Abstract
BACKGROUND
Heart failure patients with primary prevention implantable cardioverter-defibrillators (ICD) may experience an improvement in left ventricular ejection fraction (LVEF) over time. However, it is unclear how LVEF improvement affects subsequent risk for mortality and sudden cardiac death (SCD).
OBJECTIVES
We sought to assess changes in LVEF after ICD implantation and the implication of these changes on subsequent mortality and ICD shocks.
METHODS
We conducted a prospective cohort study of 538 patients with repeated LVEF assessments after ICD implantation for primary prevention of SCD. The primary endpoint was appropriate ICD shock, defined as a shock for ventricular tachyarrhythmias. The secondary endpoint was all-cause mortality.
RESULTS
Over a mean follow-up of 4.9 years, LVEF decreased in 13.0%, improved in 40.0%, and was unchanged in 47.0% of the patients. In the multivariate Cox models comparing patients with an improved LVEF to those with an unchanged LVEF, the hazard ratios were 0.33 (95% confidence interval [CI]: 0.18 to 0.59) for mortality and 0.29 (95% CI: 0.11 to 0.78) for appropriate shock, respectively. During follow-up, 25% of patients showed an improvement in LVEF to >35% and their risk of appropriate shock decreased but was not eliminated.
CONCLUSION
Among primary prevention ICD patients, 40.0% had an improved LVEF during follow-up and 25% had LVEF improved to >35%. Changes in LVEF were inversely associated with all-cause mortality and appropriate shock for ventricular tachyarrhythmia. In patients whose follow-up LVEF improved to >35%, the risk of an appropriate shock remained but was markedly decreased.
Keywords: All-cause mortality, shock, sudden cardiac death
INTRODUCTION
Implantable cardioverter-defibrillators (ICD) reduce the risk of all-cause mortality and sudden cardiac death (SCD) in patients with severe systolic heart failure (1–4). Left ventricular ejection fraction (LVEF) is a key criterion in determining eligibility for a primary prevention ICD (5). However, 25% to 40% of primary prevention ICD patients improve their LVEF to >35% after ICD implantation (6–9), calling in question whether their risk for SCD warrants ICD generator replacement especially in patients who have not experienced any appropriate ICD therapy. Additionally, it is largely unknown if improvement in LVEF affects the subsequent risk for mortality and SCD since prior studies were limited by small sample size and lack of repeated LVEF assessments during follow-up (6–9).
Using data from PROSE-ICD (Prospective Observational Study of Implantable Cardioverter-Defibrillators), we sought to assess the changes in LVEF after ICD implantation and the implication of these changes for subsequent mortality and ICD shocks.
METHODS
STUDY DESIGN AND POPULATION
PROSE-ICD is a multicenter prospective study of patients with systolic heart failure eligible for a primary prevention ICD that was conducted at 4 clinical centers in the United States from 2003 to 2013. Patients were extensively phenotyped and followed as previously described (10). Briefly, patients 18 to 80 years of age referred for primary prevention ICD implantation were enrolled if they met any of the following criteria: 1) ischemic cardiomyopathy (myocardial infarction >40 days prior to implant) with an ejection fraction of ≤30% and stable New York Heart Association (NYHA) class I to III heart failure; 2) ischemic or nonischemic cardiomyopathy with an ejection fraction ≤35% and NYHA class II or III heart failure; or 3) ejection fraction ≤35% with NYHA class II to IV heart failure undergoing guideline-indicated implantation of a cardiac resynchronization therapy device with an ICD (CRT-D). All centers obtained approval from their respective institutional review boards and all patients provided informed consent.
Among the 1,189 participants enrolled in the PROSE-ICD study, 538 had their LVEF reassessed at least once during follow-up and were selected for the current analysis. Patients without follow-up LVEF measurements were older (62.0 vs. 58.9 years), and were more likely to be male (75.3% vs. 70.1%) and to have higher baseline LVEF (22.6% vs. 21.8%), ischemic cardiomyopathy (59.3% vs. 47.6%), and more comorbidities including diabetes, hypertension, or chronic kidney disease (CKD) compared to patients with follow-up LVEF measurements (Online Table 1).
At enrollment, and prior to ICD implantation, all patients underwent a comprehensive medical history and cardiovascular examination including a digitally-recorded resting 12-lead electrocardiogram, fasting blood collection, and evaluation of LVEF. The medical history included data on NYHA class, angina class, atrial fibrillation, smoking, comorbidities, and medication use. Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation and CKD was defined as an eGFR <60 ml/min/1.73 m2.
