Abstract
Objective
Retrospective data analyses have suggested that carbonic anhydrase IX (CAIX) may have a predictive role in patients with metastatic clear cell renal cell carcinoma (ccRCC) receiving high dose interleukin-2 or sorafenib. We examined the predictive value of CAIX in estimating treatment outcome in patients receiving sorafenib vs. placebo as part of the Treatment Approaches in Renal Cancer Global Evaluation Trial (TARGET) study.
Materials and methods
Paraffin embedded tumor tissues were collected from 133 patients from the TARGET study (n _ 903). The percentage of CAIX-positive cells was assessed by a single pathologist. The impact of CAIX expression on progression-free survival (PFS, primary endpoint) and tumor shrinkage (TS, secondary endpoint) was analyzed.
Results
Clinical characteristics were similarly distributed between patients with low vs. high CAIX staining, as well as patients with available CAIX data vs. not. Median PFS for patients with high CAIX vs. low CAIX expression was 5.5 and 5.4 months, respectively, on the sorafenib arm (P _ 0.97), and 1.5 and 1.7 months on the placebo arm (P _ 0.76). Median TS for patients with high CAIX status was _14.9% vs. _12.6% in patients with low CAIX status (P _ 0.63) on the sorafenib arm, and _1.3% (high CAIX) vs. _4.8% (low CAIX) in patients on the placebo arm (P _ 0.60).
Conclusions
Despite suggestive retrospective evidence, data from the TARGET study did not find CAIX expression status to be either predictive of clinical benefit for treatment with sorafenib or of prognostic value in patients with metastatic ccRCC following cytokine therapy.
1. Introduction
Metastatic renal cell carcinoma (RCC) has been considered one of the most treatment-resistant malignancies [1]. One-third of the patients with localized RCC eventually develop metastatic disease despite surgical resection of the primary tumor. The median survival for patients with metastatic RCC has been traditionally around 13 months in the era of cytokine therapy [2]. In little over 6 years, the therapeutic landscape in metastatic RCC has dramatically changed. Currently, multiple US Food and Drug Administration (FDA)-approved systemic treatments have provided patients with new and exciting treatment options that improve clinical outcome. Such therapies are divided into 2 categories: the vascular endothelial growth factor (VEGF) pathway targeted therapies and the mammalian target of rapamycin (mTOR) inhibitors.
However, patients who receive these treatments typically have non-sustainable clinical benefit with most patients experiencing disease progression at a median of 5–11 months. Furthermore, in addition to considerable expense, these treatments can be associated with substantial short-term as well as long-term toxicities [3]. Therefore, there is an urgent need for biomarker-driven treatment selection that could provide rational guidance for optimal treatment selection, which would provide specific therapeutic agents to those patients most likely to benefit. Inactivation of the von Hippel-Lindau (VHL) gene in clear-cell RCC leads to impaired VHL protein (pVHL) and, consequently, accumulation of hypoxia-inducible factor (HIF). Increased HIF levels and the lack of functional VHL protein may further result in the induction of various HIFregulated proteins, such as VEGF, platelet derived growth factor (PDGF), and CAIX, all of which have been shown to promote angiogenesis, tumor growth, and invasion [4,5]. CAIX is a cytosolic transmembrane protein implicated in the regulation of cell survival in response to hypoxia and acidosis. It is found to be expressed in most clear cell RCC tumor tissues, but absent in normal kidney tissues [6]. High CAIX expression in the tumor cells has been reported to be associated with favorable outcome in patients with metastatic RCC [7]. In addition, several retrospective studies have linked high CAIX expression to response to treatment with interleukin-2 (IL-2) [8]. A more recent small cohort retrospective analysis suggested that sorafenib, the first approved VEGF-targeted agent, might have preferential activity in patients with high CAIX expression [1]. This study showed median tumor shrinkage of _13% in those with high CAIX expression, compared with _9% growth in patients with low CAIX expression (mean difference _22%; 95% CI, _42% to_1%; P _ 0.055). These preliminary data prompted us to examine the value of CAIX as a potential biomarker in predicting the efficacy of sorafenib, in a placebo-controlled population with metastatic clear cell RCC treated on a large randomized controlled trial (RCT).
