [PubMed] [Google Scholar]
- Tikkanen R., Enomaa N., Riikonen A., Ikonen E., Peltonen L. Intracellular sorting of aspartylglucosaminidase: the role of N-linked oligosaccharides and evidence of Man-6-P-independent lysosomal targeting. DNA Cell Biol. 1995 Apr;14(4):305–312. doi: 10.1089/dna.1995.14.305. [DOI] [PubMed] [Google Scholar]
- Tyynelä J., Palmer D. N., Baumann M., Haltia M. Storage of saposins A and D in infantile neuronal ceroid-lipofuscinosis. FEBS Lett. 1993 Sep 6;330(1):8–12. doi: 10.1016/0014-5793(93)80908-d. [DOI] [PubMed] [Google Scholar]
- Vaux D., Tooze J., Fuller S. Identification by anti-idiotype antibodies of an intracellular membrane protein that recognizes a mammalian endoplasmic reticulum retention signal. Nature. 1990 Jun 7;345(6275):495–502. doi: 10.1038/345495a0. [DOI] [PubMed] [Google Scholar]
- Veit M., Schmidt M. F. Timing of palmitoylation of influenza virus hemagglutinin. FEBS Lett. 1993 Dec 27;336(2):243–247. doi: 10.1016/0014-5793(93)80812-9. [DOI] [PubMed] [Google Scholar]
- Vesa J., Hellsten E., Verkruyse L. A., Camp L. A., Rapola J., Santavuori P., Hofmann S. L., Peltonen L. Mutations in the palmitoyl protein thioesterase gene causing infantile neuronal ceroid lipofuscinosis. Nature. 1995 Aug 17;376(6541):584–587. doi: 10.1038/376584a0. [DOI] [PubMed] [Google Scholar]
- Wedegaertner P. B., Bourne H. R. Activation and depalmitoylation of Gs alpha. Cell. 1994 Jul 1;77(7):1063–1070. doi: 10.1016/0092-8674(94)90445-6. [DOI] [PubMed] [Google Scholar]
- Wedegaertner P. B., Wilson P. T., Bourne H. R. Lipid modifications of trimeric G proteins. J Biol Chem. 1995 Jan 13;270(2):503–506. doi: 10.1074/jbc.270.2.503. [DOI] [PubMed] [Google Scholar]
- Weitz G., Proia R. L. Analysis of the glycosylation and phosphorylation of the alpha-subunit of the lysosomal enzyme, beta-hexosaminidase A, by site-directed mutagenesis. J Biol Chem. 1992 May 15;267(14):10039–10044. [PubMed] [Google Scholar]
- Witkowski A., Witkowska H. E., Smith S. Reengineering the specificity of a serine active-site enzyme. Two active-site mutations convert a hydrolase to a transferase. J Biol Chem. 1994 Jan 7;269(1):379–383. [PubMed] [Google Scholar]
- Yagel S., Warner A. H., Nellans H. N., Lala P. K., Waghorne C., Denhardt D. T. Suppression by cathepsin L inhibitors of the invasion of amnion membranes by murine cancer cells. Cancer Res. 1989 Jul 1;49(13):3553–3557. [PubMed] [Google Scholar]