Echocardiography was the main method for estimating the LVEF, accounting for 80.6% of all measurements at baseline and during study follow-up. Other methods included ventriculography (8.5%), nuclear scintigraphy (5.1%), stress test (3.7%), computed tomography (CT) scan (1.9%), and cardiac magnetic resonance (CMR) imaging (0.2%).
Patients were evaluated every 6 months after ICD implantation either in person or by phone and soon after any patient-perceived ICD therapy. The 2 study endpoints were first appropriate ICD shock and death. An appropriate ICD shock was defined as an ICD shock for ventricular tachyarrhythmias. Arrhythmic events were adjudicated by 2 clinical cardiac electrophysiologists blinded to patient demographic information. Disagreements were reconciled by a third electrophysiologist. Deaths were ascertained by phone contact with the next of kin and by searches of the National Death Index.
STATISTICAL ANALYSES
Cox proportional hazards models were used to estimate the association of changes in LVEF during follow-up with all-cause mortality and appropriate shocks. All models were stratified by enrollment center, allowing the baseline hazard functions to differ for the centers. To describe the changes in LVEF, we calculated the absolute change in LVEF as the difference between the last available LVEF measurement (for analyses of mortality) or the last LVEF measurement prior to the first appropriate shock (for analyses of appropriate shocks) minus the baseline LVEF. For survival analysis, follow-up time started at the time of the last available LVEF measurement and continued through March 18, 2015.
Two alternative model specifications were used to provide detailed dose-response analyses of the relationship between magnitude of changes in LVEF and study endpoints. First, we categorized patients into 3 groups: worsened LVEF (absolute decrease in LVEF >5%), unchanged LVEF (absolute change in LVEF −5% to 5%), and improved LVEF (absolute increase in LVEF >5%). Second, we introduced absolute change in LVEF as restricted quadratic splines with knots at the 5th, 50th, and 95th percentiles of its distribution in the Cox models to allow for a smooth yet flexible description of the relationship between change in LVEF and endpoints. For the analysis of all-cause mortality, data from all 538 patients were used while the analysis of appropriate shocks was restricted to the 464 patients with LVEF reassessed at least once prior to the first appropriate shock (for those who experienced an appropriate shock) or prior to the study’s end (for those who did not have an appropriate shock).
For all analyses, we used 2 models with progressive degrees of adjustment. The initial model was adjusted for baseline age, sex, race, and baseline LVEF. The second model was further adjusted for baseline smoking status, body mass index, NYHA class, ischemic cardiomyopathy, atrial fibrillation, diabetes, hypertension, CKD, and device type. Additional adjustment of the model for device characteristics (lowest cut-off rate, antitachycardia pacing zone used) and medication use (aspirin, angiotensin- converting enzyme (ACE) inhibitors or angiotensin receptor blockers, beta-blocker, diuretics, and aldosterone antagonist) yielded similar results (data not shown). The proportional hazards assumption was checked by plotting the log(−log(survival)) against log(time) and by using the Schoenfeld residuals.
In addition to evaluating the association between absolute changes in LVEF and mortality, we evaluated the annual rate of change in LVEF, with similar findings (not shown). We also performed separate stratified analyses in pre-specified subgroups defined by device type (ICD, CRT-D). The significance of interaction term was evaluated using Wald tests. Sensitivity analysis only including LVEF measurements by echocardiogram was also performed. All analyses were performed using STATA version 12 (StataCorp LP, College Station, Texas).