2. Materials and methods
2.1. Patient population
The phase III TARGET trial, which demonstrated significant progression-free survival advantage for sorafenib treated patients over placebo, randomized 903 patients with metastatic clear cell RCC to receive either single agent sorafenib or placebo following cytokine therapy failure. Among these patients, 133 patients with both available tissue samples and independently reviewed imaging were included in our analysis. All 133 patients have consented to an institutional review board-approved protocol for data and tissue usage for clinical research. All patients were treatment-naive to VEGFtargeted agents. Formalin-fixed, paraffin embedded, pretreatment RCC tumor sample (from prior nephrectomy specimen, or prior biopsy specimen if the nephrectomy specimen was unavailable) from each patient was retrieved for central analysis. Baseline characteristics, including the Memorial Sloan-Kettering Cancer Center (MSKCC) prognostic score were available from the prospectively established database as part of the TARGET trial. Progression-free survival (PFS) and tumor shrinkage (TS) were determined based on computed tomography (CT) or magnetic resonance imaging (MRI) assessments preformed within the last 10 days of each treatment cycle (every 6 weeks for the first 24 weeks and every 8 weeks thereafter) [9].
2.2. Central immunohistochemical analysis
Formalin-fixed, paraffin-embedded, pretreatment tumors samples were centrally reviewed by a single pathologist (S.S.), who was unaware of the clinicopathologic variables and therapeutic outcome. The tissue sections were immunostained for CAIX protein using an autostainer system (DAKO, Carpinteria, CA). The tissue sections were incubated with murine monoclonal antibody MN-75 (1:10,000 dilution), and the reaction was detected using the DAKO LSAB_ detection kit (DAKO). The CAIX staining was assessed semiquantitatively, and each specimen was scored based on the intensity and percentage of positive cell staining. The specimens were then categorized into high vs. low CAIX expression status per parameters previously described by Bui et al.; specimens with _85% of cells stained positive for CAIX were categorized as high CAIX expressing tumors, whereas those with _85% of cells stained positive for CAIX were categorized as low CAIX expressing tumors [7].
2.3. Statistical analyses
The primary endpoint was to examine the impact of CAIX expression on progression-free survival. PFS distributions were estimated using the Kaplan-Meier method and compared using the Log-rank test between CAIX expression categories for each treatment arm. The secondary endpoint was to examine the impact of CAIX expression on tumor shrinkage (reflective of disease burden and tumor size change), which was measured on a continuous scale. The distributions of change in tumor size were compared using the Wilcoxon rank-sum test between those with low vs. high CAIX expressions for each treatment arm. Patient and disease characteristic comparisons were made between patients with and without CAIX expression data, and between patients with high vs. low CAIX expression to ensure that our results are consistent with the sample size. The Wilcoxon rank-sum test was used to compare the age distribution, and the Fisher’s exact test was used to compare binary variables. Data analysis was performed using Stata/SE 11.2 (Stata-Corp., College Station, TX).
3. Results
3.1. Patient characteristics
The baseline characteristics of patients with (n _ 133) and without CAIX expression data (n _ 770) were similarly distributed (Table 1). The clinical characteristics did not significantly differ with regards to median age (P _ 0.27), gender (P _ 0.25), and MSKCC prognostic score (P _ 0.64). In addition, treatment type was also similarly distributed between patient with and without CAIX expression data; 50% of patients with CAIX data and 50% of patients without CAIX data received sorafenib as part of the TARGET trial (P _ 1.0). Sixty-eight percent of the 133 patients had high CAIX expression, 48% of these patients were treated with sorafenib, and 52% with placebo. The baseline characteristics of patients with low vs. high CAIX expression level was similarly distributed in terms of gender (P _ 0.32), MSKCC prognostic score (P _ 0.71), and treatment type (P _ 0.71) (Table 2). In comparison, a small but statistically significant difference was seen in age at therapy initiation. The median age was 62 years for patients with high CAIX expression vs.58 years in the low CAIX expressing group (P _ 0.02). However, currently there is no evidence or literature suggesting an association between age and CAIX expression level.
Table 1.
Baseline characteristics and treatment status between patients with independently reviewed PFS and CAIX data and those without
| Variable | With CAIX data (n = 133) |
No CAIX data (n = 770) |
P value |
|---|---|---|---|
| Age (year) median (range) | 61 (29–77) | 58 (19–86) | 0.27 |
| Male gender n (%) | 91 (68%) | 564 (73%) | 0.25 |
| Favorable MSKCC score n (%) |
65 (49%) | 396 (51%) | 0.64 |
| Sorafenib treatment n (%) | 66 (50%) | 385 (50%) | 1.00 |
Table 2.