RESULTS
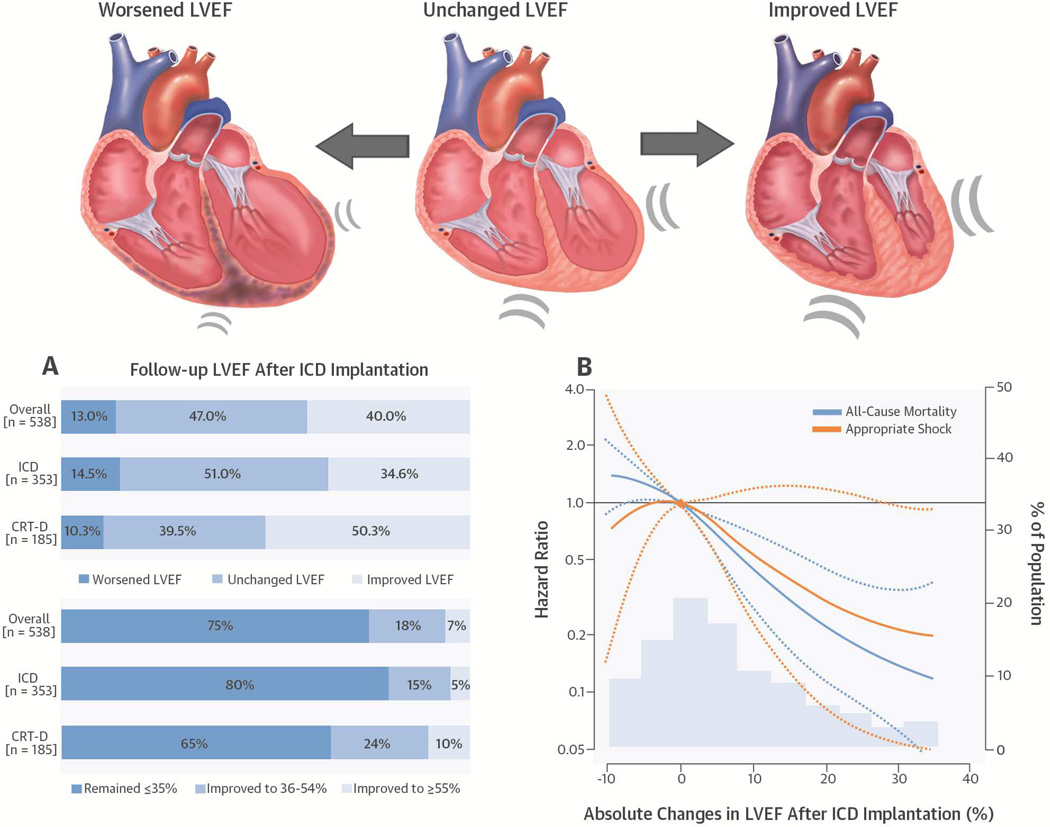
The average age of study participants at baseline was 58.9 ± 12.2 years (Table 1). Males comprised 70.1% of all subjects and 57.4% were white. During follow-up, LVEF measures were reduced in 70 (13.0%), unchanged in 253 (47.0%), and improved in 215 (40.0%) patients (Table 1, Central Illustration). The mean duration between the first and last available LVEF measurements was 4.9 years. Patients with a worsened LVEF were more likely to be older and have higher baseline LVEF, ischemic cardiomyopathy, and diabetes compared to patients whose LVEFs were unchanged or improved. Patients with an improved LVEF were more likely to have a CRT-D device (as opposed to an ICD) compared to the rest of the population (Table 2).
TABLE 1.
Changes in LVEF During Follow-up
| Overall (N = 538) |
ICD Patients (n = 353) |
CRT-D Patients (n = 185) |
|
|---|---|---|---|
| Changes in LVEF* | |||
| Worsened | 70 (13.0) | 51 (14.5) | 19 (10.3) |
| Unchanged | 253 (47.0) | 180 (51.0) | 73 (39.5) |
| Improved | 215 (40.0) | 122 (34.6) | 93 (50.3) |
| Absolute LVEF (%) | |||
| Baseline LVEF | 21.8 ± 7.2 | 22.4 ± 6.9 | 20.6 ± 7.5 |
| Last LVEF | 28.6 ± 13.7 | 27.3 ± 12.5 | 30.9 ± 15.6 |
| Average difference | 6.7 | 4.9 | 10.2 |
Values are n (%) or means ± SD.
Changes in LVEF were categorized as follow: worsened = absolute decrease in LVEF >5%; unchanged = absolute change in LVEF −5 to 5%; and improved = absolute increase in LVEF >5%.
CRT-D = cardiac resynchronization therapy device with of defibrillator capacity; ICD = implantable cardioverter-defibrillator; LVEF = left ventricular ejection fraction.
TABLE 2.
Baseline Characteristics
| Characteristic | Overall (N = 538) |
Worsened LVEF (n = 70) |
Unchanged LVEF (n = 253) |
Improved LVEF (n = 215) |
p Value* |
|---|---|---|---|---|---|
| Age, year | 58.9 ± 12.2 | 62.5 ± 13.2 | 58.6 ± 11.9 | 58.1 ± 12.1 | 0.03 |
| Sex | 0.21 | ||||
| Male | 377 (70.1) | 53 (75.7) | 182 (71.9) | 142 (66.0) | |
| Female | 161 (29.9) | 17 (24.3) | 71 (28.1) | 73 (34.0) | |
| Race | 0.48 | ||||
| White | 309 (57.4) | 44 (62.9) | 136 (53.8) | 129 (60.0) | |
| Black | 212 (39.4) | 25 (35.7) | 107 (42.3) | 80 (37.2) | |
| Other | 17 (3.2) | 1 (1.4) | 10 (4.0) | 6 (2.8) | |
| Body mass index, kg/m2 | 30.0 ± 6.6 | 30.3 ± 7.5 | 30.6 ± 6.4 | 29.3 ± 6.6 | 0.10 |
| Smoking | 0.55 | ||||
| Never | 189 (35.1) | 28 (40.0) | 94 (37.2) | 67 (31.2) | |
| Former | 249 (46.3) | 30 (42.9) | 111 (43.9) | 108 (50.2) | |
| Current | 100 (18.6) | 12 (17.1) | 48 (19.0) | 40 (18.6) | |
| Baseline LVEF, % | 21.8 ± 7.2 | 27.3 ± 5.6 | 21.6 ± 7.0 | 20.3 ± 7.1 | <0.001 |
| NYHA class | 0.36 | ||||
| Class I | 88 (16.4) | 7 (10.0) | 44 (17.4) | 37 (17.2) | |
| Class II | 245 (45.5) | 38 (54.3) | 114 (45.1) | 93 (43.3) | |
| Class III | 202 (37.5) | 24 (34.3) | 95 (37.5) | 83 (38.6) | |
| Class IV | 3 (0.6) | 1 (1.4) | 0 (0.0) | 2 (0.9) | |
| Cardiomyopathy | 0.003 | ||||
| Nonischemic | 282 (52.4) | 29 (41.4) | 122 (48.2) | 131 (60.9) | |
| Ischemic | 256 (47.6) | 41 (58.6) | 131 (51.8) | 84 (39.1) | |
| QRS duration, ms | 120.6 ± 31.7 | 120.1 ± 29.4 | 119.2 ± 31.6 | 122.3 ± 32.6 | 0.58 |
| Atrial fibrillation | 132 (24.5) | 20 (28.6) | 56 (22.1) | 56 (26.0) | 0.43 |
| Diabetes | 164 (30.5) | 25 (35.7) | 89 (35.2) | 50 (23.3) | 0.01 |
| Hypertension | 303 (56.3) | 41 (58.6) | 148 (58.5) | 114 (53.0) | 0.45 |
| Chronic kidney disease | 136 (25.3) | 23 (32.9) | 68 (26.9) | 45 (20.9) | 0.20 |
| Medications | |||||
| Aspirin | 343 (63.8) | 49 (70.0) | 161 (63.6) | 133 (61.9) | 0.47 |
| ACE-I/ARB | 380 (70.6) | 54 (77.1) | 178 (70.4) | 148 (68.8) | 0.41 |
| Beta blocker | 482 (89.6) | 62 (88.6) | 224 (88.5) | 196 (91.2) | 0.62 |
| Thiazide/loop diuretics | 385 (71.6) | 48 (68.6) | 194 (76.7) | 143 (66.5) | 0.04 |
| Aldosterone antagonist | 138 (25.7) | 13 (18.6) | 77 (30.4) | 48 (22.3) | 0.05 |
| Device type | 0.002 | ||||
| ICD | 353 (65.6) | 51 (72.9) | 180 (71.1) | 122 (56.7) | |
| CRT-D | 185 (34.4) | 19 (27.1) | 73 (28.9) | 93 (43.3) | |
| Lowest device cutoff rate (bpm) | 188.2 ± 14.1 | 186.6 ± 15.5 | 187.2 ± 13.1 | 189.8 ± 14.6 | 0.08 |
| ATP zone used | 316 (58.7) | 39 (55.7) | 164 (64.8) | 113 (52.6) | 0.03 |
Values are n (%) or means ± SD.
p values represent the comparison across the 3 groups of worsened (absolute decrease in LVEF >5%), unchanged (absolute change in LVEF −5 to 5%), and improved LVEF (absolute increase in LVEF >5%). ACE-I = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; ATP = antitachycardia pacing; NYHA = New York Heart Association; other abbreviations as in Table 1.
The mean follow-up time for endpoints since the last available LVEF measurement was 2.0 years during which 96 patients died and 27 experienced an appropriate shock. In multivariate Cox models, the hazard ratio (HR) for mortality was 0.33 (95% confidence interval [CI]: 0.18 to 0.59) comparing patients with an improved LVEF to those with an unchanged LVEF (Table 3). Similarly, the corresponding HR for appropriate shock was 0.29 (95% CI: 0.11 to 0.78). Spline regression analyses also showed a consistent inverse relationship between changes in follow-up LVEF and endpoints (Central Illustration).
TABLE 3.