Baseline characteristics and treatment status between patients with low vs. high CAIX data
| Variable | Low CAIX <85% (n = 42) |
High CAIX >85% (n = 91) |
P value |
|---|---|---|---|
| Age (year) median range) | 58 (29–73) | 62 (32–77) | 0.02 |
| Male gender n (%) | 26 (62%) | 65 (71%) | 0.32 |
| Favorable MSKCC score n (%) |
22 (52%) | 43 (47%) | 0.71 |
| Sorafenib treatment n (%) | 22 (50%) | 44 (48%) | 0.71 |
3.2. Impact of CAIX expression levels on progression-free survival
As the primary endpoint of this study, the impact of CAIX expression level on PFS was examined in patients treated with sorafenib as well as in patients on placebo (Fig. 1). As expected, patients treated with sorafenib experienced significantly longer PFS compared with patients who received placebo. However, CAIX expression levels did not correlate with a difference in PFS. Among the sorafenib treated patients (n _ 66), the median PFS was 5.4 months (95% CI, 2.7–7.8) in patients with low CAIX expression, and 5.5 months (95% CI, 3.9–6.0) in patients with high CAIX expression (P _ 0.97). Additionally, among patients who received placebo (n _67), no significant difference was noted in PFS based on CAIX expression status. Specifically, the median PFS was 1.7 months (95% CI, 1.3–3.6) in patients with low CAIX expression, and 1.5 months (95% CI, 1.3–2.8) in patients with high CAIX expression (P _ 0.76).
Fig. 1.
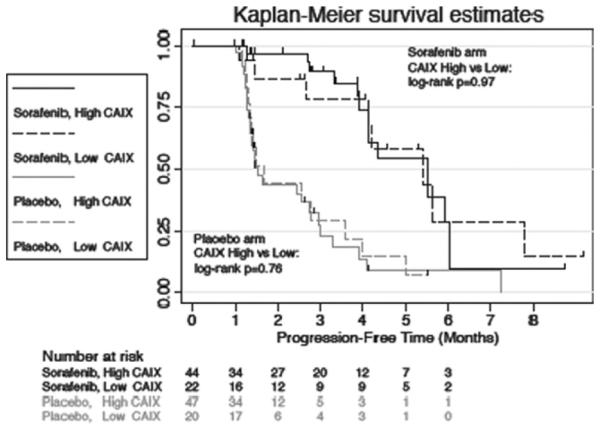
Progression-free survival estimated using the Kaplan-Meier method.
3.3. Impact of CAIX expression levels on tumor shrinkage
The secondary endpoint of this study investigated the impact of CAIX expression on change in tumor size (Fig. 2). The results showed patients treated with sorafenib experienced overall tumor shrinkage of 12.7% (95% CI, 28.2–0.5) in the low CAIX expression group, and overall shrinkage of 14.9% (95% CI, 19.1–9.6) in the high CAIX expression group (P _ 0.63). Thus, the effect of sorafenib on tumor size did not appear to vary significantly based on tumor CAIX expression status. On the placebo arm, overall tumor growth of _4.76% (95% CI, 0.0–8.1) was observed in patients with low CAIX expression status, and overall tumor growth of _1.3% (95% CI, _0.9 to 7.0) was observed in patients with high CAIX expression status. Again, no significant difference in the change in tumor size was noted between the low vs. high CAIX expression categories (P _ 0.61). Table 3 summarizes PFS and TS results for both treatment arms based on CAIX expression status. In addition, multiple CAIX expression thresholds ranging from 40% to 90% were also examined beside the 85% cutoff. None were found to be consistently prognostic or predictive of treatment outcome with sorafenib (Data not shown).
Fig. 2.
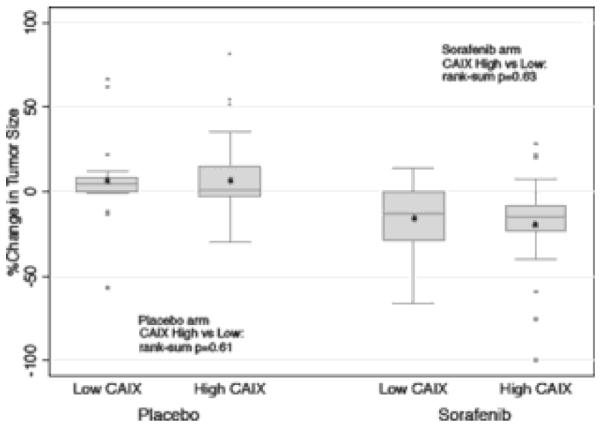
Percent change in tumor size by CAIX status and treatment arm.