Mortality and Appropriate Shock
| All-cause Mortality | Appropriate Shock | |||||||
|---|---|---|---|---|---|---|---|---|
| Events/Total Number |
Incidence rate* |
Model 1† HR (95% CI) |
Model 2‡ HR (95% CI) |
Events/Total Number |
Incidence rate* |
Model 1† HR (95% CI) |
Model 2‡ HR (95% CI) |
|
| Changes in LVEF§ | ||||||||
| Worsened | 20/70 | 20.0 | 1.48 (0.84–2.61) | 1.54 (0.87–2.75) | 1/48 | 2.3 | 0.41 (0.04–3.73) | 0.51 (0.05–5.30) |
| Unchanged | 59/253 | 11.7 | Reference | Reference | 20/219 | 6.9 | Reference | Reference |
| Improved | 17/215 | 3.8 | 0.31 (0.18–0.54) | 0.33 (0.18–0.59) | 6/197 | 2.2 | 0.33 (0.13–0.85) | 0.29 (0.11–0.78) |
Incidence rate calculated as per 100 person-years.
Model 1: Adjusted for age, sex, race, and baseline LVEF, and stratified by enrollment center.
Model 2: Further adjusted for smoking status, body mass index, NYHA class, ischemic cardiomyopathy, atrial fibrillation, diabetes, hypertension, chronic kidney disease, and device type.
Changes in LVEF were categorized into 3 groups: worsened LVEF (absolute decrease in LVEF >5%), unchanged LVEF (absolute change in LVEF −5 to 5%), and improved LVEF (absolute increase in LVEF >5%).
Among all 538 patients, 404 (75%) had an LVEF that remained ≤35%, 99 (18%) improved to 36% to 54%, and 35 (7%) improved to ≥55% (Central Illustration). Among the 464 patients who had their LVEF reassessed at least once prior to the first appropriate shock or who had not yet received an appropriate shock during follow-up, 91 (20%) had an LVEF improved to 36% to 54%, and 35 (8%) to ≥55% (Table 4). Among those whose LVEF improved to >35%, only 4 patients experienced an appropriate shock. Patients with CRT-D devices were more likely to experience improvement and normalization of LVEF compared to ICD patients (36.7% vs. 21.8%).
TABLE 4.
Incidence Rate of Appropriate Shock by Last LVEF Measurement Prior to Appropriate Shock
| Last LVEF measurement* |
Overall (n = 464) | ICD Patients (n = 298) | CRT-D Patients (n = 166) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Number (%) |
Events | Incidence Rate† |
Number (%) |
Events | Incidence Rate† |
Number (%) |
Events | Incidence Rate† |
|
| ≤35% | 338 (73) | 23 | 5.5 | 233 (78) | 13 | 4.8 | 105 (63) | 10 | 6.8 |
| 36–54% | 91 (20) | 3 | 2.4 | 49 (16) | 2 | 3.5 | 42 (25) | 1 | 1.5 |
| ≥55% | 35 (8) | 1 | 1.7 | 16 (5) | 0 | 0 | 19 (11) | 1 | 2.6 |
Prior to appropriate shock.
Incidence rate calculated as per 100 person-years.
When the analysis was stratified by device type, the association between changes in LVEF and outcomes appeared to be similar in ICD and CRT-D patients (p value for interactions = 0.99 for analysis of all-cause mortality and 0.28 for analysis of appropriate shock). Additionally, similar results were found when repeating the analyses using LVEF measurements by echocardiogram only (Supplemental Figure 1).
DISCUSSION
In a prospective cohort of patients undergoing primary prevention defibrillator implantation, LVEF worsened in 13%, improved in 40%, and was unchanged in 47% of the patients during a mean follow-up of 4.9 years post-implantation (Central Illustration). Changes in LVEF during follow-up were inversely related to all-cause mortality and appropriate ICD shock with an improved LVEF associated with reduced risk of death and appropriate shocks. During follow-up, 25% of the patients had LVEFs that improved to >35% and the risk of appropriate shock was decreased but not eliminated in these patients.
Heart failure patients may experience LVEF improvement as a result of medical therapies or the correction of reversible factors that caused the cardiomyopathy. LVEF recovery has been shown in clinical trials of heart failure therapies such as vasodilators (11), ACE inhibitors (12), beta-blockers (13), and CRT (14,15). Population and community-based studies have also demonstrated LVEF improvement in a substantial proportion of heart failure patients. The Oregon Sudden Cardiac Death Study found that among patients with severe left ventricular dysfunction (LVEF ≤35%), about one-third had an improved (36% to 54%) or normalized (≥55%) LVEF during a mean follow-up of 2 years (16). In another large cohort of 3,994 outpatient heart failure patients, 28.6% had a >10% improvement in LVEF, resulting in an average increase in LVEF from 25.8% to 32.3% at 24 months (17). Our analysis of primary prevention ICD patients found that 40% had an improved LVEF after implantation. Individuals with an improved LVEF were more likely to be younger, have a lower baseline LVEF and nonischemic cardiomyopathy, and were less likely to have diabetes. This, too, aligns with prior reports (17–19).