Table 3.
Progression-free survival and percent change in tumor size
| PFS (months) |
Percent tumor shrinkage |
|||
|---|---|---|---|---|
| Median (95% CI) | P value | Median (95% CI) | P value | |
| Sorafenib | ||||
| Low CAIX | 5.41 (2.66, 7.80) | 0.97 | −12.65% (−28.20, 0.45) | 0.63 |
| High CAIX | 5.51 (3.93, 6.03) | −14.89% (−19.08, −9.62) | ||
| Placebo | ||||
| Low CAIX | 1.67 (1.31, 3.61) | 0.76 | 4.76% (0.00, 8.10) | 0.61 |
| High CAIX | 1.54 (1.34, 2.75) | 1.34% (−0.86, 7.03) | ||
4. Discussion
Clear cell RCC, the most common RCC histologic subtype, is characterized by the inactivation of the VHL tumor suppressor gene. This genetic event leads to the subsequent up-regulation of HIF. Activated HIF promotes the overproduction of several hypoxia-regulated proteins, such as VEGF, PDGF, and CAIX, all of which play a central role in angiogenesis, tumor growth, proliferation and tumor invasion. Targeted therapies directed against pathways downstream of VHL-HIF have shown significant antitumor effects in patients with metastatic clear cell RCC [10]. Since the FDA approval of VEGF receptor-targeted agent sorafenib in 2005, 4 other VEGF-pathway inhibitors have been approved for the treatment of metastatic RCC in the USA. These include 3 VEGF tyrosine kinase inhibitors: sunitinib, pazopanib, and axitinib, as well as bevacizumab, a monoclonal antibody against the VEGF ligand. However, while data from phase III clinical trials provided general guidelines for utilizing these drugs in an average patient population, the one-size-fits-all model does not always produce optimal treatment and outcome for a specific individual. The current stratification of treatment for patients with metastatic RCC is largely based on clinical prognostic factors (good/intermediate vs. poor prognostic score) and histologic type (clear-cell vs. non-clear-cell) [11]. While such clinical characteristics-guided treatment selection is helpful, it is still based on “average” data from large sample analyses, and thus lacks predictive value for an individual patient. In an effort to advance toward personalized medicine, a growing emphasis has been placed on the use of pathologic and molecular genetic information, to help discern tumor behavior (prognosis) as well as response to various therapies (predictive factor). In patients with RCC, it is believed that baseline pathologic and molecular characteristics of the tumor, along the VHL-HIF-VEGF axis might influence its response to VEGF pathway-targeted treatment. In previous studies, Choueiri et al have noted metastatic RCC patients with VHL inactivation may have better clinical outcome from sorafenib [12]. Though the mechanism for the association of VHL mutation with benefit from sorafenib is unknown, Choueiri et al postulated that clear cell RCC tumors with inactivated VHL protein are more VEGF-dependent, thus more susceptible to a weaker VEGF receptor inhibitor, such as sorafenib [1]. However, to date, no biomarker has been identified to provide guidance for systemic targeted therapy selection in patients with metastatic RCC, and majority of prior studies did not include a placebo-arm. Interestingly, our group showed that patients with VHL inactivation have higher tumor CAIX expression [5]. This finding led to a small retrospective analysis, which suggested that high CAIX expression may be associated with better outcome from sorafenib therapy. Subsequently, we continued our preliminary work in an expanded cohort of patients treated as part of a randomized large placebo controlled clinical trial, and sought to confirm the signal detected by the previous small cohort analysis to validate the utility of CAIX as a predictive biomarker in patients receiving sorafenib. Our results showed CAIX expression does not have predictive value for treatment with sorafenib or prognostic value in patients receiving placebo in this group of 133 patients with metastatic clear cell RCC post cytokine therapy. We chose progression-free survival as our primary endpoint, a robust endpoint in RCC treatment response, which has been shown to closely correlate with overall survival based on recent analyses [13,14]. In the TARGET study, the robustness of the PFS data led to a decision to cross-over placebo-treated patients to sorafenib. In accordance with prior data, patients on sorafenib experienced significant PFS advantage over patients on placebo. However, no significant difference in median PFS was noted between patients with high vs. low CAIX expression on either treatment arm. The lack of difference in PFS on the sorafenib arm demonstrated that CAIX does not predict treatment outcome of sorafenib in this patient population. Moreover, the lack of difference in PFS on the placebo arm refuted the hypothesis that CAIX is of prognostic value in this patient population. Our secondary endpoint was change in tumor size, which was measured on a continuous scale to reflect the potential activity of sorafenib in a more precise fashion. As expected, patients treated with sorafenib experienced significantly greater overall tumor shrinkage than patients on placebo. However, no significant difference in median change in tumor size was noted between patients with high vs. low CAIX expression on either treatment arm. Of note, the relatively small median tumor growth seen on the placebo arm reflects the low and intermediate risk patient population that encompassed majority of the patients included in our analysis. Additionally, several studies have looked at CAIX expression level in the plasma as opposed to tissue, as another CAIX-based biomarker. Baseline CAIX levels by commercially available enzyme-linked immunosorbent assay (ELISA) had initially hinted its association with overall survival but not PFS only in the placebo group and on univariate analysis in one large study, but not in multivariate analysis [15]. Furthermore, a very recent study with another VEGF TKI, pazopanib, looked at several cytokines and angiokine factors (CAFs), including CAIX plasma levels. CAIX levels were not associated with PFS or tumor shrinkage in the first stage of the biomarker discovery [16]. VEGF pathway targeted therapies have evolved into standard treatments in patients with metastatic RCC. Retrospective analyses are crucial in identifying predictive and prognostic biomarkers to provide much needed guidance for systemic treatment selection. However, this study underlined the challenge of tissue biomarker research, and emphasized the importance of validating findings from small cohort analysis in large, placebo-controlled patient populations before drawing any firm conclusions. It is also possible that CAIX biomarker is relevant in RCC within other disease stages. In fact, majority of the data with regards to the CAIX biomarker is in early stage disease, with a large volume of information suggesting an independent prognostic utility in some studies, but not in others [17,18]. It is also conceivable that our population of patients who are resistant to prior cytokine therapy might have influenced subsequent response to a VEGF inhibitor, based on CAIX status compared with cytokine-naive patients. Another limitation of our study is that it is a retrospective analysis, albeit the data used were from a prospective clinical trial. Biomarker analyses would not only bring us closer to predicting treatment outcome, but might also help us understand the mechanisms of treatment resistance. Therefore, it may enable us to identify patient populations in need for nonstandard therapeutic approaches. Unfortunately, one major challenge in tissue biomarker research remains the high frequency of failure in the biomarker validation phase [19]. Continued efforts towards the collection and analyses of high quality RCC samples are essential for the development of robust predictive biomarkers that can be eventually implemented into clinical practice. Our effort in identifying biomarkers in patients with metastatic RCC will continue.
Acknowledgments
The authors acknowledge all the patients from the TARGET trial for their contribution to the findings of this study. The authors thank Dr. Carol Pena of Bayer Health-Care Pharmaceuticals for providing the database used in this study.