Few studies have evaluated the association between changes in LVEF and subsequent risk of mortality and ICD therapy. In a study of 187 nonischemic cardiomyopathy patients with LVEF <36% at baseline, patients with an improved LVEF (increased >5%) exhibited improved survival and a nonsignificant decrease in the risk of appropriate shocks compared to patients with stable (absolute change ≤5%) or decreased (<-5%) LVEF (8). In another study of 91 patients (99% male) with primary prevention ICDs, 27% had improved LVEF at generator replacement; however, the incidence rates of appropriate shock were similar between patients with improved LVEF and unchanged LVEF (6). Of note, among the 16 patients with improved LVEF and no ICD therapy before generator replacement, 3 (19%) had their first appropriate ICD therapy after generator replacement (6). With a larger and more inclusive primary prevention ICD cohort (29.9% female and 39.4% blacks), we found that changes in LVEF measurements during follow-up were inversely associated with all-cause mortality and appropriate shocks and the associations were similar among ICD and CRT-D patients. Although our study represents a large cohort of primary prevention ICD patients with follow-up LVEF measurements, only 27 patients experienced an appropriate shock among those with a follow-up LVEF measurement prior to the first appropriate shock. This is likely due to the fact that patients were more likely to have their LVEF reassessed after, but not prior to, ICD shocks.
LVEF improvement to >35% was observed in 25% of our study population, and these patients appeared to have a lower incidence of appropriate shocks compared to patients whose LVEF remained ≤35%. This was consistent with previous smaller studies that found a reduction, but not elimination, of the risk of appropriate ICD shock after LVEF improved to >35%. In a registry-based study of 157 patients with idiopathic dilated cardiomyopathy and primary prevention ICDs, 33.8% demonstrated an improvement in follow-up LVEF to >35% during a mean of 26 months and their incidence of appropriate ICD therapy was lower compared to patients whose LVEF did not improve to >35% (10 events in 53 patients who had such an improvement in LVEF vs. 34 events out of 104 patients who did not show such LVEF improvement) (7). In the DEFINITE (Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation) trial, 37% patients had a follow-up LVEF >35% during the first 2 years and they experienced a significantly lower incidence of arrhythmic events compared to patients whose LVEF remained ≤35% (4 of 70 vs. 24 of 117 events) (8). Similarly, a recent retrospective chart review of 231 patients with primary prevention ICDs found that 26% of the patients had LVEF improved to ≥40% with no prior appropriate ICD therapy. When these patients were followed over time, they received significantly fewer ICD therapies (9). Lastly, a retrospective study of 423 patients with CRT-D also found that post-implantation LVEF was inversely associated with the risk of subsequent appropriate ICD therapy and the 2-year risk of appropriate therapy was <3.3% in patients whose LVEF had improved to ≥45% (18). The observation that risk of arrhythmic events persists despite improvement in LVEF may be partly explained by the presence of a fixed substrate for ventricular arrhythmias (e.g., fibrosis, myocardial scar, heterogeneous repolarization) that does not resolve even when LVEF improves, suggesting that improvements in LVEF alone may not be enough to warrant deferring ICD generator exchange (8).
STUDY LIMITATIONS
Several limitations of our study need to be considered. This analysis was restricted to patients with repeated LVEF assessment after ICD implantation. Compared to patients included in our analysis, those who were excluded due to a lack of follow-up LVEF measurement were on average older, had a higher baseline LVEF, a higher prevalence of ischemic cardiomyopathy, higher burden of comorbidities, and a higher risk of mortality. Since excluded patients had higher baseline LVEF and, thus, were more likely to have a worsened or unchanged LVEF (Table 2), yet also higher mortality, the potential bias resulting from these exclusions was likely towards the null. In addition, LVEF was measured by different modalities including echocardiography, ventriculography, nuclear scintigraphy, CT, and CMR. Some evidence suggests that LVEF measured by different methods may differ from each other (20). However, when restricting the analysis to only include LVEF measurements by echocardiography, we found very similar results. The mode of death could not be established in many patients due to the lack of reliable records when patients died out of hospital. As a consequence, we could not examine the association between LVEF changes and cause-specific mortality. Lastly, we were not able to assess if changes in device programming during follow-up may impact our findings as this information was not available in our study. However, adjustment for device characteristics at baseline virtually did not change the results.
CONCLUSIONS
In a prospective cohort of patients undergoing primary prevention ICD implantation, 40% of the patients showed an improved LVEF during follow-up; in 25%, LVEF improved to >35%. Changes in LVEF were inversely associated with the risk of all-cause mortality and appropriate shocks. In patients whose follow-up LVEF improved to >35%, the risk of an appropriate shock was markedly decreased but still present, suggesting that improvements in EF alone may not be enough to warrant deferring ICD generator exchange.
Findings from our study indicate that repeated LVEF assessments after ICD implantation can provide additional prognostic information and may also allow for more informed decision making regarding ICD generator replacement, especially in patients whose LVEF improved to >35%. Further studies in larger populations with more frequent and prospectively-determined LVEF reassessments are needed to better understand how changes in LVEF after ICD implantation modulate the risk of mortality and appropriate shocks. More studies are also needed to provide greater guidance on which patients with LVEF improvement should actually defer generator exchange. Randomized clinical trial of generator replacement in patients whose LVEF improved to >35% would be necessary to provide the most convincing evidence as to whether ICD generator replacement has a positive or negative impact in this particular patient population.
Supplementary Material
CENTRAL ILLUSTRATION Follow-up LVEF after Primary Prevention ICD Implantation.
In this prospective cohort study of 538 patients with repeated left ventricular ejection fraction (LVEF) assessments after implantable cardioverter-defibrillators (ICD) had been inserted for primary prevention, (A) patients implanted with a cardiac resynchronization therapy device with defibrillator capacity (CRT-D) exhibited the greatest improvement both in terms of absolute increase in LVEF (upper panel) and the percentage improved to ≥35% (bottom panel). (B) Changes in LVEF were inversely associated with all-cause mortality and appropriate shock for ventricular tachyarrhythmia. The curves represent adjusted hazard ratios and their 95% confidence intervals (dashed lines) based on restricted quadratic splines for absolute change in LVEF with knots at the 5th, 50th, and 95th percentiles of its distribution. The reference values (diamond dots) were set at 0% change in LVEF. Results were obtained from Cox regression models adjusted for age, sex, race, baseline LVEF, smoking status, body mass index, New York Heart Association class, ischemic cardiomyopathy, atrial fibrillation, diabetes, hypertension, chronic kidney disease, device type, and stratified by enrollment center. Histograms represent the frequency distributions of the absolute change in LVEF. Abbreviations: Worsened LVEF = absolute decrease in LVEF >5%; unchanged LVEF = absolute change in LVEF −5 to 5%; improved LVEF = absolute increase in LVEF >5%.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE
In a prospective cohort study of patients with repeated assessments of LVEF after ICD implantation for primary prevention of lethal ventricular arrhythmias, 40% exhibited improvement in EF during a mean follow-up of 4.9 years, with LVEF exceeding 35% in 25% of patients. Changes in LVEF were inversely associated with all-cause mortality and appropriate shocks for termination of ventricular tachyarrhythmias.
TRANSLATIONAL OUTLOOK
Further studies are needed to discover the mechanisms linking changes in LVEF after ICD implantation to the risks of ventricular tachyarrhythmias and mortality, and to determine whether patients in whom EF improves might safely defer generator replacement.
Acknowledgments
Dr. Z. Eldadah received an honorarium from St. Jude Medical for participation in fellows’ educational programs. Dr. K. Ellenbogen received honoraria from Medtronic, Boston Scientific, Biotronik, served as a consultant for Medtronic, Boston Scientific, St. Jude Medical and received fellowship support from Medtronic and Boston Scientific. Dr. A. Cheng received honoraria from Boston Scientific, Medtronic and St. Jude Medical for participation in fellows’ educational programs and advisory committee participation.
Funding Support: The Donald W. Reynolds Foundation funded the initial design of the study and patient enrollment. Patient follow-up, data collection and analyses were supported by NIH R01 HL091062 (GFT) and NIH R01 HL103946 (AC).
ABBREVIATIONS AND ACRONYMS
- CKD
chronic kidney disease
- CRT
cardiac resynchronization therapy
- CT
computed tomography
- eGFR
estimated glomerular filtration rate
- ICD
implantable cardioverter-defibrillator
- LVEF
left ventricular ejection fraction
- NYHA
New York Heart Association
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest Disclosures: All other authors have no relevant disclosures to report.