References
- 1.Choueiri TK, Regan MM, Rosenberg JE, et al. Carbonic anhydrase IX and pathological features as predictors of outcome in patients with metastatic clear-cell renal cell carcinoma receiving vascular endothelial growth factor-targeted therapy. BJU Int. 2010;106:772–8. doi: 10.1111/j.1464-410X.2010.09218.x. [DOI] [PubMed] [Google Scholar]
- 2.Motzer RJ, Bacik J, Mazumdar M. Prognostic factors for survival of patients with stage IV renal cell carcinoma: Memorial Sloan-Kettering Cancer Center experience. Clin Cancer Res. 2004;10:6302S–3S. doi: 10.1158/1078-0432.CCR-040031. [DOI] [PubMed] [Google Scholar]
- 3.Appleby L, Morrissey S, Bellmunt J, et al. Management of treatmentrelated toxicity with targeted therapies for renal cell carcinoma: Evidence-based practice and best practices. Hematol Oncol Clin North Am. 2011;25:893–915. doi: 10.1016/j.hoc.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Choueiri TK, Bukowski RM, Rini BI. The current role of angiogenesis inhibitors in the treatment of renal cell carcinoma. Semin Oncol. 2006;33:596–606. doi: 10.1053/j.seminoncol.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Di Napoli A, Grisanzio C, Ghebremichael M, et al. CAIX expression correlates with VHL mutational status in sporadic clear cell carcinoma of the kidney; Proceedings of the Annual Meeting, United States and Canadian Division of the International Academy of Pathology; Denver CO. 2008. [Google Scholar]
- 6.Dorai T, Sawczuk IS, Pastorek J, et al. The role of carbonic anhydrase IX overexpression in kidney cancer. Eur J Cancer. 2005;41:2935–47. doi: 10.1016/j.ejca.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Bui MH, Seligson D, Han KR, et al. Carbonic anhydrase IX is an independent predictor of survival in advanced renal clear cell carcinoma: Implications for prognosis and therapy. Clin Cancer Res. 2003;9:802–11. [PubMed] [Google Scholar]
- 8.Atkins M, Regan M, McDermott D, et al. Carbonic anhydrase IX expression predicts outcome of interleukin 2 therapy for renal cancer. Clin Cancer Res. 2005;11:3714–21. doi: 10.1158/1078-0432.CCR-04-2019. [DOI] [PubMed] [Google Scholar]
- 9.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–34. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 10.Courtney KD, Choueiri TK. Updates on novel therapies for metastatic renal cell carcinoma. Ther adv. Med Oncol. 2010;2:209–19. doi: 10.1177/1758834010361470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molina AM, Motzer RJ. Clinical practice guidelines for the treatment of metastatic renal cell carcinoma: Today and tomorrow. Oncologist. 2011;16(Suppl 2):45–50. doi: 10.1634/theoncologist.2011-S2-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choueiri TK, Vaziri SA, Jaeger E, et al. Von Hippel-Lindau gene status and response to vascular endothelial growth factor targeted therapy for metastatic clear cell renal cell carcinoma. J Urol. 2008;180:860–5. doi: 10.1016/j.juro.2008.05.015. Discussion:5–6. [DOI] [PubMed] [Google Scholar]
- 13.Johnson JR, Williams G, Pazdur R. End points and united states food and drug administration approval of oncology drugs. J Clin Oncol. 2003;21:1404–11. doi: 10.1200/JCO.2003.08.072. [DOI] [PubMed] [Google Scholar]
- 14.Heng DY, Xie W, Bjarnason GA, et al. Progression-free survival as a predictor of overall survival in metastatic renal cell carcinoma treated with contemporary targeted therapy. Cancer. 2011;117:2637–42. doi: 10.1002/cncr.25750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peña C, Lathia C, Shan M, et al. Biomarkers predicting outcome in patients with advanced renal cell carcinoma: Results from sorafenib phase III treatment approaches in renal cancer global evaluation trial. Clin Cancer Res. 2010;16:4853–63. doi: 10.1158/1078-0432.CCR-09-3343. [DOI] [PubMed] [Google Scholar]
- 16.Tran HT, Lui Y, Zurita AJ, et al. Prognostic or predictive plasma cytokines and angiogenic factors for patients treated with pazopanib for metastatic renal-cell cancer: A retrospective analysis of phase 2 and phase 3 trials. Lancet Oncol. 2012;13:827–37. doi: 10.1016/S1470-2045(12)70241-3. [DOI] [PubMed] [Google Scholar]
- 17.Pantuck AJ, Klatte T, Seligson D, et al. Carbonic anhydrase IX as a predictive biomarker for clear cell renal cell carcinoma. J Clin Oncol. 2008;26:3105–7. doi: 10.1200/JCO.2008.16.1935. Author reply:7–9. [DOI] [PubMed] [Google Scholar]
- 18.Leibovich BC, Sheinin Y, Lohse CM, et al. Carbonic anhydrase IX is not an independent predictor of outcome for patients with clear cell renal cell carcinoma. J Clin Oncol. 2007;25:4757–64. doi: 10.1200/JCO.2007.12.1087. [DOI] [PubMed] [Google Scholar]
- 19.Bahamon B, Signoretti S. Tissue biomarkers in renal cell carcinoma: Intermediate endpoints in the selection of targeted agents for RCC. In: Rathmell K, Rini B, Figlin RA, editors. Renal cell carcinoma: Biology, prognostic factors and therapeutic targets. Springer; New York, NY: 2012. [Google Scholar]