References
- 1.Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 2.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 3.Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med. 1999;341:1882–1890. doi: 10.1056/NEJM199912163412503. [DOI] [PubMed] [Google Scholar]
- 4.Kadish A, Dyer A, Daubert JP, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151–2158. doi: 10.1056/NEJMoa033088. [DOI] [PubMed] [Google Scholar]
- 5.Epstein AE, Dimarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: executive summary. J Am Coll Cardiol. 2008;51:e1–e62. [Google Scholar]
- 6.Naksuk N, Saab A, Li JM, et al. Incidence of appropriate shock in implantable cardioverter-defibrillator patients with improved ejection fraction. J Card Fail. 2013;19:426–430. doi: 10.1016/j.cardfail.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 7.Schaer B, Theuns DA, Sticherling C, Szili-Torok T, Osswald S, Jordaens L. Effect of implantable cardioverter-defibrillator on left ventricular ejection fraction in patients with idiopathic dilated cardiomyopathy. Am J Cardiol. 2010;106:1640–1645. doi: 10.1016/j.amjcard.2010.07.024. [DOI] [PubMed] [Google Scholar]
- 8.Schliamser JE, Kadish AH, Subacius H, et al. Significance of follow-up left ventricular ejection fraction measurements in the Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation trial (DEFINITE) Heart Rhythm. 2013;10:838–846. doi: 10.1016/j.hrthm.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 9.Kini V, Soufi MK, Deo R, et al. Appropriateness of primary prevention implantable cardioverter-defibrillators at the time of generator replacement: are indications still met? J Am Coll Cardiol. 2014;63:2388–2394. doi: 10.1016/j.jacc.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng A, Dalal D, Butcher B, et al. Prospective observational study of implantable cardioverter-defibrillators in primary prevention of sudden cardiac death: study design and cohort description. J Am Heart Assoc. 2013;2:e000083. doi: 10.1161/JAHA.112.000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cintron G, Johnson G, Francis G, Cobb F, Cohn JN. Prognostic significance of serial changes in left ventricular ejection fraction in patients with congestive heart failure. The V-HeFT VA Cooperative Studies Group. Circulation. 1993;87:VI17–VI23. [PubMed] [Google Scholar]
- 12.Konstam MA, Rousseau MF, Kronenberg MW, et al. Effects of the angiotensin converting enzyme inhibitor enalapril on the long-term progression of left ventricular dysfunction in patients with heart failure. SOLVD Investigators. Circulation. 1992;86:431–438. doi: 10.1161/01.cir.86.2.431. [DOI] [PubMed] [Google Scholar]
- 13.Packer M, Colucci WS, Sackner-Bernstein JD, et al. Double-blind, placebo-controlled study of the effects of carvedilol in patients with moderate to severe heart failure. The PRECISE Trial. Prospective Randomized Evaluation of Carvedilol on Symptoms and Exercise. Circulation. 1996;94:2793–2799. doi: 10.1161/01.cir.94.11.2793. [DOI] [PubMed] [Google Scholar]
- 14.St John Sutton MG, Plappert T, Abraham WT, et al. Effect of cardiac resynchronization therapy on left ventricular size and function in chronic heart failure. Circulation. 2003;107:1985–1990. doi: 10.1161/01.CIR.0000065226.24159.E9. [DOI] [PubMed] [Google Scholar]
- 15.Linde C, Abraham WT, Gold MR, St John Sutton M, Ghio S, Daubert C. Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J Am Coll Cardiol. 2008;52:1834–1843. doi: 10.1016/j.jacc.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 16.Uy-Evanado A, Teodorescu C, Reinier K, Chugh H, Mariani R, Gunson K. Do improvements in LV ejection fraction alter risk of sudden cardiac arrest? Heart Rhythm. 2012;9:S89. [Google Scholar]
- 17.Wilcox JE, Fonarow GC, Yancy CW, et al. Factors associated with improvement in ejection fraction in clinical practice among patients with heart failure: findings from IMPROVE HF. Am Heart J. 2012;163:49–56. e2. doi: 10.1016/j.ahj.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Manfredi JA, Al-Khatib SM, Shaw LK, et al. Association between left ventricular ejection fraction post-cardiac resynchronization treatment and subsequent implantable cardioverter defibrillator therapy for sustained ventricular tachyarrhythmias. Circ Arrhythm Electrophysiol. 2013;6:257–264. doi: 10.1161/CIRCEP.112.000214. [DOI] [PubMed] [Google Scholar]
- 19.Binkley PF, Lesinski A, Ferguson JP, et al. Recovery of normal ventricular function in patients with dilated cardiomyopathy: predictors of an increasingly prevalent clinical event. Am Heart J. 2008;155:69–74. doi: 10.1016/j.ahj.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 20.Gula LJ, Klein GJ, Hellkamp AS, et al. Ejection fraction assessment and survival: an analysis of the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Am Heart J. 2008;156:1196–1200. doi: 10.1016/j.ahj.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.